Abstract
Patients with acute graft-versus-host disease (GvHD) grade I were randomized to an observation arm (n=85) or to a treatment arm (n=86) consisting of 6-methylprednisolone 1 mg/kg/day, after stratification for age and donor type. The primary end point was development of grade II–IV GvHD. The cumulative incidence of grade II–IV GvHD was 50% in the observation arm and 33% in the treatment arm (P=0.005). However, grade III–IV GvHD was comparable (13% vs. 10%, respectively; P=0.6), and this was true for sibling and alternative donor transplants. Moderate/severe chronic GvHD was also comparable (17% vs. 9%). In multivariate analysis, an early interval between transplant and randomization (<day +20) was the only negative predictor of grade III–IV GvHD. Patients in the observation arm had less infectious bacterial episodes (12 vs. 25; P=0.04), less severe infectious fungal episodes (0 vs. 3; P=0.04), and less severe adverse events (3 vs. 11; P=0.07). At five years, non-relapse mortality was 20% versus 26% (P=0.2), relapse-related mortality 25% versus 21%, and actuarial survival was 51% versus 41% (P=0.3) in the observation and treatment arms, respectively. In multivariate analysis, advanced disease phase, older age and an early onset of GvHD were significant negative predictors of survival, independent of the randomization arm. In conclusion, steroid treatment of acute grade I GvHD prevents progression to grade II but not to grade III–IV GvHD, and there is no effect on non-relapse mortality and survival. Patients treated with steroids are at a higher risk of developing infections and have more adverse events. (Trial registered as EUDTRACT 2008-000413-29).
Introduction
There is uncertainty as to whether grade I acute graft-versus-host disease (GvHD), that is a skin rash over less than 50% of the body surface, without liver or gut involvement, should be treated or not. In three prospective trials of first-line treatment, also patients with grade I acute GvHD (aGvHD) were enrolled;1–3 however, most Centers would probably treat only GvHD of grade II or over. One argument in favor of steroid treatment would be early intervention, thus possibly preventing progression to more severe GvHD. This is a general rule of medicine, but there is no evidence that this is also the case in patients with aGvHD.1,2 In one randomized study back in 1998, the Gruppo Italiano Trapianti di Midollo Osseo (GITMO) had shown that early intervention with high-dose steroid treatment (10 mg/kg) was equally effective as a conventional dose of steroids (2 mg/kg) in first-line treatment of aGvHD.3 In that study, the proportion of patients who progressed to grade III–IV was similar in the two groups, despite a median interval between transplant and treatment of 12 days, arguing against the hypothesis that early aggressive intervention would be more effective than standard therapy and would be able to modify the natural disease course.3 Similar results were seen in a more recent prospective randomized trial, once again comparing two different doses of steroids as first-line treatment, and again showing no difference in the rate of progression to severe GvHD.4 In addition, steroids cause immune deficiency and promote infectious complications.5
On the other hand, early treatment of GvHD could be beneficial. In a retrospective study of unrelated donor transplants in two different Centers, non-relapse mortality (NRM) was lower in one Center using anti-thymocyte globulin (ATG) for GvHD prophylaxis and steroid treatment of grade I aGvHD.6
In any case, whether grade I GvHD should be treated or not has not been tested in a prospective trial, and this led GITMO to undertake this trial. The aim was how to calculate the lowest and highest success rate. We used data from the previous GITMO study:2 25% of patients with grade I GvHD treated with 6methylprednisolone (6MPred) 2 mg/kg progressed to grade II–IV GvHD. We hypothesized that patients left untreated would have twice the risk of progression to grade II–IV GvHD, and 170 patients were needed to test this hypothesis.
We report the results of this trial in patients with grade I GvHD, randomized to receive steroid treatment or no treatment.
Methods
Study design
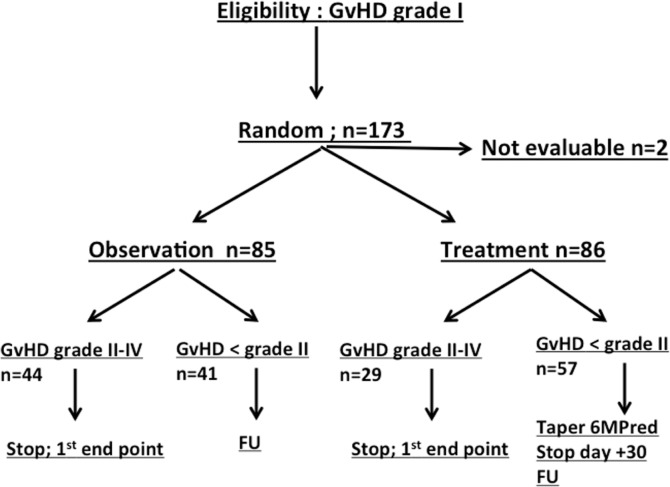
This is a Gruppo Italiano Trapianto di Midollo Osseo (GITMO) study (study name: RAMP08; registered as EUDTRACT N 2008-000413-29). The study was conducted according to Good Clinical Practice (GCP) and the Declaration of Helsinki. The study protocol was approved by all local ethical committees. Data entry was made by electronic CRF provided by Clinical Research Technolgy (CRT), Naples, Italy. The study is an open label multicenter, phase III randomized study comparing no treatment versus treatment with 6-methylprednisolone (6MPred) 1 mg/kg per day for transplanted patients with grade I aGvHD according to Gucksberg’s criteria.7 Randomization was managed centrally via the web in a 1:1 ratio. Patients were randomized using a dynamic randomization algorithm, with minimization of differences between arms A and B to no more than 2 patients overall and 3 patients per strata. Patients were stratified according to phase (early/advanced) and donor type (matched sibling/alternative donors). We applied a modified intention-to-treat analysis, and all patients with at least one day of follow up were analyzed in the arm to which they had been allocated: 173 patients were randomized, between July 2009 and August 2014, and 171 were analyzed. The study outline is shown in Figure 1. Patients randomized to the observation arm were left untreated. Patients progressing to grade II–IV GvHD were considered to have reached the primary end point of the study, independent of the interval from randomization, and were treated according to standard procedures of each Center. Patients randomized to observation and not progressing were followed up. Patients randomized to treatment received 6MPred 1 mg/kg/day for five days. Patients progressing to grade II–IV GvHD, had reached the primary end point of the sudy and were treated according to the policy of each Center. If GvHD did not progress, 6MPred was tapered as follows: 0.75 mg/kg/day on days 6–10, 0.5 mg/kg/day on days 11–15, 0.25 mg/kg/day on days 16–20, 0.12 mg/kg/day on days 21–30, and discontinued on day +30.
Figure 1.
Study outline. Patients randomized to the observation (n=85) or treatment (n=86) arms all went forward for analysis. Two patients were not evaluable because essential data were missing (1 observation arm; 1 treatment arm). 6MPred: 6 mthylprednisolone; FU: follow up.
End points
The primary end point was the cumulative incidence of patients progressing to grade II–IV aGvHD. Secondary end points were: proportion of patients with grade III–IV GvHD, proportion of bacterial infections, viral infection, fungal infections, number of adverse events and severe adverse events, cumulative incidence of non-relapse mortality (NRM), cumulative incidence of relapse, proportion of patients developing chronic GvHD (limited and extensive), actuarial overall survival (OS).
Inclusion and exclusion criteria
Study inclusion criteria were: age 0–70 years, having received an allogeneic stem cell transplant for malignant or non-malignant diseases, developing a skin rash over 10–49% of the body surface within the previous 48 hours, having received an unmanipulated graft from any donor type, and not having received previous treatment with steroids. Signed informed consent was obtained from adults or, in the case of pediatric cases, their tutors. Conventional GvHD prophylaxis was given to all patients with cyclosporin methotrexate, with the addition of ATG for unrelated donors, and post-transplant cyclophosphamide (PT-CY) for the small number (n=15) of HAPLO grafts. A skin biopsy, was recommended but not mandatory; centralized histopathology was provided (D Massi, Florence, Italy).
Exclusion criteria were: life-threatening infections, evidence of hematologic relapse, investigational drugs for GvHD prophylaxis, patients on steroid treatment (>0.5 mg/kg for 48 hours), grade II–IV GvHD. Progression to gut GvHD, but not liver GvHD, was confirmed by histology.
Patients’ characteristics
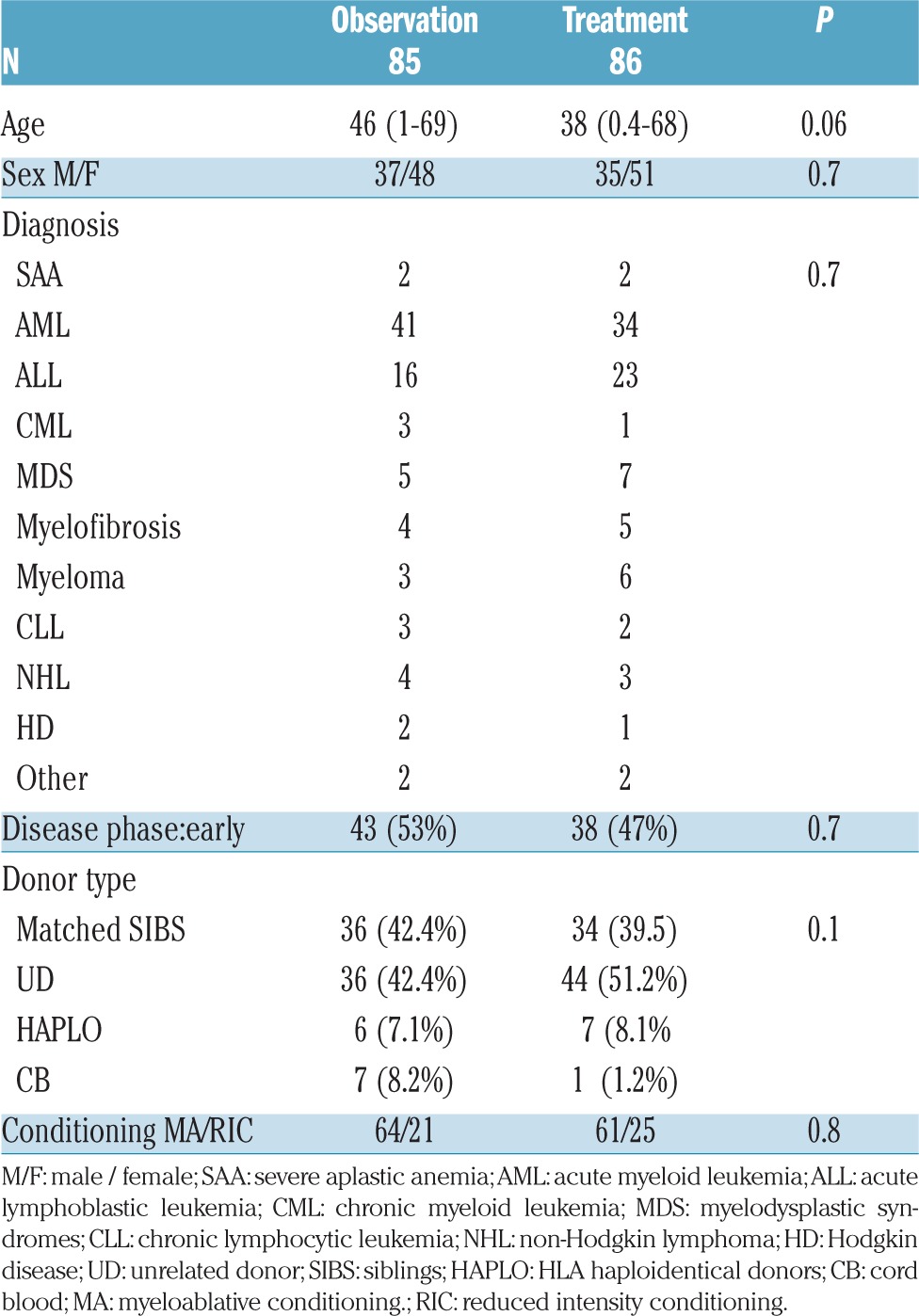
Clinical characteristics of the two groups (observation/treatment) are outlined in Table 1. Patients were well balanced in terms of diagnosis (P=0.7): the most frequent diagnosis was acute myeloid leukemia (AML) (n=75), followed by acute lymphoblastic leukemia (ALL) (n=39), and myelodysplastic syndromes (MDS) (n=12). Median age for observation/treatment was 46 years (1–69) versus 38 years (0.4–68) (P=0.06). The proportion of patients over 50 years was 51% in the observation arm and 49% in the treatment arm (P=0.8). Donor type was: HLA identical siblings n=36 and n=34, unrelated cord blood (CB) n=7 and n=1, unrelated donor (UD) n=36 and n=44, and haploidentical family donors (HAPLO) 6 and 7 (P=0.1) in the observation and treatment arms, respectively. The proportion of 1 antigen mismatched unrelated donors was 7 and 9, respectively (P=0.9). Disease phase was classified as early in 43 observation arm patients and in 38 treatment arm patients (P=0.7). The conditioning regimen was myeloablative in most patients (n=64 and n=61, respectively; P=0.8).
Table 1.
Clinical data of patients randomized.
Supportive care
Antibacterial prophylaxis with quinolones was given during the neutropenic phase. All Centers used PJV prophylaxis with cotrimoxazole and monitored cytomegalovirus (CMV) reactivation by PCR or antigenemia twice weekly. Pre-emptive therapy was given in case of CMV reactivation. Epstein Barr Virus (EBV) was monitored by PCR weekly and treated pre-emptively if positive. Aspergillus antigenemia with galactomannan was also monitored weekly; diagnosis of invasive fungal disease was performed by standard criteria and treated accordingly. Specific infectious disease policies were performed according to standard procedures of each Center.
Statistical analysis
Analysis of the primary end point was performed using the cumulative incidence (CI) of grade II–IV GvHD, calculated with mortality due to any cause as a competing risk. NRM was the competing risk for relapse-related death (RRD) and vice versa. Gray test was used to calculate difference between CI curves. Survival was calculated with Kaplan Meier curves, and the log rank test was used to test for difference between survival curves. Cox test was used for multivariate analysis. χ2 tables, Fisher exact test, and 2-sample t-test were used as appropriate; these statistical analyses were carried out using NCSS10 for Windows.
Infections and adverse events within day 100 from randomization in the two arms were assessed using Poisson or Negative Binomial (NB) regression model. Each infection type was considered as a single dependent variable and the decision on whether to use the Poisson or the NB model was based each time on a Likelihood-ratio test for overdispersion of the dependent variable considered. The treatment group indicator was considered as independent variable and the likelihood-ratio test was used to test the association with infections. The total follow up of each patient was considered as an exposure variable into the model. Stata (v.14) was used for the computation.
Sample size calculation was made using data from a previous GITMO study:2 25% of patients with grade I GvHD treated with 6methylprednisolone (6MPred) 2 mg/kg, progressed to grade II or over GvHD. We hypothesized that patients left untreated would have twice the risk of progression to grade II–IV GvHD; 170 patients were needed to reject the null hypotesis with a power of 90%.
Results
Primary end point and GvHD
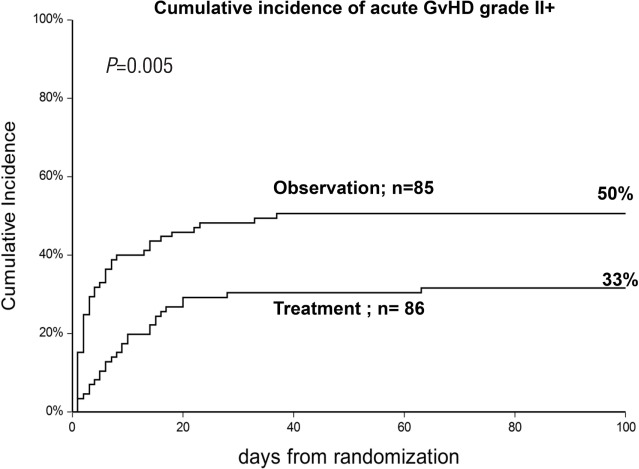
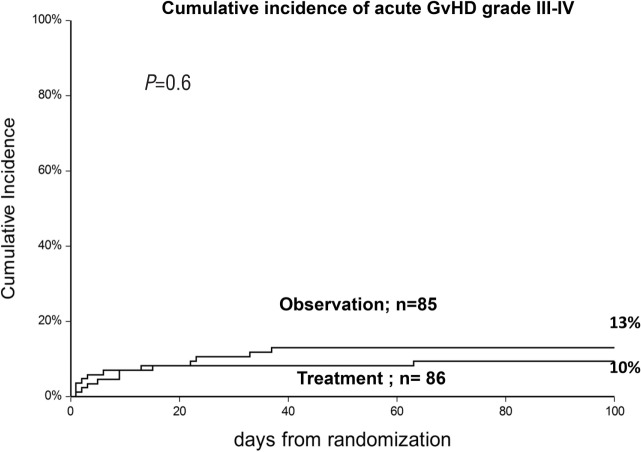
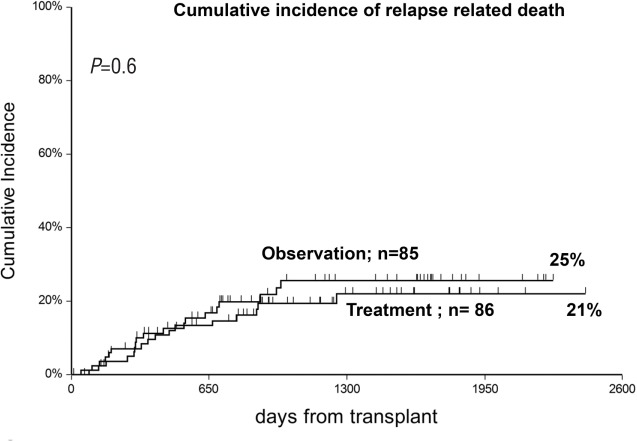
The cumulative incidence (CI) of acute GvHD grade II was 50% in the observation arm and 33% in the treatment arm (P=0.005) (Figure 2). This difference was more pronounced in sibling donor grafts (SIB) (61% vs. 32%; P=0.01), as compared to alternative donor grafts (ALT) (42% vs. 33%; P=0.1). For patients who progressed, the interval between randomization and progression was significantly shorter in the observation arm compared to the treatment arm (3 vs. 9 days; P=0.03) (Table 2). Figure 3 outlines the CI of grade III–IV GvHD in the observation versus the treatment arms (13% vs. 10%; P=0.6); it was seen in 7 vs. 4 sibling grafts, and in 4 vs. 5 alternative donor grafts, respectively (P=0.8). It was seen more frequently in patients randomized before day 20 from transplant (n=88, 17%) as compared to patients randomized later (n=83, 6%) (P=0.02) irrespective of randomization to the observation or treatment arms: 18% vs. 16% for early GvHD (<day 20), and 7% vs. 5%, for late GvHD (day 20). Moderate/severe chronic GvHD was comparable and was diagnosed in 10 patients in the observation arm versus 15 in the treatment arm (P=0.3).
Figure 2.
Cumulative incidence of grade II–IV acute graft-versus-host disease (GvHD) in patients allocated to no treatment (observation) or treatment with prednisolone 1 mg/kg (treatment).
Table 2.
Patient outcome.
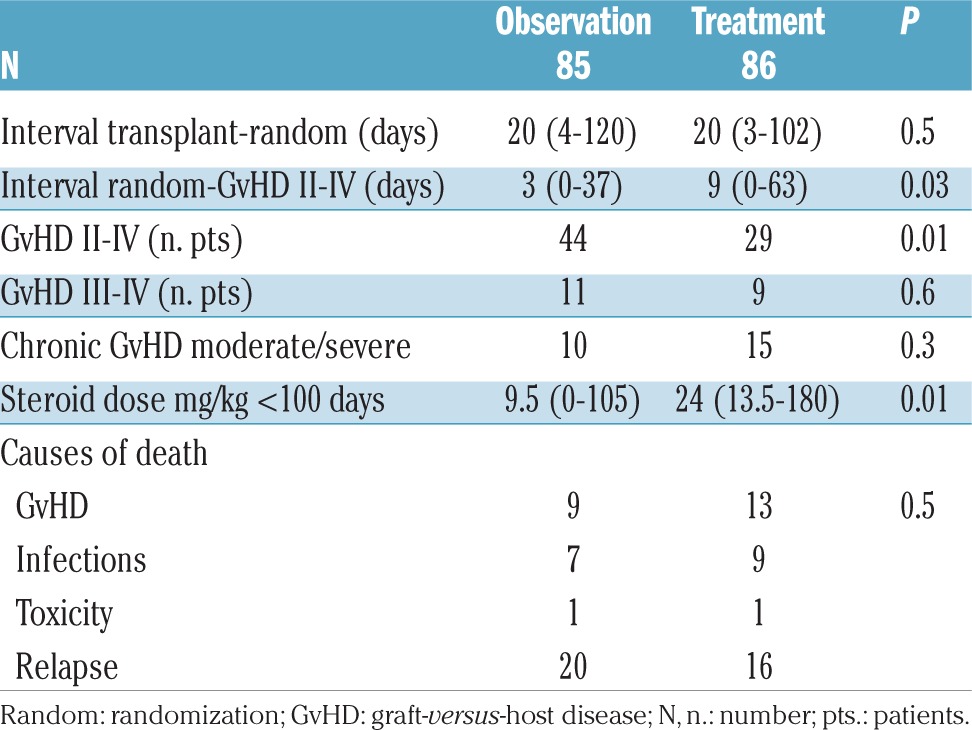
Figure 3.
Cumulative incidence of grade III–IV acute graft-versus-host disease (GvHD) in patients allocated to no treatment (observation) or treatment with prednisolone 1 mg/kg (treatment).
Greatest severity of >grade 1 GvHD
Skin GvHD, stage 2, 3 was diagnosed respectively in 22, 1 observation vs. 6, 2 treatment patients; liver GvHD stage 1,2, 3, was diagnosed in 4, 3, 1 observation vs. 7, 0, 0 treatment patients; gut GvHD stage 1, 2, 3, 4, was diagnosed respectively in 10, 3, 3, 1 observation vs. 3, 2, 3, 1 treatment patients.
Steroid dose and additional treatment
The median cumulative dose of 6MPred received in the first 100 days was 9.5 mg/kg (range 0–105) and 24 mg/kg (range 13–180) in the observation and treatment arms, respectively (P=0.01) (Table 2). Of the 86 patients in the treatment arm, 57 (66%) were off steroids by day +30, whereas 29 were on steroids having progressed to grade II–IV GvHD. Of the 85 patients in the observation arm, 41 (48%) never received steroids. The use of a second immunosuppressive drug for GvHD in addition to corticosteroids was reported in 27 and 17 treatment and observation arms, respectively (P=0.08); administration of a third drug was reported in 12 and 7 patients (P=0.2) and a fourth drug in 4 and 2 patients, respectively (P=0.4). The second drug included mycophenolate mophetil (MMF) (in 6 and 7 patients, respectively), extracorporeal photopheresis (ECP) (12 and 4 patients, respectively), or infliximab, etanercept, rituximab, basiliximab, sirolimus, anti-CD26 antibody, in a few patients each. The third added drug included ECP (3 and 2 patients, respectively), MMF (5 and 1, respectively), etanercept or anti-CD26 antibody. The fourth added drug included MMF and ECP.
Infections and adverse events
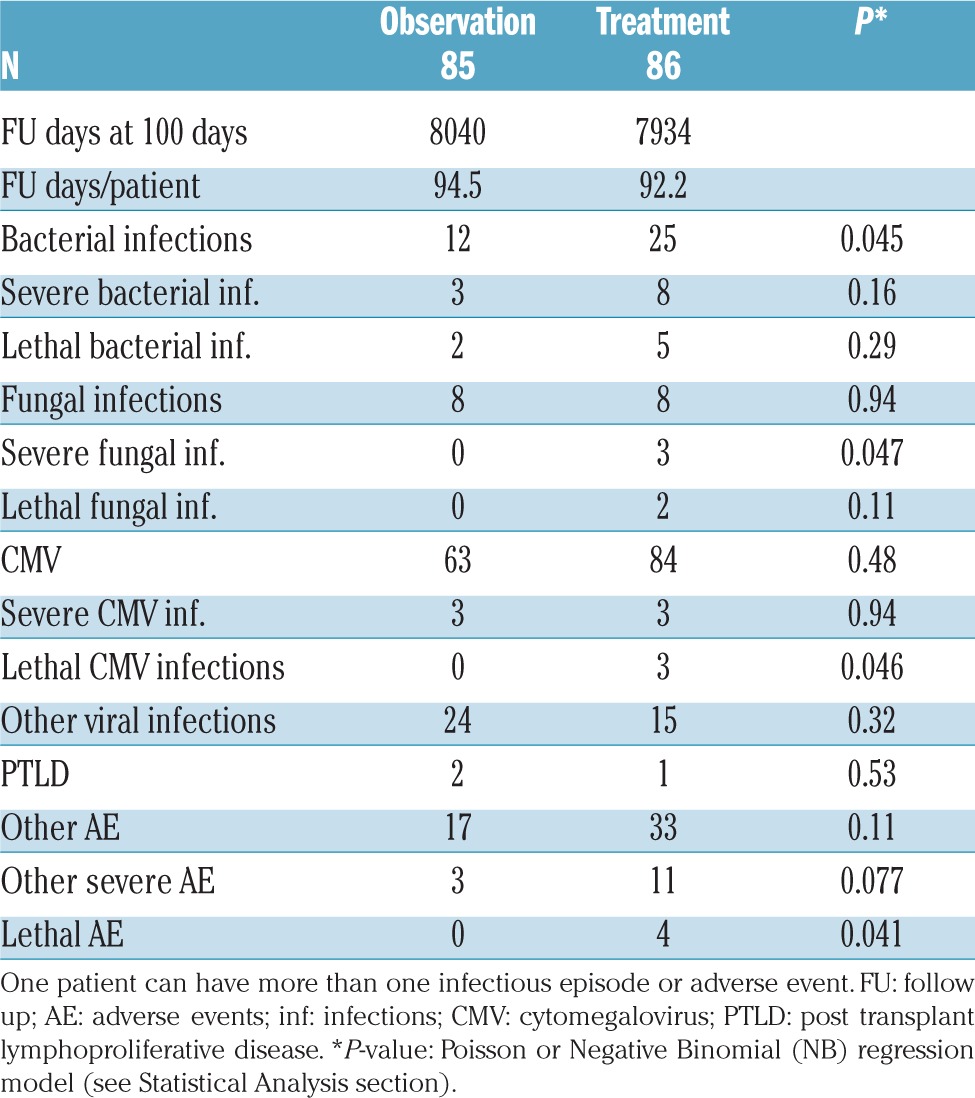
A summary of the adverse events in the two randomization arms in the first three months of treatment is shown in Table 3. The observation arm had less bacterial, fungal and CMV infections compared to the treatment arm; other adverse events and other severe adverse events were also fewer in the observation arm (Table 3), although these were mainly not statistically significant. Other adverse events were reported in 17 and 33 patients in the two arms, respectively (P=0.11), of which 3 and 11, respectively, were classified as severe (P=0.07). Adverse events included steroid-associated diabetes (0 vs. 9), acute renal failure (2 vs. 2), cystitis (5 vs. 5), hip necrosis (0 vs. 2), multi-organ failure (0 vs. 2), respiratory failure (0 vs. 3), and thrombosis (0 vs. 2) in the observation and treatment arms, respectively. Median blood counts were comparable on day +60 from randomization between the two arms. On day +60, chemistry results in the observation and treatment arms were also comparable.
Table 3.
Infectious episodes and adverse events in the two arms <100 days from randomization.
Non-relapse mortality
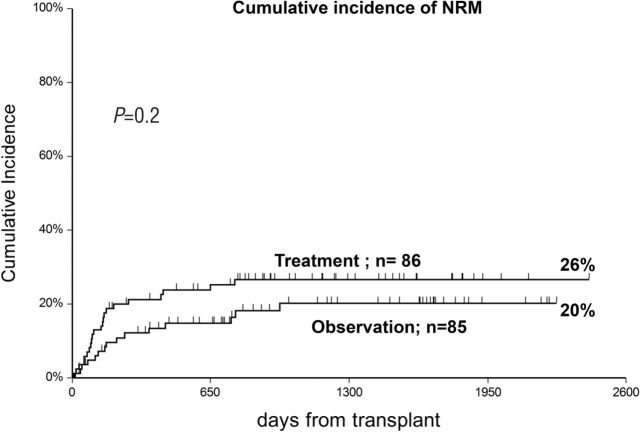
The 5-year CI of NRM was 20% (observation arm) versus 26% (treatment arm) (P=0.2) (Figure 4), and was comparable also after stratifying patients for age: <40 years (12% vs. 19%) and >40 years (28% vs. 34%). In univariate analysis, there was a very strong influence of the interval between transplant and randomization on NRM: median interval 20 days, CI of NRM 31% versus 18% (P=0.0006) for patients randomized before or after day +20 from transplant, respectively. For early randomization (<20 days from transplant) NRM was 24% vs. 46% (P=0.02) in observation versus treatment patients, due to an excess of infections in the treatment arm (2 vs. 8); for late randomization (>20 days) NRM was comparable in the two arms (22% and 14%; P=0.3).
Figure 4.
Comparable cumulative incidence of non-relapse mortality (NRM) in the two randomization groups.
Relapse-related death and survival
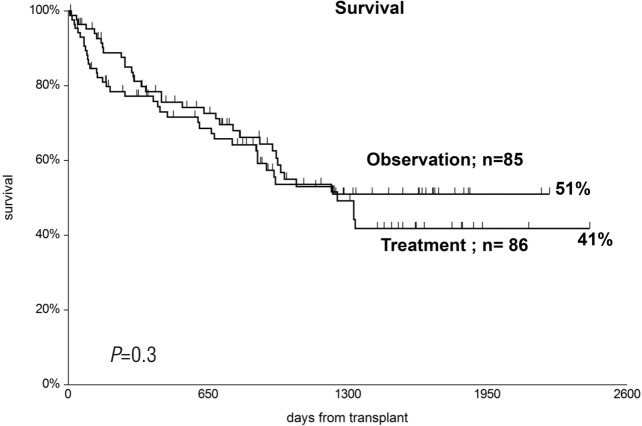
Relapse-related death was 25% in patients in the observation arm versus 21% in patients in the treatment arm (Figure 5); patients with early disease had a significantly lower probability of RRD in univariate analysis (RR 0.3, P=0.006). RRD was unaffected by the interval between transplant and randomization. Actuarial 5-year survival was 51% versus 41% in the observation and treatment arms, respectively (P=0.3) (Figure 6). Predictors of survival in univariate analysis were younger age, early disease phase, and randomization beyond day +20 from transplant. Causes of death in the two study groups were: GvHD in 9 versus 13 patients, infection in 7 versus 9 patients, toxicity in 1 patient in each group, and leukemia relapse in 20 versus 16 patients (P=0.9) (Table 2). There was no significant difference in NRM between different Centers (P=0.5).
Figure 5.
Comparable cumulative incidence of relapse-related death (RRD) in the two randomization groups.
Figure 6.
Comparable 5-year overall survival (OS) in the two randomization groups.
Skin biopsies
A skin biopsy to prove or disprove skin GvHD was not mandatory for eligibility in this trial. It was performed before randomization and reviewed centrally by one of the Authors (DM) in 38 patients. Of these, 36 (95%) were compatible with aGvHD (proven, probable, and possible in 9, 15 and 12 patients, respectively); these were equally distributed among the treatment and observation arms (P=0.7).
Multivariate analysis
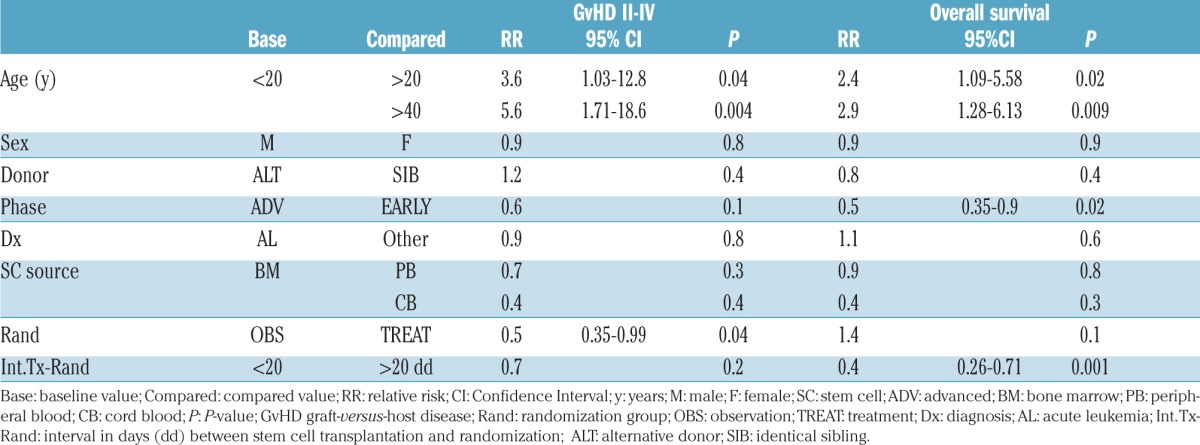
Progression to grade II–IV GvHD was predicted in a Cox analysis by age over 20 years (P=0.003) or 40 years (P=0.005), and randomization to the observation arm (P=0.02) (Table 4). A short interval between transplant and randomization (< 20 days), was the only variable predicting progression to grade III–IV GvHD (RR 0.4, 95%CI: 0.12–0.98; P=0.04) and was also associated with a higher risk of death (P=0.006). Survival was also predicted by patients’ age and disease phase. NRM was predicted only by age over 20 years (RR 2.8, 95%CI: 0.88–9.18; P=0.07) and age over 40 years (RR 3.0, 95%CI: 0.99–9.67; P=0.051), and by early onset of GvHD before day +20 from transplant (RR 2.38, 95%CI: 1.06–4.0; P=0.03).
Table 4.
Multivariate analysis.
Discussion
Treatment of aGvHD remains a difficult issue, despite several decades of studies and the many immunosuppressive/immunomodulating agents tested.8 There are difficulties not only in the treatment, but problems start with staging of involved organs and overall grading of the disease, with several possible grading options and a degree of variability according to the assessor.7–13 Despite differences in grading, and the difficulty in assessing response rates, it is recognized that mortality increases with increasing GvHD severity, and this is true both in the short and in the long term.14 In a large group of patients (n=4174), NRM at three years was 21% for grade 0-I aGvHD, 32% for grade II, 60% for grade III, and 89% for grade IV; the corresponding 3-year OS was 79%, 64%, 37% and 10%, respectively.14 This study exemplifies on one hand, the major impact of aGvHD grading on the outcome of allogeneic transplants, and on the other, the lack of effective treatment when the disease is beyond grade II. In keeping with the latter observation, a recently developed risk score for aGvHD identifies patients at high risk of mortality according to the number of involved organs and the severity of GvHD at onset.13 Mortality at six months is 22% for standard-risk and 44% for high-risk GvHD.13 A set of GvHD biomarkers have recently been described; these identify at the onset of the disease severe cases with a high risk of mortality eligible for early intervention.15
It would thus seem reasonable to try and prevent progression of aGvHD, and this may be achieved if aGvHD is treated at a very early stage (earliest being grade I, or a skin rash involving <50% of the body surface). We, therefore, asked whether steroid treatment of grade I GvHD would be beneficial, and we selected evolution to grade II or more as the primary end point of the study. Patients randomized in the observation arm would become eligible for treatment when diagnosed as grade II GvHD, also if this occurred 24 hours after randomization. This facilitated the informed consent procedure with the patients since there would be no delay in treatment once the disease had progressed to grade II. As expected, patients randomized to receive treatment at diagnosis of grade I GvHD had a significantly lower probability to progress to grade II or more GvHD compared to untreated patients (33% vs. 50%). The fact that patients in the observation arm, grafted from identical siblings, had a higher proportion of grades II–IV GvHD (61%) compared to patients in the observation arm receiving alternative donor grafts (44%) can be explained by the fact that, in the latter, GvHD prophylaxis included either ATG or PT-CY, in addition to CyA and MTX (UD grafts) or CyA and mycophenolate (HAPLO grafts). The unexpected finding was that the CI of patients progressing to severe (grade III–IV) GvHD was comparable in the two groups (13% vs. 10%). Therefore, the primary end point of the study was reached, but this was due to skin GvHD progressing from stage II to stage III in the observation arm (22 observation vs. 6 treatment patients) and stage 1 gut GvHD (10 observation vs. 3 treatment patients). On the other hand, patients with stage II–IV gut GvHD were comparable in the two randomization groups (7 and 6, respectively), and liver GvHD was seen in a few patients only.
When looking at adverse events, we found that patients in the treatment arm had more infections and more adverse events than observation patients, in particular, bacterial infections, severe fungal infections, and CMV reactivation. As a consequence of similar severe GvHD and more infections, NRM was 20% in the observation versus 26% in the treatment arm, and survival at five years was 51% versus 41%, respectively. In a multivariate Cox analysis, there was a trend for inferior survival (P=0.09) in the treatment arm, despite a median younger age (38 vs. 46 years).
Other studies have tested early treatment of GvHD.2–4 Etanercept and topical steroids have been reported by Gatza et al.16 in grade I GvHD. Of the 34 patients enrolled in that study, 3% progressed to grade III–IV, significantly lower than another group of patients receiving topical steroids alone, 18% of whom progressed to grade III–IV GvHD.16 Although Gatza et al. suggested that etanercept was able to modify the natural course of the disease, 2-year NRM was 19%,16 comparable to the 20% NRM of our observation arm, and the 26% of our treatment arm. Another non-steroid approach was tested propectively, randomizing patients to receive or not 2.5 mg/kg ATG, on day +7 after an alternative donor transplant.17 Grade III–IV GvHD was significantly reduced in the ATG group (5%) compared to the untreated group (15%), though NRM was only marginally reduced from 35% to 29% (P=ns).17 Finally, high-dose cyclophosphamide post transplant is being widely and successfully used to prevent severe GvHD,18–20 but again this is given very early (day +3) and possibly interfers with the activation phase of T cells rather than with the effector phase.
We found a strong association of early GvHD with GvHD severity and survival. Patients developing grade I GvHD within day +20 from transplant had a higher probability (RR 2.7) of developing grade III–IV GvHD, as compared to patients randomized later (17% vs. 6%; P=0.02) and a higher risk of NRM (31% vs. 18%; P=0.0006). Randomization to steroids was not beneficial in these early grade I GvHD patients, with progression to grades III–IV in 18% observation versus 16% treatment patients. In addition, more cases of infectious mortality were found in patients randomized day 20 or later to the treatment arm. Univariate and multivariate analysis predicted survival by the time of randomization; 4-year survival of patients randomized before day +20 from transplant was 33% compared to 60% for patients randomized later (P=0.001), regardless of randomization to the observation or to the treatment arms. In our data base of 2445 allogeneic transplants, the proportion of grade III–IV GvHD in patients developing GvHD within day 20, between days 21–40, or beyond day 40 is 11%, 9% and 3%, respectively (P=0.0002), and NRM is 35%, 28% and 25%, respectively (P=0.0006) (A Bacigalupo et al., 2017, unpublished data), confirming other reports on the association of early onset as a risk factor for grade III–IV GvHD.21
In conclusion, steroid treatment of grade 1 GvHD prevents progression to grade II GvHD, but not to grade III–IV GvHD, and there is no beneficial effect on NRM and survival. In addition, patients receiving steroids are at a higher risk of developing infections and have more adverse events, especially if GvHD develops within day +20 from transplant. A small proportion of patients develop life-threatening GvHD, irrespective of early steroid treatment, suggesting that the severity of GvHD is determined at onset. Early identification of high-risk patients with recently described biomarkers,22 and pre-emptive treatment with non-steroidal agents should be investigated with the aim of changing the natural course of the disease.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/12/2125
Study centers
The following Centers participated in the trial: Ospedale San Martino, Genova (A Bacigalupo); Ospedale Ferrarotto, Catania (G Milone); Ospedale San Camillo, Roma (A Locasciulli); Ospedale Civile, Pescara (A Santarone); Ospedale Regina Margherita, Torino (F Fagioli); Unviersita’ Cattolica, Roma (S Sica, P Chiusolo); Ospedale S. Croce, Cuneo (N Mordini); Ospedale Civile, Alessandria (R Sorasio).
Funding
This trial was supported by the Gruppo Italiano Trapianto di Midollo Osseo (GITMO), FARITMO, Genova, and AIRC, Milano.
References
- 1.Bolaños-Meade J, Logan BR, Alousi AM, et al. Phase 3 clinical trial of steroids/mycophenolate mofetil vs steroids/placebo as therapy for acute GVHD: BMT CTN 0802. Blood. 2014; 124(22):3221–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Lint MT, Milone G, Leotta S, et al. Treatment of acute graft-versus-host disease with prednisolone: significant survival advantage for day +5 responders and no advantage for nonresponders receiving anti-thymocyte globulin. Blood. 2006; 107(10):4177–4181. [DOI] [PubMed] [Google Scholar]
- 3.van Lint MT, Uderzo C, Locasciulli A, et al. Early treatment of acute graft-versus-host disease with high- or low-dose 6-methylprednisolone: a multicenter randomized trial from the Italian Group for Bone Marrow Transplantation. Blood. 1998; 92(7):2288–2293. [PubMed] [Google Scholar]
- 4.Mielcarek M, Furlong T, Storer BE, et al. Effectiveness and safety of lower dose prednisone for initial treatment of acute graft-versus-host disease: a randomized controlled trial. Haematologica. 2015; 100(6);842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikulska M, Raiola AM, Bruno B, et al. Risk factors for invasive aspergillosis and related mortality in recipients of allogeneic SCT from alternative donors: an analysis of 306 patients. Bone Marrow Transplant. 2009;44(6):361–370. [DOI] [PubMed] [Google Scholar]
- 6.Remberger M, Storer B, Ringdén O, Anasetti C. Association between pretransplant Thymoglobulin and reduced non-relapse mortality rate after marrow transplantation from unrelated donors. Bone Marrow Transplant. 2002;29(5):391–397. [DOI] [PubMed] [Google Scholar]
- 7.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. [DOI] [PubMed] [Google Scholar]
- 8.Deeg HJ. How I treat refractory acute GvHD. Blood. 2007;109(9):4119–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 10.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97(4):855–864. [DOI] [PubMed] [Google Scholar]
- 11.Martino R, Romero P, Subirá M, et al. Comparison of the classic Glucksberg criteria and the IBMTR Severity Index for grading acute graft-versus-host disease following HLA-identical sibling stem cell transplantation. International Bone Marrow Transplant Registry. Bone Marrow Transplant. 1999;24(3):283–287. [DOI] [PubMed] [Google Scholar]
- 12.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002; 8(7):387–394. [DOI] [PubMed] [Google Scholar]
- 13.MacMillan ML, Robin M, Harris AC, et al. A Refined Risk Score for Acute Graft-versus-Host Disease that Predicts Response to Initial Therapy, Survival, and Transplant-Related Mortality. Biol Blood and Marrow Transpl. 2015;21(4):761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gratwohl A, Brand R, Apperley J, et al. Graft vs host diseaseand outcome in HLA identical sibling transplantation, for chronic myeloid leukemia. Blood. 2002; 100(12):3877–3886 [DOI] [PubMed] [Google Scholar]
- 15.Levine JE, Braun TM, Harris AC, et al. A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study. Lancet Haematol. 2015;2(1):e21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gatza E, Braun T, Levine JE, et al. Etanercept plus topical corticosteroids as initial therapy for grade one acute graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014; 20(9):1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacigalupo A, Lamparelli T, Milone G, et al. Pre-emptive treatment of acute GVHD: a randomized multicenter trial of rabbit anti-thymocyte globulin, given on day+7 after alternative donor transplants. Bone Marrow Transplant. 2010;45:(2)385–391. [DOI] [PubMed] [Google Scholar]
- 18.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow ransplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon SR, Sizemore CA, Sanacore M, et al. Total Body Irradiatione Based Myeloablative Haploidentical Stem Cell Transplantation Is a Safe and Effective Alternative to Unrelated Donor Transplantation in Patients Without Matched Sibling Donors. Biol Blood Marrow Transpl. 2015;21(7):1299–1307 [DOI] [PubMed] [Google Scholar]
- 20.Brammer JE, Khouri I, Gaballa S, et al. Outcomes of Haploidentical Stem Cell Transplantation for Lymphoma with Melphalan-Based Conditioning. Biol Blood Marrow Transpl. 2016;22(3):493–498. [DOI] [PubMed] [Google Scholar]
- 21.Kanakry CG, Ganguly S, Zahurak M, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to post-transplantation cyclophosphamide. Sci Transl Med. 2013;5(211):211ra157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saliba RM, De Lima M, Giralt S, et al. Hyperacute GVHD: risk factors, outcomes, and clinical implications. Blood. 2007; 109(7):2751–2758. [DOI] [PubMed] [Google Scholar]
- 23.Hartwell MJ, Özbek U, Holler E, et al. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight. 2017;2(3):e89798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.