Abstract
Obese patients are more likely to encounter with difficult airway management, and supraglottic airway device has been adopted to facilitate tracheal intubation. The optimum anesthetic concentration for obese patients to insert a supraglottic airway device with spontaneous respiration has not been investigated. This study was designed to determine the end-tidal concentration of sevoflurane that would provide acceptable condition for supraglottic airway device insertion with propofol at induction in obese patients without using neuromuscular blockade.
Thirty elective obese patients [body mass index (BMI) 30–50 kg/m2] scheduled for bariatric surgery were enrolled in this study. Sevoflurane was inhaled at a concentration of 5% after infusion of 1 mg/kg propofol (within 1 minute) according to lean body weight. The target concentration of sevoflurane was initiated at 2.5% with 0.5% as a step size using a modified Dixon up-and-down method. Five minutes after target concentration achieved, the insertion of supraglottic airway device was attempted.
The minimum alveolar concentration of sevoflurane for successful insertion of supraglottic airway device calculated using up-and-down method were 2.25 (0.53) % for obese patients. The values of the effective concentration of sevoflurane for successful supraglottic airway device insertion in 50% (EC50) and 95% (EC95) of the obese patients obtained by probit regression analysis were 2.09% (95% confidence interval 1.48–2.68) and 3.31% (95% confidence interval 2.70–8.12), respectively.
We conclude that sevoflurane at a minimum alveolar concentration of 2.25% can provide optimal conditions for insertion of supraglottic airway device with spontaneous respiration in obese patients with 1 mg/kg propofol at induction.
Keywords: obesity, propofol, sevoflurane, spontaneous respiration, supraglottic airway device
1. Introduction
Obesity is considered to be a significant and growing problem facing our society. For over a decade, surgery has been established as an effective method to achieve a permanent weight reduction and concomitant disease treatment for a large amount of patients.[1] However, obese patients undergoing general anesthesia faced with a higher risk of difficult airway management and oxygen desaturation rapidly following the apnea.[2–4] Awake intubation is the gold standard in anticipated difficult airway management.[5] However, awake intubation may be difficult for obese patients to perform and bring about distress to the patient. Recently, the supraglottic airway device, as a feasible device for ventilation, has been adopted to provide oxygenation and facilitate safer tracheal intubation in obese patients.[6,7] Combes et al[8] have reported that the insertion of supraglottic airway device was performed after anesthesia induction with 2.5 mg/kg propofol and 30 μg/kg alfentanil given intravenously, and then assist safer tracheal intubation in morbidly obese patients. And Shiraishi[9] reported a safer method of airway management for obese patients by performing awake supraglottic airway device insertion (1–2 μg/kg fentanyl and 20–40 μg/kg midazolam) allowing spontaneous respiration to assist subsequent tracheal intubation. However, the optimum anesthesia induction for the insertion of supraglottic airway device with spontaneous respiration in obese patients has not been standardized.
Propofol is a common intravenous anesthetic agent, which has been demonstrated effective depressant on airway reflexes for supraglottic airway device insertion, but it has been associated with high incidence of apnea.[10] There has been reported that apnea occurred in 84% of patients induced with propofol alone for the insertion of supraglottic airway device.[11] The addition of sevoflurane during anesthesia induction reduces the dose of propofol required for supraglottic airway device insertion and provides better insertion condition. As compared with propofol, sevoflurane has lower incidence of apnea during anesthesia induction.[12] However, little is known about the optimum end-tidal concentration of sevoflurane to facilitate successful supraglottic airway device insertion with propofol at induction allowing spontaneous respiration in obese patients.
We considered that the induction of sevoflurane with a small dose of propofol may optimum the condition of supraglottic airway device insertion allowing spontaneous respiration. Therefore, we designed this study to find out the end-tidal sevoflurane concentration required for successful supraglottic airway device insertion with 1 mg/kg propofol according to lean body weight at induction in 50% of obese patients. The incidence of apnea and respiratory parameters were also investigated in this study.
2. Materials and methods
This study was approved by the Institutional Ethics Committee of Beijing Friendship Hospital, China (Ethics Committee number: 2016-P2-059-01) and registered with Chinese Clinical Trial Registry (registration NO.: ChiCTR-IPR-16009071). Written informed consent was obtained before recruitment of each patient participating in this study. From October 2016 to January 2017, 30 consecutive obese patients with body mass index (BMI) ranged from 30 to 50 kg/m2, aged 18 to 60 years, American Society of Anesthesiologists (ASA) class I-II, undergoing bariatric surgery were enrolled in this study. Patients were not recruited if they had symptoms of upper respiratory infection, a history of asthma, neck mass, neck radiation change, unstable cervical spine, interincisor distance less than 3 cm, limited or severely limited jaw protrusion, severe obstructive sleep apnea syndrome, and a history of gastroesophageal reflux disease, or if they were allergy or sensitivity to volatile anesthetics or propofol.
After the obese patient arriving in the operating room, standard anesthesia monitoring including noninvasive blood pressure, electrocardiogram, and pulse-oximeter was applied, an intravenous catheter was inserted and the lactate ringer's solution was started to infuse. The respiratory rate, tidal volume, inhaled and exhaled concentrations of sevoflurane, and end-tidal carbon dioxide were monitored breath by breath using a multiple monitoring system (S/5 CAM; Datex-Ohmeda, Louisville). The depth of anesthesia was monitored using Bispectral Index (BIS) monitor (BX42814; Aspect Medical System Inc, Norwood, MA). All patients were given 1 mg penehyclidine hydrochloride and 0.1 μg/kg sufentanil intravenously.
All participants were positioned in ramp position (the tragus of the ear level with the sternum, and the arms away from the chest)[13] and preoxygenated with 100% oxygen until the end-tidal oxygen fraction is 0.87. The circuit was primed with sevoflurane 5% at a fresh gas flow of 6 L/min for 1 minute. Anesthesia was induced with propofol at a bolus infusion of 1 mg/kg according to lean body weight within 1-minute duration. And then 5% sevoflurane was inhaled via facemask with 6 L/min of oxygen flow. After loss of eyelash reflex, the inhaled concentration of sevoflurane was changed to obtain the target end-tidal concentration (2.5% for the first patient) according to the modified Dixon up-and-down method. If the end-tidal capnography disappeared during the period of induction in case of airway obstruction, we gently placed the oropharyngeal airway and slightly lifted the jaw to maintain the airway. The ratio of the measured to target end-tidal concentration was sustained at 0.9 to 1.0 for over 5 minutes. And then, the supraglottic airway device (Blockbuster; Tuo Ren Medical Instrument Co., Ltd, Changyuan City, China) was inserted without neuromuscular blocking drugs.[14] A size 4 of the device was selected for all obese patients. After insertion of supraglottic airway device, it was connected to the respiration circuit. Patients were ventilated via the supraglottic airway device allowing spontaneous respiration. Fibreoptic bronchoscope was applied to observe the glottis counterpoint and confirm correct placement. After confirming a secure airway, general anesthesia (0.3–0.4 μg/kg sufentanil, 2 mg/kg propofol, and 0.6 mg/kg rocuronium according to ideal body weight) was induced and mechanical ventilation was started to obtain satisfactory intubation conditions. The fibreoptic-guided tracheal intubation attempt was performed with the supraglottic airway device as a channel 90 seconds after rocuronium administration.
A minimum of 3 researchers took part in the present study. A single anesthetist, who managed the study, set the target sevoflurane concentration, counted the time, and recorded related parameters. The second person, experienced in supraglottic airway device insertion and with more than 100 prior insertion of supraglottic airway device, inserted the deflated supraglottic airway device in all participants. He/she had no knowledge of the target concentration. The third person, who was unaware of target concentration, determined the reaction of supraglottic airway device insertion as either “movement” or “no movement.” Movement was defined as the appearance of coughing, bucking, laryngospasm, or gross purposeful limb movement at the first attempt or within 1 minute of supraglottic airway device insertion. We also used a scoring system of Muzi score to assess the condition of supraglottic airway device insertion based on jaw relaxation (1: fully relaxed, 2: mild resistance, 3: tight but could be opened, 4: closed requiring a dose of propofol). A Muzi score of 3 or 4 were also included in “movement.” “No movement” was defined as the paucity of above problems and a Muzi score less than 3. If any movement appeared immediately before or after supraglottic airway device insertion, additional 1 mg/kg propofol according to lean body weight was administered. However, insertion conditions were determined only for the first attempts. The additional propofol requirement was recorded. The incidence of apnea (absence of end-tidal capnography and chest movement) and apnea time (from the last spontaneous breath after sevoflurane administration to the first spontaneous respiration) according to the end-tidal capnography were recorded. We also recorded respiratory rates, tidal volume, SpO2, and BIS variables. All participants were asked whether they had any recall of events or not after the surgery.
In our study, the modified Dixon up-and-down method was adopted to identify the effective end-tidal sevoflurane concentration in 50% of obese patients during the insertion of the supraglottic airway device. Dixon up-and-down method was a staircase design which the stimulus level for the next subject is based on the response of the previous subject.[15] Dixon up-and-down design of trials yielded an evaluation of the median threshold (EC50) for all-or-none responses, which can be explored but cannot be measured directly (e.g., emesis, defibrillation, shock, and death). This design produced EC50 with as few as one-fifth the number of subjects used in more traditional designs and at least up to 6 crossover mid-points (in the EC50 curve) was suggested for this method,[15] while we set to obtain 8 crossover mid-points to be more precise. The modified Dixon up-and-down method has been used in other studies with the investigation of anesthetic concentrations during the airway device insertion depending on the response of the previous participant (movement to the airway device insertion).[16–18] One trial for each obese patient was performed and the target concentration for the first obese patient was 2.5% and the step size of the increment or decrement was 0.5%.[19,20] If the patient reacted with movement, the end-tidal sevoflurane concentration for the next patient was increased by 0.5%. If not, it was decreased by the same amount. A single measurement was obtained from each participant.
The sample size of our study was based on the fact that 8 independent pairs of participants showing a crossover point from a “movement” response to a “no-movement” response and was calculated and confirmed by the professional statisticians in our research team.[15,20] The value of sevoflurane EC50 obtained using the Dixon up-and-down method was determined by calculating the midpoint concentration of crossover point from “no-movement” to “‘movement” response. We further obtained the EC50 and EC95 of sevoflurane for suppraglottic airway device insertion with 95% confidence interval (95% CI) by using probit regression analysis. Patients’ characteristics are represented as mean (SD), median [interquartile range, IQR (range)], or number (proportion). Respiratory data were analyzed with repeated measures of analysis of variance (ANOVA). P value less than .05 was considered significant. These data were analyzed using SPSS version 17.0 (SPSS Inc., Chicago, IL).
3. Results
All of the 30 obese patients finished this study (Fig. 1). Patients’ characteristics and airway features are presented in Table 1.
Figure 1.
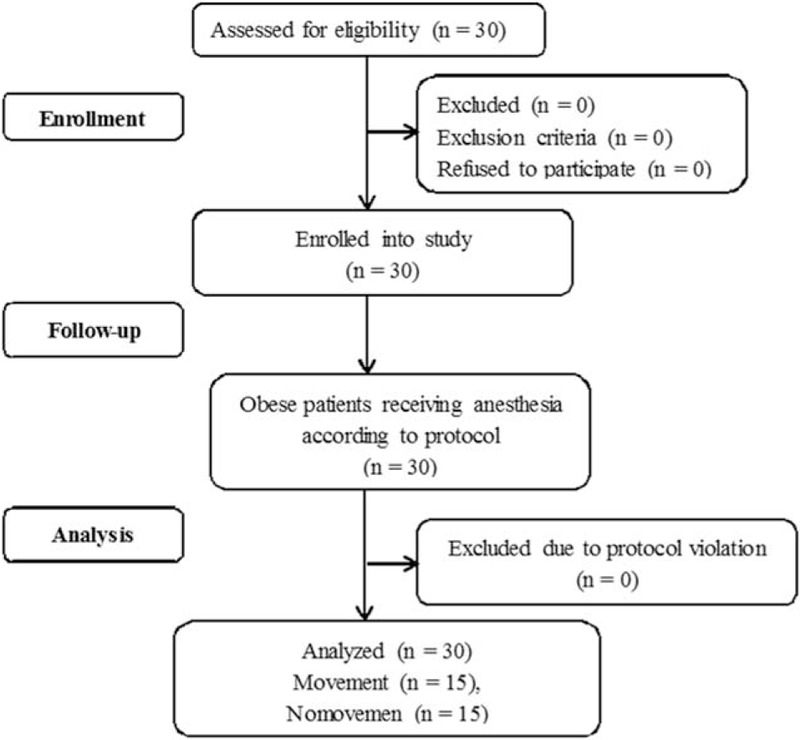
Flow diagram of obese patient recruitment.
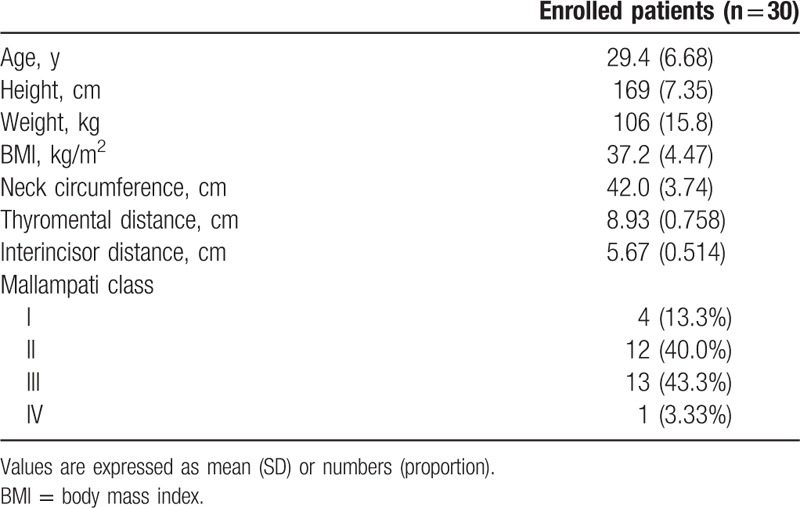
Table 1.
Baseline characteristics of obese patients receiving anesthesia induction with sevoflurane combined with 1 mg/kg propofol according to lean body weight.
Dose–response data obtained by Dixon up-and-down method are illustrated in Fig. 2. The required sevoflurane concentration calculated by the up-and-down method for successful supraglottic airway device insertion in 50% of obese patients with propofol at induction was 2.25 (0.53) %. The probit regression of the dose–response curve for each patient reflecting the probability of successful supraglottic airway device insertion versus end-tidal concentration of sevoflurane is demonstrated in Fig. 3. The predicted EC50 and EC95 for obese patients to insert a supraglottic airway device were 2.09% (95% CI 1.48–2.68) and 3.31% (95% CI 2.70–8.12), respectively.
Figure 2.
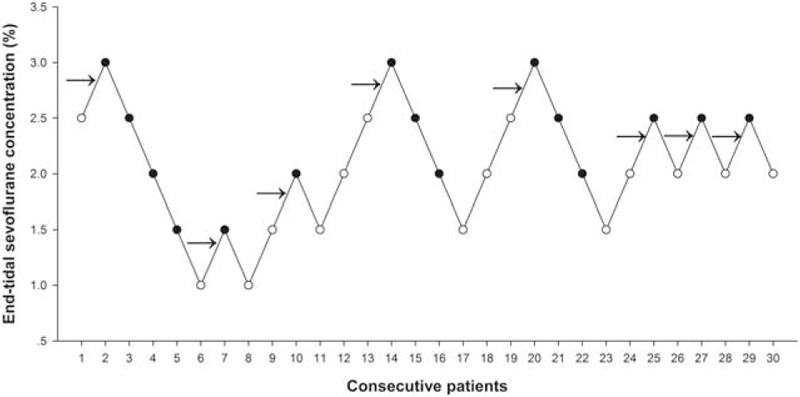
Responses of obese patients with a modified Dixon up-and-down method. Responses of 30 consecutive obese patients induced with sevoflurane combined with propofol to supraglottic airway device insertion and the end-tidal concentrations of sevoflurane in oxygen with a modified Dixon up-and-down method. Arrows indicate the mid-point dose of all independent pairs of participants who manifested cross-over from “movement” (○) to “no-movement” (●) response.
Figure 3.
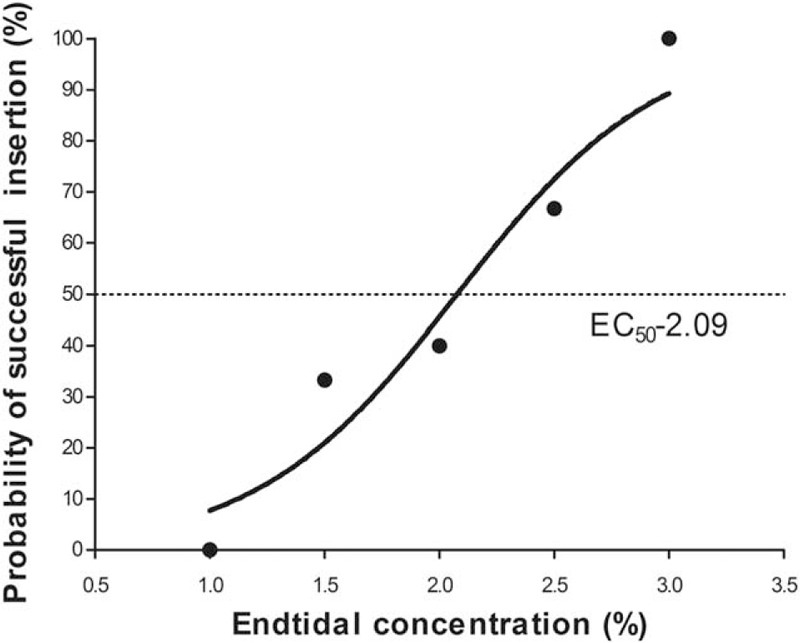
Dose–response curves of sevoflurane for supraglottic airway device insertion in obese patients. The curves plotted from probit regression analysis of individual end-tidal concentration of sevoflurane and the reactions to supraglottic airway device insertion in obese patients. The end-tidal concentrations at which there were 50% and 95% probabilities of successful supraglottic airway device insertion were 2.09% (95% CI 1.48–2.68) and 3.31% (95% CI 2.70–8.12), respectively.
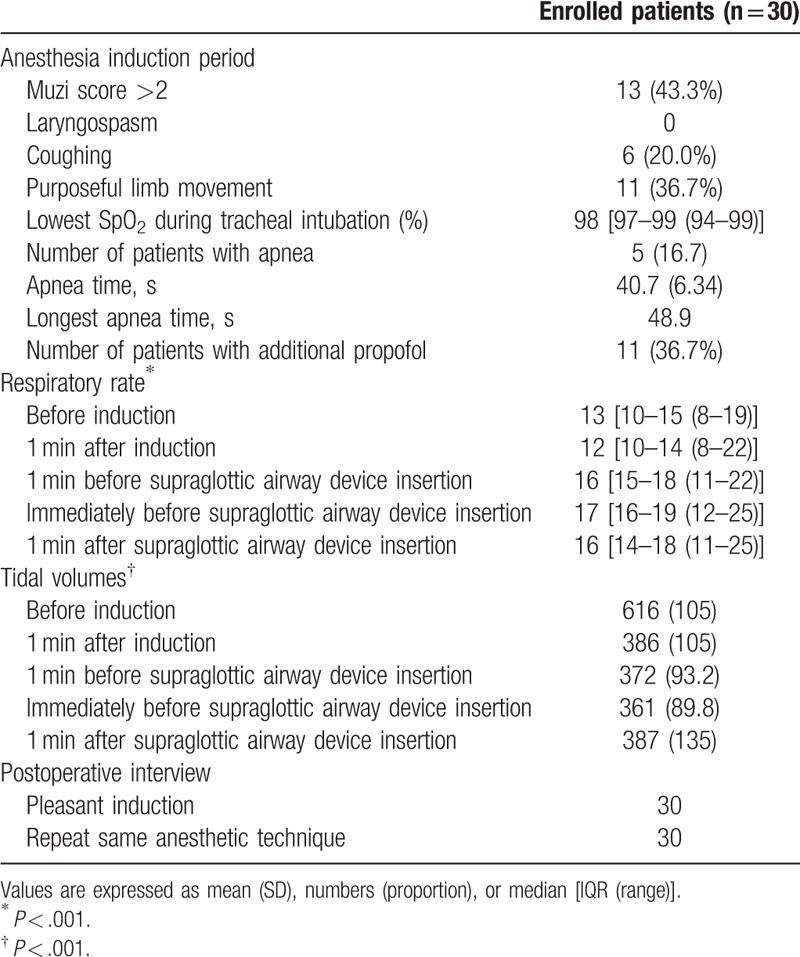
The characteristics of obese patients during anesthesia induction and supraglottic airway device insertion are summarized in Table 2. The incidence of apnea was 16.7%. However, no patient experienced oxygen desaturation less than 92%. There were no clinically significant events of laryngospasm, hypotension, hypertension, bradycardia, or tachycardia related to supraglottic airway device insertion and anesthesia induction requiring therapy.
Table 2.
Characteristics of anesthesia induction and postoperative interview in obese patients receiving anesthesia induction with sevoflurane combined with 1 mg/kg propofol according to lean body weight.
The BIS values were similar immediately before supraglottic airway device insertion in the “movement” (52 ± 10) and “no-movement” patients (48 ± 10) (P = .758). There were 8 patients who had a BIS value exceeding 60 immediately before insertion, and 2 patients who had a BIS value exceeding 70. Meanwhile, there were 7 patients whose BIS value surpassing 60 1 minute after insertion. No participants recalled any of events during supraglottic airway device insertion and anesthesia induction in this study according to the postoperative visit. All participants were satisfied with our induction method for airway management, and anesthesiologists felt comfortable.
4. Discussion
The result of this study demonstrated that anesthesia induction of sevoflurane combined with a small dose of propofol can provide optimal conditions for supraglottic airway device insertion with spontaneous respiration in obese patients. Using the Dixon up-and-down method, this study showed that the required end-tidal concentration of sevoflurane at which successful supraglottic airway device insertion is possible in 50% of obese patients with 1 mg/kg propofol at induction was 2.25 (0.53) %.
Propofol was suitable for providing acceptable conditions for supraglottic airway device insertion for its rapid induction and suppression of upper airway reflexes.[21] Kodaka et al[19] have reported that the required concentration of propofol for laryngeal mask airway Classic insertion was 3.14 μg/mL. Further study showed that optimum propofol concentration for laryngeal mask airway Classic insertion was 2.06 μg/mL combined with fentanyl 0.5 μg/kg.[22] In all of these previous studies investigating EC50 of propofol for supraglottic airway device insertion, propofol was used in the method of target-controlled infusions (TCIs). However, we adopted the method of bolus continuous infusion in this study. This is because of that there is a paucity of recommended TCI techniques for obese patients as to the most appropriate weight scalar to use with. The Marsh and Schnider formulae for TCI of propofol become unreliable for obesities weighing above 140 to 150 kg. There are no available pumps allowing input of weights over 150 kg using the Marsh model, or BMI >42 kg/m2 (male) and 35 kg/m2 (female) using the Schnider model.[13] When used alone for anesthesia induction in the method of bolus infusion, the propofol dose for supraglottic airway device insertion surpasses more than 3.0 mg/kg, which has been associated with a high incidence of apnea.[11] Therefore, 1.0 mg/kg (according to lean body weight) propofol chosen in this study is based on preliminary trial and modified with prior other studies[10,23] in consideration of avoiding high incidence of apnea. However, propofol at a small dose may not suppress airway reflexes that may prevent supraglottic airway device insertion and cause distress such as laryngospasm. Therefore, various adjunct agents such as opioids, lidocaine, midazolam, and volatile have been used with propofol to facilitate supraglottic airway device insertion.[21,23] Of these agents, sevoflurane (a nonpungent inhaled anesthetic with minimal respiratory irritant characteristics) provides the components of general anesthesia without a period of apnea. There have been a few reports demonstrated that induction with sevoflurane facilitates supraglottic airway device insertion.[20,24] Arslan et al[6] reported that 2% sevoflurane in oxygen, when administered after 3 mg/kg propofol and 0.6 mg/kg rocuronium, facilitated supraglottic airway device insertion. Also, induction of anesthesia with a single vital capacity breath of 8% sevoflurane supplemented with 1.5 mg/kg propofol improved the conditions of laryngeal mask airway insertion.[11] The required end-tidal sevoflurane concentration for 50% obese patients to successfully insert supraglottic airway device in our study was 2.25%.
The results of the present study showed that sevoflurane can provide optimal conditions for supraglottic airway device insertion allowing spontaneous ventilation in obese patients with propofol at induction with minimal adverse effects. The incidence of apnea was 16.7%. However, it did not cause oxygen desaturation obviously. Previous study has shown that apnea occurred in 16% of patients induced with sevoflurane combined with propofol.[11] Similar to the results by Siddik-Sayyid et al,[11] we found that induction of sevoflurane combined with propofol was a safer procedure for obese patients to insert supraglottic airway device without using neuromuscular blocking agents. In addition, apnea during the period of anesthesia induction with sevoflurane is associated with its mode of administration. The study by Pancaro et al[25] showed that apnea occurred in 21%, 20%, 68% of patients induced with sevoflurane at incremental concentrations of 1% (from 1% to 8%), decremental-incremental concentrations of 2% (from 8% to 4% to 8%), and a fixed concentration of 8%, respectively. The higher concentration of sevoflurane is administered, the higher is the probability that the participant will suffer with apnea. In this study, sevoflurane was inhaled up to 5% and decreased to obtain the target concentration after eyelash reflex loss, and the incidence of apnea was similar as other reported.[25]
The patients were satisfied with this method of anesthesia induction that made participants feel comfortable and avoided the distress caused by insertion of supraglottic airway device. This induction routine made anesthetists feel relax and confident in the airway management of obese patients for continuous oxygenation. Oxygenation is the primary principle in airway management. Our induction method during airway management in obese patients reflected this important principle.
There were several limitations in the present study. First, the BMI of recruited obese patients scheduled for bariatric surgery was limited to 30 to 50 kg/m2. It is uncertain whether the results could be applied to higher BMI (>50 kg/m2) patients and other surgeries. Second, the anesthetic agent of propofol was dosed to lean body weight in our study design, although adjusted body weight or total body weight may be used as the scalars for calculating anesthetic drug doses as the lack of recommendation on the criterion of commonly used anesthetic drugs for obese adults. Third, we just investigated sevoflurane combined with 1 mg/kg propofol according to lean body weight in the present study in consideration of the incidence of apnea. Finally, clinicians would be more interested in EC95 because of its greater clinical significance. The EC95 of sevoflurane for supraglottic airway device insertion was obtained using probit regression analysis, which has been adopted in literature using Dixon up-and-down method.[26–28] However, the method determines the EC95 of sevoflurane for successful placing a supraglottic airway device without regard to other parameters (respiratory index, hemodynamic values, and BIS). Therefore, further studies should be adopted to confirm the sevoflurane EC95 by a comparison with a wider range of concentrations.
In conclusion, the end-tidal concentration of sevoflurane for successful supraglottic airway device insertion allowing spontaneous respiration in 50% of obese patients was 2.25 (0.53) % during induction with 1 mg/kg propofol according to lean body weight without a neuromuscular blocking agent. From the probit regression analysis, the EC50 and EC95 of sevoflurane were 2.09% (95% CI 1.48–2.68) and 3.31% (95% CI 2.70–8.12), respectively.
Acknowledgments
The authors thank the participating investigators Dr. Jing-Dong Ke, Peng Dong, Huan-Rong Qiu, Kai Su, and general surgery team at Beijing Friendship hospital for their genuine support of the study, and Ms Na Zeng, Master of Public Health at Clinical Statistical Centre, Beijing Friendship hospital for her sincere help with statistics.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, BIS = bispectral index, BMI = body mass index, CI = confidence interval, EC50 = median threshold, TCI = target-controlled infusions.
The BlockBuster supraglottic airway device has Chinese patent number ZL201220110943.6 (http://www.sipo.gov.cn/) and was registered by the China Food and Drug Administration (Registration Number: 2660100; http://app2.sfda.gov.cn/datasearchp/index1). MT invented and held the patent of the BlockBuster supraglottic airway device, and participated in the design and development in collaboration with its manufacturer Tuo Ren Medical instrument Co (http://www.tuoren.com/html/en/products16pcon920.html); He has no financial interest in the device. The airway devices used in this study were departmental sources from the manufacturer or the local distributer. No external funding declared.
The authors have no conflicts of interest.
References
- [1].Yan Y, Sha Y, Yao G, et al. Roux-en-Y gastric bypass versus medical treatment for type 2 diabetes mellitus in obese patients: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016;95:e3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Murphy C, Wong DT. Airway management and oxygenation in obese patients. Can J Anaesth 2013;60:929–45. [DOI] [PubMed] [Google Scholar]
- [3].Kheterpal S, Healy D, Aziz MF, et al. Incidence, predictors, and outcome of difficult mask ventilation combined with difficult laryngoscopy: a report from the multicenter perioperative outcomes group. Anesthesiology 2013;119:1360–9. [DOI] [PubMed] [Google Scholar]
- [4].Riad W, Vaez MN, Raveendran R, et al. Neck circumference as a predictor of difficult intubation and difficult mask ventilation in morbidly obese patients: a prospective observational study. Eur J Anaesthesiol 2016;33:244–9. [DOI] [PubMed] [Google Scholar]
- [5].Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013;118:251–70. [DOI] [PubMed] [Google Scholar]
- [6].Arslan ZI, Ozdamar D, Yildiz TS, et al. Tracheal intubation in morbidly obese patients: a comparison of the intubating laryngeal mask airway and laryngeal mask airway CTrach. Anaesthesia 2012;67:261–5. [DOI] [PubMed] [Google Scholar]
- [7].Bindra T, Nihalani SK, Bhadoria P, et al. Use of intubating laryngeal mask airway in a morbidly obese patient with chest trauma in an emergency setting. J Anaesthesiol Clin Pharmacol 2011;27:544–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Combes X, Sauvat S, Leroux B, et al. Intubating laryngeal mask airway in morbidly obese and lean patients: a comparative study. Anesthesiology 2005;102:1106–9. discussion 1105A. [DOI] [PubMed] [Google Scholar]
- [9].Shiraishi T. Awake insertion of the air-Q intubating laryngeal airway device that facilitates safer tracheal intubation in morbidly obese patients. Br J Anaesth 2013;111:1024–5. [DOI] [PubMed] [Google Scholar]
- [10].Uzumcugil F, Canbay O, Celebi N, et al. Comparison of dexmedetomidine-propofol vs. fentanyl-propofol for laryngeal mask insertion. Eur J Anaesthesiol 2008;25:675–80. [DOI] [PubMed] [Google Scholar]
- [11].Siddik-Sayyid SM, Aouad MT, Taha SK, et al. A comparison of sevoflurane-propofol versus sevoflurane or propofol for laryngeal mask airway insertion in adults. Anesth Analg 2005;100:1204–9. [DOI] [PubMed] [Google Scholar]
- [12].Herman AG, Mahla ME. Awake intubating laryngeal mask airway placement in a morbidly obese patient with ankylosing spondylitis and unstable thoracic spine. J Clin Anesth 2016;32:62–4. [DOI] [PubMed] [Google Scholar]
- [13].Nightingale CE, Margarson MP, Shearer E, et al. Members of the Working Party. Peri-operative management of the obese surgical patient 2015: Association of Anaesthetists of Great Britain and Ireland Society for Obesity and Bariatric Anaesthesia. Anaesthesia 2015;70:859–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Su K, Gao X, Xue FS, et al. Difficult tracheal tube passage and subglottic airway injury during intubation with the GlideScope(R) videolaryngoscope: a randomised, controlled comparison of three tracheal tubes. Anaesthesia 2017;72:504–11. [DOI] [PubMed] [Google Scholar]
- [15].Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev 1991;15:47–50. [DOI] [PubMed] [Google Scholar]
- [16].Kim MK, Lee JW, Jang DJ, et al. Effect-site concentration of remifentanil for laryngeal mask airway insertion during target-controlled infusion of propofol. Anaesthesia 2009;64:136–40. [DOI] [PubMed] [Google Scholar]
- [17].Yao Y, Ni J, Yang Y, et al. The optimum dose of intranasal remifentanil for laryngeal mask airway insertion during sevoflurane induction in children: a randomized controlled trial. Int J Clin Exp Med 2015;8:21235–40. [PMC free article] [PubMed] [Google Scholar]
- [18].Goo EK, Lee JS, Koh JC. The optimal exhaled concentration of sevoflurane for intubation without neuromuscular blockade using clinical bolus doses of remifentanil: a randomized controlled trial. Medicine (Baltimore) 2017;96:e6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kodaka M, Okamoto Y, Koyama K, et al. Predicted values of propofol EC50 and sevoflurane concentration for insertion of laryngeal mask Classic and ProSeal. Br J Anaesth 2004;92:242–5. [DOI] [PubMed] [Google Scholar]
- [20].Zaballos M, Bastida E, Jimenez C, et al. Predicted end-tidal sevoflurane concentration for insertion of a Laryngeal Mask Supreme: a prospective observational study. Eur J Anaesthesiol 2013;30:170–4. [DOI] [PubMed] [Google Scholar]
- [21].Baik HJ, Kim YJ, Kim JH. Lidocaine given intravenously improves conditions for laryngeal mask airway insertion during propofol target-controlled infusion. Eur J Anaesthesiol 2009;26:377–81. [DOI] [PubMed] [Google Scholar]
- [22].Kodaka M, Okamoto Y, Handa F, et al. Relation between fentanyl dose and predicted EC50 of propofol for laryngeal mask insertion. Br J Anaesth 2004;92:238–41. [DOI] [PubMed] [Google Scholar]
- [23].Bouvet L, Da-Col X, Rimmele T, et al. Optimal remifentanil dose for laryngeal mask airway insertion when co-administered with a single standard dose of propofol. Can J Anaesth 2010;57:222–9. [DOI] [PubMed] [Google Scholar]
- [24].Ghai B, Jain K, Bansal D, et al. End-tidal sevoflurane concentration for ProSeal(TM) versus Classic(TM) laryngeal mask airway insertion in unpremedicated anaesthetised adult females. Anaesth Intensive Care 2016;44:221–6. [DOI] [PubMed] [Google Scholar]
- [25].Pancaro C, Giovannoni S, Toscano A, et al. Apnea during induction of anesthesia with sevoflurane is related to its mode of administration. Can J Anaesth 2005;52:591–4. [DOI] [PubMed] [Google Scholar]
- [26].Makkar JK, Arora S, Jain K, et al. ED50 of desflurane for laryngeal mask airway removal in anaesthetised adults. Anaesthesia 2011;66:808–11. [DOI] [PubMed] [Google Scholar]
- [27].Hui MT, Subash S, Wang CY. The 50% and 95% effective doses of desflurane for removal of the classic laryngeal mask airway in spontaneously breathing anaesthetised adults. Anaesthesia 2011;66:274–7. [DOI] [PubMed] [Google Scholar]
- [28].Ghai B, Jain K, Bansal D, et al. End-tidal concentrations of sevoflurane and desflurane for ProSeal laryngeal mask airway removal in anaesthetised adults: a randomised double-blind study. Eur J Anaesthesiol 2014;31:274–9. [DOI] [PubMed] [Google Scholar]


