Abstract
This study investigated the effects of the presence of type 2 diabetes mellitus (T2D) and/or chronic liver disease (CLD) on the incidence and prognosis of dementia during a 10-year period in Korea using a nationwide population-based dataset from the Korea National Health Insurance Service.
To assess the impact of T2D and CLD on the incidence of dementia, we included subjects aged ≥60 years without dementia, T2D, and CLD from 2003 to 2005. We created another cohort for evaluating the all-cause mortality in subjects with dementia between 2003 and 2005. The participants were categorized into 4 groups: control (neither CLD nor T2D), CLD-only, T2D-only, and T2D-and-CLD groups, and they were followed up until 2013.
The incidence of dementia was higher in the T2D-only group than in the control and CLD-only groups (2.78 vs. 2.04 and 2.00 per 1000 person-years). After adjustment for age, gender, and comorbid conditions, both T2D and CLD increased the risk of any type of dementia; however, the impact of CLD alone was much lower [hazard ratio (HR) 1.07, 95% confidence interval (CI): 1.06–1.08] than that of T2D alone (HR 1.27, 95% CI: 1.27–1.28). The risk of dementia did not significantly change in patients with the co-occurrence of T2D and CLD compared to those with T2D alone. The all-cause mortality rate was the lowest in the control group (2.59 per 1000 person-years) and the highest in the T2D-and-CLD group (3.77 per 1000 person-years). Presence of T2D or CLD alone was associated with higher mortality (HR 1.46 and HR 1.21, respectively) compared with in the absence of both the diseases. Furthermore, the presence of both the diseases further significantly increased the mortality rate compared to the presence of each disease alone (HR 1.67, 95% CI: 1.65–1.69).
In conclusion, this study found that the incidence of dementia was much higher in patients with T2D. CLD was associated with a modest increase in risk of dementia; however, there was no additive effect with T2D. In the population with dementia, however, the presence of CLD was associated with high mortality in patients with or without T2D.
Keywords: dementia, diabetes mellitus, liver diseases, type 2
1. Introduction
With the progression of population aging, dementia is becoming one of the most rapidly increasing diseases in the world.[1] Korea is one of the countries with the fastest growing elderly populations, the proportion of which is projected to be more than one third of whole population by 2050.[2] Accordingly, the number of patients with dementia in Korea is expected to increase rapidly.[3] Dementia refers to a group of symptoms that include severe memory loss and problems with thinking, solving problems, and using language. Dementia can be generally classified as Alzheimer disease or vascular dementia.[4] Furthermore, since all forms of dementia result on deteriorated quality of life and is related to mortality, it is considered as a serious health care issue.[3]
With the increase in the elderly population, the prevalence of various chronic diseases including type 2 diabetes mellitus (T2D)[5] and chronic liver disease (CLD)[6] are also rising. CLD consists of various liver diseases with recurring damage and recovery of hepatic parenchyma, resulting in fibrosis of liver and eventually leading to liver cancer.[7] Causes of CLD include toxins, viral infections, alcohol, and nonalcoholic fatty liver that increase with obesity.[7]
Many studies have investigated the relationship between diabetes mellitus and dementia.[8,9] T2D has been shown to increase the risk of Alzheimer or vascular dementia by up to 60%.[10] Another study showed poor prognosis for dementia in patients who also suffered from T2D.[11] Even though the studies were inconclusive, type 1 diabetes is also considered to increase the risk of dementia.[12] However, to date, there is a lack of data on the correlation between CLD and incidence or prognosis of dementia; likewise, the simultaneous influence of diabetes and CLD on the incidence or prognosis of dementia remains largely unexplored.
Therefore, in this study, we investigated the effects of the presence of T2D and CLD on the incidence rate of dementia during a 10-year period in Korea using a nationwide population-based dataset. Furthermore, we also studied the impacts of T2D and CLD on the all-cause mortality in patients with dementia.
2. Methods
2.1. Database used in the study
We used the national health information database maintained by National Health Insurance Service (NHIS). NHIS is the national healthcare program of Korea which covers the entire Korean population as a social insurance benefits scheme. The database contains eligibility and demographic information regarding health insurance as well as data on medical aid beneficiaries, medical bill details, medical treatment, disease histories, and prescriptions.[13] We analyzed the information for each individual with an unidentifiable code including age, sex, diagnosis, and prescribed drugs. The personal privacy of each participant was protected by deidentification of the national insurance claims data for analysis. The present study was approved by the NHIS inquiry commission and the institutional review board of the Korean National Institute for Bioethics Policy (P01-201504-21-005). Informed consent was exempted by the board. To validate the database and screen for information accuracy, the expert committee from the Korean Diabetes Association reviewed the database regularly during this analysis. The committee decided the suitability of the dataset and evaluated results of the analysis.
2.2. Study design
2.2.1. Cohort to determine the incidence of dementia
To evaluate the impact of T2D and CLD on the incidence of dementia, we included subjects aged ≥60 years without dementia, T2D, and CLD from 2003 to 2005. We categorized the subjects into 4 groups according to the absence or presence of T2D and/or CLD, namely, the neither-CLD-nor-T2D (control), CLD-only, T2D-only, and T2D-and-CLD groups. Subjects were identified using the International Classification of Diseases (ICD)-10 codes as outlined by the 10th revision of International Statistical Classification of Diseases. T2D was defined if antidiabetic drugs were prescribed at least 2 times and the ICD-10 codes with E11, E12, E13, or E14 was assigned at least 2 times per patient. CLD was defined as ICD-10 codes with B18, K70, K71.3-71.5, K71.7, K72.1, K72.7, K72.9, K73, K74, or K76 at least 2 times. This cohort was followed up until the end of the study period (i.e., December 31, 2013), or until the date of diagnosis with any type of dementia. The follow-up time in person-years was calculated for each study subject until the occurrence of dementia. Dementia was defined if antidementia drugs were prescribed at least 2 times and the codes with Alzheimer disease (ICD-10 F00 or G30), vascular dementia (ICD-10 F01.0, F01.1, F01.2, F01.3, F01.8, or F01.9), or other dementia (ICD-10 F02, F03, G23.1, G31.0, G31.1, G31.82, G31.83, G31.88, or F10.7) were assigned; antidementia drugs, belonging to the 4 classes, included rivastigmine, galantamine, memantine, and donepezil hydrochloride. The detailed diagnosis of dementia was defined as the first prescription of antidementia drugs since diagnosis. When there were both Alzheimer disease and vascular dementia (2 codes each), the final diagnosis was defined as vascular dementia. If there was neither Alzheimer disease nor vascular dementia, the diagnosis was defined as other dementia. Other considered comorbidities at the baseline were hypertension (ICD-10 I10, I11, I12, I13, or I15), dyslipidemia (ICD-10 E78), ischemic heart disease (ICD-10 I20, I21, I22, I23, I24, or I25), cardiac arrhythmia (ICD-10 I44, I45, I46, I47, or I49), heart failure (ICD-10 I50, I110, I130, or I132), peripheral arterial occlusive disease (PAOD) (ICD-10 I70 or I73.9), asthma/chronic obstructive pulmonary disease (COPD) (ICD-10 J40, J41, J42, J43, J44, J45, or J47), chronic kidney disease (CKD) (ICD-10 N18 or N19), and depression (ICD-10 F32 or F33).
2.2.2. Cohort for all-cause mortality
We made another cohort for evaluation all-cause mortality in dementia population. We selected subjects aged ≥60 years recorded to have any type of dementia between 2003 and 2005, and categorized them into 4 groups: neither-CLD-nor-T2D (control), CLD-only, T2D-only, and T2D-and-CLD groups. The definitions of dementia, T2D, and CLD were identical to those in the previous cohort. Information on mortality until 2013 was available for all subjects in this cohort. The cohort was followed up until the end of the study period (December 2013), or until the date of death for patients who died.
2.3. Statistical analysis
Continuous variables were presented as means ± standard deviation and categorical variables were presented as numbers and percentages. To compare characteristics between cohorts, analysis of variance was used for continuous variables and the chi-square test was used for binary and categorical variables. The incidence rates of dementia were calculated by dividing the number of incident cases by the total follow-up period. The incidence rates of dementia and all-cause mortality rates were presented as 1000 person-years. Multivariate Cox regression models were used to evaluate the association between the absence or presence of T2D and/or CLD and the incidence of new-onset dementia and all-cause mortality rate. Model 1 examined the unadjusted hazard ratios (HRs). Model 2 provided the age- and sex-adjusted HR for the incidence of dementia and mortality rate. Model 3 was adjusted for age, sex, classes of national health insurance system, place of residence, and other comorbidities, including hypertension, dyslipidemia, ischemic heart disease, cerebrovascular disease, heart failure, PAOD, COPD, CKD, and depression. The cumulative all-cause mortality according to the presence of T2D and/or CLD was calculated by using the Kaplan-Meier curves; the log-rank test was performed to analyze differences among the groups. The significance level was set at 2-sided P < .05. All statistical analyses were performed using SPSS version 22 and SAS version 9.4 (SAS Institute, Cary, NC).
3. Results
3.1. Baseline characteristics of the study population
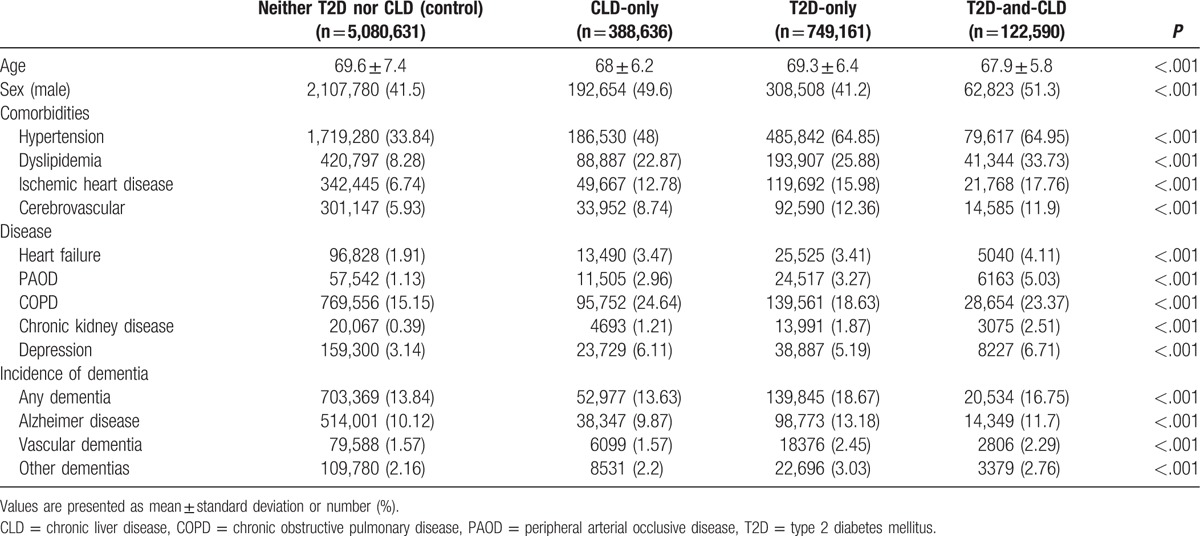
In our nationwide dataset, we identified 388,636 people who were newly diagnosed with CLD and without T2D (designated as the CLD-only group) from 2003 to 2005. Furthermore, there were 749,161 people with newly diagnosed T2D, without CLD (T2D-only group), and 122,590 people with both the diseases (CLD and T2D group). About 5 million were free of both CLD and T2D (categorized as the neither-T2D-nor-CLD or control group).
Baseline characteristics for all groups are shown in Table 1. The subjects with CLD-only and those with both T2D and CLD were slightly younger than the people without CLD. In addition, the comorbidities such as hypertension, dyslipidemia, ischemic heart disease, cerebrovascular disease, heart failure, and CKD were much more prevalent in the T2D-only and T2D-and-CLD groups than in the control or CLD-only groups.
Table 1.
Baseline characteristics of the participants according to the presence of type 2 diabetes mellitus and/or chronic liver disease.
3.2. Incidence of dementia according to the presence of type 2 diabetes or chronic liver disease
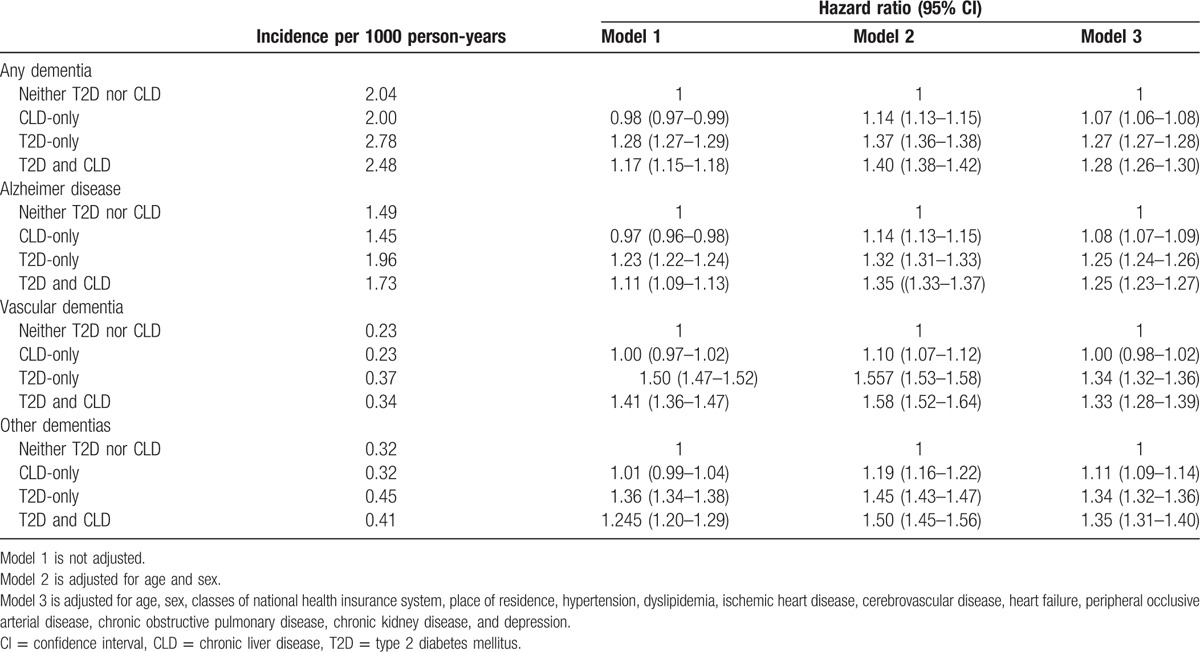
The incidence of dementia was higher in the T2D-only group than in the neither-T2D-nor-CLD group (2.78 vs 2.04 per 1000 person-years) (Table 2). The incidence of any type of dementia was lower in the CLD-only group (2.00 per 1000 person-years) compared to the former 2 groups. This could be due to the fact that subjects in the CLD-only group were significantly younger than the neither-T2D-nor-CLD group (Table 1). Therefore, we further used several statistical models to adjust for important clinical and demographic factors, including, age, gender, the classes of national health insurance system, place of residence, and the comorbid medical conditions such as hypertension, dyslipidemia, ischemic heart disease, stroke, heart failure, PAOD, COPD, CKD, and depression. After adjustment, T2D markedly increased the risk of any type of dementia [odds ratios (ORs) ranging from 1.25–1.34, with all p values < .05) (Table 2). Presence of CLD alone also significantly increased the incidence of any types of dementia, Alzheimer's disease and other types of dementia (ORs ranging from 1.07–1.11, with all p values < .05). However, the incidence of vascular dementia was not different between the CLD-only group and the neither-T2D-nor-CLD-group (OR 1.00, 95% CI: 0.98–1.02). Furthermore, the risk of dementia did not significantly change in patients with the co-occurrence of T2D and CLD compared to those with T2D alone (OR 1.27, 95% CI: 1.27–1.28 vs. OR 1.28, 95% CI: 1.26–1.30).
Table 2.
The influence of type 2 diabetes mellitus and/or chronic liver disease on the incidence of dementia.
3.3. All-cause mortality in patients with dementia based on underlying comorbidities
Next, to investigate the influence of T2D or CLD on the prognosis in patients with dementia, we analyzed the second cohort. During the follow-up period, a total 1,486,818 patients died. The incidence of all-cause mortality was the lowest in the neither-T2D-nor-CLD group (2.59 per 1000 person-years), and the highest in the T2D-and-CLD group (3.77 per 1000 person-years) (Table 3). After adjustment for age, sex, and multiple medical conditions, the presence of T2D or CLD was found to be associated with higher mortality (OR 1.46, 95% CI: 1.45–1.47, and OR 1.21, 95% CI: 1.20–1.22, respectively), and the presence of both diseases significantly increased the mortality rate compared to the neither-T2D-nor-CLD group (OR 1.67, 95% CI: 1.65–1.69) (Table 3; Fig. 1A). Because the impact of CLD could be different according to the specific causes of liver disease, we categorized CLD into 2 types based on the causative factors: alcoholic CLD and nonalcoholic CLD. Alcoholic CLD was defined as ICD-10 codes with K70, and the others were considered as nonalcoholic CLD. As expected, the mortality rate was much higher in the patients with both T2D and alcoholic CLD compared to all other groups during the 7 years of follow-up (Fig. 1B). Furthermore, there was no difference in the mortality rates between the nonalcoholic CLD-only and neither-CLD-nor-T2D groups (Fig. 1B).
Table 3.
The influence of type 2 diabetes mellitus and/or chronic liver disease on the all-cause mortality in patients with dementia.
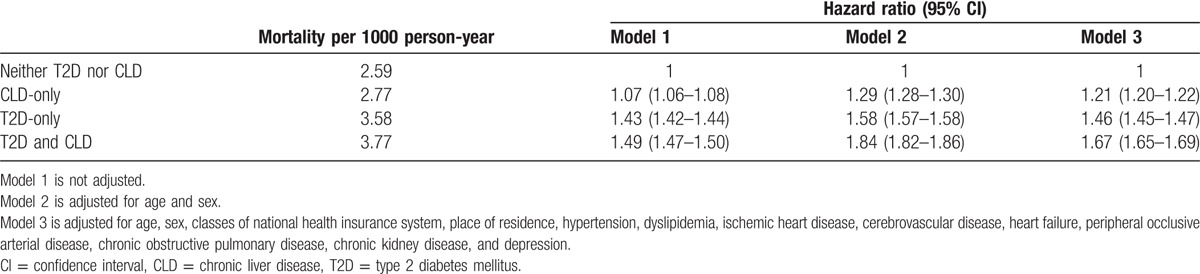
Figure 1.
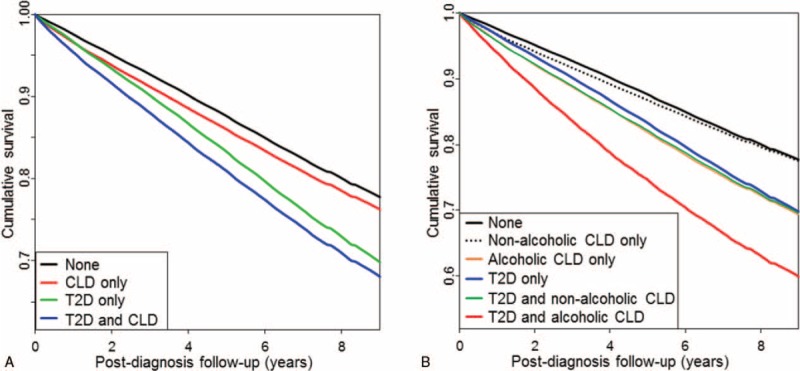
Relative survival of patients after diagnosis with dementia according to the presence of type 2 diabetes and/or chronic liver disease. Kaplan-Meier curves comparing survival in patients with dementia according to the presence of type 2 diabetes mellitus (T2D) and/or chronic liver disease (CLD) (A), and according to the presence of T2D, nonalcoholic CLD, and/or alcoholic CLD (B).
4. Discussion
In the current nationwide population-based, retrospective cohort study, CLD and T2D increased the incidence of dementia; however, the co-occurrence of the 2 did not have an additive effect compared to each disease alone. Among patients with dementia, CLD increased the all-cause mortality regardless of the occurrence of T2D. However, the impact of T2D on the all-cause mortality was much higher than that of CLD.
There have been many studies investigating the relationship between diabetes mellitus and dementia, recently. T2D, in particular, has consistently been shown to be associated with increased risk for mild cognitive impairment,[14] vascular dementia, and Alzheimer disease.[15,16] Recent meta-analysis shows that individuals with T2D are at about 60% greater risk for the development of dementia compared with those without T2D.[10] Although there are several reports on the association between type 1 diabetes and cognitive dysfunction,[9,17] this association is yet to be robustly assessed by the larger population studies. A retrospective nationwide cohort study from England showed that type 1 diabetes mellitus was associated with an elevated risk of any type dementia, Alzheimer disease, and vascular dementia.[12] Also, an association between diabetic microvascular complication, such as diabetic retinopathy, and cognitive impairment has been reported.[18,19] While the exact mechanisms underlying the association between diabetes mellitus or its complication and dementia are unclear, numerous mechanisms have been suggested for this association: brain vascular lesions,[20] insulin resistance,[21] advanced glycation end products,[22] and systemic inflammation.[23] In addition, several demographic and socioeconomic factors such as aging, education, other comorbidities such as depression, and genetic factors could also be involved.[24] This present study, however, only includes T2D, and the relative risk was approximately 1.25 to 1.34 for any dementia or each type of dementia tested. This rate was comparable with those previously reported in Korea.[25]
It remains possible that the association between CLD and dementia; however, there have been not enough data about that association between 2 disease categories. Among the various causes for CLD, the association between alcoholic liver disease and cognitive function or risk of dementia has been most frequently studied. One study revealed that patients with alcoholic liver cirrhosis had a higher risk for impaired cognitive function than in patients with liver cirrhosis from viral causes.[26] Also, patients with end-stage liver disease requiring liver transplantation showed significant cognitive impairments, and the patients with a history of alcohol abuse or dependence were found to have worse cognitive dysfunction.[27] However, controversy still remains about the relationship between alcohol consumption and cognitive impairment or dementia.[28] Viral hepatitis, one of major causes of CLD, could also be associated with brain or cognitive function. Hepatitis C viral infection, in particular, was more frequently related with cognitive impairment.[29] In addition, although primary biliary cirrhosis is a rare cause for CLD, cognitive impairment was prevalent in patients with primary biliary cirrhosis independent of the severity of liver disease.[30]
Very recently, several studies have been published which investigated the epidemiological relationship between nonalcoholic fatty liver disease (NAFLD) and cognitive function,[31] and the underlying mechanisms of NAFLD on the development of Alzheimer disease.[32] NAFLD independently associates with lower cognitive performance, independent of cardiovascular disease and its risk factors, in 4472 adults from general US population[31]. Whether CLD increases the risk of dementia is still controversial, and the pathophysiology of the relationship between CLD and cognitive impairments remains unknown. Among the several possible mechanisms, systemic inflammatory process is one of the explanations. Inflammation might be one of the major causes of dementia in patients with CLD.[33] Furthermore, various spectrums of CLD such as viral hepatitis, alcohol consumption, and expanded liver fat are also associated with increased systemic inflammation.[34–36] Therefore, it is plausible that chronic inflammation induced outside the brain could lead to the cognitive dysfunction and development of dementia.[32] Another underlying mechanism could be the NAFLD-induced insulin resistance which might contribute to the association between NAFLD and cognitive impairment or Alzheimer disease.[37,38] In our study, the presence of CLD increased the risk of dementia slightly compared to the control group; however, its co-occurrence with T2D did not have any additive effect. In the latter scenario, it is possible that the impact of CLD was masked since the influence of T2D alone on the risk of various types of dementia was much bigger than in patients with CLD alone. In addition, we did not perform subgroup analysis of the impact of the specific causes of CLD on the risk of dementia. Therefore, it is plausible that various spectrums of CLD might have contributed to the weakening of the effects observed in this study.
At present, dementia is an important cause of mortality in elderly population.[39] Many factors such as age, sex, education, ethnicity, severity of disease, and functional impairment have been reported as predictive of mortality in patients with dementia.[40] Furthermore, comorbidity is common in the older population and there have been several studies about the relationship between various comorbidities and mortality. In a multiethnic, population-based longitudinal study of patients with Alzheimer disease, the presence of hypertension or T2D was associated with increased mortality rate compared to control groups.[41] Furthermore, dementia onset occurred earlier and the mortality was greater in patients with T2D compared to the patients without T2D especially in younger population; the impact of T2D on the risk and prognosis of dementia became attenuated in the elderly group.[11] On the contrary, there has been no study investigating the influence of liver disease on the mortality in patients with dementia. In our study, the presence of only CLD markedly increased all-cause mortality compared to the control (with neither CLD nor T2D) group (HR 1.21, 95% CI: 1.20–1.22). Furthermore, co-occurrence of T2D and CLD, showed additive effect on the prognosis of dementia compared to those with T2D or CLD alone (HR 1.67, 95% CI: 1.65–1.69). We hypothesize that enhanced systemic inflammation and the liver-specific mortality in CLD population might have contributed to this additive effect; however, further studies are warranted to corroborate this possibility.
The present study has several limitations. First, this was a retrospective analysis, and the findings should be interpreted accordingly. Second, the diagnosis of T2D, CLD, and dementia was based solely on the diagnostic codes and prescription for drugs; thus, there could have been some biases or errors. Third, we could not categorize the specific causes of CLD, for example, chronic viral hepatitis, alcoholic liver disease, or NAFLD. In light of the controversy about the causal relationship between alcohol consumption and cognitive function, the use of a detailed classification of CLD might have provided greater insights on its influence on the incidence or prognosis of dementia in patients with or without T2D. Furthermore, we did not include patients with type 1 diabetes in our study. Lastly, our analyzed solely focused on the all-cause mortality, and not on the comprehensive understanding of disease-specific or dementia-related mortality.
However, our study also has a number of strengths. First, as far as we are aware, this is the first attempt to evaluate the influence of CLD and T2D on the incidence and prognosis of dementia. Second, this study used a large, national sample with a relatively long-term follow-up period; furthermore, this study accessed the national registry data with information about mortality. Lastly, we analyzed 2 different cohorts for evaluating the influence of CLD and/or T2D on the incidence of dementia and the prognosis of dementia, respectively.
In conclusion, this study found that CLD was associated with a modest increase in the risk of dementia; however, there was no additive effect with T2D on the incidence of dementia. In addition, the presence of CLD was associated with increased mortality in patients with dementia, with or without T2D. Further studies are warranted to evaluate the relationship between the specific causes of CLD, that is, NAFLD and/or alcoholic liver disease, on the risk of dementia and its prognosis.
Footnotes
Abbreviations: CKD = chronic kidney disease, CLD = chronic liver disease, COPD = chronic obstructive pulmonary disease, HR = hazard ratio, ICD = International Classification of Diseases, NAFLD = nonalcoholic fatty liver disease, NHIS = National Health Insurance Service, OR = odds ratio, PAOD = peripheral arterial occlusive disease, SD = standard deviation, T2D = type 2 diabetes mellitus.
HMK and YHL contributed equally to this study.
The authors report no conflicts of interest.
References
- [1].Prince M, Ali GC, Guerchet M, et al. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther 2016;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Beard JR, Officer AM, Cassels AK. The world report on ageing and health. Gerontologist 2016;56(suppl 2):S163–6. [DOI] [PubMed] [Google Scholar]
- [3].Park JH, Eum JH, Bold B, et al. Burden of disease due to dementia in the elderly population of Korea: present and future. BMC Public Health 2013;13:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Prince M, Bryce R, Albanese E, et al. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013;9:63–75. e62. [DOI] [PubMed] [Google Scholar]
- [5].Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care 2012;35:2650–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Premoli A, Paschetta E, Hvalryg M, et al. Characteristics of liver diseases in the elderly: a review. Minerva Gastroenterol Dietol 2009;55:71–8. [PubMed] [Google Scholar]
- [7].Frith J, Jones D, Newton JL. Chronic liver disease in an ageing population. Age Ageing 2009;38:11–8. [DOI] [PubMed] [Google Scholar]
- [8].Biessels GJ, Staekenborg S, Brunner E, et al. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006;5:64–74. [DOI] [PubMed] [Google Scholar]
- [9].Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes–systematic overview of prospective observational studies. Diabetologia 2005;48:2460–9. [DOI] [PubMed] [Google Scholar]
- [10].Chatterjee S, Peters SA, Woodward M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016;39:300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zilkens RR, Davis WA, Spilsbury K, et al. Earlier age of dementia onset and shorter survival times in dementia patients with diabetes. Am J Epidemiol 2013;177:1246–54. [DOI] [PubMed] [Google Scholar]
- [12].Smolina K, Wotton CJ, Goldacre MJ. Risk of dementia in patients hospitalised with type 1 and type 2 diabetes in England, 1998–2011: a retrospective national record linkage cohort study. Diabetologia 2015;58:942–50. [DOI] [PubMed] [Google Scholar]
- [13].Lee YH, Han K, Ko SH, et al. Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Data analytic process of a nationwide population-based study using national health information database established by national health insurance service. Diabetes Metab J 2016;40:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Luchsinger JA, Reitz C, Patel B, et al. Relation of diabetes to mild cognitive impairment. Arch Neurol 2007;64:570–5. [DOI] [PubMed] [Google Scholar]
- [15].Peila R, Rodriguez BL, Launer LJ, et al. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia aging study. Diabetes 2002;51:1256–62. [DOI] [PubMed] [Google Scholar]
- [16].Ahtiluoto S, Polvikoski T, Peltonen M, et al. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology 2010;75:1195–202. [DOI] [PubMed] [Google Scholar]
- [17].Brands AM, Biessels GJ, de Haan EH, et al. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care 2005;28:726–35. [DOI] [PubMed] [Google Scholar]
- [18].Hugenschmidt CE, Lovato JF, Ambrosius WT, et al. The cross-sectional and longitudinal associations of diabetic retinopathy with cognitive function and brain MRI findings: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 2014;37:3244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Barzilay JI, Lovato JF, Murray AM, et al. Albuminuria and cognitive decline in people with diabetes and normal renal function. Clin J Am Soc Nephrol 2013;8:1907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2672–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol 2004;3:169–78. [DOI] [PubMed] [Google Scholar]
- [22].Munch G, Schinzel R, Loske C, et al. Alzheimer's disease—synergistic effects of glucose deficit, oxidative stress and advanced glycation endproducts. J Neural Transm (Vienna) 1998;105:439–61. [DOI] [PubMed] [Google Scholar]
- [23].Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 2015;16:358–72. [DOI] [PubMed] [Google Scholar]
- [24].Ninomiya T. Diabetes mellitus and dementia. Curr Diab Rep 2014;14:487. [DOI] [PubMed] [Google Scholar]
- [25].Kimm H, Lee PH, Shin YJ, et al. Mid-life and late-life vascular risk factors and dementia in Korean men and women. Arch Gerontol Geriatr 2011;52:e117–22. [DOI] [PubMed] [Google Scholar]
- [26].Lee Y, Kim C, Suk KT, et al. Differences in cognitive function between patients with viral and alcoholic compensated liver cirrhosis. Metab Brain Dis 2016;31:369–76. [DOI] [PubMed] [Google Scholar]
- [27].Sorrell JH, Zolnikov BJ, Sharma A, et al. Cognitive impairment in people diagnosed with end-stage liver disease evaluated for liver transplantation. Psychiatry Clin Neurosci 2006;60:174–81. [DOI] [PubMed] [Google Scholar]
- [28].Panza F, Frisardi V, Seripa D, et al. Alcohol consumption in mild cognitive impairment and dementia: harmful or neuroprotective? Int J Geriatr Psychiatry 2012;27:1218–38. [DOI] [PubMed] [Google Scholar]
- [29].Weissenborn K, Tryc AB, Heeren M, et al. Hepatitis C virus infection and the brain. Metab Brain Dis 2009;24:197–210. [DOI] [PubMed] [Google Scholar]
- [30].Newton JL, Hollingsworth KG, Taylor R, et al. Cognitive impairment in primary biliary cirrhosis: symptom impact and potential etiology. Hepatology 2008;48:541–9. [DOI] [PubMed] [Google Scholar]
- [31].Seo SW, Gottesman RF, Clark JM, et al. Nonalcoholic fatty liver disease is associated with cognitive function in adults. Neurology 2016;86:1136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kim DG, Krenz A, Toussaint LE, et al. Non-alcoholic fatty liver disease induces signs of Alzheimer's disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model. J Neuroinflammation 2016;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Takeda S, Sato N, Morishita R. Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: relevance to pathogenesis and therapy. Front Aging Neurosci 2014;6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Villanova N, Moscatiello S, Ramilli S, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology 2005;42:473–80. [DOI] [PubMed] [Google Scholar]
- [35].Zampino R, Marrone A, Restivo L, et al. Chronic HCV infection and inflammation: clinical impact on hepatic and extra-hepatic manifestations. World J Hepatol 2013;5:528–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Imhof A, Froehlich M, Brenner H, et al. Effect of alcohol consumption on systemic markers of inflammation. Lancet 2001;357:763–7. [DOI] [PubMed] [Google Scholar]
- [37].Craft S. Insulin resistance and Alzheimer's disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res 2007;4:147–52. [DOI] [PubMed] [Google Scholar]
- [38].Tong M, Neusner A, Longato L, et al. Nitrosamine exposure causes insulin resistance diseases: relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and Alzheimer's disease. J Alzheimers Dis 2009;17:827–44. [PMC free article] [PubMed] [Google Scholar]
- [39].Guehne U, Riedel-Heller S, Angermeyer MC. Mortality in dementia. Neuroepidemiology 2005;25:153–62. [DOI] [PubMed] [Google Scholar]
- [40].Todd S, Barr S, Roberts M, et al. Survival in dementia and predictors of mortality: a review. Int J Geriatr Psychiatry 2013;28:1109–24. [DOI] [PubMed] [Google Scholar]
- [41].Helzner EP, Scarmeas N, Cosentino S, et al. Survival in Alzheimer disease: a multiethnic, population-based study of incident cases. Neurology 2008;71:1489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]


