Abstract
Chronic lymphocytic leukemia (CLL) cells are provided with essential survival and proliferative signals in the lymph node microenvironment. Here, CLL cells engage in various interactions with bystander cells such as T cells and macrophages. Phenotypically distinct types of tumor infiltrating macrophages can either be tumor supportive (M2) or play a role in tumor immune surveillance (M1). Although recent in vitro findings suggest a protective role for macrophages in CLL, the actual balance between these macrophage subsets in CLL lymphoid tissue is still unclear. Furthermore, the mechanism of recruitment of monocytes towards the CLL lymph node is currently unknown. Both questions are addressed in this paper. Immunofluorescence staining of lymph node samples showed macrophage skewing towards an M2 tumor-promoting phenotype. This polarization likely results from CLL-secreted soluble factors, as both patient serum and CLL-conditioned medium recapitulated the skewing effect. Considering that CLL cell cytokine secretion is affected by adjacent T cells, we next studied CLL-mediated monocyte recruitment in the presence or absence of T-cell signals. While unstimulated CLL cells were inactive, T cell-stimulated CLL cells actively recruited monocytes. This correlated with secretion of various chemokines such as C-C-motif-ligand-2,3,4,5,7,24, C-X-C-motif-ligand-5,10, and Interleukin-10. We also identified CD40L as the responsible T-cell factor that mediated recruitment, and showed that recruitment critically depended on the C-C-motif-chemokine-receptor-2 axis. These studies show that the shaping of a tumor supportive microenvironment depends on cytokinome alterations (including C-C-motif-ligand-2) that occur after interactions between CLL, T cells and monocytes. Therefore, targeted inhibition of CD40L or C-C-motif-chemokine-receptor-2 may be relevant therapeutic options.
Introduction
Chronic lymphocytic leukemia (CLL) cells strongly depend on interactions with bystander T cells and monocyte-derived cells (MDCs) within the lymph node (LN) microenvironment for their survival and resistance to therapy.1 The role of LN-residing T cells in the pathogenesis of CLL has gained much attention. It is suggested that interaction of neoplastic B cells with T cells results in skewing of the T-cell compartment towards CD40L-expressing CD4+ T cells.2 These T cells, in turn, induce both CLL cell survival and proliferation via upregulation of several pro-survival molecules as well as increased secretion of cytokines.3,4 The interaction between MDCs and CLL is less well understood, although in vitro experiments show that MDCs, in the form of Nurse-like cells, can induce CLL cell survival5 through C-X-C motif chemokine 12, B-cell activating factor and A proliferation-inducing ligand signaling.5,6
Based on data from different malignancies, there are two subgroups of tumor-associated macrophages (TAMs): 1) M2-like CD68+CD163+/CD206+ macrophages are characterized by an immunosuppressive phenotype, whereas 2 M1-like CD68+CD80+ macrophages display an immunesurveilling phenotype.7 Although there is large intratumoral and intertumoral heterogeneity, it has been suggested that M1 TAMs lead to a better and M2 TAMs lead to a worse prognosis across different tumor types.8 Tumors that are associated with M2 TAMs include breast,9 ovarian,7 and prostate10 cancers, whereas colon carcinoma TAMs are of M1 phenotype.11
With respect to CLL, ex vivo evidence shows that MDCs are present in the LN,12 and it was recently shown that MDCs contribute to CLL progression, as MDC depletion by clodronate treatment in the TCL1 CLL mouse model leads to slower CLL progression.13,14 Whether LN-residing macrophages in human CLL are indeed of a protective M2 phenotype has, however, not been directly studied. It is also not known whether circulating monocytes can actively be recruited towards the tumor-infiltrated LN.
Migration of CLL cells to the LN microenvironment depends on chemotactic gradients through the CXCL12/CXCR4,15 CXCL13/CXCR516 and CCL19,21/CCR717 axes. Upon interaction with LN-residing cells, such as T cells, CLL cells can alter their secretome,4,18,19 which, in turn, could potentially impact both skewing and migration of other cells, like MDCs. Co-operative or reciprocal signals between the triad formed by CLL cells, T cells, and MDCs could, therefore, critically contribute to the supportive microenvironment for CLL cells.
Here, we investigated both the possibly supportive differentiation of MDCs and their recruitment as a result of CLL-secreted cytokines in the context of T-cell signals. We found that CLL-secreted factors were able to differentiate macrophages towards a supporting M2 phenotype. Secondly, T cell/CD40 stimulation of CLL cells induced CLL cells to recruit monocytes; an action which critically depends on CCR2 signaling.
Methods
Patients’ samples, stimulation and conditioned medium collection
Patient material was obtained from CLL patients, after written informed consent according to the guidelines of the Medical Ethical Committee of the Academic Medical Center, Amsterdam, the Netherlands, in accordance with Declaration of Helsinki protocols. For T-cell stimulation, peripheral blood mononuclear cells (PBMCs) were isolated from either healthy donors (HDs) or from CLL patients using Ficoll gradient purification according to the manufacturer’s instructions (Lucron, Dieren, the Netherlands). These PBMCs (either magnetically sorted or not to enrichen the T-cell fraction) were added to CLL cells (in either an allogeneic or autologous fashion, as indicated) in a 1:1 ratio, each at a concentration of 1.0*106 cells/mL. Stimulating antibodies directed against CD3 (1 mg/mL, clone 1XE, Sanquin, Amsterdam, the Netherlands) and CD28 (3 μg/mL, clone 15E8, Sanquin) were added for T-cell activation. After 72 hours (h), conditioned medium was collected. For stimulation with CD40L, CLL cells were cultured at a concentration of 1.5*106 cells/mL on CD40L transfected NIH-3T3 cells or on mock transfected 3T3 cells as described previously,3 all in IMDM supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA), 100 U/mL penicillin-100 μg/mL streptomycin (Life Technologies, Austin, TX, USA), 2 mM L-glutamine (Life Technologies), and 0.00036% β-mercaptoethanol (Sigma, St. Louis, MO, USA) (IMDM+/+) for 16 h, after which conditioned medium was collected. Cell-free conditioned medium was kept at −80°C until use.
Migration assays
Conditioned or control media were diluted 1:2 in chemotaxis medium (PBS with 1% albumin, low endotoxin; Sigma). Monocytes were freshly isolated from HDs after obtaining written informed consent using negative MACS depletion as described previously20 and resuspended in chemotaxis medium. The diluted media were added in the lower chambers of a 5 μm chemotaxis assay plate (96 well ChemoTX®, NeuroProbe, Gaithersburg, MA, USA) and 100,000 monocytes were transferred to the upper chamber. After 2 h, chemotaxis was quantified by measuring the DAPI (4,6 diamidino-2-phenylindole) signal of migrated monocytes as described previously.20
When measuring inhibitor effects, both media and monocytes were incubated for 30 minutes (min) on ice with the indicated inhibitors directly prior to the migration assay. The following chemokine receptor inhibitors were used: 1 μg/mL CCR1 inhibitor BX471 (Sigma), 1 μg/mL CCR2 inhibitor INCB3284 (Tocris Bioscience, Bristol, UK), 1μM CCR3 inhibitor SB328437 (Tocris), 1 μM CCR5 inhibitor Maraviroc (Apexbio, Houston, TX, USA), 1μM CXCR4/7 inhibitor Plerixafor (Apexbio), and 0.1 μg/mL IL-10 neutralizing antibody (R&D Systems, Minneapolis, MN, USA).
Measurement of chemokine levels
Previously generated microarray profiles4 of purified (>99%) CLL cells stimulated for 16 h with activated T cells (deposited under accession number GSE50572) were normalized and analyzed using the R2 platform (http://r2.amc.nl) and data were extracted using its DataGrabber feature. When testing protein secretion, conditioned media were analyzed for the indicated chemokines by Luminex using the ProcartaPlex 9-plex chemokine immunoassay kit extended with CCL7, CCL24, CXCL5, and IL-10 (eBioscience, San Diego, CA, USA) according to the manufacturer’s instructions.
Supplementary methods
Information on monocyte isolation and in vitro differentiation, LN material and immunofluorescence, rhCD40L stimulation and intracellular CCL2 measurements, and statistical analyses can be found in the Online Supplementary Methods.
Results
LN-residing macrophages display an M2 phenotype, and both CLL cells and CLL serum induce M2 skewing
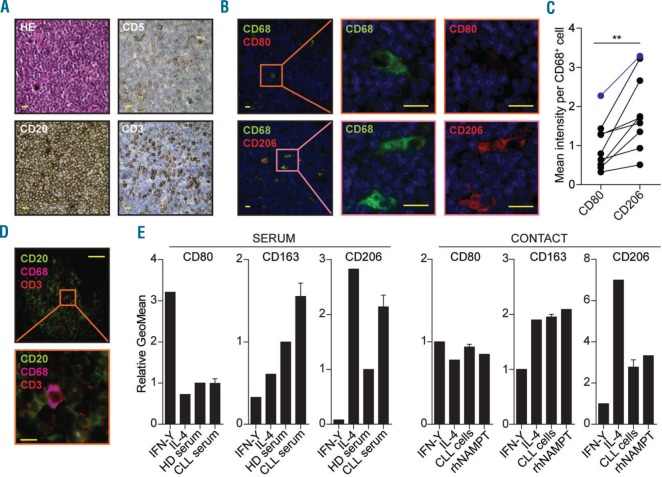
To study the phenotype of macrophages in the CLL LN, paraffin-embedded LN sections from CLL patients were stained for the pan-macrophage marker CD68 in combination with either the M1 marker CD80 or the M2 marker CD206 using immunofluorescence. The CD80/CD206 fluorescence signal per macrophage (CD68+) was then quantified using an automated cell identification pipeline in CellProfiler. CD68 positive cells were present in all samples tested and were dispersed throughout the CLL-infiltrated LNs (Figure 1A and B). Within the CD68+ cells, a higher CD206 intensity was observed as compared to CD80 (1.89 vs. 1.00 arbitrary units) (Figure 1B and C, and Online Supplementary Figure S1A).
Figure 1.
Chronic lymphocytic leukemia (CLL) cells differentiate monocytes towards an M2 phenotype. (A) Paraffin-embedded lymph node (LN) material from 9 CLL patients (for patients’ characteristics see Online Supplementary Table S1) was stained by immunohistochemistry with Hematoxylin & Eosin (HE), CLL markers CD5 and CD20, and T-cell marker CD3. One representative slide is shown. Yellow scale bars correspond to 20 μm. More information on image acquisition can be found in the Methods section. (B) The same 9 samples as in (A) were stained by immunofluorescence for pan-macrophage marker CD68 in combination with either M1 marker CD80 (top) or M2 marker CD206 (bottom). One representative slide is shown; stainings of the other slides can be found in Online Supplementary Figure S1A. Yellow scale bars correspond to 20 mm. (C) CD80 and CD206 intensity levels (both red signal) were quantified per macrophage (green signal) for each slide presented in Figure 1B and Online Supplementary Figure S1A using automated image analysis (see Methods section). Per-patient (each line) average macrophage intensity of both CD80 and CD206 are indicated (each dot). The patient presented in Figure 1B is indicated in blue. **P<0.01, paired t-tests. (D) A triple immunofluorescence staining with antibodies directed against T-cell marker CD3, CLL cell marker CD20, and macrophage marker CD68 was performed on four of the slides used for (1A). One representative slide is shown (sample CLL LN 09). Scale bars correspond to 100 mm (top) or 10 mm (bottom). (E) Healthy donor (HD) monocytes were differentiated for 72 hours (h) with IMDM containing 25% CLL serum or 25% pooled HD serum, or with complete medium containing IFN-Y (M1) or IL-4 (M2) as controls (left). In a separate experiment, monocytes were differentiated for 72 h by direct contact with CLL cells in complete medium, or with complete medium containing IFN-Y (M1), IL-4 (M2) or recombinant human (rh)NAMPT as controls (right). Monocyte differentiation was then tested by staining for M1 marker CD80 and M2 markers CD163 and CD206 using flow cytometry. Each bar represents the relative geometrical mean (GeoMean) of the fluorescence signal compared to the control condition and error bars indicate Standard Error of Mean (SEM) of n=22 (left) or n=3 (right) CLL samples.
In order to visualize spatial organization of both T cells and macrophages within CLL LN, CD3– CD68–CD20 combinatory staining was performed. CD68+ cells could be found scattered throughout the LN, and were always surrounded by CLL cells. No typical configuration of CD3 in relation to CD68 could be detected, although, on occasion, CD68+ cells were in close contact with T cells (Figure 1D).
We next studied whether the leukemic cells could account for the observed M2 polarization. First, we tested whether soluble factors present in CLL serum differentiated monocytes towards an M2 phenotype. Freshly isolated monocytes from HDs were incubated with either sera from 22 different CLL patients or pooled serum from HDs, and differentiation status was measured using flow cytometry. IFN-Y (M1) and IL-4 (M2) differentiated monocytes were included for comparison. Both M2 markers CD163 [mean relative Geomean 1.55±Standard Error of Mean (SEM) 0.16] and CD206 (2.14±0.21), but not M1 marker CD80 (1.00±0.11) were increased in CLL serum-differentiated monocytes compared to HD serum-differentiated monocytes (Figure 1E, left, and Online Supplementary Figure S1B for a representative gating strategy). Notably, no difference between serum from CLL samples from patients with either mutated or unmutated Immunoglubulin Heavy gene, or with low (<20*109) versus high (>100*109/L) leukocyte counts was observed (data not shown).
As CLL-serum components resulted in M2 differentiation, we next investigated whether the observed M2 differentiation in the LN was actuated by CLL cells. To this end, HD-isolated monocytes were differentiated for 72 h using CLL cells or positive control NAMPT.21 IFN-Y (M1) and IL-4 (M2) differentiated monocytes were again included as control. We found an upregulation of M2 markers after IL-4 stimulation. In line with the differentiation effect of CLL serum, both CLL cells and NAMPT induced an upregulation of the M2 markers, but not of the M1 marker (Figure 1E, right). Furthermore, the M2 differentiation depended on soluble factors, as conditioned medium from CLL cells likewise induced M2 differentiation (Online Supplementary Figure S1C).
Taken together these data indicate that CLL-secreted factors are able to differentiate macrophages towards an M2 phenotype.
T-cell-stimulated CLL cells secrete monocyte-attracting chemokines
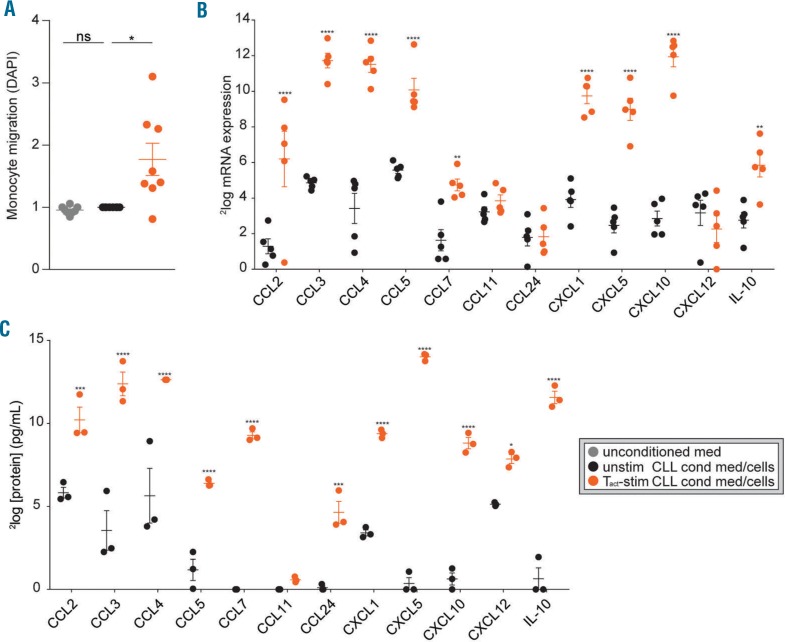
Next, we investigated whether CLL cells could direct monocyte migration. Using trans-well migration assays, we found no migration of HD monocytes towards supernatants of unstimulated CLL cells (Figure 2A). As both in vitro and ex vivo CLL LN studies strongly suggest active interaction of CLL cells with residential T cells, such as CD40L expressing follicular helper T cells within the LN22 we hypothesized that such interaction could affect CLL cytokine secretion. Therefore, supernatants of CLL cells cultured in direct contact with HD PBMCs that included αCD3/αCD28 activated T cells (Tact) (Online Supplementary Figure S2A) were compared to unstimulated CLL cells for induction of monocyte recruitment. Indeed, conditioned medium from CLL cells co-cultured in contact with activated T cells induced migration of monocytes (Figure 2A). To exclude the possibility that migration resulted from a mixed-lymphocyte reaction, we verified that autologous T cells enriched from CLL samples induced similar migration (Online Supplementary Figure S2B). In addition, we tested the migration effect of T cells without CLL cells and found some migration induction of T cells only. This effect likely results from T-cell stimulation of B/CLL cells present in these samples due to contamination, as this effect was reduced when T cells were magnetically enriched (Online Supplementary Figure S2C). To determine the candidate chemokines expressed by stimulated CLL cells that could underlie the recruitment of monocytes, we analyzed our previously generated microarray dataset (GSE50572) of purified CLL cells that were stimulated with Tact.4 Expression of several monocyte-attracting chemokines such as CCL2, 3, 4, 5, 7, CXCL1, 5, 10 and IL-1023–26 was up-regulated in CLL cells after contact with Tact (Figure 2B). To measure chemokine secretion by CLL cells, a Luminex assay was performed on three conditioned media used in Figure 2A. All chemokines that were up-regulated on the mRNA level were also significantly up-regulated on the protein level (Figure 2C).
Figure 2.
T cell-stimulated chronic lymphocytic leukemia (CLL) cells secrete monocyte-attracting chemokines. (A) Freshly isolated healthy donor (HD) monocytes were seeded in the upper chambers of a trans-well migration plate to migrate towards conditioned media (cond med) obtained from PBMC samples from CLL patients (for patients’ characteristics see Online Supplementary Table S1) that were unstimulated (unstim) or stimulated (stim) for 72 hours (h) by contact with HD PBMC T cells that were activated using α-CD3/α-CD28 antibodies. Next, the amount of migrated monocytes was quantified using DAPI staining. Each dot represents the relative (compared to the unstimulated CLL condition) DAPI signals of 8 different CLL conditioned media or 3 control media in 3 independent experiments using monocytes from 3 different donors and mean±Standard Error of Mean (SEM) are shown. All measurements were performed in triplicate. *P<0.05 in t-tests. (B) CLL cells were stimulated with α-CD3/αCD28 activated T cells or not stimulated for 16 h. RNA from CD5/CD19 FACS sorted CLL cells (>99% purity) was subjected to microarray analysis and tested for differential expression of chemokines involved in monocyte migration.23–26 Dots represent expression levels and mean±SEM are shown for 5 paired CLL samples. **P<0.01, ****P<0.0001 in a two-way ANOVA test with Bonferroni post hoc analysis. (C) Protein levels of chemokines involved in monocyte migration23–26 were determined in three conditioned media that were used to perform the migration assays in (A) by using Luminex. Dots represent protein levels and mean+SEM are shown for 3 CLL conditioned media; *P<0.05, ***P<0.001, ****P<0.0001 in a two-way ANOVA test with Bonferroni post hoc analysis.
CD40L-stimulated CLL cells attract monocytes as a result of CCR2 axis signaling
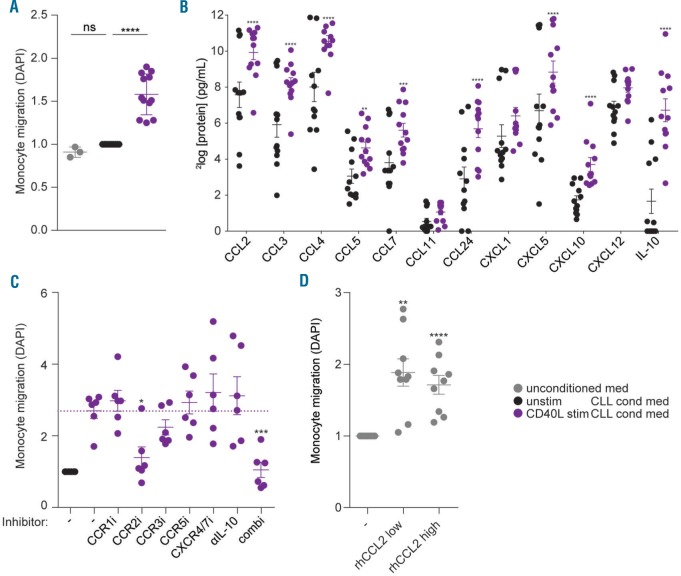
As Tact -stimulated CLL cells have highly similar gene expression profiles compared to CD40L-stimulated CLL cells,4 we investigated if CD40L stimulation similarly endows CLL cells with monocyte recruiting capacity. Comparable to the Tact results, supernatants from CD40L-stimulated CLL cells induced migration of monocytes (Figure 3A). These data indicate that a co-operative signal from Tact cells is needed for CLL cells to induce monocyte migration. Furthermore, CD40L appears to be responsible for the Tact -mediated monocyte migration. Notably, by using this T-cell free CD40L system, these data indicate that CLL-derived (rather than Tact -derived) chemokines induce recruitment of monocytes. Secreted proteins in the conditioned media from CD40L-stimulated CLL cells were measured. In line with the Tact data, several monocyte-attracting chemokines such as CCL2, 3, 4, 5, 7, 24, CXCL5, 10 and IL-10 were secreted by the CLL cells after CD40L stimulation (Figure 3B). None of the chemokines tested were detectable in supernatant from CD40L overexpressing NIH-3T3 cells alone (data not shown).
Figure 3.
CD40L-stimulated chronic lymphocytic leukemia (CLL) cells attract monocytes as a result of CCR2 axis signaling. (A) Freshly isolated healthy donor (HD) monocytes were seeded in the upper chambers of a trans-well migration plate to migrate towards conditioned media (cond med) obtained from PBMC samples from CLL patients (for patients’ characteristics see Online Supplementary Table S1) that were cultured for 16 hours (h) on CD40L-overexpressing (CD40L stim) or parental NIH-3T3 cells (unstim). Next, the amount of migrated monocytes was quantified using DAPI staining. Each dot represents the relative [compared to the unstimulated (unstim) CLL condition] DAPI signals of 12 different CLL conditioned media or 3 control media in 3 independent experiments using monocytes from 3 different donors and mean±Standard Error of Mean (SEM) are shown. All measurements were performed in triplicate. ****P<0.0001 in t-tests. (B) Protein levels of chemokines involved in monocyte migration23–26 were determined in the conditioned media that were used to perform the migration assays in (A) by using Luminex. Dots represent protein levels and mean±SEM are shown for 12 CLL conditioned media; **P<0.01, ***P<0.001, ****P<0.0001 in a two-way ANOVA test with Bonferroni post hoc analysis. (C) Freshly isolated monocytes and conditioned media were pre-incubated for 30 minutes (min) with individual small-molecule inhibitors directed against indicated chemokine receptors, with an IL-10 neutralizing antibody, or a combination of all inhibitors (combi), before performing migration assays as in (A). Each dot represents the relative (compared to the unstimulated CLL condition) DAPI signals obtained in 4 independent experiments using monocytes from 3 different donors and different CLL supernatants; mean±SEM are shown. All measurements were performed in triplicate; *P<0.05, ***P<0.001, in a one-way ANOVA test with Bonferroni post hoc analysis. (D) Monocytes were seeded in the upper chambers of a trans-well migration plate to migrate towards migration medium without or with 10 ng/mL recombinant human CCL2 (rhCCL2 low) or 100 ng/mL rhCCL2 (rhCCL2 high). Next, the amount of migrated monocytes was quantified using DAPI staining. Each dot represents the relative (compared to condition without rhCCL2) DAPI signals of 9 separate read-outs in 3 independent experiments using monocytes from 3 different donors and mean±SEM are shown; **P<0.01, ****P<0.0001 in t-tests.
To pinpoint which of the up-regulated candidate chemokines was responsible for the migration of monocytes, we applied selective small molecule inhibitors for the relevant chemokine receptors.23–26 Inhibition of CCR2 was sufficient to reduce migration to a background level. There was no additive effect of inhibition of other chemokine receptors, as a combination of the different receptor inhibitors yielded similar inhibition to CCR2 inhibition alone (Figure 3C). In a control experiment, no direct cytotoxic effect of the CCR2 inhibitor was detected after 72 h stimulation of CLL cells (Online Supplementary Figure S3A). Furthermore, supernatants from unstimulated CLL cells in combination with the different chemokine receptor inhibitors showed migration comparable to background (data not shown). We also tested if the CCR2 inhibitor could revert CLL cell-induced M2 differentiation, as observed in Figure 1, but no effect was found (data not shown). As macrophage activation depends on Bruton Tyrosine Kinase,27 we tested if migration could be reverted by inhibition via ibrutinib, but found no effect of this inhibitor (data not shown).
As CCL2 is a potent CCR2 ligand,28 we verified its induction in CD40L-stimulated CLL cells by using (cell free) recombinant CD40L (Online Supplementary Figure S3B). Furthermore, recombinant CCL2 resulted in monocyte migration (Figure 3D). Combined, these data suggest that CD40 signaling is responsible for T cell-mediated monocyte migration by CLL cells and that this migration depends on the CCL2-CCR2 axis.
Discussion
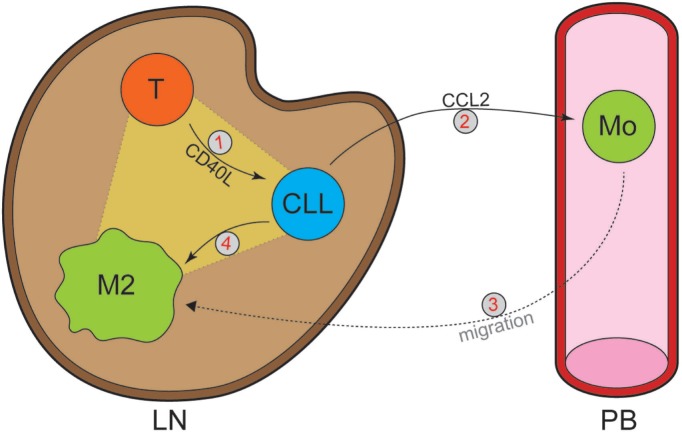
It is widely accepted that interactions with local bystander cells in the LN are critical for CLL maintenance.1 Various reports have mechanistically elucidated how bystander cells can support CLL cells, but the active role of CLL cells in shaping this supportive microenvironment is still largely unclear. In this complex interplay between the leukemic and various types of surrounding cells, we functionally addressed two key aspects: the chemo-attraction of monocytes, and the crosstalk between CLL cells and activated T cells herein. Our findings are compatible with a model (Figure 4) in which stimulation by CD40L on T cells in the LN induces CLL cells to secrete several monocyte-attracting chemokines. Of these, we found CCL2 to be the most potent chemo-attractor, suggesting that this chemokine potentially plays an important role in vivo by recruiting monocytes towards the malignant cells in the LN via CCR2. The immunofluorescence data suggest that, following engagement with CLL cells in the LN, monocytes undergo skewing towards a tumor-supportive M2 phenotype (see also below).
Figure 4.
Model of chronic lymphocytic leukemia (CLL) T-cell macrophage triad in the formation of a supportive tumor microenvironment. Stimulation of CLL cells by activated T-cell-produced CD40L (1) induces them to secrete CCL2 (2), which in turn recruits monocytes towards the lymph node (LN) (3). As a result of CLL-secreted factors, monocytes differentiate towards a tumor supporting M2 phenotype (4). Mo: monocyte; PB: peripheral blood.
Several reports that studied migration of monocytes in the context of inflammation have concluded that chemo-attraction can occur via activation of several different chemokine receptor signaling pathways.23–26 We here identified CCR2 as the receptor most likely to be responsible for monocyte recruitment towards CLL cells. The most potent chemokine that recruits monocytes via the CCR2 receptor is CCL2,28 which indeed recruited monocytes in our experiments (Figure 3D). These data are in line with the recent observation that adoptive transfer of leukemic TCL1-derived splenocytes into recipient mice that are deficient for CCR2 resulted in significantly lower percentages and numbers of monocytes in the spleen.13
Besides its importance in CLL, CCL2 has been shown to recruit monocytes towards primary tumors in prostate cancer. Furthermore, this recruitment resulted in enhanced tumor growth.29 CCR2 antagonist PF-04136309 reduced the number of monocytes and restored chemo-sensitivity in a pancreas tumor mouse model, indicating the therapeutic potential of CCL2/CCR2 inhibition.30 Our studies suggest that, also in CLL, these inhibitors can be a relevant therapeutic option, although additional in vivo studies are required.
In the light of the large number of potential interactions in the CLL LN, it is worth noting that specifically the T-cell co-stimulatory signal CD40L leads to induction of monocyte trafficking. The levels of chemokines secreted by unstimulated CLL cells are insufficient to induce migration above background (Figures 2A and 3A). Although CLL cells stimulated by monocyte-derived Nurse-like cells show increased production of CCL3 and CCL4,19 these cytokines apparently play a subordinate role in monocyte recruitment; despite their presence in the conditioned media (Figure 3B), monocyte migration is not prevented by blocking their cognate receptors CCR1 or −5 (Figure 3C). In contrast to the monocyte-attracting effect by CLL cells, it has been shown that bystander cells such as CD3+ or CD68+ cells are unable to produce CCL2 themselves.31 We have previously shown that CD40L accounts for most of the transcriptional effects of T cells on CLL cells4 and, based on our data, CD40L is sufficient to induce CCL2 production and monocyte recruitment. In this context, others have shown that another key T-cell cytokine, IL21, is not essential for CCL2 induction.32
Our observation that the large majority of macrophages in the CLL LN are of an M2 phenotype (Figure 1B and C) strongly suggests initiation of M2 differentiating signaling events once monocytes enter the CLL lymph node environment. Factors that can account for this differentiation include NAMPT21 or High mobility group box 1 (HMGB-1)12 secreted by LN-residing CLL cells. We could confirm that addition of NAMPT indeed skews monocytes towards an M2 type (Figure 1E). In addition, T-helper-2 cells that also reside in the LN22 secrete various cytokines that induce M2 differentiation, including IL-4, IL-10, and IL-13. Notably, the production of IL-10 could be complemented by CLL cells that are stimulated by T cells (Figures 2C and 3B). Together these findings indicate that the LN provides an M2-inducing milieu, which likely results in a supportive macrophage phenotype that can induce CLL cell survival and immune suppression.
Indeed, the association of M2 differentiation and tumor support has been pointed out in several other tumor types.8–11 Functionally, the tumor-promoting effects of M2 macrophages have been attributed to an increased production of direct tumor-promoting cytokines33 and a suppression of the immune response.21 M2 macrophages can, for example, induce a suppression of cytotoxic T cells, as they can up-regulate expression of PD-1 on T cells.21 In addition, they inhibit T-cell proliferation.21 Lastly, M2 macrophages suppress T-cell activation and promote the differentiation towards Treg cells.34 In the light of the recent development of T-cell therapy against CLL neoantigens,35 the subversion of T cells by macrophages is an important point to address.
In conclusion, our studies provide insight into several aspects of the complex interactions that take place in the CLL LN, and indicate how the triad of CLL cell, T cell, and macrophage potentially contributes to the shaping of the tumor-microenvironment in CLL. Finally, we identified CCR2 as a potential therapeutic target to interrupt the intercellular interplay.
Supplementary Material
Acknowledgments
The authors would like to thank all volunteers that donated blood for this study. We furthermore thank Richard Volckmann for his help with the microarray analysis and Steven Pals for providing us with the CLL LN slides.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/12/2069
Funding
This work was supported by Dutch Cancer Foundation grant number UVA 2011-5097 (APK).
References
- 1.Burger JA. Nurture versus nature: the microenvironment in chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2011;2011:96–103. [DOI] [PubMed] [Google Scholar]
- 2.Ghia P, Strola G, Granziero L, et al. Chronic lymphocytic leukemia B cells are endowed with the capacity to attract CD4+, CD40L+ T cells by producing CCL22. Eur J Immunol. 2002;32(5):1403–1413. [DOI] [PubMed] [Google Scholar]
- 3.Hallaert DY, Jaspers A, van Noesel CJ, et al. c-Abl kinase inhibitors overcome CD40-mediated drug resistance in CLL: implications for therapeutic targeting of chemoresistant niches. Blood. 2008;112(13):5141–5149. [DOI] [PubMed] [Google Scholar]
- 4.Pascutti MF, Jak M, Tromp JM, et al. IL-21 and CD40L signals from autologous T cells can induce antigen-independent proliferation of CLL cells. Blood. 2013; 122(17):3010–3019. [DOI] [PubMed] [Google Scholar]
- 5.Burger JA, Tsukada N, Burger M, et al. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000; 96(8):2655–2663. [PubMed] [Google Scholar]
- 6.Tsukada N, Burger JA, Zvaifler NJ, Kipps TJ. Distinctive features of "nurselike" cells that differentiate in the context of chronic lymphocytic leukemia. Blood. 2002; 99(3):1030–1037. [DOI] [PubMed] [Google Scholar]
- 7.Zhang QW, Liu L, Gong CY, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012; 7(12):e50946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66(2):605–612. [DOI] [PubMed] [Google Scholar]
- 9.Sousa S, Brion R, Lintunen M, et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res. 2015;17(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soki FN, Koh AJ, Jones JD, et al. Polarization of prostate cancer-associated macrophages is induced by milk fat globule-EGF factor 8 (MFG-E8)-mediated efferocytosis. J Biol Chem. 2014; 289(35):24560–24572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong SM, Tan YC, Beretta O, et al. Macrophages in human colorectal cancer are pro-inflammatory and prime T cells towards an anti-tumour type-1 inflammatory response. Eur J Immunol. 2012; 42(1):89–100. [DOI] [PubMed] [Google Scholar]
- 12.Jia L, Clear A, Liu FT, et al. Extracellular HMGB1 promotes differentiation of nurse-like cells in chronic lymphocytic leukemia. Blood. 2014;123(11):1709–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanna BS, McClanahan F, Yazdanparast H, et al. Depletion of CLL-associated patrolling monocytes and macrophages controls disease development and repairs immune dysfunction in vivo. Leukemia. 2015;30(3):570–579. [DOI] [PubMed] [Google Scholar]
- 14.Galletti G, Scielzo C, Barbaglio F, et al. Targeting Macrophages Sensitizes Chronic Lymphocytic Leukemia to Apoptosis and Inhibits Disease Progression. Cell Rep. 2016;14(7):1748–1760. [DOI] [PubMed] [Google Scholar]
- 15.O’Hayre M, Salanga CL, Kipps TJ, et al. Elucidating the CXCL12/CXCR4 signaling network in chronic lymphocytic leukemia through phosphoproteomics analysis. PLoS One. 2010;5(7):e11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burkle A, Niedermeier M, Schmitt-Graff A, et al. Overexpression of the CXCR5 chemokine receptor, and its ligand, CXCL13 in B-cell chronic lymphocytic leukemia. Blood. 2007;110(9):3316–3325. [DOI] [PubMed] [Google Scholar]
- 17.Till KJ, Lin K, Zuzel M, Cawley JC. The chemokine receptor CCR7 and alpha4 integrin are important for migration of chronic lymphocytic leukemia cells into lymph nodes. Blood. 2002;99(8):2977–2984. [DOI] [PubMed] [Google Scholar]
- 18.Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117(2):563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burger JA, Quiroga MP, Hartmann E, et al. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood. 2009;113(13):3050–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lebre MC, Vergunst CE, Choi IY, et al. Why CCR2 and CCR5 blockade failed and why CCR1 blockade might still be effective in the treatment of rheumatoid arthritis. PLoS One. 2011;6(7):e21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Audrito V, Serra S, Brusa D, et al. Extracellular nicotinamide phosphoribosyl-transferase (NAMPT) promotes M2 macrophage polarization in chronic lymphocytic leukemia. Blood. 2015; 125(1):111–123. [DOI] [PubMed] [Google Scholar]
- 22.Smit LA, Hallaert DY, Spijker R, et al. Differential Noxa/Mcl-1 balance in peripheral versus lymph node chronic lymphocytic leukemia cells correlates with survival capacity. Blood. 2007;109(4):1660–1668. [DOI] [PubMed] [Google Scholar]
- 23.Kaufmann A, Salentin R, Gemsa D, Sprenger H. Increase of CCR1 and CCR5 expression and enhanced functional response to MIP-1 alpha during differentiation of human monocytes to macrophages. J Leukoc Biol. 2001;69(2):248–252. [PubMed] [Google Scholar]
- 24.Nieto JC, Canto E, Zamora C, et al. Selective loss of chemokine receptor expression on leukocytes after cell isolation. PLoS One. 2012;7(3):e31297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghorpade A, Xia MQ, Hyman BT, et al. Role of the beta-chemokine receptors CCR3 and CCR5 in human immunodeficiency virus type 1 infection of monocytes and microglia. J Virol. 1998;72(4):3351–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thivierge M, Parent JL, Stankova J, Rola-Pleszczynski M. Modulation of formyl peptide receptor expression by IL-10 in human monocytes and neutrophils. J Immunol. 1999;162(6):3590–3595. [PubMed] [Google Scholar]
- 27.Da Roit F, Engelberts PJ, Taylor RP, et al. Ibrutinib interferes with the cell-mediated anti-tumor activities of therapeutic CD20 antibodies: implications for combination therapy. Haematologica. 2015;100(1):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White GE, Iqbal AJ, Greaves DR. CC chemokine receptors and chronic inflammation–therapeutic opportunities and pharmacological challenges. Pharmacol Rev. 2013;65(1):47–89. [DOI] [PubMed] [Google Scholar]
- 29.Mizutani K, Sud S, McGregor NA, et al. The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia. 2009;11(11):1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73(3):1128–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burgess M, Cheung C, Chambers L, et al. CCL2 and CXCL2 enhance survival of primary chronic lymphocytic leukemia cells in vitro. Leuk Lymphoma. 2012;53(10):1988–1998. [DOI] [PubMed] [Google Scholar]
- 32.De Cecco L, Capaia M, Zupo S, et al. Interleukin 21 Controls mRNA and MicroRNA Expression in CD40-Activated Chronic Lymphocytic Leukemia Cells. PLoS One. 2015;10(8):e0134706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267(2):204–215. [DOI] [PubMed] [Google Scholar]
- 34.Jitschin R, Braun M, Buttner M, et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood. 2014;124(5):750–760. [DOI] [PubMed] [Google Scholar]
- 35.Rajasagi M, Shukla SA, Fritsch EF, et al. Systematic identification of personal tumor-specific neoantigens in chronic lymphocytic leukemia. Blood. 2014;124(3):453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.