Abstract
The critical shortage of donor organs has spurred the investigation of alternative approaches to generate replacement organs or implant exogenous cells for treatment of end-stage organ failure. Non-thermal irreversible electroporation (NTIRE), which uses brief high electrical fields to induce irreversible permeabilization of cell membranes, has emerged as a technique for tumor ablation. This study introduces a new application for NTIRE that employs in situ cell ablation with NTIRE to create a niche within a solid organ for engraftment of exogenous cells in vivo. We treated the liver of mice with NTIRE and subsequently implanted exogenous congenic hepatocytes within the zone of cell ablation. Donor hepatocytes engrafted and integrated with host liver parenchyma that was pre-treated with NTIRE. This new approach may have value in studying the effects of a native matrix scaffold on in vivo cell growth and may pioneer a new aspect of minimally-invasive regenerative surgery.
Keywords: regenerative surgery, irreversible electroporation, hepatocyte transplantation, tissue engineering, decellularized scaffold
Organ transplantation is the only treatment for end-stage organ failure and is severely limited by the shortage of donor organs. The critical unmet need for alternative treatments has spurred tissue engineering and regenerative medicine approaches to generate replacement organs. Whole-organ decellularization has been developed to produce three-dimensional tissue scaffolds with native extracellular matrix (ECM) and preserved microvasculature (1). Here, we introduce a new approach to generate a native decellularized ECM scaffold in situ, which serves to facilitate the in vivo engraftment of exogenous cells in a solid organ. Our approach is based on the concept that an appropriate niche must first be created within a host organ, in order for new cells to engraft efficiently.
Non-thermal irreversible electroporation (NTIRE) is a tissue ablation technique that employs high electrical pulses that are microseconds in length to irreversibly permeabilize the cell membrane, thereby causing cell death (2). NTIRE treatment leaves behind an intact ECM on which new cells regenerate, often without scar tissue (2–7). This unique feature of NTIRE has led to the suggestion that it could be used as a method to decellularize organs (5,8,9). Inspired by the earlier work, we hypothesized that NTIRE could be utilized to remove host cells from tissues while leaving behind an intact ECM, creating a supportive niche in situ for the in vivo engraftment of exogenous cells. To test this hypothesis, we treated the liver of mice with NTIRE and subsequently implanted exogenous congenic hepatocytes within the treated volume.
All animal procedures were performed in accordance to protocols approved by the Institutional Animal Care and Use Committee at UCSF. After induction of general anesthesia, the abdomen of wild-type C57BL/6 mice was aseptically prepared and the liver exposed by making a 1cm midline incision below the xiphoid. Two needle electrodes, each 0.5mm in diameter and spaced 4mm apart, were connected to an ECM 830 Square Wave Electroporation System (BTX Harvard Apparatus, Holliston, MA) and inserted into the left lobe, perpendicular to the liver surface. Electrical pulses were applied using parameters of 500 V/cm (voltage over distance), eight 100μs pulses, and each pulse separated by 100ms. These parameters were chosen because they represented the threshold for inducing irreversible electroporation (10). Other studies have employed different electrodes that applied higher electrical fields and many more pulses (4,9). We used the lower threshold because recent studies showed that the larger numbers of pulses induced electrolysis and might not produce pure irreversible electroporation (11).
We found that the time-course of tissue injury in response to NTIRE ablation in the mouse liver was comparable to those observed in porcine and rat liver (3,4), blood vessels (5), and small intestines (12). At 3 hours after NTIRE treatment, there were no overt signs of cell death or tissue injury (Figure 1). By 24 hours, the liver parenchyma showed injured hepatocytes with vacuolated moth-eaten cytoplasm. At 3 days, there was massive ballooning degeneration of hepatocytes throughout the NTIRE-treated tissue. The pathogenesis of ballooning degeneration is not completely known, but there is evidence that they represent terminally injured cells that cannot complete the apoptotic program (13). By 7 days, the NTIRE-injured tissue had repaired itself and ballooned hepatocytes were no longer found. Importantly, there was not extensive infiltration of inflammatory cells, abnormal deposition of collagen, or scar formation after NTIRE treatment.
Figure 1.
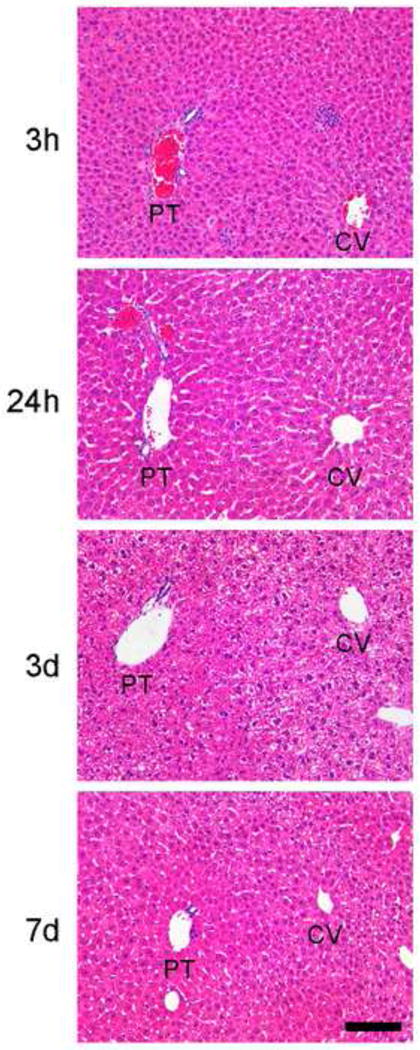
Time-course of NTIRE-treated liver tissue shows hepatocyte injury, followed by recovery, without formation of scar. There are no overt signs of cell injury at 3h. By 24h, there are injured hepatocytes with vacuolated moth-eaten cytoplasm. By 3d, hepatocytes show ballooning degeneration. By 7d, ballooned hepatocytes are no longer found. PT, portal tract; CV, central vein. Magnification 20×. Scale bar = 100μm.
The time-course studies suggested that the optimal timing of in vivo exogenous hepatocyte implantation was 3 days after NTIRE treatment, a time-point after host hepatocyte cell death peaked. Therefore, we treated the liver of mice with NTIRE as described above and then introduced donor hepatocytes by direct parenchymal implantation 3 days later. Primary hepatocytes were isolated from B6.129S7-Gt(ROSA)26Sor/J (ROSA26) mice, in which β-galactosidase was constitutively expressed in all cells, so that implanted hepatocytes could be easily identified by Xgal staining in wild-type C57BL/6 recipients. ROSA26 mice were deeply anesthetized and primary hepatocyte isolation performed by two-step perfusion (14). One million freshly isolated hepatocytes were loaded into a PE50 catheter (BD Biosciences, San Jose, CA) adapted to a glass syringe with a screw-drive (Hamilton, Reno, NV). C57BL/6 mice pretreated with NTIRE were anesthetized and the previously made midline incision was reopened. A hepatotomy was made using a 25-gauge needle in the left liver lobe, in between the two needle electrode insertion sites. The catheter loaded with hepatocytes was inserted into the hepatotomy site and donor hepatocytes were implanted by slowly turning the screw-drive of the syringe (15). Recipient wild-type mice without NTIRE pretreatment were used as controls.
At 3 and 14 days post-implantation, serial cryosections were obtained through the entire recipient liver lobe from the anterior to the posterior surface. Hepatocytes that stained positively for Xgal (blue) were manually counted in each section to determine the total number of engrafted hepatocytes in each recipient mouse. The hepatotomy performed at the time of implantation created a space within the liver parenchyma (Figure 2A, 5X). The majority of implanted cells within this space eventually die by necrosis (15). Exogenous cells that successfully engraft are at the periphery of this space and must migrate past the zone of inflammatory cells to incorporate within the host parenchyma (Figure 2A, 40X). Liver from NTIRE-treated mice showed significantly greater numbers of Xgal+ hepatocytes within and beyond the inflammatory zone compared to livers from control mice that did not receive NTIRE pre-treatment (Figure 2A, bar graph). At 14 days after implantation, Xgal+ donor hepatocytes appeared integrated with the host parenchyma near or along the scar left behind by the implantation tract (Figure 2B, 5X). Livers pretreated with NTIRE showed larger Xgal+ cell clusters compared to controls (Figure 2B, 40X). These observations suggest that, with further optimization of electroporation protocols and surgical approach, improved donor cell engraftment may be achieved in NTIRE-treated liver tissue after direct exogenous cellular implantation in vivo.
Figure 2.
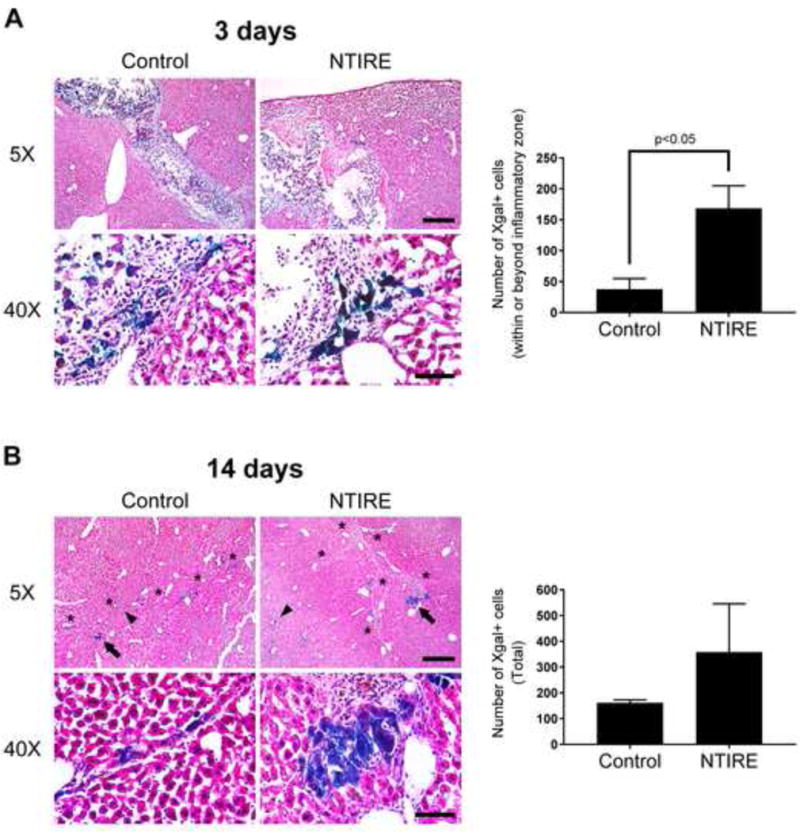
Exogenous hepatocytes implanted into NTIRE-treated liver in vivo show greater host parenchymal integration and larger clustering compared to controls without NTIRE pretreatment. (A) Day 3 - the liver of NTIRE-treated mice (n=3) showed significantly greater numbers of Xgal+ hepatocytes (blue) within and beyond the inflammatory zone compared to control (n=3). p<0.05 by two-tailed Student’s t-test. Error bars represent SEM. (B) Day 14 - individual (arrowheads) or clusters (arrows) of Xgal+ hepatocytes integrated with the host parenchyma near or along the implantation scar (asterisks). Although total numbers were not significantly increased, there were larger clusters of Xgal+ hepatocytes in the liver of NTIRE-treated (n=3) compared to control (n=3) mice. For all mice, serial cryosections were obtained through the entire implanted liver lobe from the anterior to posterior surface, and Xgal+ cells were manually counted in all sections. Scale bar = 400μm (5X) and 60μm (40X).
In conclusion, we showed that exogenous hepatocytes engrafted into the niche prepared by NTIRE pre-treatment of the liver. Our study is the first to demonstrate the feasibility of this regenerative surgical approach that may be generalizable to other organs and cell types.
METHOD SUMMARY.
We treated the liver of mice with NTIRE electrical pulses in vivo and then implanted the treated tissue volume with exogenous hepatocytes a few days later. Exogenous hepatocytes were able to engraft in liver tissue pre-treated with NTIRE. This strategy represents a novel approach to introduce new cells within a solid organ in vivo.
Acknowledgments
This work was funded by NIH K08-DK093708 and P30-DK026743 to T.T.C.
Footnotes
AUTHOR CONTRIBUTIONS
T.T.C. conceived the study, designed the experiments, performed the experiments, analyzed and interpreted the data, and wrote the manuscript. V.X.Z. performed experiments and data analysis and prepared parts of the manuscript. B.R. designed experiments and wrote the manuscript.
COMPETING INTERESTS
B.R. is a co-inventor of the NTIRE technology. The intellectual property of NTIRE is assigned to the University of California, Berkeley. B.R. has no other financial interests except as a faculty at the university.
References
- 1.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–U120. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33:223–231. doi: 10.1007/s10439-005-8981-8. [DOI] [PubMed] [Google Scholar]
- 3.Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality–clinical implications. Technol Cancer Res Treat. 2007;6:37–48. doi: 10.1177/153303460700600106. [DOI] [PubMed] [Google Scholar]
- 4.Golberg A, Bruinsma BG, Jaramillo M, Yarmush ML, Uygun BE. Rat liver regeneration following ablation with irreversible electroporation. PeerJ. 2016;4:e1571. doi: 10.7717/peerj.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips M, Maor E, Rubinsky B. Nonthermal irreversible electroporation for tissue decellularization. J Biomech Eng. 2010;132:091003. doi: 10.1115/1.4001882. [DOI] [PubMed] [Google Scholar]
- 6.Narayanan G, Froud T, Suthar R, Barbery K. Irreversible electroporation of hepatic malignancy. Semin Intervent Radiol. 2013;30:67–73. doi: 10.1055/s-0033-1333655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maor E, Ivorra A, Leor J, Rubinsky B. The effect of irreversible electroporation on blood vessels. Technol Cancer Res Treat. 2007;6:307–312. doi: 10.1177/153303460700600407. [DOI] [PubMed] [Google Scholar]
- 8.Phillips MA, Maor E, Rubinsky B, Lavee J. Extracellular matrix material created using non-thermal irreversible electroporation. 08835166. The Regents of the University of California; US Patent. 2014 Sep 16; 2014.
- 9.Sano MB, Neal RE, 2nd, Garcia PA, Gerber D, Robertson J, Davalos RV. Towards the creation of decellularized organ constructs using irreversible electroporation and active mechanical perfusion. Biomed Eng Online. 2010;9:83. doi: 10.1186/1475-925X-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miklavcic D, Semrov D, Mekid H, Mir LM. In vivo electroporation threshold determination. Proceedings of the 22nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (Cat No 00CH37143) 2000;4:2815–2818. [Google Scholar]
- 11.Rubinsky L, Guenther E, Mikus P, Stehling M, Rubinsky B. Electrolytic Effects During Tissue Ablation by Electroporation. Technol Cancer Res Treat. 2016;15:NP95–NP103. doi: 10.1177/1533034615601549. [DOI] [PubMed] [Google Scholar]
- 12.Phillips MA, Narayan R, Padath T, Rubinsky B. Irreversible electroporation on the small intestine. Br J Cancer. 2012;106:490–495. doi: 10.1038/bjc.2011.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakisaka K, Cazanave SC, Werneburg NW, Razumilava N, Mertens JC, Bronk SF, Gores GJ. A hedgehog survival pathway in ‘undead’ lipotoxic hepatocytes. J Hepatol. 2012;57:844–851. doi: 10.1016/j.jhep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai SS, Tung JC, Zhou VX, Grenert JP, Malato Y, Rezvani M, Espanol-Suner R, Willenbring H, et al. Physiological ranges of matrix rigidity modulate primary mouse hepatocyte function in part through hepatocyte nuclear factor 4 alpha. Hepatology. 2016;64:261–275. doi: 10.1002/hep.28450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou VX, Lolas M, Chang TT. Direct orthotopic implantation of hepatic organoids. J Surg Res. 2016;211:251–260. doi: 10.1016/j.jss.2016.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]


