The growing health care needs and the increase of new and expensive health care technologies are a challenge for the sustainability of health systems worldwide.1 Globally, national health systems are currently spending around US$ 100 billion per year on anticancer drugs alone.2
Biosimilars can be one of the solutions to reduce costs of cancer treatment, retaining the same efficacy and safety of originator drugs.3 The pathways of biosimilar approval have been set by the European Medicine Agency (EMA) and the U.S. Food and Drug Administration (FDA) with the purpose of checking their comparability in terms of pharmacokinetic/pharmacodynamic properties, preclinical biological activity, and physiochemical characterization as well as requiring a robust and consistent manufacturing process.4
The pathways towards market authorization are faster and less demanding for biosimilars because clinical studies on these agents are only aimed at demonstrating that safety and efficacy are comparable with those of the originator.5 However, health practitioners, owing to the limited amount of comparative data between biosimilars and originators, are often reluctant to consider these agents replaceable with one another in clinical practice.6
A recent European Public Assessment Report (EPAR) by EMA assessed the new rituximab biosimilar CT-P10 and approved this new biosimilar. On the basis of this clinical material, we performed a network meta-analysis, including not only the equivalence study comparing rituximab biosimilar versus originator in patients with advanced follicular lymphoma (AFL), but also the randomized clinical trials comparing originator versus standard of care (SOC).7,8
In the context of evidence-based studies, network meta-analysis is increasingly being used owing to its ability to compare three or more treatments with one another.9 This feature can be helpful for studying biosimilars.
Our network meta-analysis focused on the efficacy of rituximab in AFL, and aimed at comparing rituximab biosimilar CT-P10 (Truxima®) versus Mabthera/Rituxan (rituximab originator). The methodology of this assessment has already been described in a recent report.10
The clinical data on the rituximab biosimilar were extracted from the EPAR, published by EMA in 20178, whilst the meta-analysis of Gao et al.11 and that of Schulz et al.12 provided the data on both rituximab originator product and SOC. These two meta-analyses were selected according to a standard literature search on PubMed (keywords: rituximab, meta-analysis, follicular lymphoma).
Standard of care included different chemotherapy regimens such as CVP, FCM, MCP, and CHOP, which are commonly recommended in AFL. The end-point we selected was the rate of overall response (oRR) at the end of the prescribed regimens. This endpoint was a composite of CR (complete response), CRu (unconfirmed complete response), and PR (partial response).
Our network meta-analysis was based on the bayesian method proposed by the National Institute for Health and Care Excellence (NICE).13 Odds ratio (OR) for all pairwise comparisons was the output of the analysis, along with the ranking histogram and 95% credible intervals (CrIs). Since no significant heterogeneity was found in the clinical trials, the Bayesian statistics analysis was performed with a fixed effect model.
The data of oRR from the 6 randomized trials selected by Gao et al.11 and by Schulz et al.12 are shown in Table 1. Our network meta-analysis estimated an OR of 2.61 (95%CrI: 0.51 to 14.91) for the comparison biosimilar versus originator, 7.44 (95%CrI: 1.42 to 43.38) for biosimilar versus SOC, and 2.82 (95%CrI: 2.14 to 3.76) for originator versus SOC. In terms of efficacy, CT-P10 ranked: first in 88% of bayesian simulations; second in 12%, third in 0%; the originator: first, 12%; second, 88%, third, 0%; SOC always ranked third (Figure 1). The number of evaluated patients increased with this approach from 124 (EPAR) to 1,704 (network meta-analysis).
Table 1.
Rates of oRR reported in the randomized trials included in our network meta-analysis.
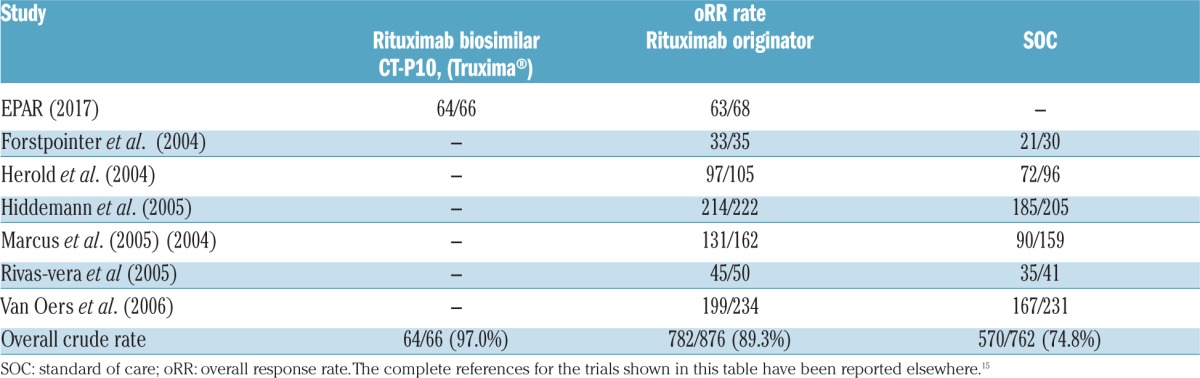
Figure 1.
Comparative effectiveness of three treatments: rituximab biosimilar plus chemotherapy, rituximab originator plus chemotherapy, and chemotherapy alone (SOC) in patients with AFL evaluated according to Bayesian network meta-analysis (7 randomized studies). The figure shows the histogram of rankings estimated for each of the three treatments according to the Bayesian probabilistic analysis (fixed-effect model). Each panel indicates, in a series of simulations, how often the concerned treatment ranked first, second or third in terms of efficacy.
The 95%CrI estimated by the bayesian meta-analysis for the comparison of biosimilar versus originator (OR=2.61; 95%CrI: 0.51 to 14.91) was close to the 95% confidence interval (CI) reported in the equivalence trial (OR=2.56; 95%CI: 0.48 to 14.28); hence, the results of our network meta-analysis concerning this comparison (together with their variability) confirmed those found in the equivalence trial.
One of the main reasons for limiting the use of biosimilar in the real world setting is the absence of RCTs involving a large number of patients.6 EMA’s EPAR reports one comparative RCT for the rituximab biosimilar CT-P10 which involved 124 patients with AFL (of whom 66 were treated with biosimilar and the others with the originator). In this framework, conducting our network meta-analysis allowed us to evaluate the efficacy endpoint of rituximab biosimilar, rituximab originator, and SOC on the basis of a total of 1,704 patients.
The results of our meta-analysis confirm the efficacy of rituximab biosimilar in treating AFL. Interestingly, the biosimilar ranked first in 88% of the bayesian simulations. Using CT-P10 in patients with AFL has a critical clinical relevance as, at least in Europe, this is the first case in which a biosimilar has been proposed for a potentially curative indication in oncologic patients.
One strength of this methodological approach is that the probability of finding a good quality meta-analysis of the originator supported by numerous RCTs is high because the originator has been available on the market for several years. On the other hand, a weakness of this method is that, when the meta-analytical results are nearly identical to those of the equivalence trial, the added value of this analysis is quite modest in practical terms. Another limitation is that our meta-analysis did not assess the issue of safety; this point is critical in the approval of biosimilars, but the EPAR of EMA8 shows that the proof confirming the safety of this biosimilar were sound.
The “total evidence” approach can provide useful information for overcoming some skepticism regarding biosimilars. In economic terms, biosimilars are typically priced at around 60–70% (or less) compared with the originator; likewise, generics generally determine savings of more than 50% in unit prices.14 In this context, sustainability of pharmaceutical expenditure in national health systems is increasingly dependent on the use of biosimilars and generics, particularly in haematology and oncology.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Sullivan R, Peppercorn J, Sikora K, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol. 2011;12(10):933–980. [DOI] [PubMed] [Google Scholar]
- 2.Prasad V, De Jesus K, Mailankody S. The high price of anticancer drugs: origins, implications, barriers, solutions. Nat Rev Clin Oncol. 2017;14(6):381–390. [DOI] [PubMed] [Google Scholar]
- 3.Schellekens H, Smolen JS, Dicato M, Rifkin RM. Safety and efficacy of biosimilars in oncology. Lancet Oncol. 2016;17(11):e502–e509. [DOI] [PubMed] [Google Scholar]
- 4.Daller J. Biosimilars: A consideration of the regulations in the United States and European union. Regul Toxicol Pharmacol. 2016;76:199–208. [DOI] [PubMed] [Google Scholar]
- 5.De Mora F. Biosimilar: what it is not. Br J Clin Pharmacol. 2015;80(5):949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck M, Michel B, Rybarczyk-Vigouret MC, et al. Rheumatologists’ perceptions of biosimilar medicines prescription: findings from a French web-based survey. BioDrugs. 2016;30(6):585–592 [DOI] [PubMed] [Google Scholar]
- 7.ClinicalTrials.gov [Internet]. Bethesda (MD, USA): National Library of Medicine (US) 2000. February 29 -. Identifier NCT02162771, To demonstrate equivalence of pharmacokinetics and noninferiority of efficacy for CT-P10 in comparison with Rituxan (rituximab); 2014 May 29 [cited 2017 Aug 21] Available from: https://clinicaltrials.gov/ct2/show/NCT02162771 [Google Scholar]
- 8.EMA European Medicine Agency - Committee for Medicinal Products for Human Use (CHMP). Truxima: EPAR - Public assessment report. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004112/WC500222695.pdf2016May;EMA/CHMP/75695/2017
- 9.Higgins JP, Welton NJ. Network meta-analysis: a norm for comparative effectiveness? Lancet. 2015;386(9994):628–630. [DOI] [PubMed] [Google Scholar]
- 10.Messori A, Trippoli S, Marinai C. “Total evidence” network meta-analysis as a tool for improving the assessment of biosimilars: application to etanercept in rheumatoid arthritis. Int J Clin Pharmacol Ther. 2017;55(6):517–520. [DOI] [PubMed] [Google Scholar]
- 11.Gao G, Liang X, Jiang J, Zhou X, et al. A systematic review and meta-analysis of immunochemotherapy with rituximab for B-cell non-Hodgkin’s lymphoma. Acta Oncol. 2010;49(1):3–12. [DOI] [PubMed] [Google Scholar]
- 12.Schulz H, Bohlius J, Skoetz N, et al. Chemotherapy plus Rituximab versus chemotherapy alone for B-cell non-Hodgkin’s lymphoma. Cochrane Database Syst Rev. 2007;17(4):CD003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NICE National Clinical Guideline Centre – Acute and Chronic Conditions (UK). Venous thromboembolism: reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. Appendix I, Winbugs code for network meta-analysis. https://www.ncbi.nlm.nih.gov/pubmed/23346611 London: Royal College of Physicians (UK), 2010. [PubMed] [Google Scholar]
- 14.Conti RM, Padula WV, Larson RA. Changing the cost of care for chronic myeloid leukemia: the availability of generic imatinib in the USA and the EU. Ann Hematol. 2015;94 Suppl 2:S249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mengato D. Rituximab biosimilar vs rituximab originator in advanced follicular lymphoma. PubMed Commons, published on 19 June 2017, available at https://www.ncbi.nlm.nih.gov/myncbi/daniele.mengato.1/comments/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


