Abstract
Head and neck cancer (HNSCC) is a devastating disease. Patients require intensive treatment that is often disfiguring and debilitating. Those who survive are often left with poor speech articulation, difficulties in chewing and swallowing, cosmetic disfigurement, as well as loss of taste. Furthermore, given that HNSCC survivors are frequently disabled and unable to return to work, the economic and societal costs associated with HNSCC are massive. HNSCC is one of many cancers that are strongly associated with tobacco use. The risk for HNSCC in smokers is approximately 10 times higher than that of never-smokers and 70–80% of new HNSCC diagnoses are associated with tobacco and alcohol use. Tobacco products have been used for centuries, however it is just within the last 60–70 years that we have developed an understanding of their damaging effects. This relatively recent understanding has created a pathway towards educational and regulatory efforts aimed at reducing tobacco use. Understanding the carcinogenic components of tobacco products and how they lead to HNSCC is critical to regulatory and harm reduction measures. To date, nitrosamines and other carcinogenic agents present in tobacco products have been associated with cancer development. The disruption of DNA structure through DNA adduct formation is felt to be a common mutagenic pathway of many carcinogens. Intense work pertaining to tobacco product constituents, tobacco use and tobacco regulation has resulted in decreased use in some parts of the world. Still, much work remains as tobacco continues to impart significant harm and contribute to HNSCC development worldwide.
Keywords: Head and neck squamous cell carcinoma, tobacco products, tobacco carcinogenesis, tobacco regulation, history of tobacco
Introduction
Head and neck cancer is the sixth most common form of cancer worldwide [1]. In 2016, it is estimated that 61,760 patients were diagnosed with head and neck cancer in the United States [2]. Worldwide, 550,000 people are diagnosed with, and about 380,000 people die from, head and neck cancer each year [3]. Tobacco products are implicated in the generation of multiple cancer types including lung, esophageal, bladder, and pancreatic cancer as well as many cardiovascular and respiratory diseases [4]. Tobacco use is also strongly associated with head and neck squamous cell carcinoma (HNSCC) [1]. Specifically, this includes tumors of the oral cavity, nasopharynx, oropharynx, hypopharynx, and larynx [4]. The last several years have witnessed steadily increasing understanding of the carcinogenic constituents contained in tobacco products as well as their roles in generating specific tumors. In this review, we aim to discuss the history of tobacco use throughout the world, the variety of tobacco products available and currently in use, specific tobacco constituents and carcinogens, and specific data which links the carcinogenic components of tobacco with cancer of the head and neck. Lastly, we will address the effects of tobacco on treatment outcomes pertaining to head and neck cancer.
History of tobacco products and use [5]
Experts believe that the tobacco plant began growing in the Americas in 6000 BCE. In 1 BCE, Americans began finding ways to use tobacco through smoking, chewing, and hallucinogenic enemas. By 1 CE, tobacco was reported to be “nearly everywhere” in the Americas. In 1492, Christopher Columbus set foot in the Americas and was greeted by natives who offered fruits, spears, and tobacco as gifts. He described the tobacco as “pungent dried leaves” and discarded them. During the 16th century, tobacco emerged as a prominent trade item and its use spread throughout the Americas and Europe.
Over the next 200 years, tobacco products experienced significant spread in use throughout the world. By 1900, there were 300,000 different cigar brands and approximately 3.5 billion cigarettes and 6 billion cigars were sold annually. The Federal Food and Drugs Act of 1906 prohibited the sale of adulterated drugs and required reporting of their content on package labels. Initially, nicotine was on the list of drugs slated to be regulated by the FDA through this Act. However, following extensive lobbying from the tobacco industry, nicotine was removed from the list of regulated substances. The lack of regulation opened to the door to product content manipulation practiced by the tobacco companies for many years before finally coming to light near the end of the 20th century. Throughout the 1950s, there was tremendous competition between tobacco companies and aggressive advertising touting their products (Figure 2). These factors played a role in the continued dramatic rise in tobacco use.
Figure 2.

Example of tobacco advertising in the 1950s [5].
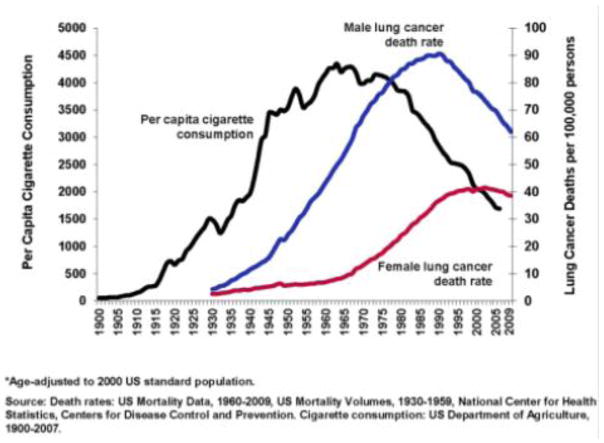
By 1950, the effects of many years of widespread smoking were beginning to become apparent as the incidence of lung cancer had increased dramatically [6]. Between 1922 and 1947, the number of deaths from lung cancer increased from 612 to 9,287, a nearly fifteen-fold increase in just 25 years (Figure 1) [6]. Several investigators began to study this dramatic rise in lung cancer. Among them, Richard Doll, a physician and statistician, and Bradford Hill, also a statistician, are credited with the first report of the association between tobacco use and lung cancer. While initially considering that environmental pollutants, such as exhaust fumes, surface dust of tarred roads, gas works, industrial plants, or coal fires, were to blame for the sudden increase in incidence of lung cancer, they quickly found a strong association with tobacco use suggesting that their initial suspicions were not related. In their monumental 1950 paper, they studied 1,732 patients with carcinoma of the lung, stomach, or large intestine and 743 general medical and surgical patients serving as controls [6]. Each of these patients was carefully queried regarding smoking habits at any period of their lives, ages at which they had started and stopped, the amount they were in the habit of smoking prior to disease onset, changes in their smoking history, the maximum they have ever been in the habit of smoking, the varying proportions smoked in pipes and cigarettes, and whether or not they inhaled. They found a clear association between smoking and the development of lung cancer [6]. Additionally, a relatively high proportion of patients with carcinoma of the lung fell into the heavier smoking category. That is, 26.0% of males and 14.6% of females with lung carcinoma reported using the equivalent of 25 or more cigarettes per day while only 13.5% of males and none of the female non-cancer control group smoked as much [6].
Figure 1.
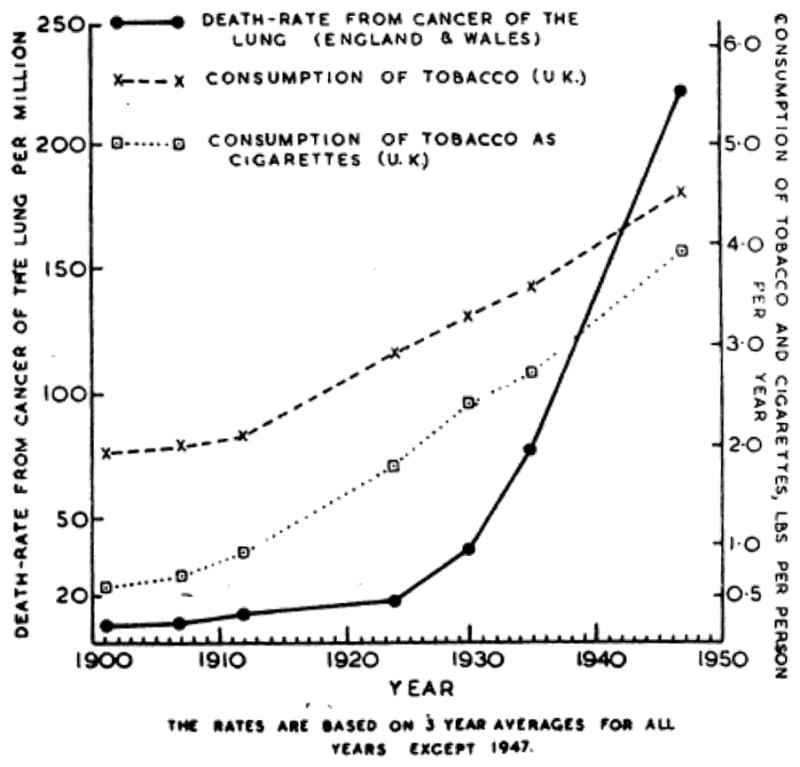
Death rate from cancer of the lung and rate of consumption of tobacco and cigarettes [6].
In 1952, Doll and Hill published an expanded report including 1,465 cases of lung cancer. They again concluded that smoking is an important factor in the production of lung cancer [7]. In 1954, Doll and Hill published the “British Doctors Study.” This study specifically investigated smoking habits and incidence of lung cancer in physicians. Doctors in the United Kingdom were asked to complete a survey describing their tobacco habits. They were asked whether they were smoking at that time, whether they had smoked but had quit, or whether they had never smoked regularly. Present smokers were asked ages at which they had started smoking, the amount of tobacco they were smoking, and the method of use. Ex-smokers were asked similar questions but relating to age at which they quit smoking. The death rate from lung cancer was found to be 0.00 per 1,000 in the non-smokers and 1.14 per 1,000 among the men categorized as heavy smokers. A similar but less pronounced effect was seen in the death rate from coronary artery thrombosis [8]. These monumental papers by Doll and Hill played a large role in the early understanding of the risks of tobacco and laid the foundation for decades of future tobacco research.
In 1954, Horn and Hammond published a large study demonstrating an increased risk of death from cancer and coronary disease in men who smoked cigarettes regularly [9]. Despite these data and other large studies, the tobacco industry continued to fight these allegations with advertisements such as the “Frank Statement to Cigarette Smokers” (Figure 3). This was a nationwide 2-page ad published in 448 newspapers across the nation [6]. It contained various statements aimed at discrediting the work associating tobacco with disease and questioning the scientists who had done the work. In addition, the “Frank Statement” communicated the Industry opinion that tobacco was not injurious to one’s health.
Figure 3.
“A Frank Statement to Cigarette Smokers.” Published in 1954 by the Tobacco Industry Research Committee.
In 1961, an alliance of private health organizations including the American Cancer Society, the American Heart Association, the National Tuberculosis Association, and the American Public Health Association called for a national commission to address the growing health issues associated with smoking [10]. The Kennedy Administration along with Luther Terry, MD, Surgeon General of the U.S. Public Health Service, responded by convening a committee to review the scientific literature on smoking. Members of the American Heart Association, National Tuberculosis Association, American Public Health Association, Food and Drug Administration, Federal Trade Commission, American Medical Association, and the Tobacco Institute (an industry trade group) were asked to nominate representatives. Ten representatives were eventually chosen representing fields such as medicine, surgery, pharmacology, and statistics. Members were only eligible if they had not previously taken a stand on tobacco use [10]. Between 1962 and 1964, the committee reviewed more than 7,000 scientific articles [10].
On January 11, 1964, Luther Terry, MD, released the Committee’s report concluding that cigarette smoking is a cause of lung and laryngeal cancer in men, a probable cause of lung cancer in women, and the most important cause of chronic bronchitis [11]. The report was presented on a Saturday to minimize the impact on financial markets while allowing the Sunday newspapers to quickly report the results. This report, which recently passed its 50th anniversary, was a watershed moment in the evolution of publicity and education regarding the harmful effects of tobacco use. What followed was a series of initiatives to inform consumers of the risks of tobacco use. The Federal Cigarette Labeling and Advertising Act of 1965 and the Public Health Cigarette Smoking Act of 1969 were important early regulatory efforts that required a health warning on cigarette packages, banned cigarette advertising on broadcasting media, and required annual reports on the health consequences of smoking [11].
Since these reports, continued measures to increase awareness of the dangers of tobacco have led to a steady decline in tobacco use. In the United States, various efforts including aggressive anti-tobacco advertising, limits in tobacco industry advertisements, increasing cigarette taxes, public smoking bans, provisions for tobacco cessation treatment, restrictions in youth access, and prevention education have helped to reduce tobacco consumption [12]. However, tobacco use continues to pose serious health risks. In 2015, 36.5 million adults in the United States smoked cigarettes [13], which, although significantly decreased from 52 million adults in 1964, continues to put millions of users at risk for multiple ailments [12]. Notably, 1.1 billion adults continue to use tobacco products worldwide [14].
Tobacco Products
Over the long history of tobacco production, many different forms have been developed. These include both combustible and smokeless products. Combustible tobacco products include cigarettes, cigars, bidis, chutta, and kretek (Table 1) [4]. Tobacco products can also be smoked using pipes or a variety of water pipes.
Table 1.
Different forms of tobacco products. Table from IARC Monograph [4].
| Cigarette | Any roll of tobacco wrapped in paper or other non-tobacco material; filter-tipped or untipped; approximately 8 mm in diameter, 70–120 mm in length. Any roll of tobacco wrapped in leaf tobacco or in any other substance containing tobacco. |
| Cigar | Types: little cigars, small cigars (‘cigarillos’), regular cigars, premium cigars. Some little cigars are filter tipped and are shaped like cigarettes. Regular cigars are up to 17 mm in diameter, 110–150 mm in length. |
| Bidi | Hand-rolled Indian cigarette; sun-dried temburni leaf rolled into a conical shape together with flaked tobacco and secured with a thread. |
| Chutta | Hand-rolled cigarette used for reverse smoking primarily by women in India. |
| Kretek | Small cigar containing tobacco (approximately 60%), cloves and cocoa. The burning blend gives a characteristic flavor and ‘honey’ taste to the smoke. |
Cigarettes and cigars use varying formulations of blended tobaccos. The specific type of tobacco blend can affect the nicotine and carcinogen content, thus impacting the toxicity of the smoke [4]. In the United States, cigarettes are the most popular form of tobacco product used. 36.5 million adults smoke cigarettes in the United States [13]. There are several varieties offered containing non-tobacco additives that appeal to different populations. Menthol and clove are the most common additives and these provide a distinct taste to the consumer. Cigarettes with mango, orange, cherry, or chocolate are also available. Waterpipes, such as hookahs, have been popular for centuries in Middle Eastern countries. However, they are becoming more popular in the United States and European countries as a social activity [15]. Water pipes are made in a variety of fashions to allow the smoke to bubble through water prior to inhalation. There is a common misconception that this process filters toxins and carcinogens, thus making waterpipe use safe. To the contrary, the use of a waterpipe has been associated with bronchogenic, oral, and bladder cancers, as well as cardiovascular and pulmonary disease [15]. Thus, evidence suggests that tobacco exposure through waterpipe use is not safer than standard combustible use.
Numerous smokeless tobacco products are also commercially available. These include chewing tobacco, snuff, snus, gutkha, and betel quid (Table 2) [15]. Many of the components that are added to smokeless products can create secondary effects or modulate the absorption rate of nicotine. Areca nut, which is commonly used in smokeless tobacco products in India and Southeast Asia, contains the alkaloid drugs arecoline, muscarine, and pilocarpine which cause cholinergic relaxing effects [15]. Betel quid is a combination of areca nut, betel leaf, and slacked lime and when tobacco is added it is called gutkha [15]. These products are chewed. The pH of a tobacco product is directly related to its rate of absorption. Buffering substances such as slacked lime or calcium carbonate are added to raise the pH, which increases the rate of nicotine absorption [15].
Table 2.
Different forms of smokeless tobacco. WHO [15].
| Chewing tobacco | Shredded like short cut grass. Generally mildly acidic and intended to be chewed throughout the day, as desired. |
| Snuff | Chopped into particles like large coffee grounds and moistened. Used by holding between gum and cheek. |
| Swedish snus | A variant of snuff that is processed differently. Typically more moist. Primarily used in India and Southeast Asia |
| Gutkha | Flavored and dry mixture of areca nut, catechu and slacked lime with tobacco and other condiments. |
| Betel quid | Tobacco added to paan. Areca nut is a common component. |
| Dry powdered tobacco | Particularly popular in England, northern Europe and parts of China in the 18th and 19th centuries. Snuffed into the nasal cavity |
Electronic cigarettes (E-cigarettes) have gained popularity at a significant pace since their patent in 2004 [16]. They are designed to simulate smoking a cigarette and often look similar to a cigarette. E-cigarettes are battery powered and heat liquid containing varying concentrations of nicotine, propylene glycol or glycerin, flavorings, and other chemicals to create a vapor that is inhaled [17]. While not recognized as a tobacco cessation agent by the US Food and Drug Administration, E-cigarettes are often marketed in this manner [16]. Since no combustion of tobacco takes place, E-cigarettes are felt to be a safer alternative to smoking cigarettes by eliminating the inhalation of harmful compounds [16]. However, multiple public health concerns exist including their potential to renormalize cigarette use in areas previously banned. In addition, there is concern regarding their potential appeal to adolescents and current nonsmokers, as well as the possibility for harmful exposure to E-cigarette constituents including flavorings, propylene glycol, or contaminants [17]. Continued active research in this area is necessary to more comprehensively understand the short and long-term effects of E–cigarette use.
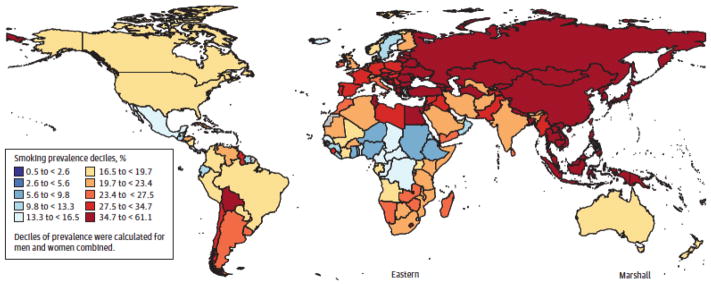
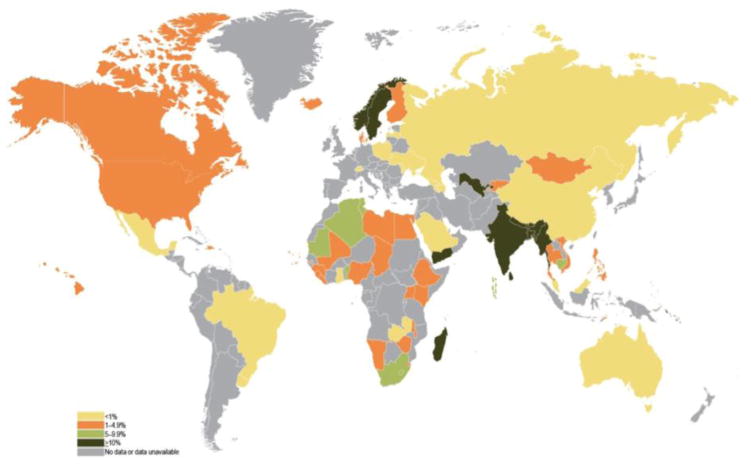
There is tremendous geographic variation in the type of tobacco product consumed worldwide. In the United States, 36.5 million adults smoke cigarettes while 8.6 million people over the age of twelve use smokeless tobacco, most of whom are between the ages of 18–24 [13,18]. The usage of smokeless tobacco has slowly been increasing in the United States [18]. Smoking prevalence varies widely throughout the world with the highest rates in Eastern European and Asian countries (Figure 6) [19]. Approximately 90% of the world’s smokeless tobacco consumption takes place in Southeast Asia (Figure 5) [18,20]. Nearly 100 million people use smokeless tobacco in India and Pakistan alone [18]. In India, the use of smokeless tobacco products is associated with many social activities. In addition, misconceptions are very common in regards to these products. For example, many smokeless products are thought to be useful as mouth fresheners and, as a result, are applied to the gingiva and teeth regularly which results in an increased risk for oral cancer. A well-known example of this phenomenon is gutkha, an Indian product that was previously espoused as a mouth freshener and is now banned in many Indian states. Still, additional smokeless products are widely available in India and are inexpensive. These two factors create a situation where everyday use by average citizens becomes widespread [20]. The cost may be so low that even school children can afford them [20]. In addition, marketing strategies aim to make these products more palatable and attractive. For example, the use of colorful packaging and various scents, sweeteners, and spices are used to attract buyers [20]. The use of appealing product names or names with religious significance, is also used to appeal to different social groups [20]. Due to the widespread use of smokeless tobacco products, 74% of the global burden of HNSCC occurs in India [21]. Indians develop HNSCC (oral cavity, lip, pharynx) at the highest rate in the world at 20 cases per 100,000 people [22].
Figure 6.
Age standardized smoking prevalence among men, 2012. Taken from JAMA. 2014;311(2):183–192 [20].
Figure 5.
Prevalence (%) of current use of smokeless tobacco in men and women. Taken from NIH Publication No. 14-7983; 2014 [19].
Epidemiological Data: Tobacco risk in Head and Neck Cancer
The odds ratio of developing HNSCC in smokers compared to never smokers is approximately 2.13 [23]. While cessation does lower the risk, it is unclear whether the risk returns to that of a never smoker [24]. Some data suggest that the risk returns to that of a never smoker after about 20 years of cessation [25].
Hashibe et al have extensively studied the carcinogenesis in the head and neck of tobacco and alcohol in humans. At least 75% of HNSCC in Europe, the United States, and other industrialized nations are attributable to the combinations of alcohol and tobacco [23]. However, the respective contributions of each risk factor can be difficult to understand as they are strongly associated with each other. In this study, Hashibe et al attempt to understand the independent associations of tobacco and alcohol with HNSCC. They performed a pooled analysis to investigate the extent to which head and neck cancer is associated with cigarette smoking in never drinkers and with alcohol drinking among never smokers. Data was pooled from 15 case control studies to include 10,244 head and neck cancer case subjects and 15,227 control subjects of whom 1,072 case subjects and 5,775 control subjects were never users of tobacco and 1,598 case subjects and 4,051 control subjects were never drinkers of alcohol. Among never drinkers, cigarette smoking was associated with an increased risk of HNSCC (OR for ever versus never smoking = 2.13, 95% CI = 1.52 to 2.98) [23]. In addition, they demonstrated a clear dose response relationship for the frequency, duration, and number of pack-years of cigarette smoking [23]. Approximately 24% (95% CI = 16% to 31%) of head and neck cancer cases among nondrinkers in this study would have been prevented if these individuals had not smoked cigarettes [23]. Clearly, tobacco smoking poses a risk for development of HNSCC in never drinkers.
Recently, Wyss et al studied the risk of cancer associated with several tobacco products. For combustible products, this revealed increased risks of HNSCC for cigarettes (OR = 3.46, 95% CI 3.24–3.70), cigars (OR = 2.54, 1.93–3.34), and pipes (OR = 2.08, 1.55–2.81) [26]. In regards to smokeless tobacco, the use of snuff has been associated with HNSCC (OR = 1.71, 1.08–2.70), particularly for oral cavity cancers (OR = 3.01, 1.63–5.55) [27]. Chewing tobacco was weakly associated with HNSCC among never smokers (OR = 1.20, 0.81–1.77) [27]. However, when restricted to oral cavity cancers, chewing tobacco had a stronger association (OR = 1.81, 1.04–3.17) [27]. Furthermore, while HPV-induced HNSCC is rising in incidence, a 2013 analysis of over 100,000 subjects showed 66% of HNSCC diagnoses were tobacco and alcohol related [28]. Thus, tobacco remains a prominent cause of HNSCC.
The synergistic effect of tobacco and alcohol is supported by analysis of pooled data from 17 European and American studies [29]. The population attributable risk was 72%, which included 4% for alcohol alone, 33% for tobacco alone and 35% attributable to both alcohol and tobacco. The odds ratios of developing HNSCC are 2.37 (1.66–3.39) for ever tobacco users among never alcohol users, 1.06 (0.88–1.28) for alcohol users among never tobacco users, and 5.73 (3.62–9.06) for alcohol and tobacco users [29]. This effect was more than multiplicative. Similar steep rises in the risk of HNSCC among alcohol and tobacco users, especially those using high amounts of each product, have been demonstrated by Dal Maso et al [30].
Tobacco Carcinogens
Since the Surgeon General’s report in 1964, there has been tremendous effort to study the carcinogenic effects of tobacco products. These research efforts have led to significant advances in understanding the constituents of tobacco products. To date, over 70 known carcinogens have been described in cigarette smoke (Table 3) [31].
Table 3.
Compounds in tobacco smoke that have been evaluated as carcinogens in either laboratory animals or humans by the International Agency for Research on Cancer. BaP, benzo[a]pyrene; NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; NNN, N′-nitrosonornicotine. Khariwala et al. 2012 [31].
| Chemical class | No. of compounds | Representative carcinogens | |
|---|---|---|---|
| Polycyclic aromatic hydrocarbons | 19 |
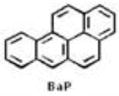
|
|
| Nitrosamines | 8 |
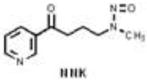
|
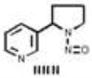
|
| Aromatic amines | 13 |
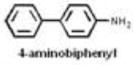
|
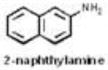
|
| Aldehydes | 2 | CH2 = 0 formaldehyde |
CH3CHO acetaldehyde |
| Various hydrocarbons | 6 |

|
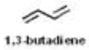
|
| Other organics | 15 |

|
|
| Inorganic compounds | 9 | cd | 2HPo |
| Total | 72 | ||
Of the many toxic and carcinogenic substances resulting from tobacco exposure, tobacco specific nitrosamines (TSNA) and polycyclic aromatic hydrocarbons (PAH) have been most heavily studied with regard to exposure and carcinogenicity. TSNA are found in tobacco and tobacco smoke and are formed during the curing and processing of tobacco. They are found in both combustible and smokeless forms of tobacco. Through various chemical reactions, TSNA can be formed (Figure 7) [32]. Seven TSNA have been identified in tobacco products: N′-nitrosonornicotine (NNN), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), N-nitrosoanatabine (NAT), N-nitrosoanabasine (NAB), 4-(methylnitrosamino)-4-(3-pyridyl)-1-butanol (iso-NNAL), and 4-(methylnitrosamino)-4-(3-pyridyl)butanoic acid (iso-NNAC) [32].
Figure 7.
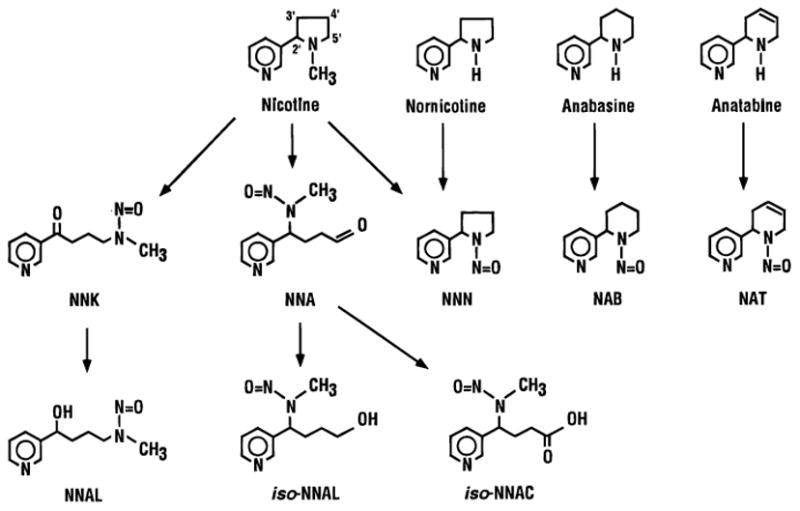
Structures of tobacco specific nitrosamines and tobacco alkaloid precursors. NNA, 4-(methylnitrosamino)-4-(3-pyridyl)butanal; NAB, N′-nitrosoanabasine; NAT, N′-nitrosoanatabine; NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; NNAC, 4-(methylnitrosamino)-4-(3-pyridyl)butanoic acid. Hecht, 1998 [32].
NNK, NNN, and NNAL are potent, well studied carcinogens [32]. NNAL is a metabolic product of NNK that is formed after a carbonyl reduction [32]. Numerous animal studies have implicated NNK and NNAL in the development of lung cancer. Administration of NNK in drinking water, by gavage, subcutaneous injection, intravesically, or by oral swabbing results in lung cancers in rats [32]. Interestingly, regardless of the method of administration, the carcinogenic effects seem to systematically target lung tissue as opposed to having local effects [32]. Administration of NNAL has also demonstrated lung carcinogenicity at similar levels as NNK, suggesting it is not solely a detoxification product of NNK [32]. Subcutaneous injection of NNK also leads to nasal tumors in rats, mainly olfactory neuroblastomas [32]. Furthermore, NNK has been demonstrated to play a role in liver and pancreas cancer development in rodents [32].
In rats, NNN reproducibly induces head and neck tumors. Subcutaneous or gavage administration of NNN to rats produces predominantly nasal tumors [32]. However, when administered in drinking water or liquid diet, NNN exposure results in oral, esophageal and nasal tumors [32]. This is likely, although not definitively demonstrated, due to direct contact with esophageal mucosa during swallowing. Interestingly, Balbo et al investigated the carcinogenic effects of NNN enantiomers, (S)-NNN and (R)-NNN, in rats [33]. Rats were administered either (S)-NNN, (R)-NNN, racemic NNN, or tap water (control group) in their drinking water. At necropsy, all rats treated with (S)-NNN developed head and neck tumors, including tongue, buccal mucosa, gingival mucosa, soft palate, epiglottis, and pharynx. They also developed a significant number of esophageal tumors. The number of tumors in the rats treated with (S)-NNN was significantly higher when compared to the (R)-NNN, and control groups. The number of tumors that developed in the (R)-NNN treated rats was not significantly different than the control group. Together, these data demonstrate that (S)-NNN is the predominant carcinogen pertaining to NNN exposure. Interestingly, tumor development in rats receiving racemic NNN in the oral cavity and esophagus was significantly greater than that in the (S)-NNN only group and greater than the added effects of (S)-NNN and (R)-NNN. This suggests that (R)-NNN acts as a co-carcinogen, synergistically enhancing the effects of (S)-NNN while itself being relatively inactive in terms of tumorgenecity [33]. This data is clinically relevant because (S)-NNN is the predominant form of NNN present in smokeless tobacco and is found at higher levels than combustible tobacco.
PAH represent a diverse group of carcinogenic compounds that share a similar structure of multiple benzene rings. Several PAH have also demonstrated carcinogenicity in animal models [4]. The most well studied constituents in this group include benzo[a]pyrene (BaP) and 1-hydroxypyrene (1-HOP) [24]. 1-HOP is a urinary metabolite of pyrene, which is always present in PAH mixtures [24]. Therefore, 1-HOP serves as a reliable biomarker for PAH exposure. While 1-HOP exposure is not specific to tobacco use, urinary levels are generally 2–3 times higher in smokers compared to non-smokers [24]. BaP has been shown to cause skin cancers when topically applied and it causes local tumors when administered subcutaneously. When given orally, PAH result in tongue and esophageal cancers, tumors of the upper respiratory tract, including nose, larynx and trachea. When given by inhalation, tumors are found in the upper digestive tract including the pharynx, esophagus, and forestomach [24].
In addition to significant work in animals, there have also been several human studies that have evaluated the risk of cancer associated with exposure to NNAL, NNN, and 1-HOP. As described above, when ingested, NNK is metabolically converted to NNAL [24]. NNAL is glucuronidated in humans to produce a mixture of glucuronides, NNAL-Glucs. Although NNK is not detectable in human urine due to its extensive metabolism, total NNAL can be detected [24].
Two prospective cohorts, the Shanghai Cohort Study and the Singapore Chinese Health Study, have allowed us to gain valuable epidemiologic data regarding the use of NNN and NNAL to inform cancer risk [34]. The Shanghai Cohort consisted with 18,244 men enrolled from January 1, 1986 to September 30, 1989, who were between 45 and 64 years of age and resided in one of four small geographically defined communities in Shanghai, China. Interviews were conducted to obtain information on tobacco use, alcohol use, diet, and medical history. In addition, a 10 mL blood sample and urine sample was obtained. The Singapore Chinese Health Study included 63,257 Cantonese and Hokkien Chinese men and women in Singapore that were enrolled between April 1, 1993 and December 31, 1998. These subjects were between 45 and 74 years of age and resided in government-built housing estates. Each subject in these cohorts was interviewed using a structured questionnaire that requested information on demographics, tobacco use, alcohol use, physical activity, reproductive histories, occupational exposure, medical history, family history of cancer, dietary habits. Blood and urine samples were also obtained. In 2000, follow up biospecimens were obtained and follow up surveys were obtained to update tobacco, alcohol, medical, and medication information. Both of these cohorts have been followed to monitor for cancer development.
Yuan et al demonstrated a relationship between urinary NNAL levels and development of lung cancer [34]. 246 cases of incident lung cancer and 245 matched controls were identified using the Shanghai Cohort Study and the Singapore Chinese Health Study data. Control subjects were matched for smoking status, age, dialect group, date of original interview, and date of biospecimen collection. Study subjects were divided into tertiles based on urinary NNAL levels. After adjusting for self-reported smoking history, smokers in the highest tertiles of urinary total NNAL and cotinine exhibited a 8.5-fold increased risk for lung cancer relative to smokers with comparable smoking history [34].
A similar study investigated the role of NNN in the carcinogenesis of esophageal cancer. Using the Shanghai Cohort Study, 77 patients with esophageal cancer and 223 individually matched controls were studied. Urinary total NNN was significantly higher, whereas the percentage of its detoxification product (NNN-N-glucuronide) was significantly lower, in patients with esophageal cancer [35]. After adjusting for smoking intensity and duration, odds ratios of esophageal cancer in the second and third tertiles of urinary NNN were 3.99 (1.25–12.7) and 17.0 (3.99–72.8), respectively [35]. This suggests that both increased exposure and reduced detoxification are important considerations in determining tobacco-induced cancer risk. Mechanistically, this work suggests that less detoxification, perhaps due to genetic predisposition, in some smokers increases the risk of esophageal carcinoma.
More recently, a matched control study was performed to study tobacco-related carcinogens in HNSCC. Urinary levels of 1-HOP, NNN, and NNAL were measured in smokers with a new diagnosis of HNSCC and compared to smokers without cancer. Levels of 1-HOP and NNN were elevated in the smokers with HNSCC compared to controls matched on multiple variables, including age, gender, self-reported cigarettes used per day [24]. Total NNAL did not differ between the smokers with and without HNSCC. While the sample size was relatively small, the data suggest that 1-HOP and NNN may play a greater role in HNSCC than NNAL.
Additional data obtained in patients with HNSCC examined the relationship between urinary tobacco-related carcinogens and self-reported tobacco use. This study also included an examination of urinary cotinine, which as a metabolite of nicotine, as the most robust measure of actual tobacco exposure [36]. This study of 84 smokers with HNSCC showed that self-reported tobacco use was a poor measure of actual carcinogen exposure in this population. Instead, urinary cotinine was more strongly correlated with urinary levels of NNN, NNAL and 1-HOP [36]. The reasons for this are likely multiple but relate to the inherent loss of accuracy when employing self-report: subjects may misremember or misrepresent for a variety of reasons. This study also showed correlation between the carcinogens (NNN, NNAL, 1-HOP) themselves, suggesting that exposure is proportional in this population.
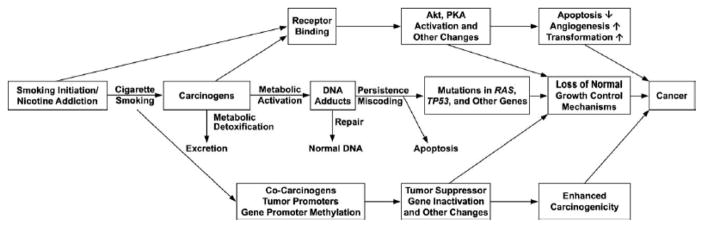
While these data clearly implicate tobacco in the carcinogenicity of HNSCC, not all people who use tobacco products will develop a HNSCC. There are complex mechanisms of genetic predisposition, carcinogen metabolism and excretion, immunologic competency, and genetic alterations that allow some tobacco users to progress along the carcinogenesis pathway (Figure 8) to develop a cancer while others are able to exit this pathway, for example by excreting the carcinogens or repairing the DNA damage[37].
Figure 8.
Complex relationship between tobacco, metabolism, genetic alterations, and DNA repair in carcinogenesis. Hecht, 2003 [37]
Processes such as biotransformation, detoxification, and elimination of carcinogens, together with DNA repair mechanisms and apoptotic pathways are the most important mechanisms of defense against carcinogenesis [38]. Genetic alterations in any of the above defense mechanisms can alter the development of HNSCC. Polymorphisms in the genes encoding for enzymes involved in biotransformation of carcinogens, such as cytochrome P-450s, glutathione S-transferases, UDP glucoronyltransferases, aldehyde dehydrogenase, and alcohol dehydrogenase, have been associated with HNSCC risk [38]. Additionally, polymorphisms of the DNA repair mechanisms, base excision repair, nucleotide excision repair, mismatch repair, and recombination repair, are associated with cancers [38]. Similar associations have been identified with polymorphism of genes involved with apoptosis [38]. Mutations of p53, CDK4, EGFR, PI3K, mTOR, NOTCH, among many others have also been associated with risk of HNSCC [39].
A common downstream mechanism for most carcinogens is the binding of DNA to form DNA adducts. DNA adducts are created when a cancer causing agent binds and disrupts the double-helical DNA structure. If left unrepaired, DNA adducts can cause miscoding and permanent mutations which can activate oncogenes such as K-ras, or inactivate tumor suppressor genes such as p53 [31]. 2′ hydroxylation of NNN is thought to be the major mechanism of metabolic activation of NNN. Once activated, NNN leads to formation of pyridyloxobutyl (POB)-DNA adducts [40]. Under strong acid hydrolysis conditions, the majority of POB-DNA adducts decompose to release 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB) [41–43]. In vitro and laboratory animal studies have demonstrated the importance of HPB-releasing adducts in NNK- and NNN-induced carcinogenesis [32,44]. Earlier, we described the enhanced carcinogenicity of (S)-NNN compared to (R)-NNN. It has been demonstrated that (S)-NNN is preferentially metabolized by 2′ hydroxylation whereas there is no preference for 2′ hydroxylation versus 5′ hydroxylation in the metabolism of (R)-NNN. This allows (S)-NNN to produce more (POB)-DNA adducts than (R)-NNN [40]. NNK, NNAL, and 1-HOP also form DNA adducts in their pathway towards carcinogenesis [31,45].
We have recently quantified the level of DNA adduct formation occurring in the oral cavity of smokers who have developed HNSCC. An analysis of the DNA in buccal cells was performed in a cohort of smokers with HNSCC to determine the level of HPB-releasing DNA adducts present at the time of diagnosis. These levels in HNSCC patients were compared to that found in cancer-free smokers. Our results demonstrated significantly higher levels of DNA adduct formation in those smokers who have developed a HNSCC (Geometric mean comparing cancer patients to cancer-free controls= 20) [46]. This information suggests that some smokers are more susceptible to the carcinogens in tobacco products than others. Further analysis of the carcinogen exposure in each group revealed that smokers in each group were not different with respect to their nitrosamine exposure (manuscript in preparation). Analysis of cotinine was similar in both groups as well, again suggesting equal exposure to nicotine in both groups. Thus, it appears that the difference in DNA adduct levels in smokers with HNSCC is due to an intrinsic difference in carcinogen processing, DNA adduct formation or DNA repair in some smokers. Future work in this area will aim to address the genetic variation among smokers that may lead to a reduced ability to detoxify the carcinogen exposure that occurs following tobacco use. It is possible that future genetic analysis as it pertains to tobacco processing may help to identify those smokers who are at greatest risk of HNSCC.
Tobacco-related effects on the treatment of head and neck cancer
In addition to the carcinogenic effects of tobacco, numerous studies have demonstrated negative effects on a variety of treatment-related outcomes among smokers with HNSCC. These include radiation efficacy, surgical outcomes, and wound complications. Chen et al conducted a matched control study of patients undergoing radiation therapy for HNSCC and evaluated the effects of smoking on treatment outcomes. They identified 101 patients with newly diagnosed HNSCC who continued to smoke during radiation therapy between 1999 and 2008. Each of these patients was matched to a control patient who had quit smoking prior to beginning radiation therapy. Subjects were matched on tobacco use, primary site of cancer, age, sex, performance status, disease stage, radiation dose, chemotherapy use, year of treatment, and whether surgical resection was performed. Never smokers were excluded from this study. Patients who continued smoking throughout radiation demonstrated significantly inferior 5-year overall survival (23% vs. 55%), locoregional control (58% vs. 69%), and disease-free survival (42% vs. 65%) [47].
In addition to impacting disease outcomes, tobacco use can also affect wound healing and more acute surgical outcomes. Hatcher et al investigated the risk of tobacco use on post-operative surgical outcomes. In their study, they included 129 patients with newly diagnosed HNSCC who were scheduled to undergo a major surgical procedure from June 2011 to October 2012. These patients were asked to complete a questionnaire to assess demographic factors, history of smoking, current cigarette use, ever use of other tobacco products (smokeless, pipes, cigars, and others), and current use of other tobacco products. On the day of surgery, recent tobacco use was assessed by urinary cotinine. Patients undergoing surgical treatment for HNSCC who were current or former smokers were 6 times more likely to have a complication (vascular, pulmonary, renal, acute blood loss anemia, ethanol withdrawal, wound complications, and other complications such as urinary tract infection or delirium) and a longer length of stay than never smokers [48].
Similarly, Marin et al studied wound healing in smokers undergoing free tissue transfer. Smoking has been demonstrated to impair wound healing and end organ oxygen delivery [49]. Researchers believe that smoking impairs endothelial migration at surgical anastomosis and can lead to luminal thrombosis after free tissue transfer [49]. Nicotine also has direct vasoconstrictive effects on the microvascular circulation [49]. Eighty-nine patients undergoing 101 free flap reconstructions were enrolled in a prospective epidemiologic study of HNSCC (oral cavity, oropharynx, larynx, hypopharynx). Prior to treatment, all patients completed a questionnaire regarding demographics and tobacco exposure information. Patients classified themselves as never smoker (<100 cigarettes in lifetime), former smoker (quit at least one year previously), or current smoker. Each patient also donated blood for preoperative biomarker testing. Patients with a high serum cotinine concentration (>50 ng/mL) had the highest complication rates (59.3%) and those with intermediate concentration (11 to 50 ng/mL) had a modestly elevated complication rate (30.8%) (p=0.045) [49]. The authors concluded that a serum cotinine concentration greater than 10 ng/ml may predict an increased risk of wound complication in head and neck flap reconstruction [49]. Interestingly, there was no association between self-reported tobacco use and wound complications [49].
Conclusion
Worldwide, head and neck cancer is a significant problem. Tobacco products, both smokeless and combustible, play a large role in the development of HNSCC in addition to leading to poorer treatment outcomes. Data in the 20th century has helped to usher in an era of increased regulation and decreased product consumption. Still, tobacco is used throughout the world and, in some pockets, at very high rates. Future efforts to study and regulate tobacco production and sale continue to serve vital importance.
Figure 4.
Trends in tobacco use and lung cancer deaths in the United States, 1900–2009. ACS 2013.
Footnotes
Conflicts of Interest: None
References
- 1.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371(9625):1695–1709. doi: 10.1016/s0140-6736(08)60728-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridge J. Head and Neck Tumors. 2016 http://www.cancernetwork.com/cancer-management/head-and-neck-tumors.
- 3.Global Burden of Disease Cancer C. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 5.Borio G. Tobacco Timeline. 2004 http://archive.tobacco.org/History/Tobacco_History.html.
- 6.Doll R, Hill AB. Smoking and carcinoma of the lung; preliminary report. Br Med J. 1950;2(4682):739–748. doi: 10.1136/bmj.2.4682.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doll R, Hill AB. A study of the aetiology of carcinoma of the lung. Br Med J. 1952;2(4797):1271–1286. doi: 10.1136/bmj.2.4797.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doll R, Hill AB. The mortality of doctors in relation to their smoking habits; a preliminary report. Br Med J. 1954;1(4877):1451–1455. doi: 10.1136/bmj.1.4877.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammond EC, Horn D. The relationship between human smoking habits and death rates: a follow-up study of 187,766 men. J Am Med Assoc. 1954;155(15):1316–1328. doi: 10.1001/jama.1954.03690330020006. [DOI] [PubMed] [Google Scholar]
- 10. [Accessed 1/24/2017];The 1964 report on smoking and health. https://profiles.nlm.nih.gov/ps/retrieve/Narrative/NN/p-nid/60.
- 11.Office on Smoking and Health, N. C. f. C. D. P. a. H. P. [Accessed 1/24/2017];History of the surgeon generals reports on smoking and health. https://www.cdc.gov/tobacco/data_statistics/sgr/history/
- 12.Medicine, T. I. o. Ending the tobacco problem: A blueprint for the nation. The National Academic Press; 2007. [Google Scholar]
- 13.Office on Smoking and Health, N. C. f. C. D. P. a. H. P. Current Cigarette Smoking Among Adults in the United States. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/
- 14.Data, G. H. O. Prevalance of Tobacco Smoking. [Accessed 1/24/2017]. [Google Scholar]
- 15.Organization, W. H. Tobacco: deadly in any form or disguise. World Health Organization; 2006. [Google Scholar]
- 16.Franck C, Budlovsky T, Windle SB, Filion KB, Eisenberg MJ. Electronic cigarettes in North America: history, use, and implications for smoking cessation. Circulation. 2014;129(19):1945–1952. doi: 10.1161/circulationaha.113.006416. [DOI] [PubMed] [Google Scholar]
- 17.Rigotti NA. e-Cigarette Use and Subsequent Tobacco Use by Adolescents: New Evidence About a Potential Risk of e-Cigarettes. Jama. 2015;314(7):673–674. doi: 10.1001/jama.2015.8382. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J, Michaud DS, Langevin SM, McClean MD, Eliot M, Kelsey KT. Smokeless tobacco and risk of head and neck cancer: evidence from a case-control study in New England. Int J Cancer. 2013;132(8):1911–1917. doi: 10.1002/ijc.27839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. Jama. 2014;311(2):183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 20.Smokeless Tobacco and Public Health: A Global Perspective. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Institutes of Health; 2014. (NIH Publication No. 14-7983) [Google Scholar]
- 21.Siddiqi K, Shah S, Abbas SM, Vidyasagaran A, Jawad M, Dogar O, et al. Global burden of disease due to smokeless tobacco consumption in adults: analysis of data from 113 countries. BMC Med. 2015;13:194. doi: 10.1186/s12916-015-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coelho KR. Challenges of the oral cancer burden in India. J Cancer Epidemiol. 2012;2012:701932. doi: 10.1155/2012/701932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99(10):777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 24.Khariwala SS, Carmella SG, Stepanov I, Fernandes P, Lassig AA, Yueh B, et al. Elevated levels of 1-hydroxypyrene and N′-nitrosonornicotine in smokers with head and neck cancer: A matched control study. Head Neck. 2013;35(8):1096–1100. doi: 10.1002/hed.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winn DM, Lee YC, Hashibe M, Boffetta P. The INHANCE consortium: toward a better understanding of the causes and mechanisms of head and neck cancer. Oral Dis. 2015;21(6):685–693. doi: 10.1111/odi.12342. [DOI] [PubMed] [Google Scholar]
- 26.Wyss A, Hashibe M, Chuang SC, Lee YC, Zhang ZF, Yu GP, et al. Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Am J Epidemiol. 2013;178(5):679–690. doi: 10.1093/aje/kwt029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wyss AB, Hashibe M, Lee YA, Chuang SC, Muscat J, Chen C, et al. Smokeless Tobacco Use and the Risk of Head and Neck Cancer: Pooled Analysis of US Studies in the INHANCE Consortium. Am J Epidemiol. 2016 doi: 10.1093/aje/kww075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashibe M, Hunt J, Wei M, Buys S, Gren L, Lee YC. Tobacco, alcohol, body mass index, physical activity, and the risk of head and neck cancer in the prostate, lung, colorectal, and ovarian (PLCO) cohort. Head Neck. 2013;35(7):914–922. doi: 10.1002/hed.23052. [DOI] [PubMed] [Google Scholar]
- 29.Hashibe M, Brennan P, Chuang SC, Boccia S, Castellsague X, Chen C, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(2):541–550. doi: 10.1158/1055-9965.epi-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dal Maso L, Torelli N, Biancotto E, Di Maso M, Gini A, Franchin G, et al. Combined effect of tobacco smoking and alcohol drinking in the risk of head and neck cancers: a re-analysis of case-control studies using bi-dimensional spline models. Eur J Epidemiol. 2016;31(4):385–393. doi: 10.1007/s10654-015-0028-3. [DOI] [PubMed] [Google Scholar]
- 31.Khariwala SS, Hatsukami D, Hecht SS. Tobacco carcinogen metabolites and DNA adducts as biomarkers in head and neck cancer: potential screening tools and prognostic indicators. Head Neck. 2012;34(3):441–447. doi: 10.1002/hed.21705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11(6):559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 33.Balbo S, James-Yi S, Johnson CS, O’Sullivan MG, Stepanov I, Wang M, et al. (S)-N’-Nitrosonornicotine, a constituent of smokeless tobacco, is a powerful oral cavity carcinogen in rats. Carcinogenesis. 2013;34(9):2178–2183. doi: 10.1093/carcin/bgt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan JM, Koh WP, Murphy SE, Fan Y, Wang R, Carmella SG, et al. Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Res. 2009;69(7):2990–2995. doi: 10.1158/0008-5472.can-08-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan JM, Knezevich AD, Wang R, Gao YT, Hecht SS, Stepanov I. Urinary levels of the tobacco-specific carcinogen N’-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis. 2011;32(9):1366–1371. doi: 10.1093/carcin/bgr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khariwala SS, Carmella SG, Stepanov I, Bandyopadhyay D, Nelson HH, Yueh B, et al. Self-reported Tobacco use does not correlate with carcinogen exposure in smokers with head and neck cancer. Laryngoscope. 2015;125(8):1844–1848. doi: 10.1002/lary.25290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3(10):733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 38.Lacko M, Braakhuis BJ, Sturgis EM, Boedeker CC, Suarez C, Rinaldo A, et al. Genetic susceptibility to head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2014;89(1):38–48. doi: 10.1016/j.ijrobp.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 39.Riaz N, Morris LG, Lee W, Chan TA. Unraveling the molecular genetics of head and neck cancer through genome-wide approaches. Genes Dis. 2014;1(1):75–86. doi: 10.1016/j.gendis.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lao Y, Yu N, Kassie F, Villalta PW, Hecht SS. Analysis of pyridyloxobutyl DNA adducts in F344 rats chronically treated with (R)- and (S)-N′-nitrosonornicotine. Chem Res Toxicol. 2007;20(2):246–256. doi: 10.1021/tx060208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hecht SS, Spratt TE, Trushin N. Evidence for 4-(3-pyridyl)-4-oxobutylation of DNA in F344 rats treated with the tobacco-specific nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and N′-nitrosonornicotine. Carcinogenesis. 1988;9(1):161–165. doi: 10.1093/carcin/9.1.161. [DOI] [PubMed] [Google Scholar]
- 42.Wang M, Cheng G, Sturla SJ, Shi Y, McIntee EJ, Villalta PW, et al. Identification of adducts formed by pyridyloxobutylation of deoxyguanosine and DNA by 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone, a chemically activated form of tobacco specific carcinogens. Chem Res Toxicol. 2003;16(5):616–626. doi: 10.1021/tx034003b. [DOI] [PubMed] [Google Scholar]
- 43.Stepanov I, Muzic J, Le CT, Sebero E, Villalta P, Ma B, et al. Analysis of 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB)-releasing DNA adducts in human exfoliated oral mucosa cells by liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem Res Toxicol. 2013;26(1):37–45. doi: 10.1021/tx300282k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hecht SS, Stepanov I, Carmella SG. Exposure and Metabolic Activation Biomarkers of Carcinogenic Tobacco-Specific Nitrosamines. Acc Chem Res. 2016;49(1):106–114. doi: 10.1021/acs.accounts.5b00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S, Wang M, Villalta PW, Lindgren BR, Upadhyaya P, Lao Y, et al. Analysis of pyridyloxobutyl and pyridylhydroxybutyl DNA adducts in extrahepatic tissues of F344 rats treated chronically with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem Res Toxicol. 2009;22(5):926–936. doi: 10.1021/tx900015d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma B, Ruszczak C, Jain V, Khariwala SS, Lindgren B, Hatsukami DK, et al. Optimized Liquid Chromatography Nanoelectrospray-High-Resolution Tandem Mass Spectrometry Method for the Analysis of 4-Hydroxy-1-(3-pyridyl)-1-butanone-Releasing DNA Adducts in Human Oral Cells. Chem Res Toxicol. 2016;29(11):1849–1856. doi: 10.1021/acs.chemrestox.6b00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen AM, Chen LM, Vaughan A, Sreeraman R, Farwell DG, Luu Q, et al. Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int J Radiat Oncol Biol Phys. 2011;79(2):414–419. doi: 10.1016/j.ijrobp.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 48.Hatcher JL, Sterba KR, Tooze JA, Day TA, Carpenter MJ, Alberg AJ, et al. Tobacco use and surgical outcomes in patients with head and neck cancer. Head Neck. 2016;38(5):700–706. doi: 10.1002/hed.23944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marin VP, Pytynia KB, Langstein HN, Dahlstrom KR, Wei Q, Sturgis EM. Serum cotinine concentration and wound complications in head and neck reconstruction. Plast Reconstr Surg. 2008;121(2):451–457. doi: 10.1097/01.prs.0000297833.53794.27. [DOI] [PubMed] [Google Scholar]