Abstract
Background:
Emergence agitation (EA) occurs frequently after nasal surgery. N-methyl-d-aspartate (NMDA) receptor antagonists and analgesics, such as fentanyl, have been shown to prevent EA. Nefopam inhibits the NMDA receptor and shows a potent analgesic effect. We investigated the effects of nefopam on EA in patients undergoing nasal surgery.
Methods:
In this prospective, double-blind study, 100 adult patients were allocated randomly to 1 of 2 groups (each n = 50). Patients received 20 mg of nefopam in 98 mL of saline for 20 minutes immediately after induction of anesthesia (nefopam group) or 100 mL of saline (control group) in the same manner. After surgery, the incidence and degree of EA, time for extubation, hemodynamic parameters, and adverse events were evaluated by an observer blinded to the group allocation.
Results:
The overall incidence of EA was lower in the nefopam group than in the control group (34% [17/50] vs 54% [27/50], respectively; P = .044). The incidence of severe EA was also lower in the nefopam group than in the control group (8% [4/50] vs 38% [19/50], respectively; P = .001). Heart rate (HR) was higher in the nefopam group than in the control group from the end of surgery to 3 minutes after extubation (P = .008). Time for extubation and adverse events were similar between groups.
Conclusions:
Nefopam infusion is effective in preventing and reducing the severity of EA after nasal surgery without a delay in extubation. However, caution is required regarding the increase in HR.
Keywords: nasal surgical procedures, nefopam, N-methyl-D-aspartate, psychomotor agitation, receptors
1. Introduction
General anesthesia for ear, nose, and throat (ENT) surgery has frequently been associated with emergence agitation (EA).[1] In particular, patients undergoing nasal surgery commonly complain of a sense of suffocation due to intranasal packing and manifest agitation during emergence.[2–4] EA can cause serious problems, such as reoperation due to bleeding of the surgical site, falling out of bed, injury to the patient or medical staff, and unplanned endotracheal tube extubation.[5] Several pharmacological methods, including opioid (fentanyl, remifentanil), propofol, benzodiazepine (midazolam), α2-aderenoreceptor agonist (clonidine, dexmedetomidine), and N-methyl-d-aspartate (NMDA) receptor antagonist (ketamine, magnesium sulfate) administration, have been introduced to mitigate EA.[3,6,7] However, opioids and propofol can induce respiratory depression and delayed recovery.[8–10] Conflicting results have been reported regarding the effects of benzodiazepine and dexmedetomidine on EA.[3,11,12] An NMDA receptor antagonist, ketamine, effectively reduced the incidence of EA in children, but caused delayed recovery.[6] Infusion of another NMDA receptor antagonist, magnesium sulfate, also reduced the incidence of EA in children undergoing sevoflurane anesthesia without neuromuscular blocking agent,[13] but intraoperative magnesium sulfate infusion can increase the risk of residual neuromuscular block or recurarization in patients receiving general anesthesia with nondepolarizing muscle relaxant.[14]
Although EA can occur after minimal or nonpainful surgeries, pain is a major risk factor of EA.[1,2,11,15] The centrally acting, non-narcotic analgesic drug, nefopam, has anti-NMDA properties in common with ketamine and magnesium sulfate and can be useful in treating neuropathic, and also nociceptive pain. Furthermore, nefopam has opioid-sparing, antishivering, antidepressant, and anticonvulsant effects, without causing depression of the central nervous and respiratory systems.[16]
We postulated that nefopam would probably reduce the postanesthetic EA response. Therefore, we designed this study to assess the hypothesis that nefopam infusion after induction of anesthesia would reduce EA in adult patients undergoing nasal surgery.
2. Materials and methods
After receiving approval from the Institutional Review Board of Konyang University Hospital, Daejeon, Korea (KYUH 2015-10-018-001), we obtained written informed consent from all patients before the study. This prospective, double-blind, randomized controlled study was registered at the Korea Clinical Research Information Service (http://cris.nih.go.kr; permit number, KCT 0002215). Inclusion criteria were age >19 years, American Society of Anesthesiologists physical status I to II, and patients scheduled for elective nasal surgery under general anesthesia. Exclusion criteria were known adverse response to nefopam, history of convulsive disorders, tachyarrhythmia, coronary artery disease, glaucoma, dysuria, prostatitis, and cognitive dysfunction.
Patients were allocated randomly (allocation ratio 1:1), using sealed envelopes indicating the allocation, to receive 20 mg/2 mL of nefopam (Acupan; Pharmbio Korea, Seoul, Korea) mixed with 98 mL of normal saline (nefopam group) or 100 mL of normal saline alone (control group).
All patients arrived at the operating room without premedication. Routine monitoring, including pulse oximetry, noninvasive automated blood pressure monitoring, electrocardiography, and bispectral index (BIS), was started.
Before induction of anesthesia, the study drugs (nefopam 20 mg/2 mL in 98 mL of normal saline for the nefopam group or 100 mL of normal saline for the control group) were prepared by an anesthetic nurse who was blinded to the group allocation.
Anesthesia was induced with intravenous propofol (2 mg/kg) and end-tidal concentration of 5% to 7% desflurane. Endotracheal intubation was facilitated by rocuronium bromide (0.6 mg/kg). Anesthesia was maintained with nitrous oxide:oxygen (1:1) and desflurane. Controlled mechanical ventilation and end-tidal concentration of desflurane were adjusted to maintain an end-tidal carbon dioxide concentration of 30 to 35 mm Hg and BIS 40 to 60 during surgery, respectively. Immediately after induction of anesthesia, the study drugs were infused intravenously and administered for 20 minutes. Study drug infusion and anesthesia management during surgery were conducted by an anesthesiologist who was blinded to both the patient's group allocation and prepared study drugs. Various types of nasal surgery were performed after induction of anesthesia, ending with intranasal packing using Guardcel (Genewel Co., Seongnam, Korea). After completion of nasal packing, desflurane and nitrous oxide were turned off, and manual ventilation was performed with 100% oxygen at 6 L/min. Reversal of neuromuscular block was performed with pyridostigmine (0.2 mg/kg) and glycopyrrolate (0.01 mg/kg). Extubation was performed after confirming the response to verbal stimulation, spontaneous respiration (tidal volume >5 mg/kg, respiratory rate >12/min), and BIS >75. The durations of anesthesia and surgery, and time to extubation (time from turning off desflurane to extubation) were recorded.
Emergence was defined as the time interval from turning off desflurane to 3 minutes after extubation. EA was evaluated using the Richmond Agitation-Sedation Scale (RASS: +4, combative; +3, very agitated; +2, agitated; +1, restless; 0, alert and calm; −1, drowsy; −2, light sedation; −3, moderate sedation; −4, deep sedation; −5, unarousable),[17] and each patient's highest score during emergence was recorded. EA was defined as any RASS score ≥+2, with severe EA defined as RASS ≥+3. Cough during emergence was assessed on a 4-point scale (0 = no cough; 1 = single cough; 2 = more than 1 episode of unsustained [≤5 seconds] coughing, grade 3; sustained [>5 seconds] bouts of coughing).[18]
After arrival at the postanesthesia care unit (PACU), residual sedation (RASS ≤−2) and postoperative pain using numerical rating scale (NRS) scores (0 = no pain; 10 = worst pain imaginable) were assessed continuously. Fentanyl (1 μg/kg) was injected intravenously when the NRS score was >5, or on a patient's request for analgesics in the PACU.
During the time from induction of anesthesia to discharge from the PACU, persistent (>3 minutes) hypotension (fall in mean blood pressure [MBP] >30% of preanesthetic MBP or MBP <60 mm Hg) and hypertension (increase in MBP >30% of preanesthetic MBP) were treated with intravenous ephedrine and nicardipine in increments of 5 and 0.5 mg, respectively. Similarly, sustained (>3 minutes) bradycardia (heart rate [HR] <45 beats/min) and tachycardia (HR >120 beats/min) were treated with intravenous atropine and esmolol in increments of 0.5 and 10 mg, respectively.
Mean blood pressure and HR were recorded before induction of anesthesia (baseline), at the end of surgery, immediately after extubation, and 3 minutes after extubation. All adverse events were assessed before discharge from the PACU. If patients complained of nausea and required antiemetics in the PACU, they were given 10 mg of metoclopramide intravenously.
2.1. Statistical analysis
The primary outcome of this study was the incidence of EA. Based on a previous study that reported a 55.4% EA incidence rate after ENT surgery under general anesthesia,[1] we used power analysis (α = 0.05 [2-sided], power = 0.80) to calculate a sample size of 49 patients in each group required to detect a 50% reduction in the incidence of EA. Therefore, considering possible dropouts, we enrolled 50 patients per group.
Statistical analysis was performed using SPSS (ver. 18.0 for Windows; SPSS Inc., Chicago, IL). The normal distribution of continuous variables was assessed with the Kolmogorov–Smirnov test. Continuous variables were analyzed using Student t test or the Mann–Whitney U test, when appropriate. Categorical variables were compared using the chi-square test or Fisher exact test, as appropriate. RASS score and grade of cough were analyzed using the chi-square test for trend (linear-by-linear association). MBP and HR were evaluated using repeated-measures analysis of variance, followed by the t test with Bonferroni correction. All values are presented as number of patients (%), mean ± standard deviation (SD), or median (range). In all analyses, P < .05 was taken to indicate statistical significance.
3. Results
In all, 103 patients were assessed for eligibility, and 3 were excluded; 1 patient showed tachyarrhythmia and 2 patients declined to participate in the study. Thus, 100 patients were randomly allocated into the control or nefopam group and completed this study (Fig. 1).
Figure 1.
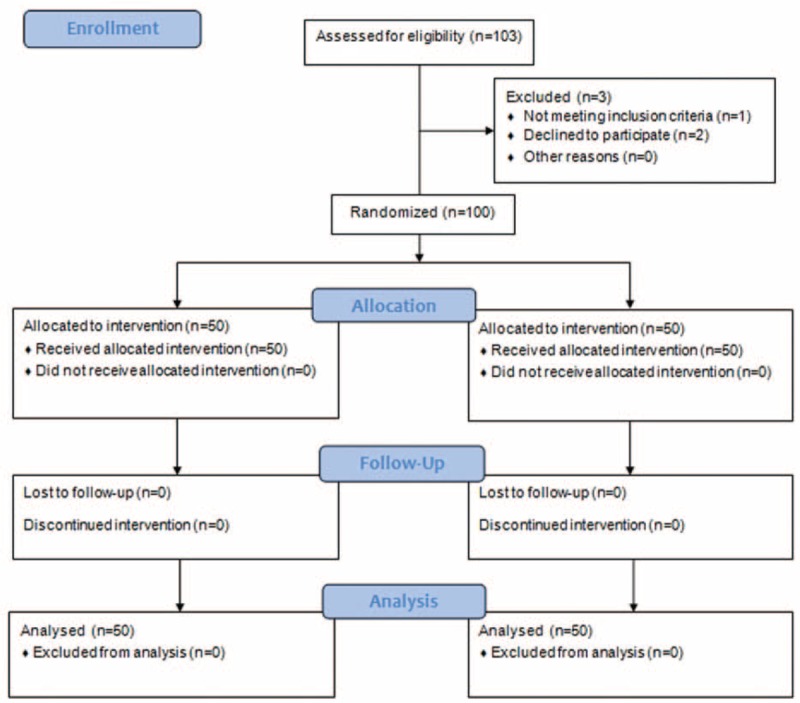
Flow chart.
Patient characteristics and types of surgery were similar between the 2 groups (Table 1).
Table 1.
Demographic and operative data.

The overall incidence of EA in the nefopam group was lower than in the control group (34% [17/50] vs 54% [27/50], respectively; relative risk 0.66; 95% confidence interval (CI) 0.4–1.0, P = .044). The incidence of severe EA was significantly lower in the nefopam group compared with the control group (8% [4/50] vs 38% [19/50], respectively; relative risk 0.29, 95% CI 0.1–0.7, P = .001) (Fig. 2). RASS score was significantly different between the 2 groups (P = .002) (Table 2).
Figure 2.

Incidence rates of overall and severe emergence agitation. Emergence was defined as the period from turning off desflurane to 3 minutes after extubation. EA and severe EA were defined on the Richmond Agitation-Sedation Scale as ≥+2 and ≥+3 during emergence, respectively. EA = emergence agitation.
Table 2.
Recovery profile.
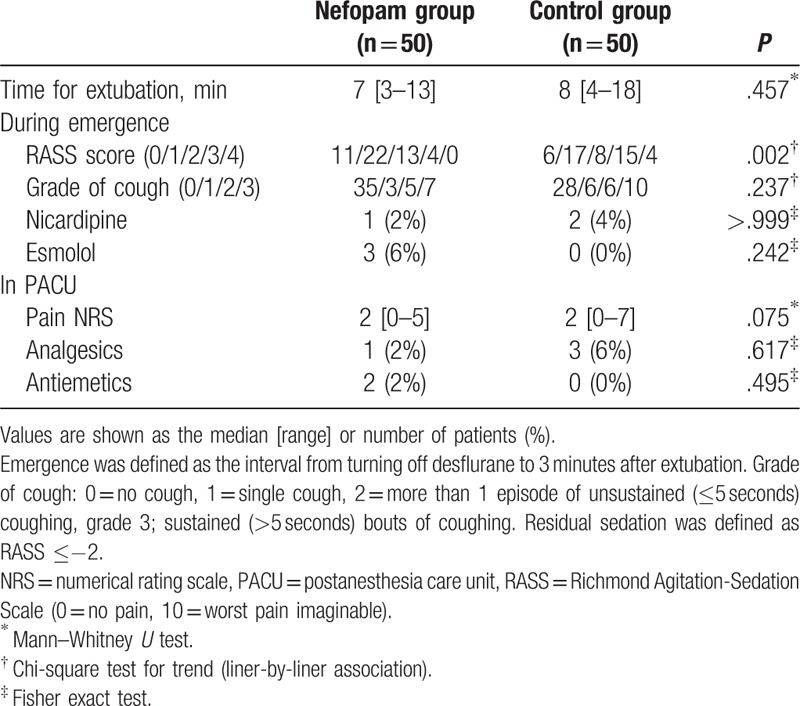
Changes in HR were significantly different between groups (P = .008). HR during emergence was significantly higher in the nefopam group than in the control group (P < .05, Bonferroni-corrected), whereas the changes in MBP values were comparable in both groups (Fig. 3). Other operative data and recovery profiles are presented in Table 2 and did not show intergroup differences.
Figure 3.
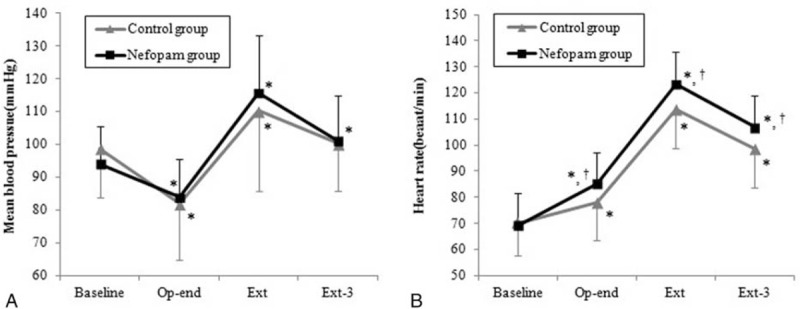
Mean blood pressure and heart rate changes during emergence. Data are presented as means ± standard deviation. ∗P < .05, versus baseline in each group (Bonferroni-corrected). †P < .05, versus control group (Bonferroni-corrected). Baseline = before anesthesia induction, Ext = at extubation, Ext-3 = 3 minutes after extubation, Op-end = end of surgery.
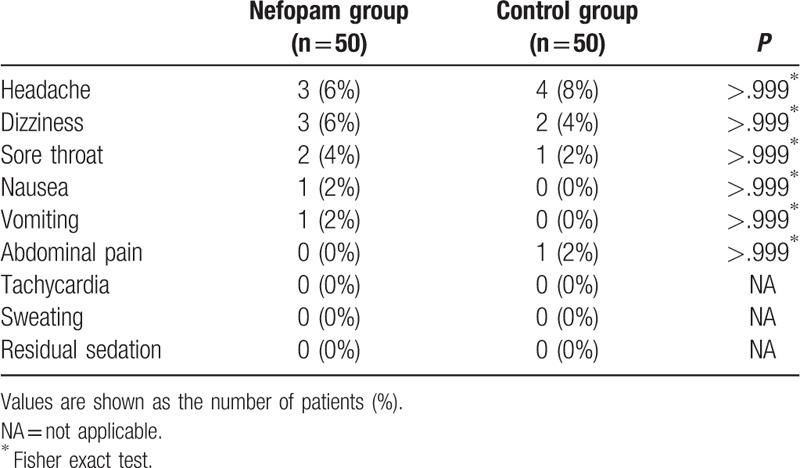
There were no significant differences in adverse events in the PACU between the 2 groups (Table 3).
Table 3.
Adverse events in postanesthesia care unit.
4. Discussion
This study demonstrated that nefopam infusion after induction of anesthesia effectively reduced the incidence and severity of EA in patients undergoing nasal surgery without delay of extubation time. However, during emergence, HR was increased to a greater extent in the nefopam group than in the control group, although esmolol requirements were comparable between the 2 groups.
Previous studies using nefopam focused mainly on its analgesic efficacy or opioid-sparing effect.[15,19–21] To our knowledge, no study has evaluated the preventive efficacy of nefopam on EA to date. Through this study, we identified the efficacy of nefopam in preventing EA.
Despite its high incidence rate after general anesthesia, the precise etiopathogenic mechanism of EA has yet to be elucidated. A possible explanation is that NMDA postsynaptic potential-induced excitatory hyperactivity at the thalamolateral nucleus of the amygdala synapse enhances the uncomfortable stimuli-induced behavior.[22]
The presence of an endotracheal tube, male sex, inhalation anesthetics, age (<40 and >64 years), postoperative pain (NRS ≥5), benzodiazepine premedication, administration of doxapram, recent smoking, history of social drinking, type (oral cavity and ENT surgery) and duration of surgery, chronic lung disease, voiding urgency, and the presence of a urinary catheter and/or gastric tube are known to be etiological risk factors of EA in adults,[1,2,5,11,15] although there is controversy regarding study design (prospective or retrospective), type of surgery, and assessment parameter of EA (eg, RASS, Riker sedation-agitation score, Aono scale). In previous studies,[1,2,5] the presence of an endotracheal tube was shown to be 1 of the most important risk factors for EA. In addition, the incidence of EA was higher when assessed in the operating room during extubation than in the PACU after extubation.[5] Most previous studies regarding EA in pediatric[6,9,13,23–25] and adult patients[1,2,12,15] involved evaluation at the PACU. However, this study showed that administration of nefopam prevents EA during endotracheal tube extubation in the operation room where the risk of EA is relatively high. Although EA can lead to serious problems, it resolves spontaneously as the patient recovers consciousness. Thus, prevention of EA is more important than medication therapy after its occurrence.
The pathophysiological mechanism underlying the reduction in incidence of EA by nefopam is uncertain, but may be explained as follows. Nefopam has a potent analgesic effect by inhibiting serotonin and noradrenaline reuptake, and by modulating glutaminergic transmission via inhibition of postsynaptic NMDA receptors.[26] Consistent with our results, the administration of NMDA receptor antagonists, such as ketamine and magnesium sulfate, showed a reduction in incidence of EA compared with placebo controls.[6,13] However, despite the direct activation of NMDA receptors by remifentanil,[27] infusion of remifentanil resulted in smooth awakening from anesthesia and was also effective in decreasing the incidence of EA.[4,25] These findings suggest that potent analgesic activity may be more important in preventing EA than NMDA receptor inhibition. Previous studies support this suggestion. Adequate postoperative pain control using potent analgesics, such as fentanyl or remifentanil, showed efficacy in reducing EA,[1,4] but weak analgesics and nonsteroidal anti-inflammatory drugs (such as ketorolac) alone did not reduce EA after anesthesia.[24] Notwithstanding the EA-preventive effect of nefopam infusion during emergence, pain score and analgesic requirements in the PACU were similar between the 2 groups in the present study. This may have been due to the low pain score in the control group (median value of NRS 2) and the target sample size calculation, which was performed to detect a difference in the incidence of EA, but not pain score in the PACU. Similar to our results, in a previous study,[3] dexmedetomidine infusion decreased the incidence of EA compared with the placebo group after nasal surgery, but showed no difference in pain NRS or need for analgesics in the PACU.
Apart from its analgesic effect, nefopam has anticonvulsant properties, although convulsions and seizures can occur when administered at an excessive dose.[16] The anticonvulsant properties of nefopam may contribute to the reduction of EA. Abdulatif et al[13] postulated that the anticonvulsant potential of magnesium sulfate could prevent EA by impacting the inhalation anesthetic-induced seizure activity. Furthermore, antidepressant and antishivering effects of nefopam may contribute to reducing EA, but the specific roles of these effects in reduction of EA are still not clear. Further studies are required to elucidate the pathophysiology of nefopam on EA.
The timing and methods of administration (bolus or continuous infusion), and also dosage, are important to gain the maximum expected effect while reducing drug-related side effects. In a previous study performed in a pediatric population, a bolus injection of propofol at induction of anesthesia was not protective, whereas continuous infusion with a bolus injection of propofol at the end of surgery was effective at preventing EA.[7] Moreover, a bolus of 0.03 mg/kg midazolam before the end of surgery had similar efficacy in preventing EA while reducing the emergence time compared with a dose of 0.05 mg/kg.[25] Intravenous nefopam at a dose of 20 mg has an analgesic efficacy equipotent to 6 to 12 mg of morphine.[20] The onset of action, peak effect, and duration of action of nefopam are 15 to 30 minutes, 30 to 60 minutes, and 4 to 6 hours, respectively.[19] Bolus intravenous injection or rapid infusion and use during the postoperative period have been shown to increase the rate of adverse events.[16,19] In this study, a dose of 20 mg slowly infused over 20 minutes immediately after induction of anesthesia was used, taking into consideration the pharmacological properties of nefopam and anticipated duration of surgery.
Patients receiving nefopam may manifest anticholinergic or sympathomimetic effects, such as tachycardia, sweating, hypertension, dilated pupils, voiding difficulty, dry mouth, nausea, vomiting, hallucinations, confusion, and seizure, as adverse drug reactions.[28] Among these, signs of overdose present as neuropsychiatric symptoms and cardiac conduction abnormalities, including tachycardia.[28] In the present study, although HR was higher in the nefopam group than in the control group during emergence, we do not believe that it was induced by nefopam overdose or rapid infusion, because there were no cases of tachycardia in the PACU in the nefopam group. Instead, we propose that this was a transient phenomenon caused by the elevated sympathomimetic potential of nefopam due to rapid awakening from anesthesia. If increased HR resulted from overdose, tachycardia should have occurred in the PACU when considering the duration of anesthesia and duration of action of nefopam. In addition, despite the slow infusion of 20 mg of nefopam for 30 minutes in the intensive care unit (ICU), HR was increased by >15% compared with the baseline in 29% of patients in a previous study.[19] On the contrary, before induction of anesthesia, 40 mg of nefopam infusion for 20 minutes did not lead to tachycardia within 24 hours postoperatively.[21] However, caution is required regarding administration of nefopam in patients with limited coronary reserve, such as coronary artery disease, because of its potential to increase HR. If a patient is not contraindicated for nefopam, slowly infusing 20 mg nefopam for more than 20 minutes after induction of anesthesia may be appropriate for preventing EA and reducing nefopam-related side effects in a patient who is at risk for EA.
This study had some limitations. First, differences in the sex ratio, age, type of surgery, and duration of surgery between the groups may have influenced the outcome of this study,[2,11,15] despite the lack of statistically significant differences in these factors between the groups. In addition, we did not assess potential factors contributing to the incidence of EA,[2,15] such as preoperative anxiety, history of smoking or drinking alcohol, or presence of chronic lung disease before patient allocation. However, these factors should have had little influence on our results because patients were allocated at random, and so their influence would be equivalent in both groups. Second, although the validity and interobserver reliability of RASS for assessing agitation-sedation in adult ICU patients have been confirmed,[17] its validity is uncertain for assessing EA in the operating room during awakening from general anesthesia. There is still no gold standard for EA assessment in adult patients during emergence. However, unlike previous studies that assessed EA in the PACU, our study excluded the effects of rescue analgesics or antiemetics on EA because we evaluated the incidence and severity of agitation during emergence in the operation room.
5. Conclusions
In conclusion, infusion of 20 mg of nefopam over 20 minutes immediately after induction of anesthesia is effective at reducing the incidence and severity of EA in patients undergoing nasal surgery without a delay in time for extubation. However, caution is required regarding the potential risk of tachycardia, especially in vulnerable patients.
Footnotes
Abbreviations: BIS = bispectral index, EA = emergence agitation, ENT = ear, nose, and throat, HR = heart rate, ICU = intensive care unit, MBP = mean blood pressure, NMDA = N-methyl-D-aspartate, NRS = numerical rating scale, PACU = postanesthesia care unit, RASS = Richmond Agitation-Sedation Scale.
Presentation: This report was presented, in part, at the 94th Annual Scientific Meeting of the Korean Society of Anesthesiologists, November 2 to 4, 2017, The-K Hotel, Seoul, Korea.
This study was registered at the Clinical Research Information Service, Republic of Korea (http://cris.nih.go.kr; permit number, KCT 0002215).
The authors report no conflicts of interest.
References
- [1].Yu D, Chai W, Sun X, et al. Emergence agitation in adults: risk factors in 2,000 patients. Can J Anaesth 2010;57:843–8. [DOI] [PubMed] [Google Scholar]
- [2].Kim HJ, Kim DK, Kim HY, et al. Risk factors of emergence agitation in adults undergoing general anesthesia for nasal surgery. Clin Exp Otorhinolaryngol 2015;8:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim SY, Kim JM, Lee JH, et al. Efficacy of intraoperative dexmedetomidine infusion on emergence agitation and quality of recovery after nasal surgery. Br J Anaesth 2013;111:222–8. [DOI] [PubMed] [Google Scholar]
- [4].Polat R, Peker K, Baran I, et al. Comparison between dexmedetomidine and remifentanil infusion in emergence agitation during recovery after nasal surgery: a randomized double-blind trial. Anaesthesist 2015;64:740–6. [DOI] [PubMed] [Google Scholar]
- [5].Munk L, Andersen G, Moller AM. Post-anaesthetic emergence delirium in adults: incidence, predictors and consequences. Acta Anaesthesiol Scand 2016;60:1059–66. [DOI] [PubMed] [Google Scholar]
- [6].Chen JY, Jia JE, Liu TJ, et al. Comparison of the effects of dexmedetomidine, ketamine, and placebo on emergence agitation after strabismus surgery in children. Can J Anesth 2013;60:385–92. [DOI] [PubMed] [Google Scholar]
- [7].Dahmani S, Stany I, Brasher C, et al. Pharmacological prevention of sevoflurane- and desflurane-related emergence agitation in children: a meta-analysis of published studies. Br J Anaesth 2010;104:216–23. [DOI] [PubMed] [Google Scholar]
- [8].Chang CH, Lee JW, Choi JR, et al. Effect-site concentration of remifentanil to prevent cough after laryngomicrosurgery. Laryngoscope 2013;123:3105–9. [DOI] [PubMed] [Google Scholar]
- [9].Aouad MT, Yazbeck-Karam VG, Nasr VG, et al. A single dose of propofol at the end of surgery for the prevention of emergence agitation in children undergoing strabismus surgery during sevoflurane anesthesia. Anesthesiology 2007;107:733–8. [DOI] [PubMed] [Google Scholar]
- [10].van Hoff SL, O’Neill ES, Cohen LC, et al. Does a prophylactic dose of propofol reduce emergence agitation in children receiving anesthesia? A systematic review and meta-analysis. Pediatr Anesth 2015;25:668–76. [DOI] [PubMed] [Google Scholar]
- [11].Rim JC, Kim JA, Hong JI, et al. Risk factors of emergence agitation after general anesthesia in adult patients. Anesth Pain Med 2016;11:410–6. [Google Scholar]
- [12].Ham SY, Kim JE, Park C, et al. Dexmedetomidine does not reduce emergence agitation in adults following orthognathic surgery. Acta Anaesthesiol Scand 2014;58:955–60. [DOI] [PubMed] [Google Scholar]
- [13].Abdulatif M, Ahmed A, Mukhtar A, et al. The effect of magnesium sulphate infusion on the incidence and severity of emergence agitation in children undergoing adenotonsillectomy using sevoflurane anaesthesia. Anaesthesia 2013;68:1045–52. [DOI] [PubMed] [Google Scholar]
- [14].Czarnetzki C, Lysakowski C, Elia N, et al. Time course of rocuronium-induced neuromuscular block after pre-treatment with magnesium sulphate: a randomized study. Acta Anaesthesiol Scand 2010;54:299–306. [DOI] [PubMed] [Google Scholar]
- [15].Kim HC, Kim E, Jeon YT, et al. Postanaesthetic emergence agitation in adult patients after general anaesthesia for urological surgery. J Int Med Res 2015;43:226–35. [DOI] [PubMed] [Google Scholar]
- [16].Kim KH, Abdi S. Rediscovery of nefopam for the treatment of neuropathic pain. Korean J Pain 2014;27:103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA 2003;289:2983–91. [DOI] [PubMed] [Google Scholar]
- [18].Minogue SC, Ralph J, Lampa MJ. Laryngotracheal topicalization with lidocaine before intubation decreases the incidence of coughing on emergence from general anesthesia. Anesth Analg 2004;99:1253–7. [DOI] [PubMed] [Google Scholar]
- [19].Chanques G, Sebbane M, Constantin JM, et al. Analgesic efficacy and haemodynamic effects of nefopam in critically ill patients. Br J Anaesth 2011;106:336–43. [DOI] [PubMed] [Google Scholar]
- [20].Mok MS, Lippmann M, Steen SN. Comparison of intravenous nefopam versus morphine for the relief of postoperative pain. Clin Pharmacol Ther 1979;25:237–8. [Google Scholar]
- [21].Yoo JY, Lim BG, Kim H, et al. The analgesic effect of nefopam combined with low dose remifentanil in patients undergoing middle ear surgery under desflurane anesthesia: a randomized controlled trial. Korean J Anesthesiol 2015;68:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McLott J, Jurecic J, Hemphill L, et al. Development of an amygdalocentric neurocircuitry-reactive aggression theoretical model of emergence delirium in posttraumatic stress disorder: an integrative literature review. AANA J 2013;81:379–84. [PubMed] [Google Scholar]
- [23].Na HS, Song IA, Hwang JW, et al. Emergence agitation in children undergoing adenotonsillectomy: a comparison of sevoflurane vs. sevoflurane-remifentanil administration. Acta Anaesthesiol Scand 2013;57:100–5. [DOI] [PubMed] [Google Scholar]
- [24].Kim D, Doo AR, Lim H, et al. Effect of ketorolac on the prevention of emergence agitation in children after sevoflurane anesthesia. Korean J Anesthesiol 2013;64:240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cho EJ, Yoon SZ, Cho JE, et al. Comparison of the effects of 0.03 and 0.05 mg/kg midazolam with placebo on prevention of emergence agitation in children having strabismus surgery. Anesthesiology 2014;120:1354–61. [DOI] [PubMed] [Google Scholar]
- [26].Novelli A, Diaz-Trelles R, Groppetti A, et al. Nefopam inhibits calcium influx, cGMP formation, and NMDA receptor dependent neurotoxicity following activation of voltage sensitive calcium channels. Amino Acids 2005;28:183–91. [DOI] [PubMed] [Google Scholar]
- [27].Hahnenkamp K, Nollet J, Van Aken HK, et al. Remifentanil directly activates human N-methyl-d-aspartate receptors expressed in Xenopus laevis oocytes. Anesthesiology 2004;100:1531–7. [DOI] [PubMed] [Google Scholar]
- [28].Durrieu G, Olivier P, Bagheri H, et al. Overview of adverse reactions to nefopam: an analysis of the French Pharmacovigilance database. Fundam Clin Pharmacol 2007;21:555–8. [DOI] [PubMed] [Google Scholar]


