Abstract
Purpose
This study seeks to clarify the modern prognostic significance of visceral pleura invasion (VPI) in stage IB (T2aN0M0) non-small cell lung cancer (NSCLC) within the context of the 7th Edition TNM classification using the data set from a recent prospective multicenter trial.
Patients and methods
1111 early-stage NSCLC patients participating in the ACOSOG Z0030 trial (1990–2004) underwent curative pulmonary resection. After excluding T2b tumours (>5cm and <=7cm) and non-size-based T2 factors other than VPI, 289 patients were categorized as Stage IB NSCLC –T2aN0M0-according to the AJCC 7th edition classification. The patients were divided into three groups according to size and VPI: tumours <=3cm with VPI (group I, “VPI-alone”, n=83), tumours >3cm and <=5cm without VPI (group II, “Size-alone”, n=156), and tumours >3cm and <=5cm with VPI (group III, “VPI+Size”, n=50). Multivariate Cox regression analysis was used to assess the association of VPI and size with survival, adjusting for age, gender, histology and type of resection.
Results
VPI in stage IB was identified in 133 patients (46.0%). Survival analysis in these patients identified an optimal cutpoint for survival based on size of 3.1 cm. Group III (VPI+Size) had a 5-year survival rate of 55.0% significantly shorter when compared to group I (VPI-alone=68.3%, p=0.009), and group II (Size-alone=67.2%, p=0.021). No difference was found between Groups I and II. Multivariable analysis showed that VPI associated with size was an independent negative prognostic factor of long-term survival, along with older age and limited resection.
Conclusions
Stage IB patients with VPI and tumours >3cm and <=5cm have significantly worse prognosis than those with ‘T2a’ tumours on the basis of VPI or tumour size alone. This finding would suggest upstaging these patients from the current IB status to stage IIA.
Keywords: Visceral pleura, Non-small cell lung cancer, 7th Edition TNM classification, Prognostic factor
INTRODUCTION
Visceral pleura invasion (VPI) appeared in the mid 1970s as a specific entity in the TNM classification of non-small cell lung cancer (NSCLC) (1). Since that time, it has remained unchanged: a tumour of any size that invades the visceral pleura is classified as T2 (unless it is classified as T3 or T4 for other factors). Recently however, the T factor subcommittee of the International Staging Committee (ISC) of the International Association for the Study of Lung Cancer (IASLC), whose objective was the revision of the UICC and AJCC TNM classification for NSCLC staging was unable to perform an analysis of primary data on this criterion due to the absence of detailed pathologic information related to VPI (2,3). The prognostic role of VPI, in patients with stage I NSCLC, and more specifically its relationship with the size of the tumour, therefore remains unclear (4).
We set out to determine the prognostic role of VPI in stage IB (T2aN0M0) patients utilizing the data collected prospectively the American College of Surgeons Oncology Group (ACOSOG) Z0030 trial database (5). Z0030 is a randomized prospective multicenter trial comparing mediastinal lymph node sampling to complete mediastinal lymphadenectomy at the time of pulmonary resection in patients with N0 or non-hilar N1 NSCLC.
PATIENTS AND METHODS
Overall, the Z0030 trial included 1111 consecutive patients who all had complete resection of their early stage NSCLC with systematic mediastinal lymph node dissection or lymph node sampling from 63 institutions across North America (5). We reviewed the data collected prospectively on all patients who underwent lung resection for pathologic stage IB (T2aN0M0) NSCLC (1999–2004), as defined by the 7th edition TNM classification (2,3). In this definition, T2a tumours are defined as tumours >3 cm and <=5 cm, or tumours <=3 cm with VPI, and T2b as tumours >5 cm and <=7 cm, with or without VPI. In our analysis, we excluded T2b tumours and non-size-based T2 factors other than VPI, such as: main bronchus involvement >= 2 cm distal to the carina and atelectasis or obstructive pneumonitis extending to the hilar region but not involving the entire lung. We also excluded N1 and N2 patients. In accordance with the 7th edition classification, when defining VPI, we referred to the modified Hammar’s diagram (see Table 1) and defined VPI as tumour invasion beyond the elastic layer (PL1 and PL2) (6,7). The use of specific elastic stains to assist with determination of VPI was not mandated and therefore variable in the Z0030 trial. Also, specific information about visceral pleural involvement was not required in data submission for the ACOSOG Z0030 trial. Previous to the start of the study all the pathology reports of the patients involved were reviewed in order to be sure that information about VPI was available and therefore the study feasible.
Table 1.
Hammar´s diagram classifying visceral pleura involvement
| PX | Tumours situated within the lung parenchyma with no relationship to the pleura. |
| PL0 | Tumours with no pleural involvement or that reach the visceral pleura but do not extend beyond its elastic layer. |
| PL1 | Tumours that extend beyond the elastic layer of the visceral pleura but are not exposed on the pleural surface |
| PL2 | Tumours that are exposed on the pleural surface but do not involve the parietal pleura |
The patients were divided into three groups according to size and VPI: tumours <=3cm with VPI (group I, “VPI-alone”), tumours >3cm and <=5cm without VPI (group II, “Size-alone”), and tumours >3cm and <=5cm with VPI (group III, “VPI+Size”).
Operative mortality was defined as any death occurring within 30 days of operation or during the initial hospitalization. Late mortality was defined as any subsequent death. Follow-up data were obtained from patients’ clinic visits, and when needed, by telephone interview. The median follow-up for patients not known to be deceased was 80 months (range: 0–121 months).
The Chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables were used to compare patients’ characteristics across groups. Cumulative survival probabilities were estimated using the Kaplan-Meier method. The log rank test was used to compare survival of groups. Univariable Cox proportional hazards regression models were used to investigate the association of selected variables with survival. Multivariable Cox regression analysis was used to assess the association of VPI and size with survival, adjusting for age, gender, histology and type of resection. In all cases p-values <0.05 were considered statistically significant. This study was approved by the Mayo Clinic Institutional Review Board.
RESULTS
289 patients were categorized as stage IB NSCLC (T2aN0M0). There were 152 men (52.6%) and 137 women (47.4%). The mean age was 67.7±9.2 years old. Two-hundred and sixty-six (92%) of the subjects underwent a lobectomy, bilobectomy, or pneumonectomy, while 23 (8%) underwent a sublobar resection (segmentectomy or wedge resection). One hundred and twenty-four patients (42.9%) had adenocarcinoma, while 72 patients (24.9%) had squamous cancer, and 93 patients (32.2%) had other NSCLC histologies. An operative mortality occurred in 5 patients (1.7%). Table 2 presents the baseline demographics by T2a categories.
Table 2.
Stage IB (T2aN0M0) patients characteristics
|
Group I (VPI Alone) VPI Tumours <3cm (N=83) |
Group II (Size Alone) No VPI 3cm<Tumours<5cm (N=156) |
Group III (VPI+Size) VPI 3cm<Tumours<5cm (N=50) |
Total (N=289) |
p value* |
|
|---|---|---|---|---|---|
| Age | 0.61 | ||||
| Mean (SD) | 68.3 (9.13) | 67.3 (9.50) | 68.2 (8.15) | 67.7 (9.16) | |
| Median | 69.5 | 67.9 | 68.7 | 68.5 | |
| Range | (42.4–89.2) | (41.0–88.3) | (52.7–86.2) | (41.0–89.2) | |
| Sex | 0.026 | ||||
| Male | 35 (42.2%) | 84 (53.8%) | 33 (66%) | 152 (52.6%) | |
| Female | 48 (57.8%) | 72 (46.2%) | 17 (34%) | 137 (47.4%) | |
| Surgical type | 0.26 | ||||
| Lobectomy/pneumectomy/bilobectomy | 73 (88%) | 146 (93.6%) | 47 (94%) | 266 (92%) | |
| Segmentectomy/Wedge resection | 10 (12%) | 10 (6.4%) | 3 (6%) | 23 (8%) | |
| Histology | 0.002 | ||||
| Squamous | 8 (9.6%) | 49 (31.4%) | 15 (30%) | 72 (24.9%) | |
| Adenocarcinoma | 47 (56.6%) | 55 (35.3%) | 22 (44%) | 124 (42.9%) | |
| Other | 28 (33.7%) | 52 (33.3%) | 13 (26%) | 93 (32.2%) | |
| Operative mortality | 0.001 | ||||
| Missing | 0 (0%) | 1 (0%) | 0 (0%) | 1 | |
| Yes | 1 (1.2%) | 0 (0%) | 4 (8%) | 5 (1.7%) | |
| No | 82 (98.8%) | 155 (100%) | 46 (92%) | 283 (98.3%) |
Kruskal Wallis or Chi-Square test as appropriate
Percentages may not add to 100 due to rounding
VPI in stage IB was identified in 133 patients (46.0%). Survival analysis in these patients identified an optimal cutpoint for survival based on size of 3.1 cm. We analyzed the overall effect of VPI in all patients (n=289) and we did not find a statistically significant increase in the risk of death (HR=1.11; 95% CI: 0.78–1.58; p=0.55). However, the survival analysis in the 133 subjects with VPI found that, in those patients with VPI and tumours > 3.1 cm, the risk of death was significantly higher (HR=1.31; 95% CI: 1.03–1.66; p=0.026).
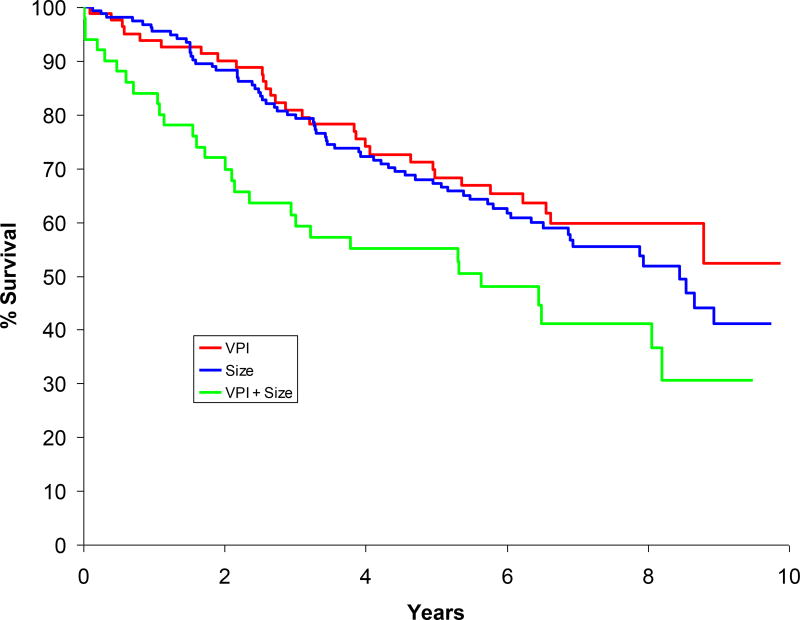
When analyzing the groups based on size and VPI, the median and 5-year survival estimates were as follows: group I, “VPI-alone”, median = not achieved, 5-year survival = 68.3%; group II, “Size-alone”, median = 8.4 years, 5-year survival = 67.2%; and group III, “VPI+Size”, median = 5.6 years, 5-year survival = 55.0% (see Figure 1). Groups I and III (p=0.009) and groups II and III (p=0.021) were significantly different from each other while groups I and II did not differ (p=0.43).
Figure 1.
Survival curves of stage IB groups (based on size and VPI).
Paired-wise test Visceral Pleura Invasion vs. Size: P-value=0.432.
Visceral Pleura Invasion vs. Visceral Pleura/Size: P-value=0.009.
Size vs. Visceral Pleura/Size: P-value=0.021.
Sex, age, operative procedure, tumour histology, tumour size and VPI were evaluated in a univariable analysis to find predictors of a poor prognosis. As a result, segmentectomy/wedge resection (Hazard ratio (HR)=2.37; 95% CI: 1.39–4.01; P=0.001), age at time of surgery (HR=1.04; 95% CI: 1.02–1.06; P<0.001), and T2a category (Size alone: HR=1.19; 95% CI: 0.77–1.83; p=0.43; VPI and Size: HR=1.98; 95% CI: 1.19–3.30; p=0.009; VPI alone was used as the reference point; overall p=0.021) were found to be statistically significant predictors of a poor prognosis (see Table 3). Multivariable analysis showed that T2a category (both by VPI and/or size) was an independent negative prognostic factor of long-term survival (p=0.015), along with older age (p=0.003) and limited resection (p=0.003) (Table 4).
Table 3.
Univariable Survival Analysis on T2a/IB patients
| Variable | Hazard Ratio |
95% Hazard Ratio Confidence Limits |
P-value |
|---|---|---|---|
| T2a Category (Visceral Pleural Invasion vs. Size vs. Visceral Pleura/Size) | 0.021 | ||
| Visceral Pleura | 1.0 | - | - |
| Size | 1.19 | 0.77, 1.83 | 0.43 |
| Visceral Pleura/Size | 1.98 | 1.19, 3.30 | 0.009 |
| Sex (Male vs. Female) | 0.77 | 0.54, 1.09 | 0.14 |
| Age | 1.04 | 1.02, 1.06 | <0.001 |
| Histology (Squamous vs. Adenocarcinoma vs. Other) | 0.14 | ||
| Adenocarcinoma | 0.69 | 0.46, 1.05 | 0.086 |
| Other | 0.66 | 0.42, 1.05 | 0.077 |
| Surgical Resections (Segmentectomy/Wedge vs. Lobectomy/Bilobectomy/Pneumonectomy) | |||
| Segmentectomy/Wedge | 2.37 | 1.39, 4.01 | 0.001 |
Table 4.
Multivariable Survival Analysis on T2a/IB patients
| Variable | Hazard Ratio |
95% Hazard Ratio Confidence Limits |
P-value |
|---|---|---|---|
| T2a Category (Visceral Pleural Invasion vs. Size vs. Visceral Pleura/Size) | 0.015 | ||
| Visceral Pleura | 1.0 | - | - |
| Size | 1.22 | 0.78, 1.91 | 0.38 |
| Visceral Pleura/Size | 2.07 | 1.23, 3.50 | 0.006 |
| Sex (Male vs. Female) | 0.80 | 0.56, 1.16 | 0.24 |
| Age | 1.03 | 1.01, 1.06 | 0.003 |
| Histology (Squamous vs. Adenocarcinoma vs. Other) | 0.25 | ||
| Adenocarcinoma | 0.76 | 0.49, 1.17 | 0.21 |
| Other | 0.69 | 0.43, 1.09 | 0.11 |
| Surgical Resections (Segmentectomy/Wedge vs. Lobectomy/Bilobectomy/Pneumonectomy) | |||
| Segmentectomy/Wedge | 2.34 | 1.34, 4.08 | 0.003 |
DISCUSSION
In the 6th edition TNM classification, all T2 tumours were categorized as stage IB (T2N0M0), while in the 7th edition, the new T2b tumour subset (>5cm <= 7cm) has been upstaged to stage IIA, leaving the new stage IB cases consisting of T2aN0M0 tumours. The 7th edition TNM classification has proposed revisions to the T descriptor in early-stage NSCLC primarily based on a finer subdivision of tumour size. However, data for the non-size-based T2 descriptors were less robust given the small number of patients available and a lack of statistical validation (2,3).
VPI, known as an important and “interest generator” descriptor remains unchanged, although in this new TNM revision, emphasis has been placed on it as an important T factor for lung cancer (7). There are several reports demonstrating the prognostic significance of VPI in NSCLC (8–13). Kang and coworkers (8) demonstrated that VPI was an adverse prognostic factor in T2 NSCLC and it correlated with more extensive mediastinal lymph node involvement. Shimizu and colleagues (9) showed that VPI was a significant independent adverse prognostic factor in NSCLC with or without lymph node metastases. Although there are multiple studies regarding the global role of VPI in NSCLC survival, there is a paucity of published literature on early stage patients staged solely due to size and VPI. Furthermore, when stratifying prognostic significance of VPI by tumour size, studies have not shown homogeneous results (14–17).
Some reports analyzing VPI and size have suggested that VPI is an adverse prognostic factor in early stage NSCLC independent of the size of the tumour. Yoshida and coauthors (14) from the Japanese Joint Committee for Lung Cancer Registration suggested that all the tumours, 7 cm or less, with VPI should be upgraded to the next T level in the 7th edition of the TNM classification. Conversely, several studies have reported VPI as being an adverse factor only in tumours > 3cm.
Ou and colleagues (15) with data from the California Cancer Registry suggested that VPI was an adverse prognostic factor along with tumour size but with a greater significance in tumours > 3 cm as compared with smaller tumours. Manac´h and coinvestigators (16) reported that the 5-year survival rate of patients with VPI whose tumours were < 3 cm compared favorably to patients with VPI and tumours > 3cm in size. Martini and coworkers (13) did not find VPI to be a significant adverse prognostic factor in overall stage I NSCLC cases, yet in tumours over 3 cm in size it was significant.
As a consequence of these discrepancies, to date, the question of the specific effect of tumour size on the impact of VPI in early stage NSCLC has appeared indeterminate (6). Our study focused on the effect of VPI on the new stage IB category (T2aN0M0) from the 7th edition revisions and sought to evaluate the homogeneity in survival in this subset of patients according to their size and presence of VPI. A potential strength of our report is that we restricted our analysis only to stage IB NSCLC patients with VPI; excluding N1 and N2 patients as well as other non-size-based T factors in this way we eliminated other confounders included in previous reports (4). Using the ACOSOG Z0030 data we observed that stage IB patients with VPI and tumours >3cm and <=5cm had significantly worse prognosis than those with ‘T2a’ tumours classified on the basis of VPI alone or tumour size alone. The survival analysis performed in the 133 VPI subjects to identify the optimal cutpoint for survival based on size found that this was 3.1 cm, just 0.1 cm over the adopted TNM staging threshold of 3.0 cm, supporting our finding regarding the influence of size on VPI in stage IB patients.
It is known from previous published work such as that of Taube and colleagues that use of elastic stains in the diagnosis of VPI and can upstage close to around 20% of presumed stage IA tumours to stage IB (18). Travis and colleagues reviewed six articles that addressed reported survival data using elastic stains to assess for VPI. In five of the six, survival was shown to be significantly worse for VPI (7). A potential limitation of our study is that given that the initial and main purpose of the Z0030 trial was not the evaluation of VPI but related rather to lymph node dissection versus sampling, the use of elastic stains to evaluate for VPI was not mandated and therefore variable. This may have the effect of underestimating the true rate of VPI in the Z0030 data set.
Our results, from a prospective multicenter ACOSOG trial database, thus further corroborate findings from other previous reports that have highlighted the significant adverse effect of VPI solely in tumours > 3cm. We believe that our results may warrant further in-depth analyses exploring the link between size and VPI. Interpretation of the prognostic importance of VPI may be better when considered in association with tumour size rather than as a stand alone factor in future refinements of the NSCLC staging system. Patients with VPI and a tumour size between 3 and 5 cm may warrant upstaging from stage IB to IIA, while those with VPI and tumour size smaller than 3 cm would remain classified as stage IB.
Acknowledgments
This project was not funded.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
All authors disclose any actual of potential conflict of interest including any financial, personal of other relationships with other people or organizations that could inappropriately influence (bias) their work.
References
- 1.Brewer LA. Patterns of survival in lung cancer. Chest. 1977;71:644–50. doi: 10.1378/chest.71.5.644. [DOI] [PubMed] [Google Scholar]
- 2.Rami-Porta R, Ball D, Crowley J, Giroux DJ, Jett J, Travis WD, Tsuboi M, Vallières E, Goldstraw P International Staging Committee; Cancer Research and Biostatistics; Observers to the Committee. The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:593–602. doi: 10.1097/JTO.0b013e31807a2f81. [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L International Association for the Study of Lung Cancer International Staging Committee. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 4.Hung JJ, Liu JS, Wu YC, Hsu WH. The effect of tumor size on non-size-based descriptors in staging of stage I non-small cell lung cancer. Chest. 2009;135:1695–6. doi: 10.1378/chest.09-0406. [DOI] [PubMed] [Google Scholar]
- 5.Allen MS, Darling GE, Pechet TT, Mitchell JD, Herndon JE, 2nd, Landreneau RJ, Inculet RI, Jones DR, Meyers BF, Harpole DH, Putnam JB, Jr, Rusch VW ACOSOG Z0030 Study Group. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg. 2006;81:1013–9. doi: 10.1016/j.athoracsur.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 6.Hammar SP. Common Tumors. In: Dail DH, Hammar SP, editors. Pulmonary Pathology. 1. New York: Springer-Verlag; 1988. pp. 727–845. [Google Scholar]
- 7.Travis WD, Brambilla E, Rami-Porta R, Vallières E, Tsuboi M, Rusch V, Goldstraw P International Staging Committee. Visceral pleural invasion: pathologic criteria and use of elastic stains: proposal for the 7th edition of the TNM classification for lung cancer. J Thorac Oncol. 2008;3:1384–90. doi: 10.1097/JTO.0b013e31818e0d9f. [DOI] [PubMed] [Google Scholar]
- 8.Kang JH, Kim KD, Chung KY. Prognostic value of visceral pleura invasion in non-small cell lung cancer. Eur J Cardiothorac Surg. 2003;23:865–9. doi: 10.1016/s1010-7940(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu K, Yoshida J, Nagai K, Nishimura M, Ishii G, Morishita Y, Nishiwaki Y. Visceral pleural invasion is an invasive and aggressive indicator of non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;130:160–5. doi: 10.1016/j.jtcvs.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Osaki T, Nagashima A, Yoshimatsu T, Yamada S, Yasumoto K. Visceral pleural involvement in nonsmall cell lung cancer: prognostic significance. Ann Thorac Surg. 2004;77:1769–73. doi: 10.1016/j.athoracsur.2003.10.058. [DOI] [PubMed] [Google Scholar]
- 11.Ichinose Y, Yano T, Asoh H, Yokoyama H, Yoshino I, Katsuda Y. Prognostic factors obtained by a pathologic examination in completely resected non-small-cell lung cancer. An analysis in each pathologic stage. J Thorac Cardiovasc Surg. 1995;110:601–5. doi: 10.1016/S0022-5223(95)70090-0. [DOI] [PubMed] [Google Scholar]
- 12.Padilla J, Calvo V, Peñalver JC, Zarza AG, Pastor J, Blasco E, París F. Survival and risk model for stage IB non-small cell lung cancer. Lung Cancer. 2002;36:43–8. doi: 10.1016/s0169-5002(01)00450-0. [DOI] [PubMed] [Google Scholar]
- 13.Martini N, Bains MS, Burt ME, Zakowski MF, McCormack P, Rusch VW, Ginsberg RJ. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109:120–9. doi: 10.1016/S0022-5223(95)70427-2. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida J, Nagai K, Asamura H, Goya T, Koshiishi Y, Sohara Y, Eguchi K, Mori M, Nakanishi Y, Tsuchiya R, Miyaoka E Japanese Joint Committee for Lung Cancer Registration. Visceral pleura invasion impact on non-small cell lung cancer patient survival: its implications for the forthcoming TNM staging based on a large-scale nation-wide database. J Thorac Oncol. 2009;4:959–63. doi: 10.1097/JTO.0b013e3181a85d5e. [DOI] [PubMed] [Google Scholar]
- 15.Ou SH, Zell JA, Ziogas A, Anton-Culver H. Prognostic significance of the non-size-based AJCC T2 descriptors: visceral pleura invasion, hilar atelectasis, or obstructive pneumonitis in stage IB non-small cell lung cancer is dependent on tumor size. Chest. 2008;133:662–9. doi: 10.1378/chest.07-1306. [DOI] [PubMed] [Google Scholar]
- 16.Manac´h D, Riquet M, Medioni J, Le Pimpec-Barthes F, Dujon A, Danel C. Visceral pleura invasion by non-small cell lung cancer: an underrated bad prognostic factor. Ann Thorac Surg. 2001;71:1088–93. doi: 10.1016/s0003-4975(00)02649-7. [DOI] [PubMed] [Google Scholar]
- 17.Shim HS, Park IK, Lee CY, Chung KY. Prognostic significance of visceral pleural invasion in the forthcoming (seventh) edition of TNM classification for lung cancer. Lung Cancer. 2009;65:161–5. doi: 10.1016/j.lungcan.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Taube JM, Askin FB, Brock MV, Westra W. Impact of elastic staining on the staging of peripheral lung cancers. Am J Surg Pathol. 2007;31:953–956. doi: 10.1097/PAS.0b013e31802ca413. [DOI] [PubMed] [Google Scholar]