Abstract
In the European Intergroup EURO-LB02 trial, children and adolescents with lymphoblastic lymphoma underwent the non-Hodgkin lymphoma Berlin-Frankfurt-Münster protocol without prophylactic cranial radiotherapy. The primary aims of this trial were to test whether replacing prednisone with dexamethasone during induction increases event-free survival in the subgroups with T-cell lymphoblastic lymphoma and whether therapy duration could be reduced from 24 to 18 months (factorial design, randomizations). These questions could not be answered due to premature closure of the trial. Here we report on the secondary aims of the trial: whether the results of the NHL-BFM90 study could be reproduced and evaluation of disease features and prognostic factors. Three hundred and nineteen patients (66 with precursor B-cell lymphoblastic lymphoma, 233 with T-cell lymphoblastic lymphoma, 12 with mixed phenotype, 8 not classifiable) were enrolled. In induction, 215 patients received prednisone and 104 patients received dexamethasone. The median follow-up was 6.8 years (range, 3.0–10.3). The 5-year event-free survival was 82±2% [12 toxic deaths, 5 secondary malignancies, 43 non-response/relapse (central nervous system n=9; all received prednisone during induction)]. The event-free survival rate was 80±5% for patients with precursor B-cell lymphoblastic lymphoma, 82±3% for those with T-cell lymphoblastic lymphoma, and 100% for patients with a mixed phenotype. During induction, significantly more grade III/IV toxicities were observed in patients receiving dexamethasone, resulting in significant treatment delays. The number of toxic deaths did not differ significantly. The only variable associated with outcome was performance status at diagnosis. The 90% event-free survival rate for patients with T-cell lymphoblastic lymphoma shown in study NHL-BFM90 was not replicated, mainly due to more toxic deaths and central nervous system relapses. Dexamethasone in induction may prevent central nervous system relapse more effectively than prednisone but produces a higher burden of toxicity. (#NCT00275106).
Introduction
Although lymphoblastic lymphoma (LBL) is an orphan disease, it is the second most frequent non-Hodgkin lymphoma (NHL) observed in children and adolescents. Acute lymphoblastic leukemia (ALL)-type therapeutic strategies have been demonstrated to be efficacious in LBL. Treatment protocols derived from the Berlin-Frankfurt Münster (BFM) group ALL strategy result in event-free survival rates of 75% to 90%. These survival rates are, however, achieved at the expense of considerable toxicity.1–7 On the other hand, children who do not respond to this treatment or who relapse after it still have an extremely poor chance of surviving.8 Thus, many questions regarding optimal treatment of childhood LBL remain to be clarified.
Although LBL and ALL are biologically similar, they probably represent different diseases, as suggested by recent research,9–12 and advances achieved in the optimization of childhood ALL treatment cannot be adopted as LBL treatment strategies. In particular, meaningful parameters allowing risk-adapted therapies, such as chromosomal translocations and minimal residual disease monitoring,13,14 are not yet available for LBL.
The lack of meaningful prognostic parameters for childhood LBL is mostly explained by the nature of the disease and the limited number of patients who are treated uniformly. In contrast to ALL, peripheral blood and bone marrow are limited sources of tumor cells. More extensive surgery to obtain appropriate tumor material for biological studies, may not be feasible given the often life-threatening condition of the patients at initial presentation. Thus, even the immunological classification of the lymphoma is not exact in some cases.
The EURO-LB 02 study was the second inter-group clinical trial of the newly established European Intergroup Cooperation on Childhood and Adolescent NHL (EIC-NHL). The patients were diagnosed and classified according to a standardized work-up, including a central pathology review. The treatment strategy of the trial was adapted from the NHL-BFM90 study, with the omission of prophylactic cranial irradiation.3,15 The primary aims of the EURO-LB02 study were to test, in a randomized manner, whether replacing prednisone with dexamethasone during the induction phase increases event-free survival (EFS) in the subgroup of patients with T-cell LBL (T-LBL) and whether therapy duration could be reduced from 24 to 18 months (factorial design). We could not answer these questions because the trial had to be closed prematurely due to a substantial number of toxic deaths.
Here we report on the results of the secondary aims of this study: to determine whether the outstanding results for patients with T-LBL in the NHL-BFM90 study could be reproduced in a large European inter-group trial, to assess features of the disease, and to evaluate prognostic factors. Furthermore, we provide comprehensive information on toxicity and specific caveats regarding this treatment strategy, which is currently the backbone of LBL therapy in many countries. The trial is registered at http://www.clinicaltrials.gov (clinicaltrials.gov identifier: #NCT00275106).
Methods
Patients
Patients <22 years old with newly diagnosed LBL were eligible for entry into the study. Patients with T-LBL (local assessment) were eligible for the first randomization. For participation in the second randomization, T-LBL had to have been confirmed by the national reference laboratory. Further exclusion criteria are listed in Online Supplement 2.
Diagnostic work-up
The diagnosis of LBL was established by tumor biopsy and/or cytological and immunological examinations of malignant effusions. A review by national reference pathologists or national reference cytomorphology/immunophenotyping laboratories was requested for all patients. Minimal diagnostic requirements and staging procedures are outlined in Online Supplements 3 and 4.
Treatment plan and responses
Chemotherapy was based on the NHL-BFM90 protocol (Table 1).5 Patients with non-T-cell LBL received standard treatment stratified according to stage and central nervous system (CNS) status, whereas the treatments were randomized in patients with T-LBL (Table 1 and Figure 1A,B). CNS-positive patients received cranial radiotherapy after re-intensification. Online Supplements 5 and 6 contain recommendations on infection prophylaxis and details regarding the randomization process. Response criteria are outlined in Online Supplement 7.
Table 1.
Treatment protocol.
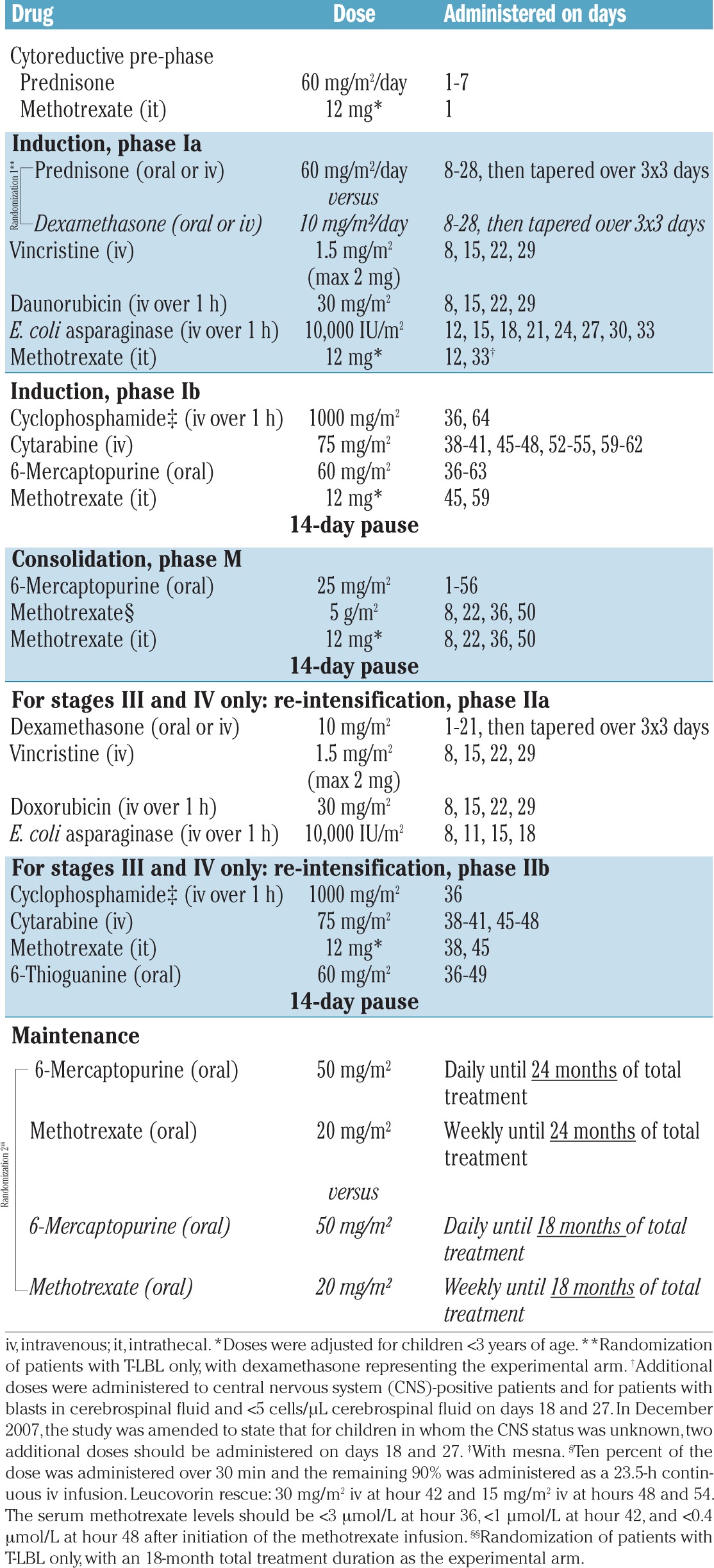
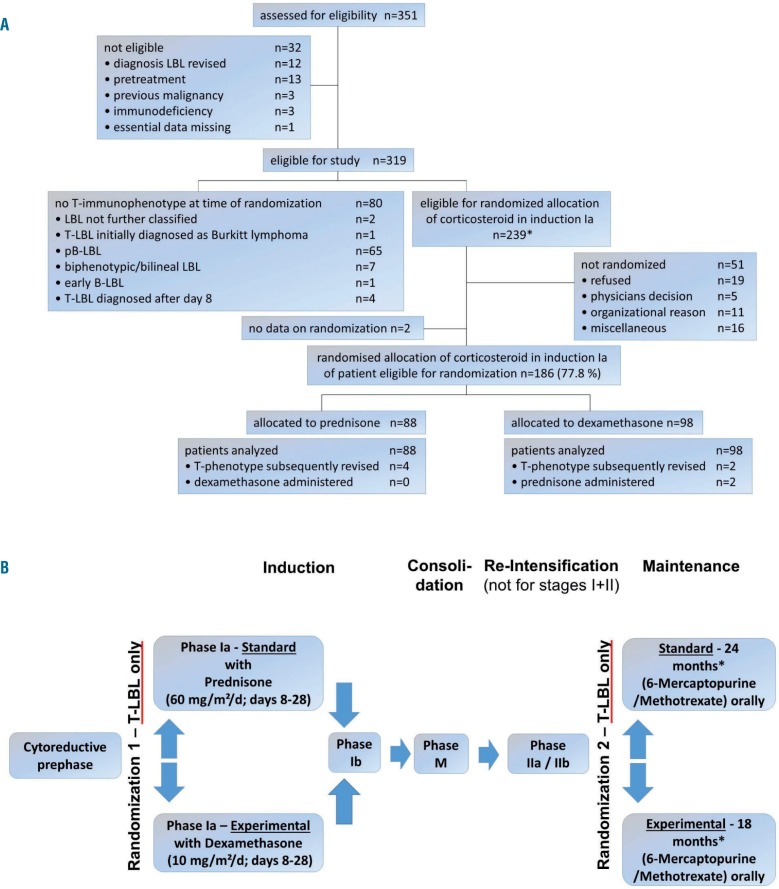
Figure 1.
Consort flow diagram and flow chart of the EURO-LB02 trial. (A) CONSORT flow diagram. *In 11 patients, the diagnosis of T-LBL was subsequently revised (precursor B-cell LBL, n=1; biphenotypic/bilineal LBL, n=5; T-NHL not further classified, n=3; undifferentiated lymphoma, n=2). Six of these patients were randomized. (B) Flow chart of the EURO-LB02 trial. Patients with precurcor B-cell-LBL received the standard arm. *Resulting in a total therapy duration of 18 or 24 months.
Study design and statistics
EURO-LB02 was conducted by eight national/multinational cooperative study groups in 14 European countries.
The primary endpoint for both study questions was EFS, computed as the time from randomization to date of the last follow-up visit or the first event, i.e., a non-response on day 33 (defined as >5% blasts in the bone marrow and/or blasts in the cerebrospinal fluid) and/or <35% tumor regression), relapse, secondary malignancy, or death from any cause.
The power and sample size calculations are detailed in Online Supplement 8.
The secondary endpoints were overall survival and toxicity. Overall survival was calculated as the time from randomization to the date of death or the last follow-up visit.
EFS and overall survival were estimated using the Kaplan-Meier method. Differences were compared with the log-rank test. Cumulative incidence functions for relapse/non-response and toxic death were constructed according to the method reported by Kalbfleisch and Prentice16 and were compared using Gray test.17 The multivariate analysis of prognostic factors was conducted using a Cox regression analysis with backward elimination.18
Toxicity was assessed after each treatment phase using the National Cancer Institute Common Toxicity Criteria (NCI-CTC).19 Differences in the distribution of individual parameters between subsets of patients were analyzed using the chi-square test or Fisher exact test for categorized variables and the Mann-Whitney U test for continuous variables. A P-value <0.05 was considered statistically significant.
The incidence of toxic deaths was monitored with a Wald sequential plan assuming a toxic death rate of 1% as acceptable, based on previous NHL-BFM studies. In an amendment the stopping rule was set to accept a toxic death rate of 2%. Details of the toxic death monitoring procedure are provided in Online Supplement 9.
The analysis was based on follow-up data available as of March 2014. The data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Signed informed consent was obtained. The study was performed after receiving approval from the responsible ethics committees and was conducted in accordance with the Declaration of Helsinki of 1975 and its revision in 2000.
Results
Patients’ enrollment and premature trial closure
When a fifth case of toxic death occurred among 115 patients with a follow-up duration of at least 30 weeks or a toxic death, patients were no longer enrolled in the study because the stopping rule was met. After an amendment to the protocol that included auditing of the participating centers, advice on the particular toxicity of the induction and re-induction phases, and setting P0 of the Wald sequential plan for monitoring the incidence of toxic deaths to 2% (Online Supplement 9), enrollment was re-opened 9 months after the initial termination. Enrollment was ultimately closed 2 years later, when the 12th case of toxic death occurred among 268 patients with a follow-up duration of at least 30 weeks or a toxic death. Up to that time point, 351 patients had been assessed for eligibility (Figure 1A), and 319 patients were eligible for study entry and included in the subsequent analyses.
Patients’ characteristics
The patients’ characteristics are presented in Table 2. The diagnoses were T-LBL in 233 (73%) patients, precursor B-cell LBL (pB-LBL) in 66 patients (21%), mixed phenotype LBL in 12 patients (4%), and other constellations in eight patients, as specified in Table 2.
Table 2.
Protocol patients: characteristics and events.
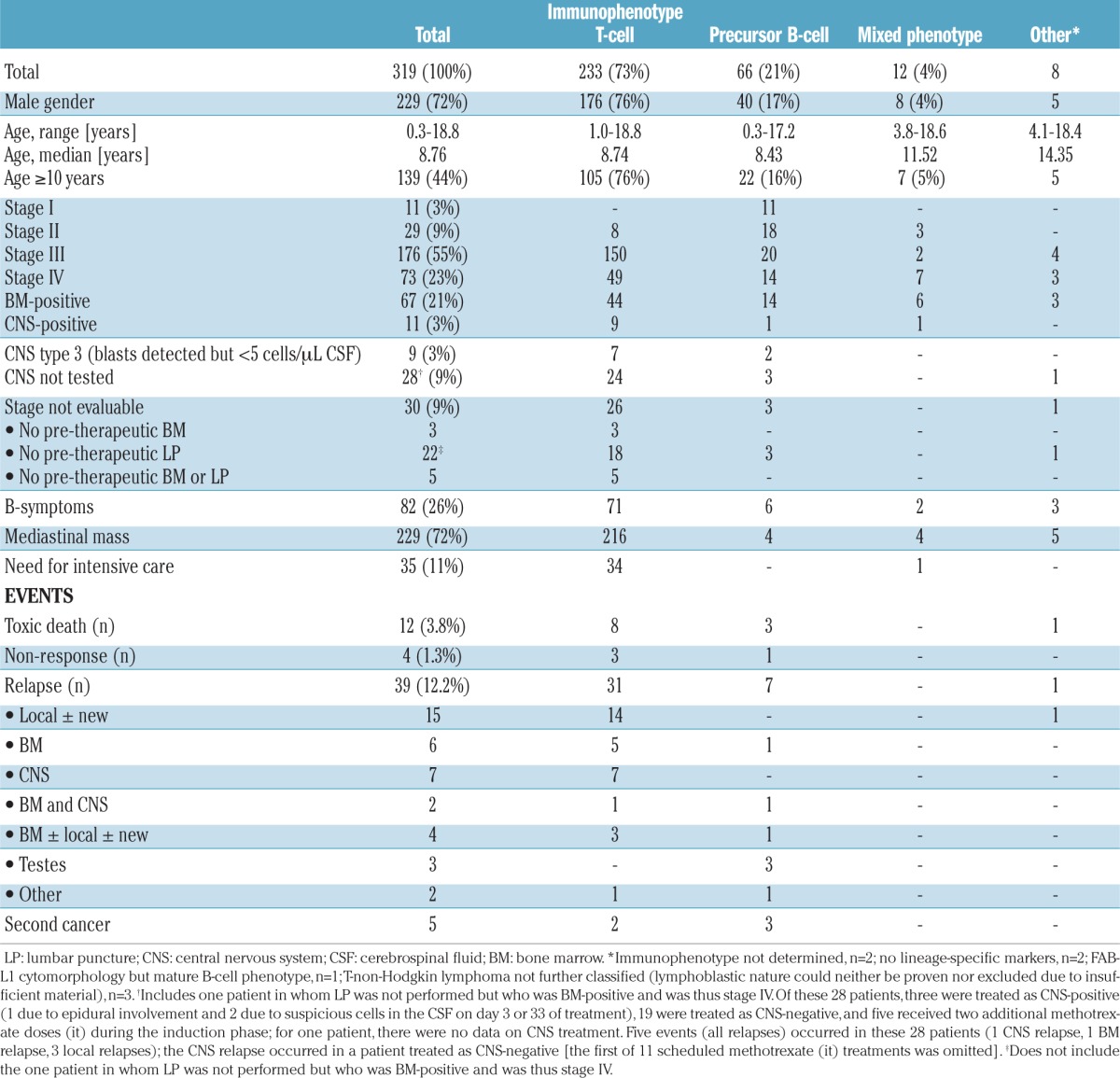
In 30 patients (9%), the disease stage could not be precisely determined due to the lack of pre-therapeutic bone marrow collection and/or lumbar puncture. In 28 cases, this process was consistent with the recommendation to postpone invasive measures when a critical mediastinal tumor syndrome was present and to initiate treatment immediately.
Treatment
Consistent with the protocol, 35 of the 40 patients with stage I/II tumors received induction, consolidation M and maintenance therapy, whereas 279 patients with stage III, IV tumors or an unknown stage received additional re-intensification therapy. Five of the 40 patients with stage I/II tumors also received re-intensification in violation of the protocol due to individual decisions.
Of the 319 patients, 239 were eligible for the randomization treatment with prednisone or dexamethasone in induction phase Ia (Figure 1A). One hundred and eighty-six of these 239 patients were randomized; 88 were selected to receive prednisone and 98 were selected to receive dexamethasone, two of whom received prednisone due to individual decisions. Of the 53 non-randomized patients, three received dexamethasone. Of the 80 patients who were not eligible for randomization, five were given dexamethasone based on individual decisions. Thus, a total of 215 patients received prednisone and 104 patients received dexamethasone in induction phase Ia.
Outcomes
With a median follow-up period of 6.8 years (range, 3.0–10.3), the 5-year EFS rate for the 319 eligible patients was 82±2%. Sixty events were reported (Table 2). Twelve patients (3.8%) died from toxicity. Four patients had not responded by day 33 of induction therapy, 39 patients relapsed and five developed secondary cancers. Secondary malignancies included: acute myeloid leukemia, myelodysplastic syndrome progressing to acute myeloid leukemia, colorectal adenocarcinoma, ALL, and Epstein-Barr virus-associated lymphoma.
A local lack of response or relapse was the predominant failure in patients with T-LBL, whereas systemic relapse and testicular relapse were the predominant sites of failure in patients with pB-LBL. The median time from diagnosis to relapse was 12 months (range, 1–77 months) for patients with T-LBL and 34 months (range, 3–70 months) for patients with pB-LBL. CNS relapses occurred early (at a median of 12 months from diagnosis; range, 3–27 months). All nine patients who had a CNS relapse had received prednisone during induction phase Ia.
The 5-year overall survival rate was 87±2%. The causes of death in the 41 patients who died were disease progression or relapse in 27 patients, toxicity in 12 patients and secondary malignancies in two patients.
Toxicity
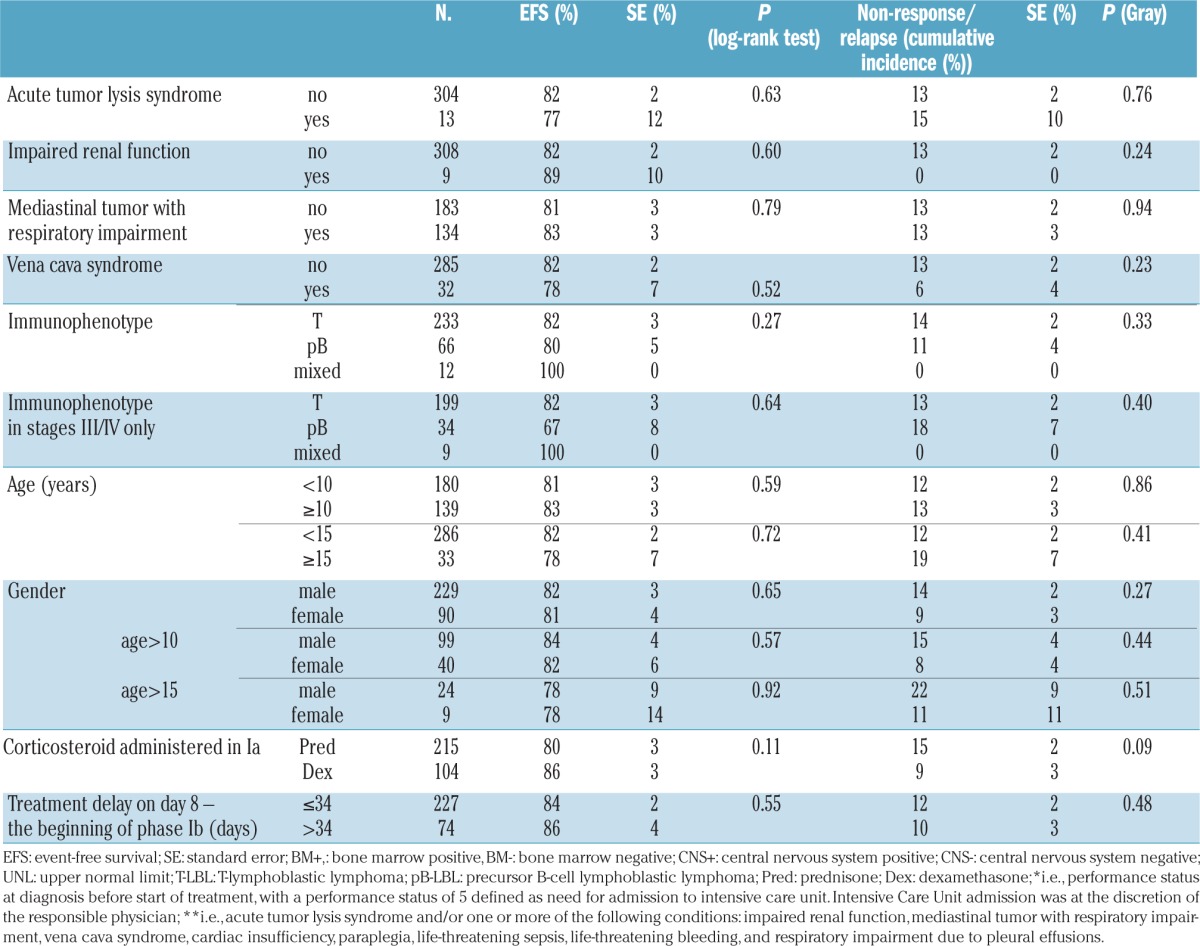
Eight of the 215 patients given prednisone (3.7%) and four of the 104 patients (3.8%) given dexamethasone in induction phase Ia died due to toxicity. Five toxic deaths occurred during induction phase Ia (prednisone, n=2 and dexamethasone n=3). One patient died in induction phase Ib, two patients died during consolidation phase M, three patients died during re-induction phase IIa and one patient died during the maintenance phase. Detailed causes of death are provided in Table 3. The toxic death rate per study group ranged from 0% to 9.7%. Some of the participating study groups had not previously used the BFM strategy. The toxic death rate was higher in study groups that had not previously used the BFM strategy (4 toxic deaths among 78 patients, cumulative incidence of toxic death, 5±3%) than in the groups in which the BFM strategy had already been established prior to the EURO-LB 02 study (8 toxic deaths among 241 patients; cumulative incidence of toxic death, 3±1%); this difference was not statistically significant (P=0.46).
Table 3.
Treatment-related mortality.
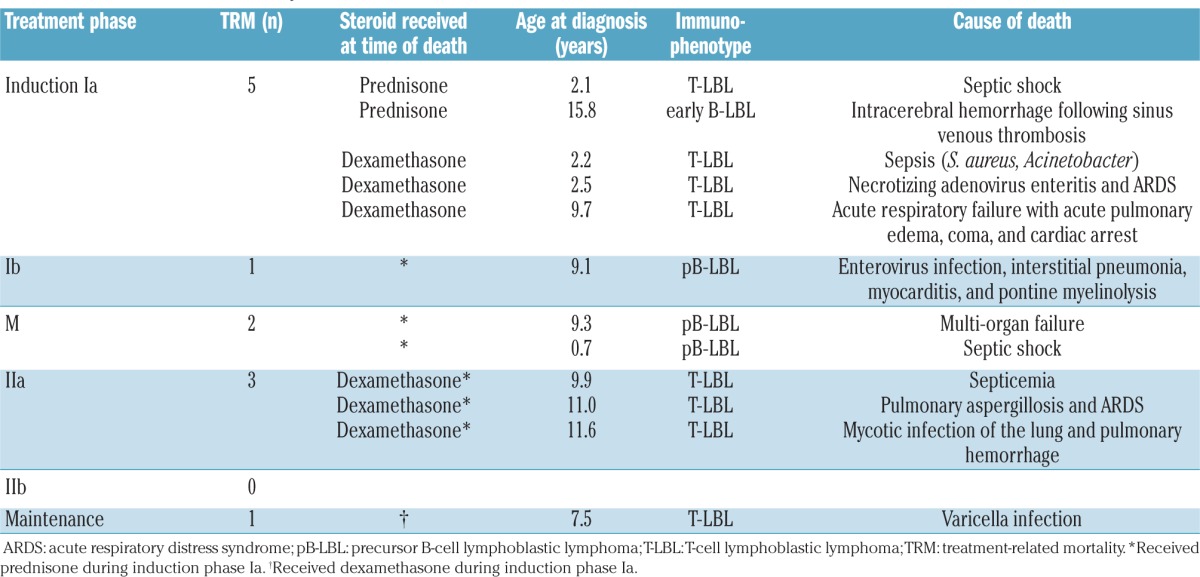
A total of 65 non-fatal severe adverse events were reported in 51 patients. The number of such events did not differ significantly between patients receiving prednisone in induction Ia (19%, 41/215 patients) and those receiving dexamethasone (23%, 24/104 patients). The incidence of non-fatal severe adverse events also did not differ significantly among the patients randomized to receive either prednisone or dexamethasone in induction Ia (data not shown).
Among all patients, 29 of the severe adverse events occurred during induction Ia [12 in 215 patients receiving prednisone (6%) and 17 in 104 patients receiving dexamethasone (16%)], three during induction Ib, ten during consolidation phase M, 16 during re-intensification phase IIa, four during re-intensification phase IIb and three during maintenance. Details are provided in Online Supplement 10.
Grade III and IV NCI-CTC toxicities observed in each treatment phase, with the exception of the maintenance phase, are shown in Online Supplements 11 and 12. The most frequently reported toxicities were hematologic toxicity, coagulation problems, infection, and liver toxicity. Hematologic toxicity was the most frequent toxicity observed during all phases, with the exception of the cytoreductive prephase. Toxicity due to coagulation and thrombosis most frequently occurred in induction phase Ia and re-intensification phase IIa. Regarding the corticosteroid administered in induction phase Ia, the following grade III and IV toxicities were reported significantly more frequently in patients given dexamethasone than in patients given prednisone: hematologic toxicity, infection, stomatitis, thrombosis, arrhythmia and peripheral neurotoxicity. Details are given in Online Supplements 11 and 12. In the subset of patients randomized to receive either prednisone or dexamethasone in induction phase Ia, only grade III/IV hematologic toxicity, infection and peripheral neurotoxicity occurred significantly more frequently in patients given dexamethasone than in those given prednisone (Online Supplement 13). In the total group and in the subset of randomized patients, the higher frequency of grade III/IV toxicities observed in patients receiving dexamethasone in induction phase Ia was associated with a significantly greater delay in the initiation of induction phase Ib (a median of 4 days for patients receiving dexamethasone in induction phase Ia and a median of 1 day for patients receiving prednisone in induction phase Ia; P=0.0003 for the total group, P=0.01 in the subset of randomized patients only; Online Supplement 14).
Prognostic factors
Results of univariate analyses of variables with possible impacts on treatment outcomes in the entire group are presented in Table 4 and by immunophenotypic subgroup in Online Supplements 15 and 16.
Table 4.
Univariate analyses of prognostic factors in all patients.
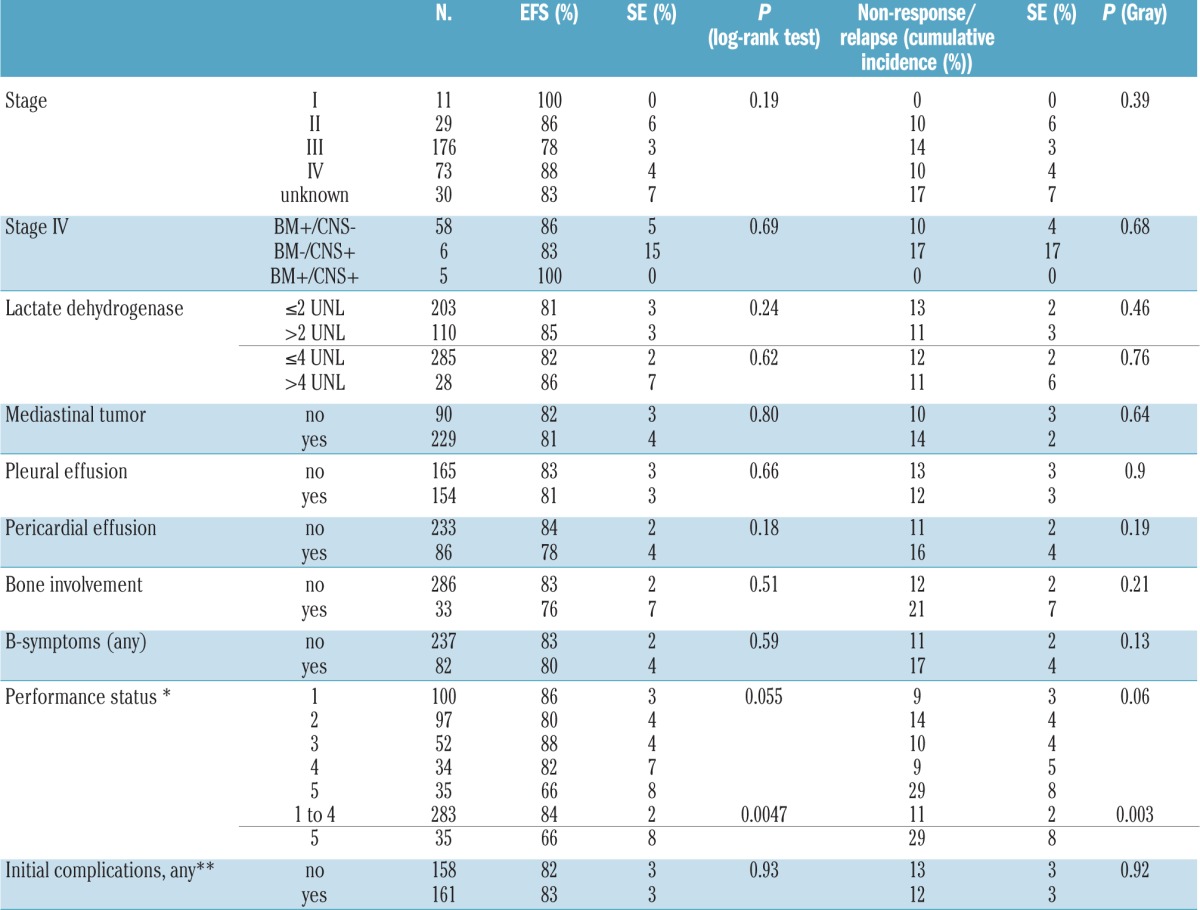
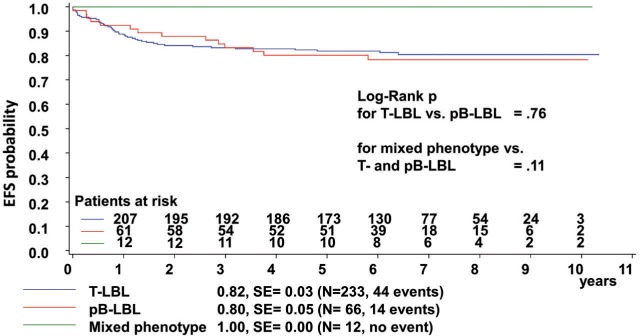
The patients’ immunophenotypes had no statistically significant impact on outcomes (Figure 2). The disease stage had no statistically significant impact on the entire group or on patients with T-LBL. In the subset of patients with pB-LBL, stage III disease was associated with a significant reduction in EFS, whereas the cumulative incidences of relapse or non-response were not significantly different (Online Supplement 16). The nine events occurring among the 20 patients with stage III pB-LBL were four relapses, one non-response, one toxic death, and three secondary malignancies (myeloproliferative disease: n=1, Epstein-Barr virus associated lymphoma: n=1, and mixed pre-T/common-ALL: n=1). Performance status 5 (i.e., a need for intensive care treatment) at diagnosis before the start of treatment was associated with poor outcomes and a higher incidence of relapse or non-response in the entire group and in patients with T-LBL. Performance status at diagnosis was documented for 232 of 233 patients with T-LBL. Thirty-four of the 35 patients with performance status 5 had T-LBL. Their outcomes were significantly worse than those of the 198 patients with T-LBL and performance status 1–4: EFS 66±8% versus 85±3%, P=0.003; cumulative incidence of non-response/relapse 29±8% versus 11±2%, P=0.006.
Figure 2.
The 5-year event-free survival (EFS, from diagnosis) of protocol patients with T-cell, precursor B-cell and biphenotypic lymphoblastic lymphoma. EFS: event-free survival SE: standard error. The median time to an event was 0.9 and 2.3 years (P=0.21) in patients suffering from T-LBL and pB-LBL, respectively.
In the univariate analyses, none of the other factors examined had an impact on outcomes, either in the entire group or in the subgroups of patients with T-LBL or pB-LBL.
In the multivariate analysis (Cox regression with backward elimination) of the entire group, which included the variables immunophenotype, age (<10 versus ≥10 years), gender, stage (<III versus ≥III), bone involvement, bone marrow involvement, CNS involvement, lactate dehydrogenase levels (< 2 versus ≥2x the normal value), B-symptoms, mediastinal mass, superior vena cava syndrome, pleural effusion, pericardial effusion, tumor lysis syndrome, impaired renal function, and performance status (<5 versus 5), only a performance status of 5 remained in the models for EFS (hazard ratio: 3.0; 95% confidence interval: 1.3–6.9; P=0.01) and for cumulative incidence of non-response/relapse (hazard ratio: 2.9; 95% confidence interval: 1.4–6.9; P=0.004). This finding was also true when the analysis was restricted to only patients with T-LBL: EFS hazard ratio 3.1; 95% confidence interval: 1.3–7.2; P=0.01, incidence of non-response/relapse hazard ratio 2.8; 95% confidence interval: 1.3–6.9; P=0.006).
Patients who received dexamethasone in induction phase Ia had a lower cumulative incidence of non-response or relapse than patients who received prednisone, with the difference being of borderline statistical significance (Table 4). Regarding the site of failure, the cumulative incidence of CNS relapses at 5 years was 4±1% among the 215 patients who received prednisone in the induction phase compared to 0% in the 104 patients who received dexamethasone (P=0.03), whereas the cumulative incidences of non-CNS relapse or non-response at 5 years were 10±2% and 9±3%, respectively (P=0.45).
Discussion
We report data from 319 children and adolescents suffering from LBL enrolled in the EURO-LB02 trial. We derived important conclusions and useful information from this large multi-group, multinational study, although neither randomized study questions could be answered in the confirmatory analysis because the trial had to be closed prematurely due to an excess of toxic deaths.
Firstly, this trial was instrumental in establishing a large European network of inter-group collaborators in the field of childhood and adolescent NHL, which will foster future progress in the understanding and treatment of this orphan disease.12
The reference treatment arm of the EURO-LB02 trial was the protocol of the NHL-BFM90 study, except that preventive cranial irradiation was omitted.5 The outstanding result of a 5-year EFS of 90% for patients with T-LBL reported in the BFM group NHL-BFM90 trial5 was not replicated in the inter-group EURO-LB02 study. The lower 5-year EFS of 82% for patients with T-LBL in the EURO-LB02 trial was primarily due to higher rates of toxic death (0% in NHL-BFM 90, 3.4% in EURO-LB02) and CNS relapse (cumulative incidence of CNS relapse at 5 years: 0% in NHL-BFM 90 and 3±1% in EURO-LB02; P=0.05), but the cumulative incidence of non-CNS relapse/non-response was comparable (cumulative incidence of non-CNS relapse/non-response at 5 years: 8±3% in NHL-BFM 90 and 10±2% in EURO-LB02; P=0.27).
The higher incidence of toxic deaths in the EURO-LB02 trial compared to the previous NHL-BFM studies3,5 cannot be clearly ascribed to a distinct reason.
At the premature stopping of the EURO-LB02 trial, the rate of toxic deaths in the 319 enrolled patients was 3.8% (no further toxic deaths occurred during follow-up) and was higher than expected from previous BFM group studies, since no toxic deaths were observed in the NHL-BFM90 trial5 and the rate of such deaths in the NHL-BFM95 trial was 1.3%.3 Infection was the most frequent cause of death, and induction phase Ia and re-intensification phase IIa were clearly the most dangerous phases of this therapy.
According to several ALL studies, compared to prednisone, dexamethasone increases the toxicity of induction therapy.20–23 In the randomized ALL AIEOP-BFM 2000 trial, toxic deaths also occurred significantly more frequently in patients who were randomized to receive dexamethasone in the induction phase than in patients randomized to receive prednisone.24
In the EURO-LB02 study, there was no significant difference in the number of toxic deaths between patients receiving dexamethasone or prednisone in induction phase Ia, either in the number of toxic deaths observed during induction phase Ia itself or in the total number of toxic deaths. However, non-fatal grade III and IV toxicity occurred significantly more frequently in patients receiving dexamethasone, resulting in a significant delay in subsequent treatment phases.
Some of the participating study groups had not previously used the BFM strategy. Although the toxic death rate was higher in those study groups than in the groups in which the BFM strategy had been established prior to the EURO-LB02 study, this difference was not statistically significant. Nevertheless, familiarity with treatment protocols should be considered when planning further multi-group trials.
An unexpectedly high number of CNS relapses was observed in the EURO-LB02 study; all of these relapses occurred in patients who had received prednisone during induction phase Ia. The prevention of CNS relapse remains an unsolved problem. The A5971 trial by the Children’s Oncology Group (COG)25 showed comparable outcomes when CNS prophylaxis depended on the frequent delivery of intrathecal methotrexate compared to high-dose methotrexate. Thus, additional intrathecal methotrexate applications might represent a promising approach. Additional high-dose methotrexate infusions are another promising approach. In the SIOP LMT96 trial,26 ten courses of high-dose methotrexate (3 g/m2) were administered. Only one of the 79 trial participants experienced a CNS relapse. The administration of additional high-dose methotrexate infusions during the maintenance phase has also been shown to treat CNS disease effectively in patients with T-LBL.7 Based on our data, the use of dexamethasone during induction phase Ia is more efficacious than prednisone in preventing CNS relapse, but is more toxic. The higher rate of infections observed in the dexamethasone group is of particular concern. This finding is consistent with the results from several,20–24,27 but not all,28,29 trials in patients with ALL. However, some studies have reported higher EFS and overall survival, despite higher rates of severe infection, in patients treated with dexamethasone than in patients treated with prednisone.23,27 An important point to consider is the dose equivalence between dexamethasone and prednisone with regard to anti-tumor efficacy and toxicity. Data regarding these drugs’ relative anti-leukemic activity are inconsistent, and data on their toxic potential are lacking.30,31 In animal models, the cerebrospinal fluid penetration of dexamethasone is greater than that of prednisone.32 Thus, a dose of dexamethasone lower than 10 mg/m2/day, which was used in this study, might be less toxic but more efficacious in protecting against CNS toxicity, as has been shown in children suffering from ALL.29 A shorter duration of dexamethasone treatment might be another reasonable option for taking advantage of the efficacy of dexamethasone at a tolerable toxicity.
The administration of dexamethasone for 2 weeks versus prednisone for 4 weeks in patients with high-risk precursor-B-ALL during induction therapy was recently shown to result in better efficacy of dexamethasone in patients younger than 10 years, but not in patients aged 10 years or older. The rate of CNS relapses was lower in patients treated with dexamethasone.33
In the EURO-LB02 study, patients with stage I or II disease did not receive re-intensification therapy. Given the biological similarities between LBL and ALL, we reasonably asked whether patients with LBL would also benefit from delayed intensification therapy, like patients with ALL.34 Notably, the 5-year EFS rates for patients with stage I/II disease were 90% in both the EURO-LB02 study (5-year EFS, 90±5%, n=40) and the COG A5971 study35 (5-year EFS, 90%, n=51). However, delayed intensification therapy for patients with stage I/II disease was only administered in the COG A5971 study, resulting in a higher cumulative dose of anthracycline and cyclophosphamide.
Improving the outcome for pediatric/adolescent patients with LBL remains a challenge. The toxic death rate of 3.8% in our study indicates a critical balance between treatment efficacy and risks. However, the salvage of relapsed patients has been shown to be poor.1,8,25 Thus, treatment optimization involves finding highly predictive markers that enable the early identification of patients who are not curable with current treatments. These patients could be designated to alternative treatment strategies, whereas all others could be protected from the risk of experimental treatments.
We performed a comprehensive analysis of variables with possible impacts on outcomes in this large group of children who were classified and treated uniformly.
Apart from localized versus advanced stage, the only variable associated with decreased EFS was a poor performance status at diagnosis. Researchers have not clearly determined whether a poor performance status reflects disease with a more aggressive biology or difficulties in treatment realization. In any case, this parameter is not very useful for risk stratification. Nevertheless, this finding might alert physicians to pay special attention to these patients.
The EURO-LB02 study contributed reliable up-to-date information on the classification of patients with LBL. A central pathology review was performed in as many as 90.4% of the cases. Additional detailed immunohistochemical analyses of this large series were performed by the European Childhood Lymphoma Panel. The results enabled the development of a step-wise and material-sparing diagnostic approach as a complementary strategy to the strategy recommended by the World Health Organization.36 Based on this reliable classification, we also showed that the immunophenotype lineage had no additional impact on outcome. A novel observation from the EURO-LB02 study was that lineage promiscuity was present in as many as 4% of all patients diagnosed with LBL. Given the aggressive course observed in children with leukemia of an ambiguous lineage,37 the outcomes of the 12 patients with mixed phenotype LBL in the EURO-LB02 study were rather favorable, with no events observed among this subgroup.
The prognostic power of new biomarkers must be evaluated for the standardized classification and treatment of patients. NOTCH1 and FBXW7 mutations38,39 and loss of heterozygosity at chromosome 6q38,40 were recently shown to be promising prognostic biomarkers of pediatric T-LBL. Other candidates for prospective evaluation may be parameters of the kinetics of the response to treatment, such as those determined by positron emission tomography and minimal disseminated disease and its monitoring during treatment.41,42 The network of collaborators established in the EURO-LB02 study may provide a platform for this purpose.
Supplementary Material
Acknowledgments
An independent Data Safety and Monitoring Committee (DSMC) monitored the progress of this study and received reports on the current status of the trial every 6 months. The authors would like to thank the members of the external DSMC, P. Brice (Paris, France), D. Hasenclever (Leipzig, Germany), M. Hudson (Memphis, USA), M. Link (Palo Alto, USA), M. Parmar (London, UK), and D. Poplack (Houston, USA), for their critical review of the study progress and their advice. This work was supported by the Deutsche Krebshilfe (grants 102595 and 107813) (ARe), PHRC 2003 (national grant) (YB) and the Kinderkrebsinitiative, Buchholz, Holm-Seppensen (KKI) (WK). The authors would like to thank the European Intergroup Cooperation for Childhood Non-Hodgkin Lymphoma for its participation in this study. A complete membership list appears in the “Online Supplement”. The authors would also like to thank the doctors, nurses and data managers at the participating hospitals who cared for these sick children and supplied data. Finally, the authors would particularly like to thank the children and their caregivers for participating in the trial.
Footnotes
Some results were presented at the 4th International Symposium on Childhood, Adolescent and Young Adult Non-Hodgkin Lymphoma, New York, NY, USA, Nov. 1–3, 2012. Part of the data were presented at the 14th International Conference on Malignant Lymphoma in Lugano, Switzerland, June 17–20, 2015.
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/12/2086
References
- 1.Abromowitch M, Sposto R, Perkins S, et al. Shortened intensified multi-agent chemotherapy and non-cross resistant maintenance therapy for advanced lymphoblastic lymphoma in children and adolescents: report from the Children’s Oncology Group. Br J Haematol. 2008;143(2):261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amylon MD, Shuster J, Pullen J, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia. 1999;13(3):335–342. [DOI] [PubMed] [Google Scholar]
- 3.Burkhardt B, Woessmann W, Zimmermann M, et al. Impact of cranial radiotherapy on central nervous system prophylaxis in children and adolescents with central nervous system-negative stage III or IV lymphoblastic lymphoma. J Clin Oncol. 2006;24(3):491–499. [DOI] [PubMed] [Google Scholar]
- 4.Pillon M, Piglione M, Garaventa A, et al. Long-term results of AIEOP LNH-92 protocol for the treatment of pediatric lymphoblastic lymphoma: a report of the Italian Association of Pediatric Hematology and Oncology. Pediatr Blood Cancer. 2009;53(6):953–959. [DOI] [PubMed] [Google Scholar]
- 5.Reiter A, Schrappe M, Ludwig WD, et al. Intensive ALL-type therapy without local radiotherapy provides a 90% event-free survival for children with T-cell lymphoblastic lymphoma: a BFM group report. Blood. 2000;95(2):416–421. [PubMed] [Google Scholar]
- 6.Sandlund JT, Pui CH, Zhou Y, et al. Effective treatment of advanced-stage childhood lymphoblastic lymphoma without prophylactic cranial irradiation: results of St Jude NHL13 study. Leukemia. 2009;23(6):1127–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uyttebroeck A, Suciu S, Laureys G, et al. Treatment of childhood T-cell lymphoblastic lymphoma according to the strategy for acute lymphoblastic leukaemia, without radiotherapy: long term results of the EORTC CLG 58881 trial. Eur J Cancer. 2008;44(6):840–846. [DOI] [PubMed] [Google Scholar]
- 8.Burkhardt B, Reiter A, Landmann E, et al. Poor outcome for children and adolescents with progressive disease or relapse of lymphoblastic lymphoma: a report from the Berlin-Frankfurt-Muenster group. J Clin Oncol. 2009;27(20):3363–3369. [DOI] [PubMed] [Google Scholar]
- 9.Bonn BR, Huge A, Rohde M, et al. Whole exome sequencing hints at a unique mutational profile of paediatric T-cell lymphoblastic lymphoma. Br J Haematol. 2015;168(2):308–13. [DOI] [PubMed] [Google Scholar]
- 10.Burkhardt B, Moericke A, Klapper W, et al. Pediatric precursor T lymphoblastic leukemia and lymphoblastic lymphoma: differences in the common regions with loss of heterozygosity at chromosome 6q and their prognostic impact. Leuk Lymphoma. 2008;49(3):451–461. [DOI] [PubMed] [Google Scholar]
- 11.Feng H, Stachura DL, White RM, et al. T-lymphoblastic lymphoma cells express high levels of BCL2, S1P1, and ICAM1, leading to a blockade of tumor cell intravasation. Cancer Cell. 2010;18(4):353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minard-Colin V, Brugieres L, Reiter A, et al. Non-Hodgkin lymphoma in children and adolescents: progress through effective collaboration, current knowledge, and challenges ahead. J Clin Oncol. 2015;33(27): 2963–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206–3214. [DOI] [PubMed] [Google Scholar]
- 14.Schrappe M, Valsecchi MG, Bartram CR, et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood. 2011;118(8): 2077–2084. [DOI] [PubMed] [Google Scholar]
- 15.Reiter A, Schrappe M, Tiemann M, et al. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: a report of the Berlin-Frankfurt-Munster group trial NHL-BFM 90. Blood. 1999;94(10):3294–3306. [PubMed] [Google Scholar]
- 16.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Wiley, New York, 1980. [Google Scholar]
- 17.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 18.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34(2):187–220. [Google Scholar]
- 19.Common Toxicity Criteria V 2, 1999. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf NC.
- 20.De Moerloose B, Suciu S, Bertrand Y, et al. Improved outcome with pulses of vincristine and corticosteroids in continuation therapy of children with average risk acute lymphoblastic leukemia (ALL) and lymphoblastic non-Hodgkin lymphoma (NHL): report of the EORTC randomized phase 3 trial 58951. Blood. 2010;116(1):36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurwitz CA, Silverman LB, Schorin MA, et al. Substituting dexamethasone for prednisone complicates remission induction in children with acute lymphoblastic leukemia. Cancer. 2000;88(8):1964–1969. [PubMed] [Google Scholar]
- 22.Igarashi S, Manabe A, Ohara A, et al. No advantage of dexamethasone over prednisolone for the outcome of standard- and intermediate-risk childhood acute lymphoblastic leukemia in the Tokyo Children’s Cancer Study Group L95-14 protocol. J Clin Oncol. 2005;23(27):6489–6498. [DOI] [PubMed] [Google Scholar]
- 23.Vrooman LM, Stevenson KE, Supko JG, et al. Postinduction dexamethasone and individualized dosing of Escherichia coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study–Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. J Clin Oncol. 2013;31(9):1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moricke A, Zimmermann M, Valsecchi MG, et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood. 2016;127(17):2101–2112. [DOI] [PubMed] [Google Scholar]
- 25.Termuhlen AM, Smith LM, Perkins SL, et al. Disseminated lymphoblastic lymphoma in children and adolescents: results of the COG A5971 trial: a report from the Children’s Oncology Group. Br J Haematol. 2013;162(6):792–801. [DOI] [PubMed] [Google Scholar]
- 26.Bergeron C, Coze C, Segura C, et al. Treatment of childhood T-cell lymphoblastic lymphoma-long-term results of the SFOP LMT96 trial. Pediatr Blood Cancer. 2015;62(12):2150–2156. [DOI] [PubMed] [Google Scholar]
- 27.Schrappe M, Zimmermann M, Moricke A, et al. Dexamethasone in induction can eliminate one third of all relapses in childhood acute lymphoblastic leukemia (ALL): results of an international randomized trial in 3655 patients (Trial AIEOP-BFM ALL 2000). ASH Annual Meeting Abstracts. 2008;112(11):7. [Google Scholar]
- 28.Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 2003;101(10):3809–3817. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell CD, Richards SM, Kinsey SE, Lilleyman J, Vora A, Eden TO. Benefit of dexamethasone compared with prednisolone for childhood acute lymphoblastic leukaemia: results of the UK Medical Research Council ALL97 randomized trial. Br J Haematol. 2005;129(6):734–745. [DOI] [PubMed] [Google Scholar]
- 30.Ito C, Evans WE, McNinch L, et al. Comparative cytotoxicity of dexamethasone and prednisolone in childhood acute lymphoblastic leukemia. J Clin Oncol. 1996;14(8):2370–2376. [DOI] [PubMed] [Google Scholar]
- 31.Kaspers GJ, Veerman AJ, Popp-Snijders C, et al. Comparison of the antileukemic activity in vitro of dexamethasone and prednisolone in childhood acute lymphoblastic leukemia. Med Pediatr Oncol. 1996;27(2):114–121. [DOI] [PubMed] [Google Scholar]
- 32.Balis FM, Lester CM, Chrousos GP, Heideman RL, Poplack DG. Differences in cerebrospinal fluid penetration of corticosteroids: possible relationship to the prevention of meningeal leukemia. J Clin Oncol. 1987;5(2):202–207. [DOI] [PubMed] [Google Scholar]
- 33.Larsen EC, Devidas M, Chen S, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: a report from Children’s Oncology Group study AALL0232. J Clin Oncol. 2016;34(20):2380–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tubergen DG, Gilchrist GS, O’Brien RT, et al. Improved outcome with delayed intensification for children with acute lymphoblastic leukemia and intermediate presenting features: a Childrens Cancer Group phase III trial. J Clin Oncol. 1993;11(3):527–537. [DOI] [PubMed] [Google Scholar]
- 35.Termuhlen AM, Smith LM, Perkins SL, et al. Outcome of newly diagnosed children and adolescents with localized lymphoblastic lymphoma treated on Children’s Oncology Group trial A5971: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2012;59(7):1229–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oschlies I, Burkhardt B, Chassagne-Clement C, et al. Diagnosis and immunophenotype of 188 pediatric lymphoblastic lymphomas treated within a randomized prospective trial: experiences and preliminary recommendations from the European childhood lymphoma pathology panel. Am J Surg Pathol. 2011;35(6):836–844. [DOI] [PubMed] [Google Scholar]
- 37.Gerr H, Zimmermann M, Schrappe M, et al. Acute leukaemias of ambiguous lineage in children: characterization, prognosis and therapy recommendations. Br J Haematol. 2010;149(1):84–92. [DOI] [PubMed] [Google Scholar]
- 38.Bonn BR, Rohde M, Zimmermann M, et al. Incidence and prognostic relevance of genetic variations in T-cell lymphoblastic lymphoma in childhood and adolescence. Blood. 2013;121(16):3153–3160. [DOI] [PubMed] [Google Scholar]
- 39.Callens C, Baleydier F, Lengline E, et al. Clinical impact of NOTCH1 and/or FBXW7 mutations, FLASH deletion, and TCR status in pediatric T-cell lymphoblastic lymphoma. J Clin Oncol. 2012;30(16):1966–1973. [DOI] [PubMed] [Google Scholar]
- 40.Burkhardt B, Bruch J, Zimmermann M, et al. Loss of heterozygosity on chromosome 6q14-q24 is associated with poor outcome in children and adolescents with T-cell lymphoblastic lymphoma. Leukemia. 2006;20(8): 1422–1429. [DOI] [PubMed] [Google Scholar]
- 41.Coustan-Smith E, Sandlund JT, Perkins SL, et al. Minimal disseminated disease in childhood T-cell lymphoblastic lymphoma: a report from the Children’s Oncology Group. J Clin Oncol. 2009;27(21):3533–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mussolin L, Buldini B, Lovisa F, et al. Detection and role of minimal disseminated disease in children with lymphoblastic lymphoma: the AIEOP experience. Pediatr Blood Cancer. 2015;62(11):1906–1913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.