Abstract
Up to 90% of patients with a myelodysplastic syndrome require red blood cell transfusion; nevertheless, comprehensive data on red cell alloimmunization in such patients are limited. This study evaluates the incidence and clinical impact of red cell alloimmunization in a large cohort of patients with myelodysplastic syndrome registered in the statewide South Australian-MDS registry. The median age of the 817 patients studied was 73 years, and 66% were male. The cumulative incidence of alloimmunization was 11%. Disease-modifying therapy was associated with a lower risk of alloimmunization while alloimmunization was significantly higher in patients with a revised International Prognostic Scoring System classification of Very Low, Low or Intermediate risk compared to those with a High or Very High risk (P=0.03). Alloantibodies were most commonly directed against antigens in the Rh (54%) and Kell (24%) systems. Multiple alloantibodies were present in 49% of alloimmunized patients. Although 73% of alloimmunized patients developed alloantibodies during the period in which they received their first 20 red cell units, the total number of units transfused was significantly higher in alloimmunized patients than in non-alloimmunized patients (90±100 versus 30±52; P<0.0001). In individual patients, red cell transfusion intensity increased significantly following alloimmunization (2.8±1.3 versus 4.1±2.0; P<0.0001). A significantly higher proportion of alloimmunized patients than non-alloimmunized patients had detectable autoantibodies (65% versus 18%; P<0.0001) and the majority of autoantibodies were detected within a short period of alloimmunization. In conclusion, this study characterizes alloimmunization in a large cohort of patients with myelodysplastic syndrome and demonstrates a signficant increase in red cell transfusion requirements following alloimmunization, most probably due to development of additional alloantibodies and autoantibodies, resulting in subclinical/clinical hemolysis. Strategies to mitigate alloimmunization risk are critical for optimizing red cell transfusion support.
Introduction
Myelodysplastic syndromes (MDS) are clonal diseases characterized by peripheral cytopenias, ineffective hematopoiesis and an increased risk of leukemic transformation.1 They are among the most commonly diagnosed myeloid malignancies2 with the median age of affected individuals at diagnosis being 72 years.3 Management options include disease-modifying therapies and supportive measures such as red blood cell (RBC) and platelet transfusions, antimicrobials and growth factors.4,5 Transfusion support remains a cornerstone of management for most MDS patients. Up to 90% of patients require RBC transfusions during the course of their disease6 and 30–45% become dependent on RBC transfusions.7,8
Importantly, RBC transfusion dependency is associated with a significantly worse survival independently of the revised International Prognostic Scoring System (IPSS-R) risk score. MDS patients are at high risk of developing transfusion-associated complications such as iron overload and acute or delayed hemolytic transfusion reactions, resulting in significant morbidity and mortality.9 True morbidity and mortality burden from RBC alloimmunization is likely higher than reported to hemovigilance programs.10,11 Alloimmunization may also drive RBC autoantibody formation and subclinical/serological hemolytic transfusion reactions.12 Although RBC autoantibody formation after alloimmunization can occur in any transfused patient, reported rates are much higher in transfused patients with thalassemia or sickle cell disease with a cumulative incidence of 6–10%.13–16 In a study of 717 patients with autoantibodies, 200 (28%) patients had both autoantibodies and alloantibodies, and the majority were detected simultaneously and were induced by transfusion.17 We have observed clinically that some MDS patients have increased RBC transfusion requirement following development of alloimmunization. For the transfusion service, identifying and characterizing allo- and autoantibodies can be time-consuming, laborious and expensive and can cause difficulties and delay in finding compatible units.
Despite the high prevalence of MDS, data on alloimmunization in chronically transfused MDS patients mostly reflect experience from single centers with limited numbers of patients and often relatively short follow-up.18–20 Highly variable alloimmunization rates have been reported.18–20 Larger studies are required to better understand alloimmunization in MDS, including the complex interplay between disease- and patient-related factors, and transfusion burden. This study characterizes RBC alloimmunization in a large series of well-annotated patients with MDS followed in a state-wide MDS registry.
Methods
The South Australian MDS Registry has been described previously.7 Briefly, it is a comprehensive, state-wide database of adult patients with MDS, MDS/myeloproliferative neoplasm overlap syndrome, acute myeloid leukemia (<30% blasts) and therapy-related myeloid neoplasm from six participating hospitals across the public and private sectors (Online Supplementary Methods). Institutional ethics committee approval was obtained from all participating institutions and procedures were performed in accordance with the revised Helsinki Declaration.
Demographic, clinical, laboratory and treatment (including transfusions) details of patients diagnosed between 1990 and 2015 enrolled in the registry and with at least 6 months of follow-up were analyzed. Disease-modifying therapies included azacitidine, lenalidomide, intensive chemotherapy and allogeneic hematopoietic stem cell transplantation.
RBC transfusion dependency was defined as the requirement of at least one RBC unit every 8 weeks over a 4-month period.7,8 As some patients received RBC transfusions before their MDS diagnosis had been established, we assessed the serial blood counts, clinical profile and RBC transfusion requirements of all patients before and after MDS diagnosis. RBC units transfused before the diagnosis of MDS were considered MDS-related if a patient was transfused to alleviate persistent or progressive anemia due to MDS. RBC units transfused before the MDS diagnosis for other causes, such as gastrointestinal bleeding, surgery or trauma, were considered unrelated.
To minimize the influence of clinical variables such as infection, bleeding, disease-modifying therapies and invasive procedures, we evaluated transfusion intensity (number of RBC units transfused per month) in patients requiring regular RBC transfusion before and after alloimmunization during the entire study period, and over a fixed period of 8 months (4 months before and 4 months after first documentation of alloimmunization). Patients who received disease-modifying therapies, died or progressed to acute myeloid leukemia within 4 months of alloimmunization were excluded, as these variables would influence RBC transfusion intensity. Patients who developed alloantibodies before MDS-related transfusion, developed alloantibodies after only one or two episodes of RBC transfusion, received only intermittent RBC transfusions, or did not receive further RBC transfusion after alloantibodies had been detected were also excluded from this analysis as RBC transfusion intensity could not be calculated accurately in these patients.
Laboratory data included patients’ ABO/Rh type, antibody screening results, direct antiglobulin test and alloantibody and autoantibody specificities (where specificity was documented). Data concerning transfusion reactions were obtained from the hospitals’ transfusion records (Online Supplementary Methods). A delayed serological transfusion reaction was considered to have occurred if all the following criteria were satisfied: (i) a new antibody was detected; (ii) there was a new positive direct antiglobulin test, (iii) RBC elution identified the presence of the same antibody that was identified in the serum; and (iv) phenotyping of the patient’s RBC demonstrated mixed-field typing or negativity for the antigen towards which the alloantibody in the patient’s serum/eluate was directed. A delayed hemolytic transfusion reaction was defined as having occurred when a patient with a delayed serological transfusion reaction had clinical evidence of hemolysis.21,22
The cumulative incidence of alloimmunization was analyzed by competing-risks regression using the Fine and Gray method. Factors associated with RBC alloantibody formation were investigated using random survival forest, recursive partitioning and competing risk regression analyses (Online Supplementary Methods).
Results
Patient and clinical characteristics
The clinical and laboratory records of 836 MDS patients were reviewed. Nineteen patients were ineligible for further analysis because of inadequate follow up and/or incomplete transfusion history. The median age of the 817 patients eligible for analysis was 73 years (range, 19–98 years); 536 (66%) patients were male. MDS-multilineage dysplasia, MDS with excess blasts-1 and MDS with excess blasts-2 were the most frequent subtypes (Table 1). The majority of patients received supportive care alone (605; 74%) while 204 (25%) patients received disease-modifying therapies (Table 1).
Table 1.
Clinical features of patients included in the analysis.
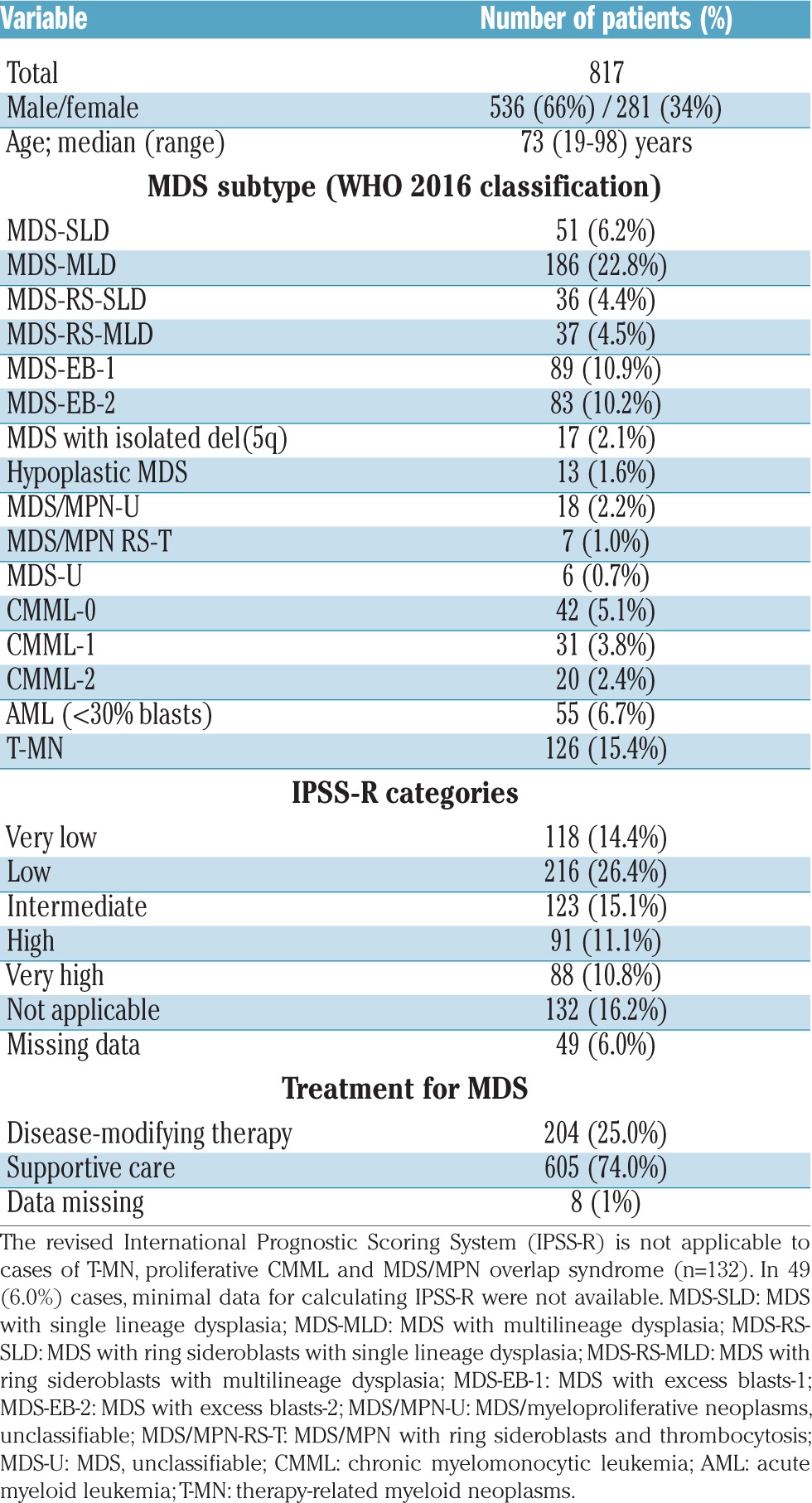
According to IPSS-R score, 457 (56%) patients were classified in the Very Low, Low and Intermediate risk groups, while 179 (22%) patients were classified in the High and Very High risk groups, with significant differences in survival and cumulative incidence of RBC transfusion dependency between the groups (Table 1; Online Supplementary Figure S1A,B). The 132 (16%) patients with therapy-related myeloid neoplasm or proliferative MDS/myeloproliferative neoplasm overlap were not eligible for IPSS-R calculations. IPSS-R could not be assessed in 49 (6%) patients because of missing data or failed metaphase cytogenetics.
Incidence of red blood cell alloimmunization
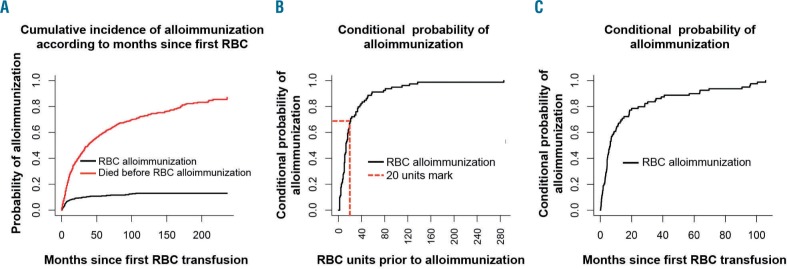
During the study 695 (85%) patients received at least one unit of RBC, and 98 (12%) patients developed 175 alloantibodies. Of these, seven patients developed antibodies before their first documented RBC transfusion and 11 patients developed alloantibodies following MDS-unrelated RBC transfusion before the diagnosis of MDS (range, 0.23 to 146 months prior) (Online Supplementary Table S1). The remaining 80 patients (including six patients who were also transfused before MDS diagnosis) developed alloantibodies following MDS-related RBC transfusion (Online Supplementary Table SI). Thus, the cumulative incidence of RBC alloimmunization with death as a competing risk was 11% at 50 months following the first MDS-related RBC transfusion (Figure 1A). Importantly, 73% and 50% of alloimmunized patients developed alloantibodies following transfusions of fewer than 20 RBC units (Figure 1B; Online Supplementary Figure S1C) and during the first 6 months following commencement of RBC transfusions (Figure 1C), respectively.
Figure 1.
Cumulative incidence of red blood cell alloimmunization and time to development of alloantibodies. (A) Probability of alloimmunization with death as competing risk by months following first RBC transfusion; (B) 73% of alloimmunized patients developed alloantibodies within the period of transfusion of their first 20 units of RBC; (C) 50% of alloimmunized patients developed alloantibodies within 6 months after their first RBC transfusion.
Of the 98 patients who developed RBC alloantibodies, 50 (51%) patients developed one alloantibody, while 48 (49%) developed multiple alloantibodies, including four patients who developed four antibodies each, and one patient who developed six antibodies in total. Fourteen and three patients developed two and three antibodies simultaneously, respectively (Table 2 and Online Supplementary Table S1).
Table 2.
Alloantibody specificities.
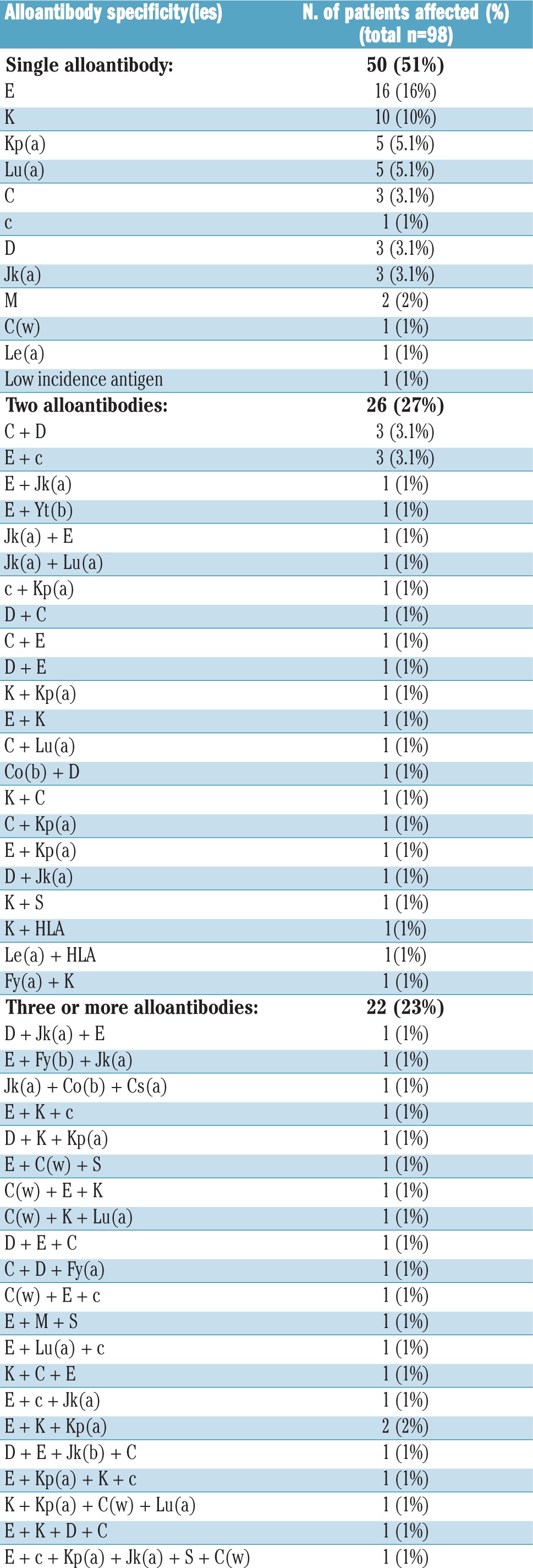
Rh (54%) and Kell (24%) system antibodies were the most frequent, followed by Kidd (7.5%) and Lutheran (5.7%) system antibodies (Table 2 and Online Supplementary Table S1). Within the Rh group, anti-E (46%) was the most frequent, followed by anti-C (17%) and anti-D (17%) (Table 2). Despite our state-wide policy of providing RhD-compatible RBC transfusions, 16 RhD-negative patients developed anti-D. These patients’ transfusion records were reviewed for the period prior to anti-D alloimmunization. Anti-D was detected following RhD-positive platelet transfusions in eight patients (one to two units of platelets), and three patients received RhD-positive RBC units due to a clinical emergency. In three female patients, the anti-D alloimmunization was most probably pregnancy-related. For the remaining two patients, we could find no record of RhD-positive platelet or RBC transfusion. Administration of anti-D Rh immunoglobulin remains a possibility, but we could not find any record of it in the participating institutions.
Autoantibody formation following alloimmunization
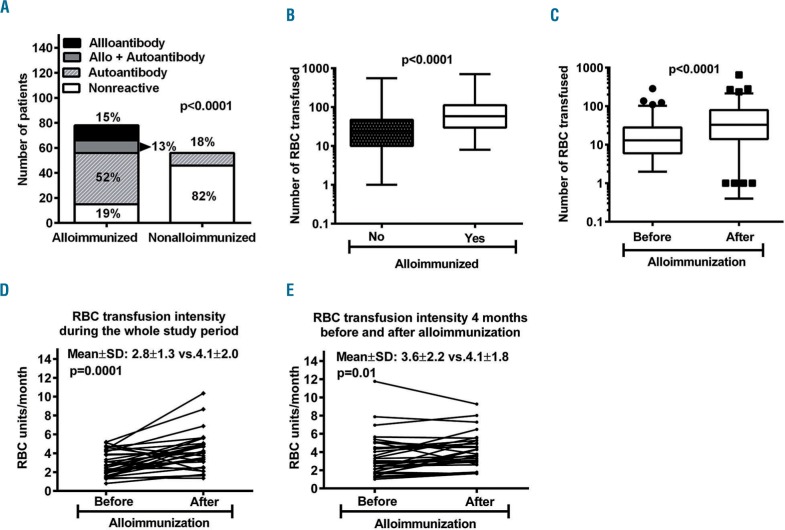
During the study period, 327 (40%) patients had a direct antiglobulin test performed and the test was positive at least once in 157 (48%) patients. Alloimmunized patients had much higher rates of positive direct antiglobulin test (84% versus 33%, P<0.0001; Online Supplementary Figure S1D) and reactive eluates (80% versus 18%, P<0.0001, Figure 2A) than non-alloimmunized patients. RBC eluates showed pan-agglutination due to non-specific autoantibody, an alloantibody, a combination of an allo- and autoantibody, and non-reactivity in 52%, 15%, 13%, and 19% of alloimmunized patients, respectively. Thus, a significantly higher proportion of alloimmunized patients had detectable autoantibody compared to non-alloimmunized patients (65% versus 18%; P<0.0001) (Figure 2A). Circulating free autoantibody was also detected in 31/51 (61%) of alloimmunized patients.
Figure 2.
Alloimmunization is associated with autoantibody formation and increased red blood cell transfusion requirement. (A) Autoantibodies were detected in a significantly higher number of alloimmunized patients than in non-alloimmunized ones (65% vs. 18%; P<0.0001) (B) The total number of RBC units transfused was significantly higher in alloimmunized patients than in non-alloimmunized patients (P<0.0001) (C) In alloimmunized patients, the total number of RBC units transfused was significantly higher after alloimmunization (D) RBC transfusion intensity was significantly higher following alloimmunization during the whole study period (E) RBC transfusion intensity compared over 8 months (4 months before and 4 months after alloimmunization) also confirmed that RBC transfusion intensity increases significantly following alloimmunization.
In the 88% of alloimmunized patients who developed autoantibodies, these were detected either at the time of alloimmunization or within the 5 months preceding or following alloimmunization (Online Supplementary Figure S1E). In two cases in which autoantibody was detected 74 and 18 months prior to alloantibody detection, the patients were diagnosed with warm autoimmune hemolytic anemia on the background of non-Hodgkin lymphoma or chronic lymphocytic leukemia. In one female patient, the autoantibody was detected at the time that chronic lymphocytic leukemia was diagnosed, 107 months after the alloantibody had first been detected. In this case, the alloantibody was most probably related to a previous pregnancy.
Clinical consequences of antibody formation
Two patients developed significant hemolysis after alloimmunization. A 51-year old male patient developed an alloantibody resulting in life-threatening delayed hemolytic transfusion reactions, cardiac arrest, multiorgan failure and a prolonged stay in the Intensive Care Unit (Online Supplementary Figure S2A–C). Another 83-year old male patient developed hemolysis after 6 months of RBC transfusion. Investigations revealed alloantibody, panagglutinating autoantibody and autoimmune hemolysis, which responded to steroids (Online Supplementary Figure S2D–F). Of the 79 alloimmunized cases with a positive direct antiglobulin test, 22 (28%) satisfied the definition of delayed serological transfusion reaction but were not reported to the transfusion services as having had such a reaction or a delayed hemolytic transfusion reaction. Other transfusion reactions were generally infrequent, mild in nature and limited to febrile non-hemolytic transfusion reactions or allergic reactions. Fifteen alloimmunized patients had 18 episodes of transfusion reactions including 13 febrile non-hemolytic transfusion reactions and five allergic reactions.
Alloimmunization increases red blood cell transfusion intensity
The total number of RBC units transfused in alloimmunized patients was significantly higher than in non-alloimmunized patients (90±100 versus 30±52; P<0.0001) (Figure 2B). In alloimmunized patients the total number of RBC transfused after alloimmunization was significantly higher than the number before alloimmunization (P<0.0001) (Figure 2C). RBC transfusion intensity was compared in 33 individual patients eligible for analysis before and after alloimmunization (Online Supplementary Figure S3). RBC transfusion intensity was significantly higher following documentation of alloimmunization than prior to alloantibody formation (2.8±1.3 versus 4.1±2.0 units per month; P<0.0001) (Figure 2D). To further minimize the impact of other variables, we also compared RBC transfusion intensity over a period of 8 months (4 months before and after detection of alloimmunization). In this analysis, RBC transfusion intensity was also significantly higher following alloimmunization (3.6±2.2 versus 4.1±1.8 units per month; P=0.01) (Figure 2E). Reticulocyte response, as expected, was poor. In these patients, there was no clinically significant different variation in platelet and neutrophil counts during the period of analysis (data not shown). Lactate dehydrogenase and bilirubin levels transiently increased in some patients after alloimmunization (data not shown). Haptoglobin results were not available.
Risk factors for alloimmunization
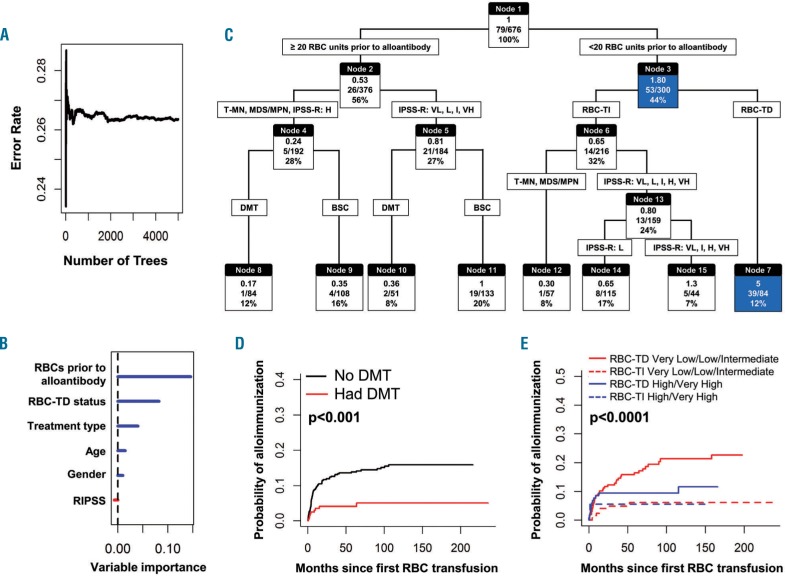
To identify potential predictors of alloimmunization 676 patients, including 80 who developed alloantibodies following MDS-related RBC transfusions, were included in random survival forest and recursive partitioning analyses. The number of RBC units transfused before alloantibody formation was the most important predictor of alloimmunization, followed by RBC transfusion dependency status, treatment type, and age (Figure 3A–C). A classification tree using recursive partitioning suggested that 46% (39/84) patients who were dependent on RBC transfusions developed an alloantibody within the period they were given their first 20 units of RBC (hazard ratio 5; node 7). In the random survival forest analysis, the predicted error rate was 26%, indicating a 74% chance of correctly predicting alloimmunization risk with a tree constructed from the five factors shown in Figure 3A,B.
Figure 3.
Recursive partition and random forest analysis to identify patients at higher risk of alloimmunization. (A-B) The random forest analysis predicted an error rate of 26%, thus indicating a 74% chance of correctly predicting alloimmunization risk. RBC units transfused prior to alloantibody, RBC-TD status and treatment type are major predictors of alloimmunization (C) Recursive partition analysis incorporating these variables produced a classification tree demonstrating that the majority of alloimmunized patients developed antibody within the first 20 units of RBC transfusion. Alloimmunization risk was highest in RBC-TD patients within the initial 20 units of RBC (hazard ratio 5; node 7) (D) Cumulative incidence of alloimmunization was significantly lower in patients treated with DMT (E) Cumulative incidence of alloimmunization was significantly higher in RBC-TD IPSS-R Very Low, Low, intermediate risk groups compared to RBC-TD High and Very High risk groups, while alloimmunization was significantly lower in RBC-TI patients in both groups. In Figure 3C: top, middle and bottom values in each node (box) indicate hazard ratio (HR), number of cases developing alloantibody divided by number of cases in that group and percentage of total cases, respectively. For example in node 7, the HR of developing an alloantibody was 5 (top number), 39/84 cases developed alloantibodies and this node represents 12% of the total cases. IPSS-R categories are Very Low (VL), Low (L), Intermediate (I), High (H), and Very High (VH). T-MN: therapy-related myeloid neoplasm; MDS/MPN overlap myelodysplastic/myeloproliferative neoplasm overlap syndrome. DMT: disease-modifying therapy; BSC: best supporting care; RBC-TD: RBC transfusion dependency; RBC-TI: RBC transfusion independent.
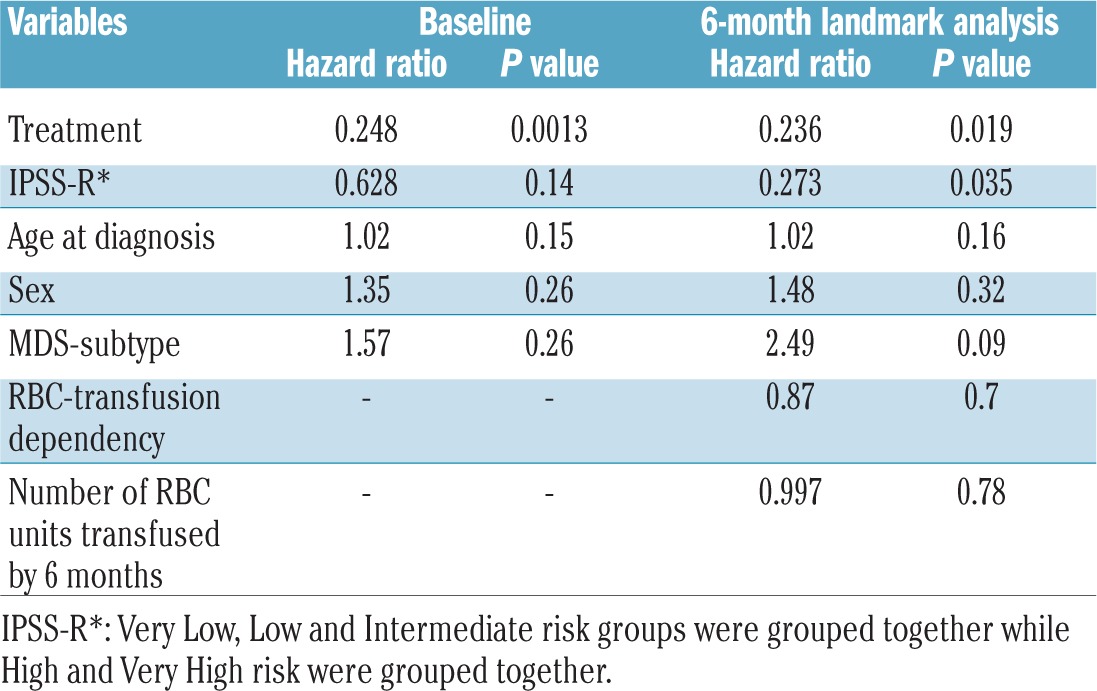
As 73% and 50% of alloimmunized patients developed alloantibodies within the initial 20 units of RBC and within 6 months after their first RBC transfusion, we performed landmark analyses using a competing risk regression model both at baseline and at 6 months following the start of RBC transfusions. For the baseline analysis age, sex, WHO subtype, IPSS-R risk groups and type of treatment were included. For the 6-month analysis numbers of RBC units transfused within 6 months and RBC transfusion dependency status at 6 months were included, in addition to all the baseline factors.
At the baseline and 6-month landmark analyses, alloimmunization risk was significantly lower in patients treated with disease-modifying therapies (hazard ratio 0.24; P=0.0013) (Table 3). The cumulative incidences of alloimmunization at 50 months (4% versus 14%) and 100 months (5% versus 15%) were significantly lower in patients treated with disease-modifying therapies than in those given supportive care only (P<0.001) (Figure 3D) and appeared to be unrelated to number of RBC transfusions, as patients treated with disease-modifying therapies received significantly more RBC units prior to alloantibody detection than patients treated with supportive care (42.5±44 versus 35±54.6; P<0.0001) (Online Supplementary Figure S4A). Within the group given disease-modifying therapies, the rate of alloimmunization was significantly higher in patients treated with azacitidine/lenalidomide than in those treated with intensive chemotherapy and/or allogeneic hematopoietic stem cell transplantation (2.9% versus 0% at 12 months, P=0.02) (Online Supplementary Figure S4B). At the 6-month landmark, IPSS-R groups also predicted alloimmunization; the cumulative incidence of alloimmunization was significantly higher in patients in the combined IPSS-R Very Low, Low and Intermediate risk groups than in those in the combined High and Very High risk groups (P=0.03). The cumulative incidence of alloimmunization was significantly higher in RBC transfusion-dependent patients in IPSS-R Very Low, Low and Intermediate risk groups than in RBC transfusion-dependent patients in IPSS-R High and Very High risk groups. The incidence of alloimmunization was significantly lower in RBC-transfusion-independent groups (Figure 3E).
Table 3.
Competing risk regression analysis for alloimmunization.
Discussion
Post-transfusion RBC alloimmunization rates vary from 2.5–3.3% for surgical patients to 9–13% in patients with hematologic malignancies.18,23–25 In a large study of more than 21,000 previously non-transfused patients who received RBC transfusions without extended matching, alloantibodies were detected in 2.2% of all transfused patients with a cumulative alloimmunization incidence of 7.7% after 40 units.26 In MDS, highly variable alloimmunization rates have been reported, ranging from 15 to 59%,18–20,27–30 which may reflect small cohorts of patients, inconsistent inclusion criteria and variable follow-up periods. Of these, studies with smaller numbers of patients reported higher alloimmunization rates of 44 to 57%,18,20,30 while a study of 272 patients reported an alloimmunization rate of only 15%.19 This is similar to the 11% cumulative incidence of alloimmunization in our study, which, to the best of our knowledge, is the largest of its type and, crucially, was also able to distinguish between alloimmunization due to MDS-related and unrelated RBC transfusions. Importantly, this is the first study demonstrating a significant increase in RBC transfusion requirements following alloimmunization in MDS patients.
In our study 76% of alloimmunized patients developed antibodies against antigens in the Rh and Kell systems, similar to the 62% reported in MDS by Sanz et al.,19 and consistent with observations made in studies of patients with sickle cell disease and thalassemia31,32 and medical patients.26 Differences in immunogenic RBC antigens between donors and recipients also play a role in alloimmunization. These disparities are unlikely to be a major contributor to alloimmunization in our cohort of patients with MDS as the vast majority of the recipients and donors in our cohort were Caucasian.
The life expectancy of some higher risk MDS patients is short and, overall, only 11% of transfused MDS patients developed alloantibodies. It is, however, of considerable interest from clinical and cost-effectiveness standpoints to identify the patients at highest risk of RBC alloimmunization, because they would be the ones to benefit most from a policy of extended antigen-matched RBC transfusions. Although the number of RBC units transfused increases the risk of alloimmunization,19 RBC transfusion requirement is dynamic. We found that 73% of patients developing alloantibodies did so within the period of receiving their first 20 units of RBC and 50% of patients within 6 months of their first RBC transfusion. Hence, it is critical to identify patient- and disease-related factors that will differentiate between “responders” and “non-responders” to RBC antigens.
In our study, disease-modifying therapies predicted alloimmunization risk at both the baseline and 6-month landmark analyses. Interestingly, the cumulative incidence of alloimmunization was significantly lower in patients treated with intensive chemotherapy and/or allogeneic hematopoietic stem cell transplantation compared to that in patients treated with azacitidine/lenalidomide, possibly due to the greater degree of immunosuppression. Lower alloimmunization rates in IPSS-R High and Very High risk groups compared to Very Low, Low and Intermediate risk groups could be due to the shorter median overall survival, larger proportion of patients requiring disease-modifying therapies, and greater degree of immunosuppression in higher risk groups. Within each group, alloimmunization risk was significantly higher among the RBC transfusion-dependent group compared to the transfusion-independent group, while alloimmunization rate was similarly low in RBC transfusion-independent regardless of risk and treatment assignment. The number of regulatory T cells, known to inhibit alloimmunization, is significantly lower in IPSS low risk patients than in IPSS high risk patients.33,34
This study focused on the clinical implications of alloimmunization, but alloimmunization also leads to increased laboratory workloads and poses the challenge of securing appropriate RBC units in a timely fashion. The clinical consequences of alloimmunization in our study included at least two cases of severe delayed hemolytic transfusion reaction, 22 cases of delayed serological transfusion reaction, and increased RBC transfusion requirements. RBC transfusion requirement increased following alloimmunization, most likely due to the development of additional alloantibodies and autoantibodies, resulting in subclinical serological hemolytic transfusion reactions and/or autoimmune hemolysis. Notably, autoantibodies were detected before, simultaneously or within a short time after alloimmunization. This suggests that alloimmunization drives autoantibody formation. Young et al. detected autoantibodies in 121/2618 (4.6%) individuals with a positive direct or indirect antiglobulin test.12 Interestingly, 41 (34%) of these individuals had both alloantibodies and autoantibodies, and at least 34% of cases developed RBC autoantibodies after previous blood transfusion and in association with alloimmunization.12 Similarly, other studies reported that 8% to 25% of multiply transfused patients with sickle cell dis ease16 or thalassemia13 have IgG autoantibodies, mostly associated with alloimmunization. RBC autoantibody formation has also been described in both animal and human experimental models of RBC transfusion.15 The pathophysiological mechanisms are not yet fully understood.35 The implication of autoantibodies is two-fold. Firstly, they pose challenges to the transfusion laboratory. Resolution of these complex cases translates into a heavy workload, delay in provision of transfusions and increased cost. Secondly, autoantibodies can be pathological, causing shortened RBC survival and autoimmune hemolysis, which may be severe.13 In MDS patients, hemolysis assessment can be complicated by higher (disease-related) baseline lactate dehydrogenase and poor reticulocyte response due to dyserythropoiesis. Since RBC transfusion can also influence lactate dehydrogenase, haptoglobin and bilirubin levels, a high degree of clinical suspicion is required. The integration of RBC genotyping can minimize or even potentially eliminate labor-intensive serological testing and provide better matched RBC units. Investigators at the Wisconsin Blood Center genotyped 42 blood group antigens in 43,066 blood donors and were able to provide antigen-negative RBC for more than 94% of requests. There were no cases of acute hemolytic transfusion reaction, delayed hemolytic transfusion reaction, alloimmunization or other adverse reactions.36
In summary, this large registry-based study shows that RBC alloimmunization is not uncommon in MDS, occurs early after commencing transfusion, and has important clinical consequences, including an association with increased RBC transfusion requirements. Provision of extended phenotype-matched RBC units from initiation of a transfusion program for MDS patients can minimize alloimmunization30 and its complications and may be of benefit.
Supplementary Material
Acknowledgments
The authors would like to thank the Royal Adelaide Hospital Research Fund, Contributing Haematologists’ Committee, Royal Adelaide Hospital and Novartis Pharmaceuticals Australia Pty Limited for research funding support for the SA-MDS Registry.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/12/2021
References
- 1.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. [DOI] [PubMed] [Google Scholar]
- 2.Brunning RD, Orazi A, Germing U, et al. Myelodysplastic syndromes/neoplasms, overview. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. 4th ed. Lyon: International Agency for Research on Cancer; 2008. p. 88–93. [Google Scholar]
- 3.Visser O, Trama A, Maynadie M, et al. Incidence, survival and prevalence of myeloid malignancies in Europe. Eur J Cancer. 2012;48(17):3257–3266. [DOI] [PubMed] [Google Scholar]
- 4.Sekeres MA, Cutler C. How we treat higher-risk myelodysplastic syndromes. Blood. 2014;123(6):829–836. [DOI] [PubMed] [Google Scholar]
- 5.Fenaux P, Ades L. How we treat lower-risk myelodysplastic syndromes. Blood. 2013; 121(21):4280–4286. [DOI] [PubMed] [Google Scholar]
- 6.Hellstrom-Lindberg E. Management of anemia associated with myelodysplastic syndrome. Semin Hematol. 2005;42(2 Suppl 1):S10–13. [DOI] [PubMed] [Google Scholar]
- 7.Hiwase DK, Singhal D, Strupp C, et al. Dynamic assessment of RBC-transfusion dependency improves the prognostic value of the revised-IPSS in MDS patients. Am J Hematol. 2017;92(6):508–514. [DOI] [PubMed] [Google Scholar]
- 8.Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25(23):3503–3510. [DOI] [PubMed] [Google Scholar]
- 9.FDA. Fatalities Reported to FDA Following Blood Collection and Transfusion. US Department of Health and Human Services; [cited 2017 August 20]. Available from: https://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TransfusionDonationFatalities. [Google Scholar]
- 10.Telen MJ, Afenyi-Annan A, Garrett ME, et al. Alloimmunization in sickle cell disease: changing antibody specificities and association with chronic pain and decreased survival. Transfusion. 2015;55(6pt2):1378–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nickel RS, Hendrickson JE, Fasano RM, et al. Impact of red blood cell alloimmunization on sickle cell disease mortality: a case series. Transfusion. 2016;56(1):107–114. [DOI] [PubMed] [Google Scholar]
- 12.Young PP, Uzieblo A, Trulock E, et al. Autoantibody formation after alloimmunization: are blood transfusions a risk factor for autoimmune hemolytic anemia? Transfusion. 2004;44(1):67–72. [DOI] [PubMed] [Google Scholar]
- 13.Singer ST, Wu V, Mignacca R, et al. Alloimmunization and erythrocyte autoimmunization in transfusion-dependent thalassemia patients of predominantly Asian descent. Blood. 2000;96(10):3369–3373. [PubMed] [Google Scholar]
- 14.Castellino SM, Combs MR, Zimmerman SA, et al. Erythrocyte autoantibodies in paediatric patients with sickle cell disease receiving transfusion therapy: frequency, characteristics and significance. Br J Haematol. 1999;104(1):189–194. [DOI] [PubMed] [Google Scholar]
- 15.Garratty G. Autoantibodies induced by blood transfusion. Transfusion. 2004;44(1):5–9. [DOI] [PubMed] [Google Scholar]
- 16.Aygun B, Padmanabhan S, Paley C, et al. Clinical significance of RBC alloantibodies and autoantibodies in sickle cell patients who received transfusions. Transfusion. 2002;42(1):37–43. [DOI] [PubMed] [Google Scholar]
- 17.Ahrens N, Pruss A, Kahne A, et al. Coexistence of autoantibodies and alloantibodies to red blood cells due to blood transfusion. Transfusion. 2007;47(5):813–816. [DOI] [PubMed] [Google Scholar]
- 18.Stiegler G, Sperr W, Lorber C, et al. Red cell antibodies in frequently transfused patients with myelodysplastic syndrome. Ann Hematol. 2001;80(6):330–333. [DOI] [PubMed] [Google Scholar]
- 19.Sanz C, Nomdedeu M, Belkaid M, et al. Red blood cell alloimmunization in transfused patients with myelodysplastic syndrome or chronic myelomonocytic leukemia. Transfusion. 2013;53(4):710–715. [DOI] [PubMed] [Google Scholar]
- 20.Novaretti MC, Sopelete CR, Velloso ER, et al. Immunohematological findings in myelodysplastic syndrome. Acta Haematol. 2001;105(1):1–6. [DOI] [PubMed] [Google Scholar]
- 21.Winters JL, Richa EM, Bryant SC, et al. Polyethylene glycol antiglobulin tube versus gel microcolumn: influence on the incidence of delayed hemolytic transfusion reactions and delayed serologic transfusion reactions. Transfusion. 2010;50(7):1444–1452. [DOI] [PubMed] [Google Scholar]
- 22.Vamvakas EC, Pineda AA, Reisner R, et al. The differentiation of delayed hemolytic and delayed serologic transfusion reactions: incidence and predictors of hemolysis. Transfusion. 1995;35(1):26–32. [DOI] [PubMed] [Google Scholar]
- 23.Schonewille H, Haak HL, van Zijl AM. Alloimmunization after blood transfusion in patients with hematologic and oncologic diseases. Transfusion. 1999;39(7):763–771. [DOI] [PubMed] [Google Scholar]
- 24.Heddle NM, Soutar RL, O’Hoski PL, et al. A prospective study to determine the frequency and clinical significance of alloimmunization post-transfusion. Br J Haematol. 1995;91(4):1000–1005. [DOI] [PubMed] [Google Scholar]
- 25.Evers D, Zwaginga JJ, Tijmensen J, et al. Treatments for hematological malignancies in contrast to those for solid cancers are associated with reduced red cell alloimmunization. Haematologica. 2016;102(1):52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evers D, Middelburg RA, de Haas M, et al. Red-blood-cell alloimmunisation in relation to antigens’ exposure and their immunogenicity: a cohort study. Lancet Haematol. 2016;3(6):e284–e292. [DOI] [PubMed] [Google Scholar]
- 27.Guelsin GA, Rodrigues C, Visentainer JE, et al. Molecular matching for Rh and K reduces red blood cell alloimmunisation in patients with myelodysplastic syndrome. Blood Transfus. 2015;13(1):53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortiz S, Orero MT, Javier K, et al. Impact of azacitidine on red blood cell alloimmunisation in myelodysplastic syndrome. Blood Transfus. 2017;15(5):472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozovski U, Ben-Tal O, Kirgner I, et al. Increased incidence of red blood cell alloantibodies in myelodysplastic syndrome. Isr Med Assoc J. 2015;17(10): 624–627. [PubMed] [Google Scholar]
- 30.Lin Y, Saskin A, Wells RA, et al. Prophylactic RhCE and Kell antigen matching: impact on alloimmunization in transfusion-dependent patients with myelodysplastic syndromes. Vox Sang. 2017;112(1):79–86. [DOI] [PubMed] [Google Scholar]
- 31.Matteocci A, Pierelli L. Red blood cell alloimmunization in sickle cell disease and in thalassaemia: current status, future perspectives and potential role of molecular typing. Vox Sang. 2014;106(3):197–208. [DOI] [PubMed] [Google Scholar]
- 32.Rosse WF, Gallagher D, Kinney TR, et al. Transfusion and alloimmunization in sick le cell disease. The Cooperative Study of Sickle Cell Disease. Blood. 1990;76(7): 1431–1437. [PubMed] [Google Scholar]
- 33.Kordasti SY, Afzali B, Lim Z, et al. IL-17-producing CD4(+) T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br J Haematol. 2009;145(1):64–72. [DOI] [PubMed] [Google Scholar]
- 34.Kotsianidis I, Bouchliou I, Nakou E, et al. Kinetics, function and bone marrow trafficking of CD4+CD25+FOXP3+ regulatory T cells in myelodysplastic syndromes (MDS). Leukemia. 2009;23(3):510–518. [DOI] [PubMed] [Google Scholar]
- 35.Kaminski ER, Hows JM, Goldman JM, et al. Lymphocytes from multi-transfused patients exhibit cytotoxicity against autologous cells. Br J Haematol. 1992;81(1):23–26. [DOI] [PubMed] [Google Scholar]
- 36.Flegel WA, Gottschall JL, Denomme GA. Integration of red cell genotyping into the blood supply chain: a population-based study. Lancet Haematol. 2015;2(7):e282–e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.