Abstract
Purpose of review
SH2 domain-containing tyrosine phosphatase 2 (SHP2), encoded by PTPN11 plays an important role in regulating signaling from cell surface receptor tyrosine kinases during normal development as well as oncogenesis. Herein we review recently discovered roles of SHP2 in normal and aberrant hematopoiesis along with novel strategies to target it.
Recent findings
Cell autonomous role of SHP2 in normal hematopoiesis and leukemogenesis has long been recognized. The review will discuss the newly discovered role of SHP2 in lineage specific differentiation. Recently, a non-cell autonomous role of oncogenic SHP2 has been reported in which activated SHP2 was shown to alter the bone marrow microenvironment resulting in transformation of donor derived normal hematopoietic cells and development of myeloid malignancy. From being considered as an ‘undruggable’ target, recent development of allosteric inhibitor has made it possible to specifically target SHP2 in receptor tyrosine kinase driven malignancies.
Summary
SHP2 has emerged as an attractive target for therapeutic targeting in hematological malignancies for its cell autonomous and micro-environmental effects. However a better understanding of the role of SHP2 in different hematopoietic lineages and its crosstalk with signaling pathways activated by other genetic lesions is required before the promise is realized in the clinic.
Keywords: SHP2, Ptpn11, myeloid malignancy, hematopoiesis
Introduction
SH2 domain-containing tyrosine phosphatase 2 (SHP2), a non-receptor tyrosine phosphatase is encoded by PTPN11 gene. It is a positive regulator of signaling downstream of several receptor tyrosine kinases such as cKIT and FLT3 [1,2*]. Recruitment of SHP2 to an activated receptor releases the self-inhibitory conformation and leads to catalytic activation of its phosphatase domain. In addition to its function as a phosphatase, SHP2 also serves as a docking protein to recruit other signaling intermediates through the two amino terminus SH2 domains. Since SHP2 is a positive regulator of cellular signaling leading to proliferation, differentiation and survival, its constitutive activation is associated with oncogenesis.
SHP2 in Hematopoiesis
Normal hematopoietic development and homeostasis is maintained by cell to cell interactions between cells of the hematopoietic system and their surroundings as well as through soluble mediators that include growth factors, cytokines and chemokines. Collectively these factors constitute a complex niche in which hematopoietic stem cells reside and wherein their function is governed by both cell autonomous genetic programs and niche properties [3]. Given that SHP2 plays a role in signaling through multiple tyrosine kinases in response to different cytokines, deregulation of SHP2 has broad consequences on hematopoiesis [4,5]. Mouse models with conditional deletion of Ptpn11, the gene encoding SHP2 have conclusively established an indispensable role for SHP2 in regulating normal hematopoietic stem cell (HSC) function [6,7]. According to general consensus, SHP2 is a positive regulator of hematopoiesis and loss of SHP2 or decrease in its catalytic activity is associated with reduced stem and progenitor cell numbers and function. Reciprocal transplantation experiments have shown that the defects in HSC function due to loss of SHP2 are primarily cell autonomous with no significant involvement of the bone marrow microenvironment [6,7]. Likewise, in human CD34+ cord blood cells, knockdown of SHP2 has been associated with decrease in cell growth and colony formation [8]. A similar reduction in colony formation were observed upon expression of SHP2 with a point mutation, resulting in loss of phosphatase function in human CD34+ cord blood cells [9]. Conversely, a gain of phosphatase function mutant of SHP2 promoted colony formation in this study. Interestingly, expression of a phosphatase domain truncated version of SHP2 with adaptor function intact functioned in a dominant negative manner [9]. These mouse models and human cord blood studies indicate that phosphatase function of SHP2 is integral to normal HSC function.
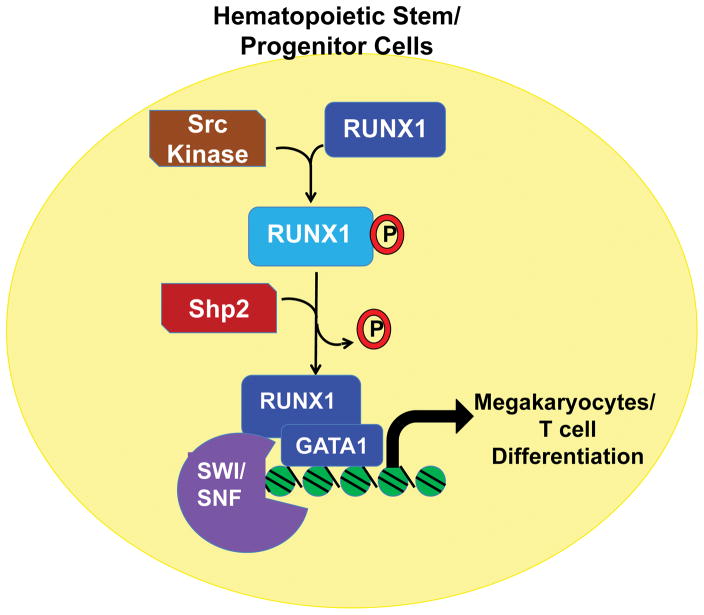
At the molecular level, modulation in the expression of transcription factors such as GATA2, C/EBPα and induction of p53 independent apoptosis in the stem and progenitor cell compartment have been implicated in deregulation of HSC number and function in response to loss of SHP2 [6,7]. Thus far, downregulation of the Ras- extracellular regulated kinase (ERK) signaling axis in the absence of SHP2 is considered as the major mediator of the above described molecular changes. Recently RUNX1, a master regulator of hematopoiesis, has been identified as a direct target of SHP2 phosphatase activity [10]. In the progenitor cells, RUNX1 is phosphorylated by Src family kinases (SFK) and removal of this phosphorylation by SHP2 is required during megakaryocyte differentiation [10]. The dephosphorylation of RUNX1 enabled its association with the transcription factors such as GATA1 and SWI/SNF chromatin remodeling complex for execution of megakaryocyte terminal differentiation program (Figure 1) [10]. Consistent with these findings, conditional deletion of Ptpn11 in megakaryocyte lineage cells is associated with suppression of thrombopoietin and integrin signaling, reduction in proplatelets and development of macrothrombocytopenia [11]. In addition to the megakaryocyte lineage, dephosphorylation of RUNX1 is also required for differentiation of T cells into CD8+ single positive cells [10]. Thymocytes with deletion of Ptpn11 show increase in RUNX1 phosphorylation and defects in the development of CD8+ cells [10]. Thus SHP2 plays an important role in regulating the transcriptional activity of RUNX1 during cellular differentiation through its phosphatase activity. Level of RUNX1 activity then determines the differentiation of the progenitor cells along either megakaryocyte or erythroid lineage. While RUNX1 promotes development of megakaryocytes, inhibits erythroid development through repression of KLF1 [12].
Figure 1.
SHP2 regulates RUNX1 interaction with GATA1 and SWI/SNF chromatin remodeling complex during megakaryocyte and T cell differentiation. The auto-inhibitory domain of RUNX1 is phosphorylated by SFK in progenitor cells and is dephosphorylated by SHP2.
In line with this, expression of oncogenic Ptpn11 that is constitutively active blocks erythrocyte differentiation and maturation suggesting that downregulation of SHP2 activity is required for erythrocyte differentiation [13]. Interestingly, a similar block in erythrocyte differentiation was observed as a result of loss of SHP2 activity in the context of phosphatase and tensin homolog (PTEN) deficiency [14*]. Contrasting results have been reported on the role of MAPK\ERK activation in erythropoiesis due to loss or gain of function of SHP2. In one study, MEK inhibitor U0126 inhibited the hyperproliferation of erythroblasts [13] while the other study using trametinib, another MEK inhibitor, phenocopied the loss of SHP2 mediated defect in erythropoiesis [14*]. It is possible that these differences are due to off target effects of the different MEK inhibitors used in the two studies. Further studies are needed to clarify these contradictory observations and further clarify the role SHP2/ERK axis in erythropoiesis. These cell lineage specific functions of SHP2 are going to be valuable while designing strategies to target SHP2 in hematological malignancies.
SHP2 in Leukemogenesis
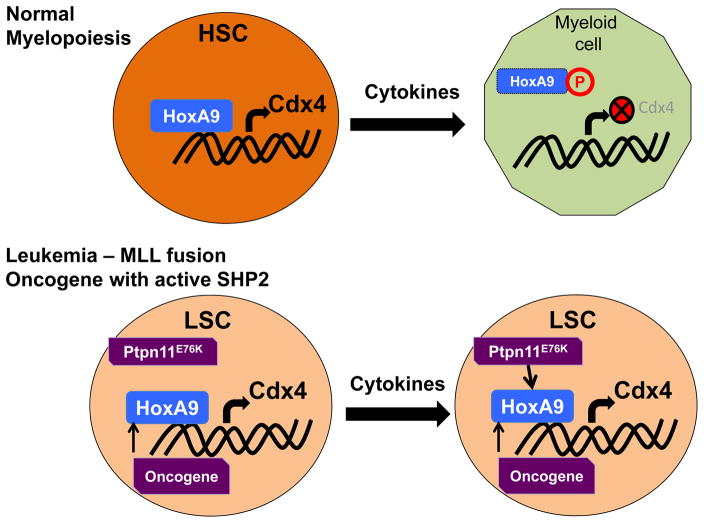
SHP2 is the first phosphatase to be recognized as a bonafide oncogene. Constitutive activation of SHP2 in the hematopoietic stem and progenitor compartment due to point mutations in its N-terminus SH2 domain results in the development of cell autonomous leukemia in different cell lineages independent of the stage of differentiation [15]. In addition to the hematopoietic compartment, PTPN11 has been recognized as an oncogene in 41 different cancer types with Q510, A72, E76 and G503 identified as hotspots of mutation in a panel of 119 tumors [16]. Interestingly, all these mutations have a positive impact on the phosphatase activity of SHP2 through either reducing regulatory N-SH2 domain (A72, E76) interaction with the catalytic domain or alter the active site (G503, Q510) [17,18]. Mutations in PTPN11 that lead to alterations in the catalytic activity of SHP2 have been implicated in pathogenesis of Noonan syndrome (NS), Leopard syndrome (LS) and juvenile myelomonocytic leukemia (JMML) [4,5]. PTPN11 is also frequently mutated in secondary acute myeloid leukemia (AML) [19], relapsed pediatric AML [20] and acute lymphoblastic leukemia (ALL) [21]. The frequency of PTPN11 mutations in AML is higher in patients older than 60yrs [22] and also has prognostic significance. Secondary AML patients carrying mutations in PTPN11 show rapid disease progression and reduced overall survival [19]. In AML, PTPN11G503A mutation has been reported to co-occur with mixed lineage leukemia (MLL) translocation, MLL-AF10. In a mouse model co-expression of MLL-AF10 and PTPN11G503A resulted in accelerated disease development as compared to MLL-AF10 alone [23**]. Increase in leukemia stem cell frequency along with rapid AML development is also seen when Ptpn11E76K is co-expressed with MLL-AF9 fusion oncogene [24]. In presence of mutant PTPN11, the leukemic cells show increase in the transcription of colony stimulating factor 1 (Csf1) and secretion of macrophage colony stimulating factor (M-CSF) resulting in enhanced differentiation of HSCs into myeloid lineage cells [23**]. Although co-expression of MLL-AF9 with constitutively active SHP2 does not alter the expression of Meis1 or HoxA9 [24], it can reverse cytokine induced tyrosine phosphorylation of HoxA9 and HoxA10 leading to sustained expression of CDX4, a homeodomain transcription factor (Figure 2) [25]. During normal myelopoiesis CDX4 is downregulated as HSCs differentiate into myeloid cells. Thus its sustained high expression would inhibit differentiation and contribute to more stem cell like phenotype of leukemic cells. AML cells co-expressing mutant Ptpn11 with MLL fusion oncogenes are also more resistant to Mcl-1 inhibitor and daunorubicin [23**,24]. These studies provide insights into how co-existence of PTPN11 mutations can alter disease progression and drug resistance.
Figure 2.
Constitutive activation of SHP2 suppresses the phosphorylation of HoxA9 in response to differentiation inducing cytokines during myelopoiesis. This leads to increased expression of CDX4 transcription factor and block in differentiation.
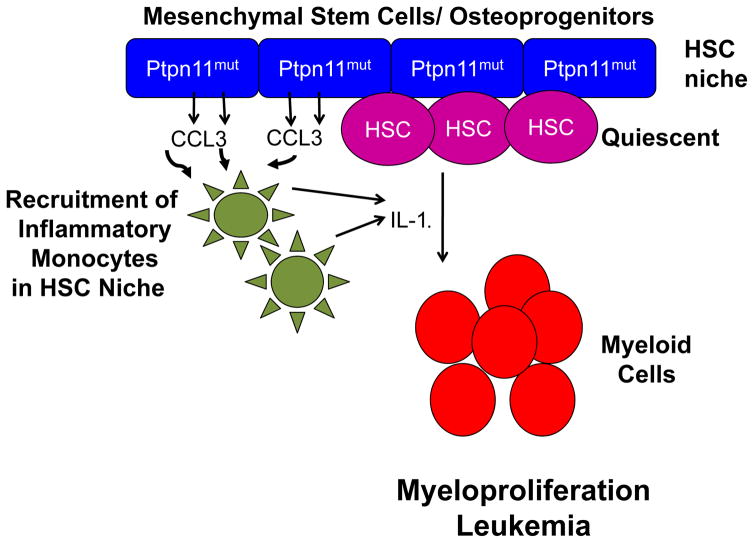
Recent studies have shed novel light on our understanding of the non-cell autonomous role of constitutively active SHP2 in leukemogenesis. Bone marrow transplant recipients are known to develop donor cell derived secondary leukemia. One of the contributing factor resulting in this observation likely relates to the oncogenic signaling initiated by the microenvironment [3,26]. The microenvironment induced leukemogenic effects could be a result of the presence of genetic aberrations in the microenvironmental cells or modulation of the niche by leukemic cells [3,26]. Dong et al [27**] have shown that expression of Noonan syndrome associated leukemogenic Ptpn11 mutations in mesenchymal stem and osteoprogenitor cells can modulate chemokine and cytokine secretion resulting in a favorable environment for recruitment of monocytes to the HSC niche (Figure 3). The inflammatory nature of such modified stem cell niche promotes myeloid hyperproliferation and development of donor derived myeloproliferative neoplasm (MPN). Using conditional activation of leukemogenic Ptpn11, authors demonstrated that donor cell derived MPN was observed only when the mutant Ptpn11 was expressed in mesenchymal stem and bone progenitor cells [27**]. Expression of mutant Ptpn11 in more differentiated cells such as osteoblasts or endothelial cells did not induce donor derived MPN. In addition, antagonist of CCL3 receptor mitigated development of donor derived MPN confirming the pivotal role of modulation of secreted factors in driving cellular transformation. These studies underline the importance of targeting the alterations in the microenvironment for successful therapy of diseases involving germline PTPN11 mutations.
Figure 3.
Expression of mutant Ptpn11 in MSC and osteo-progenitor cells leads to donor cell derived myeloproliferation. Mutant Ptpn11 expressing MSC and osteo-progenitor cells have increased secretion of chemokine, CCL3 which recruits inflammatory IL-1β secreting monocytes to the HSC niche. These changes in the niche contribute to loss of HSC quiescence and increased myeloid lineage commitment.
Targeting SHP2 in Hematological Malignancy
Targeting the aberrant signaling through tyrosine kinase inhibitors (TKI) led to initial success but subsequent acquisition of resistance to TKI due to additional mutations has been a major challenge in the long-term management of these malignancies [28,29]. Therefore, efforts have shifted to target further downstream mediators of the oncogenic signals [29]. While constitutively active SHP2 leads to the development of hematological malignancies such as JMML, wild type SHP2 is required by oncogenic tyrosine kinases for cellular transformation [30–32]. Thus, SHP2 has emerged as a therapeutic target not only for diseases involving PTPN11 mutations but also in malignancies driven by receptor tyrosine kinases such as AML, MPN. However, traditionally phosphatases have been considered as undruggable in part due to the inability to efficiently and selectively target their catalytic site with cell permeable small molecule inhibitors [33]. Protein phosphatases have highly conserved residues around catalytic sites, and therefore attaining selectivity has been a formidable challenge. Over the course of last several years, efforts have been made to develop inhibitors of SHP2 for clinical use based on their activity in vitro and in vivo [34]. However, despite promising pre-clinical results, little progress has been made in translating these findings into clinic. Thus far, only one SHP2 inhibitor, Sodium Stibogluconate (SB), also known as Pentostam has entered clinical trials. It is one of the first known compounds to irreversibly inhibit SHP2 along with related phosphatases such as SHP1 and PTP1B in hematopoietic cells [35]. Phase I/II clinical trials were conducted with co-administration of SB with IFN- α2B in advanced cancers and melanoma with or without chemotherapy (NCT00629200 and NCT00498979). Though the drug combination was well tolerated in patients with decrease in target phosphoproteins in peripheral blood, no objective disease regression was observed in any of the patients [36].
Development of allosteric inhibitors has been a major step in overcoming non-specificity in targeting phosphatases. Since allosteric inhibitors do not bind to the catalytic site, they can be targeted to the non-conserved domains of the protein to alter the active site structure and function. The pharmaceutical company, Novartis has recently been successful in identifying a small molecule allosteric inhibitor of SHP2 from a library of compounds [37,38**]. SHP099, obtained after a series of optimization steps, is a potent, orally bioavailable allosteric inhibitor of SHP2 that selectively inhibits the proliferation of receptor tyrosine kinase driven cancer cell lines in vitro without impacting BRAF or KRAS driven cancer cells [38**]. SHP099 was also shown to be effective in vivo models of tumor xenograft including in AML model. Inhibition of growth of cancer cells correlated with the ability of SHP099 to downregulate ERK signaling pathway with no significant impact on the phosphatidylinositol-3-kinase (PI3K)/AKT pathway. Interestingly, SHP099 was as effective as the FDA approved EGFR tyrosine kinase inhibitor, Erlotinib in inhibiting activation of ERK and tumor growth in xenograft models [38**]. Additionally, negligible activity against a broad panel of phosphatases, kinases and other potential off target effectors strongly suggest that SHP099 is highly specific for SHP2 [38**]. However, it still remains to be seen whether allosteric inhibition will be as successful against leukemogenic SHP2 wherein the self-inhibitory state is perturbed. In addition to the chemically synthesized inhibitors, recently Fumosorinone (Fumos) has been isolated from entomogenous fungi and characterized as an effective inhibitor of SHP2 [39]. Similar to SHP099, Fumos showed significant inhibition of the RAS/ERK pathway downstream of EGFR but was ineffective in the presence of oncogenic RAS or phorbol myristate acetate (PMA) [39]. With the development and availability of such specific and potent inhibitors of SHP2, the likelihood of moving these drugs in clinic seems imminent.
Some unintended consequences in non-myeloid lineage cells due to targeting SHP2 for myeloid malignancies have also been described. Zhu and colleagues [14*] have shown that inhibition of SHP2 using either a genetic or a chemical approach though successful in ameliorating myeloid cell hyperproliferation induced by loss of PTEN, appeared to have a negative impact on red blood cells (RBC), resulting in anemia and shortened overall life span. In these studies, a similar effect was observed when mice were treated with MEK inhibitor, trametinib highlighting the differential role of the MEK/ERK pathway in myeloid versus erythroid lineages. While proliferation was suppressed in myeloid lineage cells; in the erythroid progenitor cells differentiation into mature RBCs was blocked [14*]. Interestingly, treatment with antioxidants partially rescued these mice from development of anemia by extending the lifespan of erythrocytes but had no significant impact on differentiation of erythroblasts [14*]. Though the molecular mechanisms for the differential impact of inhibition of Shp2/MEK/ERK on myeloid versus erythroid lineage cells are not clear, AKT hyperphosphorylation may be one of the factors. AKT phosphorylation is abrogated in myeloid cells due to loss of SHP2 in the context of PTEN deficiency; however it remained elevated in TER119+ erythroblasts [14*]. Therefore, it may be essential to investigate the impact of SHP2 inhibition in cells that are not intended to be targeted especially in the context of additional genetic aberrations present. These deleterious effects on erythroid lineage were not observed by loss of SHP2 alone but only in the presence of concurrent loss of PTEN and SHP2. To this end, PI3K/AKT inhibitors are being actively pursued in pre-clinical and clinical trials in various hematological malignancies [40]. Association of GAB2 with mutant SHP2 can also activate the PI3K/AKT/mTOR pathway and inhibition of mTOR by rapamycin ameliorates myeloid cell expansion [41*]. Similar results have been shown with inactivation of the PI3K catalytic unit p110δ or the regulatory unit coded by Pik3r1 in oncogenic Ptpn11 dependent model of JMML [42,43]. In these studies, hypersensitivity to GM-CSF is lost in the absence of activation of the PI3K/AKT pathway. Consistent with these observations, a combination of SHP2 inhibitor IIB08 and PI3K inhibitor, LY294002 shows synergism in inhibiting cell proliferation and prolonging survival in receptor tyrosine kinase driven lung carcinoma and MPN models [30,44]. Similar benefits of combined targeting have been observed in vitro with SHP2 inhibitor, IIB08 and SYK inhibitor R406 through their effects on STAT5 pathway in FLT3-ITD AML cells [45]. With the availability of newer more specific, potent and well tolerated SHP2 inhibitor such as SHP009, it should be possible to further explore the combinations that give best effect with least toxicity and off target effects in hematological malignancies.
Conclusion
In conclusion, SHP2 is a crucial node for integration of signals from different cell surface receptors with all the major cellular signaling pathways within the cells. Therefore, while aberrant activation of SHP2 triggers activation of signaling pathways leading to the development of hematological malignancies; it also provides a vulnerable therapeutic target. However, given its ubiquitous expression and functional role in different tissues and organs further studies are needed before SHP2 targeted therapeutics enter the clinic.
Key points.
PTPN11 mutations are frequently present in hematological malignancies and carry prognostic significance.
Constitutive activation of SHP2 has both cell autonomous and non-cell autonomous role in pathogenesis of myeloid hyperproliferation.
Highly selective small molecule allosteric SHP2 inhibitor, SHP099 is effective in suppressing growth of receptor tyrosine kinase driven tumor cells both in vitro and in vivo.
Acknowledgments
Financial Support and Sponsorhip
RP is funded by NIH T32DK-07519 and research in RK lab is supported by R01HL077177, R01HL081111, R01CA173852, R01CA134777 and Riley Children’s foundation.
Footnotes
Conflicts of Interest
none
References
- 1.Grossmann KS, Rosario M, Birchmeier C, Birchmeier W. The tyrosine phosphatase Shp2 in development and cancer. Adv Cancer Res. 2010;106:53–89. doi: 10.1016/S0065-230X(10)06002-1. [DOI] [PubMed] [Google Scholar]
- 2*.Tajan M, de Rocca Serra A, Valet P, et al. SHP2 sails from physiology to pathology. Eur J Med Genet. 2015;58:509–525. doi: 10.1016/j.ejmg.2015.08.005. Recent detailed review of SHP2 function in normal development and in disease pathogenesis. [DOI] [PubMed] [Google Scholar]
- 3.Schepers K, Campbell TB, Passegue E. Normal and leukemic stem cell niches: insights and therapeutic opportunities. Cell Stem Cell. 2015;16:254–267. doi: 10.1016/j.stem.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X, Qu CK. Protein Tyrosine Phosphatase SHP-2 (PTPN11) in Hematopoiesis and Leukemogenesis. J Signal Transduct. 2011;2011:195239. doi: 10.1155/2011/195239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nabinger SC, Chan RJ. Shp2 function in hematopoietic stem cell biology and leukemogenesis. Curr Opin Hematol. 2012;19:273–279. doi: 10.1097/MOH.0b013e328353c6bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan G, Cheung LS, Yang W, et al. Essential role for Ptpn11 in survival of hematopoietic stem and progenitor cells. Blood. 2011;117:4253–4261. doi: 10.1182/blood-2010-11-319517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu HH, Ji K, Alderson N, et al. Kit-Shp2-Kit signaling acts to maintain a functional hematopoietic stem and progenitor cell pool. Blood. 2011;117:5350–5361. doi: 10.1182/blood-2011-01-333476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Modi H, McDonald T, et al. A critical role for SHP2 in STAT5 activation and growth factor-mediated proliferation, survival, and differentiation of human CD34+ cells. Blood. 2011;118:1504–1515. doi: 10.1182/blood-2010-06-288910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broxmeyer HE, Etienne-Julan M, Gotoh A, et al. Hematopoietic colony formation from human growth factor-dependent TF1 cells and human cord blood myeloid progenitor cells depends on SHP2 phosphatase function. Stem Cells Dev. 2013;22:998–1006. doi: 10.1089/scd.2012.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang H, Woo AJ, Waldon Z, et al. A Src family kinase-Shp2 axis controls RUNX1 activity in megakaryocyte and T-lymphocyte differentiation. Genes Dev. 2012;26:1587–1601. doi: 10.1101/gad.192054.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazharian A, Mori J, Wang YJ, et al. Megakaryocyte-specific deletion of the protein-tyrosine phosphatases Shp1 and Shp2 causes abnormal megakaryocyte development, platelet production, and function. Blood. 2013;121:4205–4220. doi: 10.1182/blood-2012-08-449272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuvardina ON, Herglotz J, Kolodziej S, et al. RUNX1 represses the erythroid gene expression program during megakaryocytic differentiation. Blood. 2015;125:3570–3579. doi: 10.1182/blood-2014-11-610519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Usenko T, Chan G, Torlakovic E, et al. Leukemogenic Ptpn11 allele causes defective erythropoiesis in mice. PLoS One. 2014;9:e109682. doi: 10.1371/journal.pone.0109682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Zhu HH, Luo X, Zhang K, et al. Shp2 and Pten have antagonistic roles in myeloproliferation but cooperate to promote erythropoiesis in mammals. Proc Natl Acad Sci U S A. 2015;112:13342–13347. doi: 10.1073/pnas.1507599112. SHP2 can have differential effects in differnt hematopoietic lineage cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu D, Liu X, Yu WM, et al. Non-lineage/stage-restricted effects of a gain-of-function mutation in tyrosine phosphatase Ptpn11 (Shp2) on malignant transformation of hematopoietic cells. J Exp Med. 2011;208:1977–1988. doi: 10.1084/jem.20110450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang MT, Asthana S, Gao SP, et al. Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. 2016;34:155–163. doi: 10.1038/nbt.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tartaglia M, Martinelli S, Stella L, et al. Diversity and functional consequences of germline and somatic PTPN11 mutations in human disease. Am J Hum Genet. 2006;78:279–290. doi: 10.1086/499925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu ZH, Zhang RY, Walls CD, et al. Molecular basis of gain-of-function LEOPARD syndrome-associated SHP2 mutations. Biochemistry. 2014;53:4136–4151. doi: 10.1021/bi5002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makishima H, Yoshizato T, Yoshida K, et al. Dynamics of clonal evolution in myelodysplastic syndromes. 2017;49:204–212. doi: 10.1038/ng.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrar JE, Schuback HL, Ries RE, et al. Genomic Profiling of Pediatric Acute Myeloid Leukemia Reveals a Changing Mutational Landscape from Disease Diagnosis to Relapse. Cancer Res. 2016;76:2197–2205. doi: 10.1158/0008-5472.CAN-15-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oshima K, Khiabanian H, da Silva-Almeida AC, et al. Mutational landscape, clonal evolution patterns, and role of RAS mutations in relapsed acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2016;113:11306–11311. doi: 10.1073/pnas.1608420113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai CH, Hou HA, Tang JL, et al. Genetic alterations and their clinical implications in older patients with acute myeloid leukemia. Leukemia. 2016;30:1485–1492. doi: 10.1038/leu.2016.65. [DOI] [PubMed] [Google Scholar]
- 23**.Fu JF, Liang ST, Huang YJ, et al. Cooperation of MLL/AF10(OM-LZ) with PTPN11 activating mutation induced monocytic leukemia with a shorter latency in a mouse bone marrow transplantation model. Int J Cancer. 2017;140:1159–1172. doi: 10.1002/ijc.30515. Mutant Ptpn11 co-operates with MLL fusion oncogene to increase M-CSF secretion, accelerates AML development and modulates drug resistance. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Chen W, Mysliwski M, et al. Mutated Ptpn11 alters leukemic stem cell frequency and reduces the sensitivity of acute myeloid leukemia cells to Mcl1 inhibition. Leukemia. 2015;29:1290–1300. doi: 10.1038/leu.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bei L, Shah C, Wang H, et al. Regulation of CDX4 gene transcription by HoxA9, HoxA10, the Mll-Ell oncogene and Shp2 during leukemogenesis. Oncogenesis. 2014;3:e135. doi: 10.1038/oncsis.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krause DS, Scadden DT. A hostel for the hostile: the bone marrow niche in hematologic neoplasms. Haematologica. 2015;100:1376–1387. doi: 10.3324/haematol.2014.113852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27**.Dong L, Yu WM, Zheng H, et al. Leukaemogenic effects of Ptpn11 activating mutations in the stem cell microenvironment. Nature. 2016;539:304–308. doi: 10.1038/nature20131. This paper for the first time reports non-cell autonomous role in development of myeloid neoplasm by oncogenic Ptpn11 expression in the HSC niche cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuwano M, Sonoda K, Murakami Y, et al. Overcoming drug resistance to receptor tyrosine kinase inhibitors: Learning from lung cancer. Pharmacol Ther. 2016;161:97–110. doi: 10.1016/j.pharmthera.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Buet D, Gallais I, Lauret E, et al. Cotargeting signaling pathways driving survival and cell cycle circumvents resistance to Kit inhibitors in leukemia. Blood. 2012;119:4228–4241. doi: 10.1182/blood-2011-07-368316. [DOI] [PubMed] [Google Scholar]
- 30.Mali RS, Ma P, Zeng LF, et al. Role of SHP2 phosphatase in KIT-induced transformation: identification of SHP2 as a druggable target in diseases involving oncogenic KIT. Blood. 2012;120:2669–2678. doi: 10.1182/blood-2011-08-375873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nabinger SC, Li XJ, Ramdas B, et al. The protein tyrosine phosphatase, Shp2, positively contributes to FLT3-ITD-induced hematopoietic progenitor hyperproliferation and malignant disease in vivo. Leukemia. 2013;27:398–408. doi: 10.1038/leu.2012.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noel LA, Arts FA, Montano-Almendras CP, et al. The tyrosine phosphatase SHP2 is required for cell transformation by the receptor tyrosine kinase mutants FIP1L1-PDGFRalpha and PDGFRalpha D842V. Mol Oncol. 2014;8:728–740. doi: 10.1016/j.molonc.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barr AJ. Protein tyrosine phosphatases as drug targets: strategies and challenges of inhibitor development. Future Med Chem. 2010;2:1563–1576. doi: 10.4155/fmc.10.241. [DOI] [PubMed] [Google Scholar]
- 34.Butterworth S, Overduin M, Barr AJ. Targeting protein tyrosine phosphatase SHP2 for therapeutic intervention. Future Med Chem. 2014;6:1423–1437. doi: 10.4155/fmc.14.88. [DOI] [PubMed] [Google Scholar]
- 35.Pathak MK, Yi T. Sodium stibogluconate is a potent inhibitor of protein tyrosine phosphatases and augments cytokine responses in hemopoietic cell lines. J Immunol. 2001;167:3391–3397. doi: 10.4049/jimmunol.167.6.3391. [DOI] [PubMed] [Google Scholar]
- 36.Yi T, Elson P, Mitsuhashi M, et al. Phosphatase inhibitor, sodium stibogluconate, in combination with interferon (IFN) alpha 2b: phase I trials to identify pharmacodynamic and clinical effects. Oncotarget. 2011;2:1155–1164. doi: 10.18632/oncotarget.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia Fortanet J, Chen CH, Chen YN, et al. Allosteric Inhibition of SHP2: Identification of a Potent, Selective, and Orally Efficacious Phosphatase Inhibitor. J Med Chem. 2016;59:7773–7782. doi: 10.1021/acs.jmedchem.6b00680. [DOI] [PubMed] [Google Scholar]
- 38**.Chen YN, LaMarche MJ, Chan HM, et al. Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature. 2016;535:148–152. doi: 10.1038/nature18621. The paper describes biological characterization of effectiveness of first allosteric inhibitor of SHP2 in receptor tyrosine kinase driven malignacnies through downregulation of ERK pathway. [DOI] [PubMed] [Google Scholar]
- 39.Chen C, Cao M, Zhu S, et al. Discovery of a Novel Inhibitor of the Protein Tyrosine Phosphatase Shp2. Sci Rep. 2015;5:17626. doi: 10.1038/srep17626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandey R, Kapur R. Targeting phosphatidylinositol-3-kinase pathway for the treatment of Philadelphia-negative myeloproliferative neoplasms. Mol Cancer. 2015;14:118. doi: 10.1186/s12943-015-0388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Liu W, Yu WM, Zhang J, et al. Inhibition of the Gab2/PI3K/mTOR signaling ameliorates myeloid malignancy caused by Ptpn11 (Shp2) gain-of-function mutations. Leukemia. 2017 doi: 10.1038/leu.2016.326. Inhibition of PI3K pathway is effective in suppressing oncogenic SHP2 driven myeloid malignancy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodwin CB, Yang Z, Yin F, et al. Genetic disruption of the PI3K regulatory subunits, p85alpha, p55alpha, and p50alpha, normalizes mutant PTPN11-induced hypersensitivity to GM-CSF. Haematologica. 2012;97:1042–1047. doi: 10.3324/haematol.2011.046896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodwin CB, Li XJ, Mali RS, et al. PI3K p110delta uniquely promotes gain-of-function Shp2-induced GM-CSF hypersensitivity in a model of JMML. Blood. 2014;123:2838–2842. doi: 10.1182/blood-2013-10-535104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu J, Zeng LF, Shen W, et al. Targeting SHP2 for EGFR inhibitor resistant non-small cell lung carcinoma. Biochem Biophys Res Commun. 2013;439:586–590. doi: 10.1016/j.bbrc.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richine BM, Virts EL, Bowling JD, et al. Syk kinase and Shp2 phosphatase inhibition cooperate to reduce FLT3-ITD-induced STAT5 activation and proliferation of acute myeloid leukemia. Leukemia. 2016;30:2094–2097. doi: 10.1038/leu.2016.131. [DOI] [PMC free article] [PubMed] [Google Scholar]