Abstract
The aim of the study is to investigate the association between olfactory dysfunction (OD), hearing loss, and dysphonia.
The cross-sectional data for 17,984 adults who completed the Korea National Health and Nutrition Examination Surveys (2010−12) were analyzed. OD, hearing loss, and dysphonia were assessed using self-reporting questionnaires. The association of OD with hearing loss and dysphonia was evaluated.
Hearing loss and dysphonia were significantly more prevalent in patients with OD than in those without OD (hearing loss, 28.1% vs 11.3%; dysphonia, 11.1% vs 5.9%; both P < .0001). After adjusting for confounders, including mental stress and metabolic syndrome, the risk of OD was significantly associated with hearing loss and dysphonia, and was greater in those with combined hearing loss and dysphonia than in both patients without these dysfunctions and in those with a single dysfunction (odds ratio 3.115, 95% confidence interval 1.973–4.917).
OD was significantly associated with hearing loss and dysphonia.
Keywords: hearing, olfaction, surveys and questionnaires, voice
1. Introduction
Olfactory dysfunction (OD), defined as an impaired perception of smell, affects about one-fifth of the general population. The main causes of olfactory loss are post-viral upper respiratory infection, sinonasal disease, and head trauma, which are frequently encountered in patients visiting otorhinolaryngology clinics.[1] The prevalence of subjective OD in the Korean population was reported to be 5.0% in 2010–11, and another study reported that 9% to 14% of self-rating patients in Asia had OD.[2,3] Impairment of the sense of smell leads to disturbances in important areas of everyday life, such as enjoyment of food and detection of harmful foods and smoke, and to some extent affects functioning in the social and work settings.[1] Several reports have described an association between OD and depressive disorder/anxiety-like psychiatric problems.[4,5] Given the high prevalence of OD and the growing concern about mental health issues, OD is regarded as a potentially serious public health problem.
Mild but progressive olfactory impairment, hearing loss, and weakening of the voice commonly occur with aging and are often encountered by otolaryngologists.[6] These age-related changes may go unnoticed for some time, but have a serious cumulative effect on a person's ability to communicate as well as confidence and independence, and may trigger mental health problems, such as depression. The components of the metabolic syndrome (MetS), such as high glucose and triglyceride levels and hypertension, have been reported to be associated with OD. Previously, we reported an epidemiologic association between MetS and OD.[7]
We hypothesized that OD is associated with hearing loss and dysphonia (difficulties in speaking arising from physical disorders of the mouth or vocal cords), and that these dysfunctions would be associated with mental health problems or MetS. A previous nationwide study focused on the association between a dysfunction and its risk factors; however, there have been no reports on the combined association of OD, hearing loss, and dysphonia in the Korean population. The aims of this study were to assess this association using the Fifth Korea National Health and Nutrition Examination Survey (KNHANES) in 2010–2012 and to investigate its impact on mental health and MetS.
2. Materials and methods
2.1. Ethics statement
The study protocol was approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (approval numbers 2010-02CON-21-C, 2011-02CON-06-C, and 2012-01EXP-01-2C). The participants provided written informed consent at baseline.
2.2. Study population
The study was based on data collected during the 2010–12 KNHANES. The KNHANES is a nationwide survey that has been conducted by the Division of Chronic Disease Surveillance under the Korea Centers for Disease Control and Prevention since 1998, and is designed to accurately assess national health and nutrition levels. The survey team consisted of an otolaryngologist and nurse examiners, who performed interviews related to OD, hearing loss, and dysphonia. The KNHANES methodology has been described in detail elsewhere.[8–10] The study sample included 17,984 subjects ≥19 years old; we focused on this population because OD becomes progressively worse in adults beyond this age.[11]
2.3. Survey of OD, hearing loss, and dysphonia
OD, hearing loss, and dysphonia were assessed using self-reporting questionnaires. The olfactory questionnaire inquired whether the participants had had problems with their sense of smell during the previous 3 months. Participants with positive or negative responses were considered hyposmic or normosmic, respectively. The hearing loss questionnaire inquired whether the participants had experienced hearing problems, and their responses were categorized into 4 grades: no discomfort (HL0), mild discomfort (HL1), severe discomfort (HL2), and deaf (HL3). The dysphonia questionnaire inquired whether the participants had vocal problems, and the responses were categorized into 3 grades: no vocal problem (DP0), vocal problems lasting <3 weeks (DP1), and vocal problems lasting >3 weeks (DP2).
2.4. Demographic characteristics and lifestyle habits
Data on medical history, demographic characteristics (age, sex), and lifestyle habits were collected using self-reporting questionnaires. Patients were categorized according to smoking history as current smokers, ex-smokers, or nonsmokers. Participants who drank >30 g alcohol per day were deemed to be heavy drinkers. Regular exercise was defined as strenuous physical activity performed for at least 20 minutes at a time, at least 3 times/wk. Job was defined as an employed state, and participants who had life partners or were married were designated as having a spouse. Residence was categorized as urban or rural according to the official address of the subject. The category of “low income” corresponded to the lowest quartile of annual household income. The education level of the subject was classified as high if the participant had completed education beyond university. Physical and mental health status included levels of perceived stress (“light or none” or “some or heavy”), the experience of feeling depressed for at least 2 weeks (“yes” or “no”), and suicidal ideation in the past 12 months (“yes” or “no”).
2.5. Anthropometric and laboratory measurements
Weight, waist circumference, and height were measured by well-trained medical professionals. Body mass index (BMI) was calculated as weight (kg)/height (m2). Obesity was defined as a BMI ≥25 kg/m2, as recommended by the International Obesity Task Force (IOTF) and the World Health Organization (WHO) Regional Office for the Western Pacific Region for Asian individuals.[12] Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in the right arm. To assess serum biochemical marker levels, a blood sample was obtained from the antecubital vein of each participant after a 10 to 12-h overnight fast. Serum levels of fasting blood glucose, total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol were measured using an enzymatic method (Hitachi Automatic Analyzer 7600, Hitachi, Tokyo, Japan).
2.6. Definition of MetS
MetS was defined according to the criteria proposed by the American Heart Association and the National Heart, Lung, and Blood Institute together with the International Diabetes Federation in 2009.[13] MetS was diagnosed if a participant had at least 3 of the following: waist circumference ≥90 cm in men and ≥80 cm in women, according to the International Diabetes Federation criteria for Asian countries; fasting blood glucose ≥100 mg/dL or use of medication for elevated glucose; fasting triglycerides ≥150 mg/dL or use of cholesterol-lowering medication; HDL cholesterol <40 mg/dL in men and <50 mg/dL in women or use of cholesterol-lowering medication; and SBP ≥130 mm Hg and/or DBP ≥85 mm Hg or use of antihypertensive drugs in patients with a history of hypertension.
2.7. Statistical analysis
The statistical analyses were performed using Statistical Analysis Software (SAS) version 9.3 (SAS Institute, Cary, NC). To calculate unbiased national estimates representing the general Korean population, we used KNHANES sample weights to adjust for the unequal probability of selection. For the complex sampling design, we used cluster adjustment and stratification with the SAS PROC SURVEY module, as recommended in a reference article.[8] Continuous and categorical variables were described using means (and standard errors) and proportions (and standard errors), respectively. For the differences in general characteristics between subjects with and without OD, continuous variables were evaluated using the t test in the SURVEYREG procedure, while categorical variables were tested with the Rao-Scott chi-square test in the SURVEYFREQ procedure. Using multiple logistic regression analysis in the SURVEYLOGISTIC procedure, we tested the association of OD with hearing loss and dysphonia after adjustment for confounding factors. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated after adjustment for potential confounders. We first adjusted for age and sex (model 1), and then for the variables in model 1 plus smoking status, alcohol intake, regular exercise, household income, and education level (model 2), and then for model 2 plus BMI, MetS, diabetes, hypertension, and mental stress (model 3). All P values were 2-tailed, and the level of significance was set at P < .05, including for multivariate analysis and interaction analysis. The prevalence rates of MetS and mental health problems (severe stress, depressed mood, and suicidal ideation) were calculated according to the presence or absence of OD, hearing loss, and dysphonia.
3. Results
3.1. General characteristics of the study population
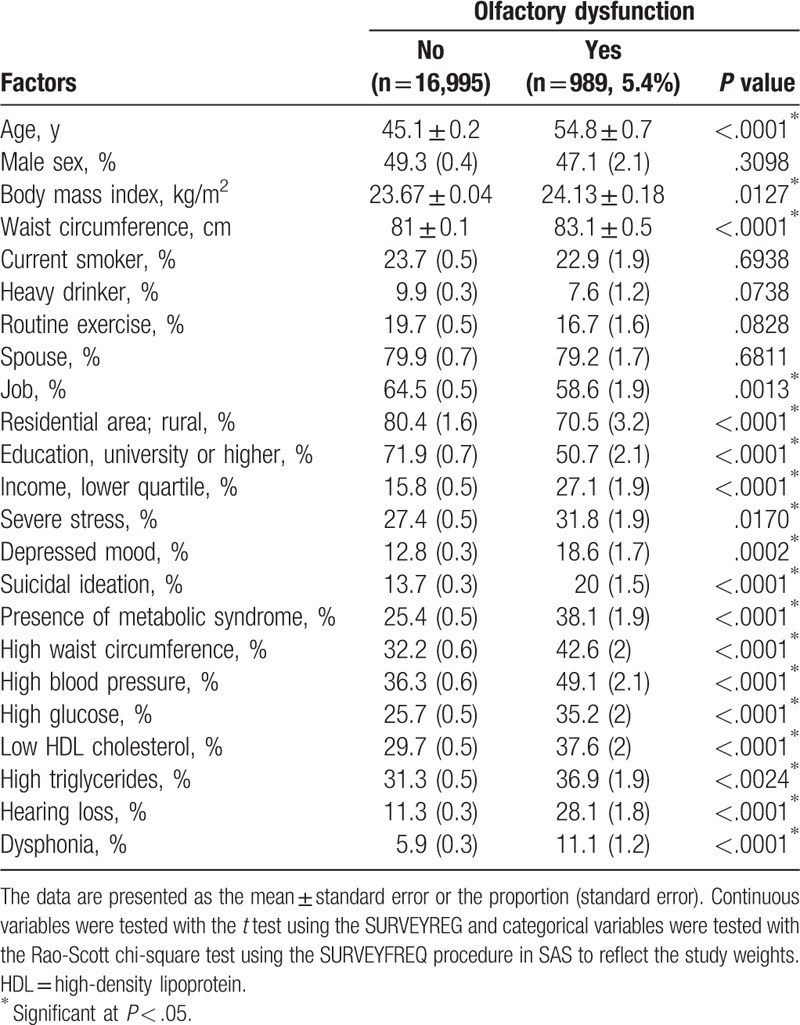
Nine hundred eighty-nine (5.4%) of the 17,984 study participants had experienced OD (men, 47.1%; women, 52.9%). The baseline characteristics of the study participants according to whether or not they had OD are shown in Table 1. Age, waist circumference, BMI, job, residential area, education level, household income, severe stress, depressed mood, suicidal ideation, MetS, hearing loss, and dysphonia were significantly associated with OD. The prevalence of hearing loss was significantly higher in subjects with OD than in subjects without OD (28.1% vs 11.3%, P < .001). The prevalence of dysphonia was also significantly higher in subjects with OD than in those without OD (11.1% vs 5.9%, P < .001).
Table 1.
Analysis of factors potentially associated with olfactory dysfunction (n = 17,984).
3.2. Association of OD, hearing loss, and dysphonia with metabolic syndrome and mental health problems
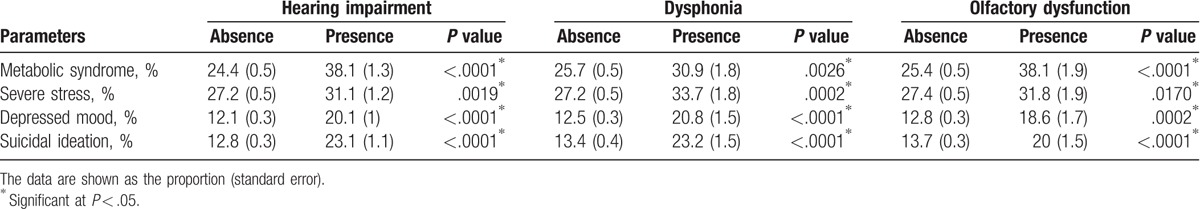
Previously, we reported on the relationships among OD, MetS, and mental health problems.[1] To assess the interrelationships between these health disorders and the causes for these relationships, the data for the components of MetS and mental health problems were evaluated in the group with hearing loss, dysphonia, and OD. MetS, severe stress, depressed mood, and suicidal ideation were significantly associated with hearing loss, dysphonia, and OD (Table 2).
Table 2.
Prevalence of metabolic syndrome and mental health problems according to the presence of hearing loss, dysphonia, and olfactory dysfunction.
3.3. Multivariate analyses of the association of OD with hearing loss and dysphonia
Table 3 shows the association of hearing loss and dysphonia with OD, after adjusting for confounders. To avoid issues with multicollinearity, we included “stress” as one of the confounders. Stress was selected from the 3 mental health symptoms of severe stress, depressed mood, and suicidal ideation listed in Table 2. Depressed mood and suicidal ideation were excluded because these symptoms usually show overlap in the clinical setting. There was a significant association of OD with all grades of hearing loss and dysphonia. The risk of OD increased with the grade of hearing loss (OR 3.372, 95% CI 1.167–9.744 for the deaf group, model 3). Dysphonia lasting <3 weeks showed a stronger association with OD than dysphonia lasting 3 weeks or longer (OR 1.761, 95% CI 1.177–2.634 for dysphonia lasting ≥3 weeks, model 3) after adjustment for confounders.
Table 3.
Logistic regression models of hearing loss, dysphonia, and combined dysfunctions for olfactory dysfunction.
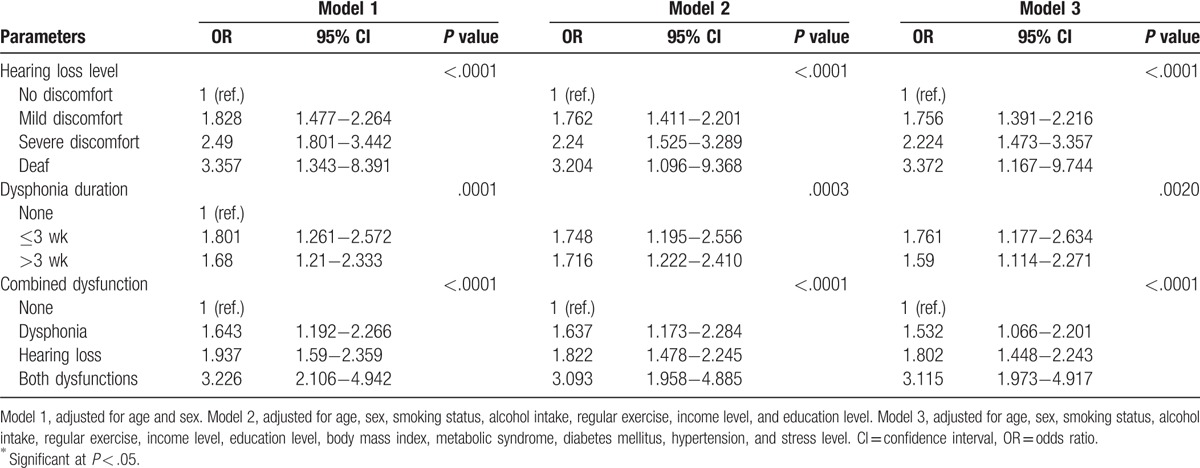
We also assessed the impact of a combination of hearing loss and dysphonia on the risk of OD. Subjects with either or both of these dysfunctions had a higher risk of OD than those with neither dysfunction (OR 3.115, 95% CI 1.973–4.917 for a combination of hearing loss and dysphonia, model 3).
To assess the influence of a combination of hearing loss and dysphonia on the risk of OD in relation to age and sex, model 3 was stratified by these factors and reanalyzed (Fig. 1). The ORs showed a greater increase in subjects with both hearing loss and dysphonia (3.96 in the younger age group [20−65 years], 2.29 in the older age group [≥65 years], 1.941 in men, and 3.812 in women) than the OR in the reference group. However, the differences were not statistically significant between the stratified groups. The P value for interaction was .14 when stratified by age and .14 when stratified by sex.
Figure 1.
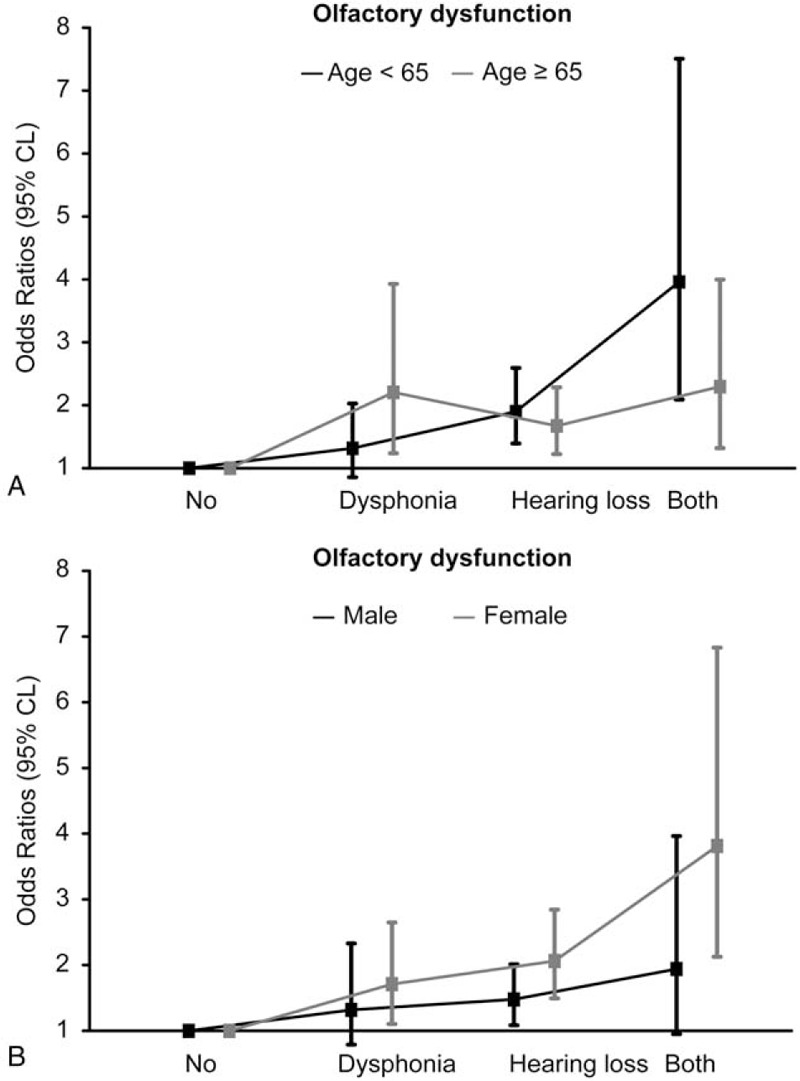
Odds ratios for olfactory dysfunction alone or combined with hearing loss and dysphonia and stratified by age (A) and sex (B).
4. Discussion
In this study, we sought to determine whether hearing loss and dysphonia were associated with OD in the adult Korean population. When combined, these dysfunctions can aggravate mental health problems because of their functional importance for communication. Asian countries, including Korea, have undergone rapid westernization and economic development during the past 2 decades. In such rapidly changing environment, mental health and lifestyle diseases, such as MetS, are of increasing clinical concern. Relationships between OD and comorbid depressive and anxiety disorders have already been described in several reports.[4,5] Previously, we have reported an epidemiologic association between MetS and OD.[7] Therefore, we conducted this study to determine the association of hearing loss and dysphonia with OD and to assess their association with mental problems and MetS. In addition, our sample was taken from a nationally representative sample of the general Korean adult population. To estimate the association of hearing loss and dysphonia with OD, we generated 3 models that considered potential confounders, including demographic characteristics, socioeconomic status, and comorbid diseases. A statistically significant relationship remained even after adjustment for these potential confounding factors. We also performed a subgroup analysis of OD according to age and sex and found a nonsignificant difference between the subgroups and an association of OD with hearing loss and dysphonia in that these 2 dysfunctions in combination showed a stronger association with OD than either or neither of these dysfunctions.
There have been several studies of these 3 common otolaryngologic complaints and their relationship with mental health problems. Collins studied the combined degenerative changes that occur in the ear, nose, and throat with aging.[6] Age-related hearing loss is common and results in impaired understanding of normal speech. Voice production is dependent on the lung, larynx, and resonant cavity. Forced expiratory volume and vocal fold vibration decreases as the body ages, leading to presbyphonia. Olfactory cells in the nose are stimulated by odors, and a large proportion of elderly people (over the age of 60 years) lose their olfactory function. Olfactory disorders impair quality of life and can trigger depression because of decreased enjoyment of food and reduced social opportunities because of the implications of loss of sense of smell in terms of personal hygiene. Moreover, there is reduced input from the olfactory bulb via the amygdala to the limbic circuit.[1] A significant association of hyposmia and poor psychosocial outcome has been reported in patients who have suffered a severe traumatic brain injury.[14] Mild and progressive sensory impairments are common, and have a cumulative detrimental effect on the ability to communicate as well as self-confidence and independence. These detrimental effects can result in mental health problems, which were often associated with the otolaryngologic dysfunctions investigated in our study. For deaf people, communication for emotional and practical support is the key to maintaining mental health.[15] From the standpoint of vocal dysfunction, psychosocial conditions, socioeconomic status, and quality of life can be diminished, especially in individuals who overuse their voice, such as teachers.[16]
There have been several reports on the association of MetS with hearing loss, dysphonia, and OD, and we have previously demonstrated an association between MetS and OD.[7] The peripheral and central neuronal pathways related to olfaction are affected by various circulating molecules, such as leptin, insulin, and glucose, all of which are associated with nutritional status. Other potential causes of the relationship between OD and MetS are cognitive problems induced by obesity or dyslipidemia, diabetes-induced microvascular complications, and dietary salt-induced hypertension. Food choices are changing in response to the changes in economic and nutritional status in Korea, and lifestyle diseases such as MetS are becoming a public health concern. There have also been several reports on the association of hearing loss with MetS. A significant association between the presence of a greater number of MetS components and a reduced hearing threshold has been reported.[17] Basic research on degeneration of the stria vascularis in guinea pigs fed a lipid-rich diet suggests an association between dyslipidemia and reduced hearing.[18,19] However, research on the relationship between voice dysfunction and MetS is scarce. We have reported a significant association between MetS and chronic laryngitis in Korean women,[20] and gastroesophageal reflux disease is known to be common in patients with obesity and chronic laryngitis. Patients with MetS would be expected to develop chronic laryngitis and gastroesophageal reflux disease in association with dysphonia.
In our study, hearing loss and dysphonia were correlated with OD after adjustment for several characteristics, including age, which has previously been identified as an influential factor. Even after adjustment for comorbid mental health impairment (ie, stress) and MetS, the association of hearing loss and dysphonia with OD remained statistically significant. Thus, our results support the association of these 2 impairments with OD. Moreover, the risk of OD increased in the presence of hearing loss and dysphonia but without a significant interaction of age and sex. Although further study is needed, we hypothesize that the possible cause of this relationship involves comorbid impairment of communication related to hearing loss and dysphonia, which may have a detrimental psychosocial effect, affecting memory and worsening OD.[21] Another possible common factor is infection, which can cause OD, hearing loss, and laryngitis.
Our study has several limitations. Its cross-sectional nature meant that temporal relationships between the 3 conditions could not be determined. Our findings are only associational. However, the results of the study may be generalizable in view of the nationwide population-based nature of the research. The study also contained an element of recall bias, because the dysfunctions were self-reported and not diagnosed objectively, such as by pure tone audiometry, multidimensional voice program analysis, and the Sniffin Sticks Test.
This nationwide population-based study indicates that OD is associated with hearing loss and dysphonia, even after adjusting for potential confounders. All 3 otolaryngologic impairments were associated with poor mental health and MetS. The results of this population-based study on the prevalence of OD and its association with hearing loss and dysphonia lay the foundations for prevention and management of these conditions in the Korean population.
Footnotes
Abbreviations: BMI = body mass index, CIs = confidence intervals, DBP = diastolic blood pressure, DP0 = no vocal problem, DP1 = vocal problems lasting <3 weeks, DP2 = vocal problems lasting >3 weeks, HDL = high-density lipoprotein, HL1 = no hearing loss, HL2 = mild discomfort, HL3 = severe discomfort, HL4 = deaf, IOTF = International Obesity Task Force, KNHANES = Korean National Health and Nutrition Examination Survey, LDL = low-density lipoprotein, MetS = metabolic syndrome, ORs = odds ratios, SBP = systolic blood pressure, SE = standard error/proportion, WHO = World Health Organization.
JHP and HKB contributed equally to this study
This work was supported by the Soonchunhyang University Research Fund.
The authors have no conflicts of interest to disclose.
References
- [1].Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life—an updated review. Chem Senses 2014;39:185–94. [DOI] [PubMed] [Google Scholar]
- [2].Joo YH, Hwang SH, Han KD, et al. Relationship between olfactory dysfunction and suicidal ideation: The Korea National Health and Nutrition Examination Survey. Am J Rhinol Allergy 2015;29:268–72. [DOI] [PubMed] [Google Scholar]
- [3].Gaines AD. Anosmia and hyposmia. Allergy Asthma Proc 2010;31:185–9. [DOI] [PubMed] [Google Scholar]
- [4].Kohli P, Soler ZM, Nguyen SA, et al. The association between olfaction and depression: a systematic review. Chem Senses 2016;41:479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Burón E, Bulbena A. Olfaction in affective and anxiety disorders: a review of the literature. Psychopathology 2013;46:63–74. [DOI] [PubMed] [Google Scholar]
- [6].Collins J. Letter from the editor: “ageENTs of change”. Semin Roentgenol 2013;48:1–2. [DOI] [PubMed] [Google Scholar]
- [7].Hwang SH, Kang JM, Seo JH, et al. Gender difference in the epidemiological association between metabolic syndrome and olfactory dysfunction: The Korea National Health and Nutrition Examination Survey. PLoS One 2016;11:e0148813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim Y, Park S, Kim NS, et al. Inappropriate survey design analysis of the Korean National Health and Nutrition Examination Survey may produce biased results. J Prev Med Public Health 2013;46:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Park HA. The Korea national health and nutrition examination survey as a primary data source. Korean J Fam Med 2013;34:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Park SH, Lee KS, Park HY. Dietary carbohydrate intake is associated with cardiovascular disease risk in Korean: analysis of the third Korea National Health and Nutrition Examination Survey (KNHANES III). Int J Cardiol 2010;139:234–40. [DOI] [PubMed] [Google Scholar]
- [11].Lee WH, Wee JH, Kim DK, et al. Prevalence of subjective olfactory dysfunction and its risk factors: Korean National Health and Nutrition Examination Survey. PLoS One 2013;8:e62725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Oh SW, Shin SA, Yun YH, et al. Cut-off point of BMI and obesity-related comorbidities and mortality in middle-aged Koreans. Obes Res 2004;12:2031–40. [DOI] [PubMed] [Google Scholar]
- [13].Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–5. [DOI] [PubMed] [Google Scholar]
- [14].Osborne-Crowley K, McDonald S. Hyposmia, not emotion perception, is associated with psychosocial outcome after severe traumatic brain injury. Neuropsychology 2016;30:820–9. [DOI] [PubMed] [Google Scholar]
- [15].Du Feu M, Fergusson K. Sensory impairment and mental health. Adv Psych Treatment 2003;9:95–103. [Google Scholar]
- [16].Bermúdez de Alvear R, Martínez-Arquero G. Teachers’ voice disorders collateral effects. Otorynolaryngologia 2009;8:129–35. [Google Scholar]
- [17].Sun YS, Fang WH, Kao TW, et al. Components of metabolic syndrome as risk factors for hearing threshold shifts. PLoS One 2015;10:e0134388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Saito T, Sato K, Saito H. An experimental study of auditory dysfunction associated with hyperlipoproteinemia. Arch Otorhinolaryngol 1986;243:242–5. [DOI] [PubMed] [Google Scholar]
- [19].Satar B, Ozkaptan Y, Sürücü HS, et al. Ultrastructural effects of hypercholesterolemia on the cochlea. Otol Neurotol 2001;22:786–9. [DOI] [PubMed] [Google Scholar]
- [20].Kim CS, Lee SS, Han KD, et al. Metabolic syndrome and chronic laryngitis: The Korean National Health and Nutrition Examination Survey 2008 to 2010. Medicine 2015;94:e1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pellegrino R, Hähner A, Bojanowski V, et al. Olfactory function in patients with hyposmia compared to healthy subjects—an fMRI study. Rhinology 2016;54:374–81. [DOI] [PubMed] [Google Scholar]


