Abstract
Background
Sample quality is critical for biomarker detection in oncology, and platelet degradation and contamination in plasma have a remarkable impact on the ability to accurately quantify many blood-based biomarkers. Platelet factor 4 (PF4) can be used as an indicator to monitor sample quality. This multicenter study aimed to determine the impact of critical components of the blood sample handling process on platelet degradation/contamination and to establish an optimal method for collecting platelet-poor plasma samples.
Methods
At each of six participating centers, blood samples were drawn from 12–13 healthy volunteers. Serum and plasma samples were prepared from whole blood samples using nine different methods that have been commonly used in ongoing multicenter trials. PF4 levels in the prepared samples were measured by enzyme-linked immunosorbent assay (ELISA). Paired t-tests were used for statistical analysis.
Results
Blood samples were collected from 74 subjects enrolled in six centers. PF4 levels were significantly higher in serum samples than in plasma samples (P<0.001), in plasma samples from blood that sat at room temperature for 5 minutes (P=0.021), in plasma samples prepared at an insufficient centrifugal force (P<0.001), and in plasma samples prepared from blood that sat for longer than 4 hours on ice (P=0.001). For each method, the PF4 levels did not differ significantly among the centers or between Chinese and American subjects. The methods that resulted in normal levels of PF4 involved keeping blood samples on ice for 30 minutes to <4 hours and centrifugation at 2,500–3,000 ×g for 30 min.
Conclusions
This multicenter study evaluated multiple blood sample handling conditions for minimizing platelet degradation during plasma serum preparation and determined an optimal method for preparing platelet-poor plasma. The findings of this study can be applied in future blood biomarker studies.
Keywords: Plasma, platelet factor 4 (PF4), serum, biomarker study
Introduction
Appropriate blood sample handling is crucial for blood-based biomarker studies including proteomics investigation. However, there are many pre-analytical steps that could interfere with the accurate measurement of potential circulating biomarkers (1), and platelet activation and contamination have significant impacts on the accurate quantification of many molecules including cytokine profiles. Thus, a suboptimal blood sample preparation process can invalidate study results. For example, results from laboratory studies and a few small human series have shown that transforming growth factor-beta (TGF-β1) is a radiation-inducible cytokine involved in the early changes in molecular signaling in radiation-induced lung damage and that a change in the plasma TGF-β1 level may precede the occurrence of radiation pneumonitis and therefore can be used as a predictive marker (2-7). However, data regarding TGF-β1 levels from human studies are inconsistent regarding the association between the circulating TGF-β1 level and the risk of radiation pneumonitis (8-10). The reported TGF-β1 levels in normal human subjects varied from series to series, and the variation found in plasma and serum samples from normal control subjects using the same enzyme-linked immunosorbent assay (ELISA) methodology was obviously related to the sample collection and handling process (11,12). Notably, TGF-β1 levels are 40–100-fold higher in platelets than in any other normal tissue in the body (13), and thus, activation or degranulation of platelets in plasma or serum samples can significantly interfere with the TGF-β1 concentration in those samples (2,11).
With technological advances, proteome signature and biomarker identification techniques have become available for cancer research (14). Studies have shown that for the analysis of low molecular weight proteins, platelet contamination/activation can also significantly influence the proteome profile, as platelet-poor plasma is used for these tests. Obtaining good samples is crucial for accurate proteome analysis (15,16). Similarly, tests determining cytokine profiles will also be affected by the blood sample handling process.
The primary goal of this study was to establish an optimal method for preparing plasma samples for accurate estimation of cytokine profiles to ensure sample quality for future biomarker studies. The specific aims of this study were: to study the result variations generated from various temperature settings and spinning gravities; to establish reliable blood sample preparation methods for obtaining platelet-poor plasma and serum samples; and to determine whether platelet-poor plasma samples prepared using different methods have reproducible plasma cytokine profiles.
Wakefield et al. (17) and Jeon et al. (18) demonstrated that platelet factor 4 (PF4) levels could be used to monitor the quality of plasma samples for TGF-β1 measurement. We hypothesized that levels of PF4 reflect the activation and degranulation of platelets, and thus, can be used as a marker of platelet contamination in plasma and serum samples. Other components of the sample handling process that do not directly impact on platelet degranulation may also have impact on the accuracy of protein quantification. Thus, we also hypothesized that: samples with normal PF4 levels may have varying levels of cytokines other than TGF-β1 due to the various sample handling procedures such as duration of time and/or the temperature at which blood samples stand prior to preparation.
Methods
Study design
This was a multicenter study, and all of the centers utilized the same protocol which included detailed blood collection methods. All centers received approval from their respective Institutional Review Boards prior to subject enrollment. Only healthy adults aged more than 18 years were eligible for this study. Pregnant women were excluded. Subjects diagnosed with cancer within 2 years prior to the study or with any evidence of acute illness were also excluded. All subjects signed a study-specific, written, informed consent form. Each center collected blood from normal controls and delivered the samples according to protocol instructions (samples from U.S. centers were delivered to Dr. Kong’s lab and samples from Chinese centers were delivered to Dr. Wang’s lab).
Blood draws and sample preparation
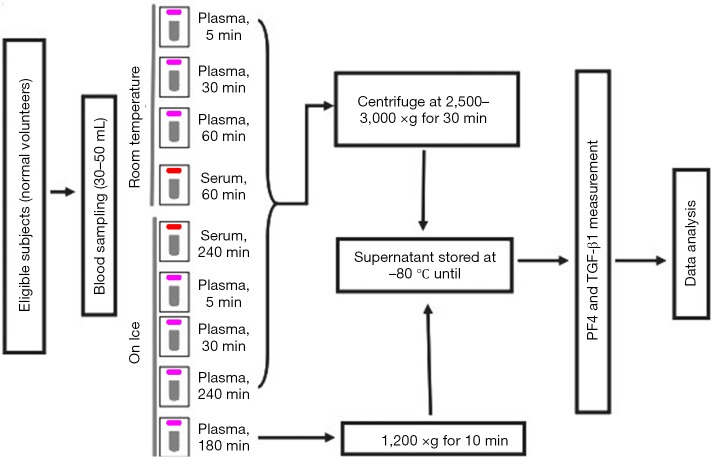
A schema of the study blood draw and sample handling process is shown in Figure 1. A total of 9 tubes (5 mL each) of blood were obtained from each subject, 7 for plasma and 2 for serum analyses. Needles of 19–21 gauge were used. The blood was collected into vacutainers containing K2 Ethylenediaminetetraacetic acid (EDTA) as anticoagulant for the preparation of plasma (purple top) or into non-additive tubes for the preparation of serum (red top). Although it is commonly used as an anticoagulant during the collection of blood samples, heparin was not allowed for measurement TGF-β1, as it will bind to and enhance the activity of antithrombin III and lead to a higher TGF-β1 level in plasma due to platelet activation and the release of other proteins stored in platelets. EDTA does not have a direct effect on platelet activation and was thus used as an anticoagulant in blood collection for plasma samples of TGF-β1. Collected blood samples were mixed by gentle inversion and immediately placed on ice or kept at room temperature according to the study design. Specifically, six plasma tubes were kept at room temperature (RT) for 5, 30, or 60 min or on ice for 5, 30, or 240 min before centrifugation at 2,500–3,000 ×g for 30 min (5-min-RT, 30-min-RT, and 60-min-RT plasma; or 5-min-on-ice, 30-min-on-ice, and 240-min-on-ice plasma, respectively). The remaining plasma tube was kept on ice for 180 min until centrifugation at 1,200 ×g for 10 min [low-relative centrifugal field (RCF) plasma]. The two serum tubes were kept at room temperature for 60 min or on ice for 240 min before centrifugation at 2,500–3,000 ×g for 30 min (RT-serum or on-ice-serum, respectively).
Figure 1.
Blood collection procedures. All plasma tubes were collected in EDTA pre-coated purple-top tubes, and serum samples in red-top tubes without any additives. EDTA, Ethylenediaminetetraacetic acid.
All centrifugation processes were performed at 4 °C. The upper one-third of the plasma or serum supernatant was collected, taking care not to touch the buffy coat. The plasma or serum samples were aliquoted and stored at −80 °C until sample delivery on dry ice.
Cytokine measurement and proteomic analysis
Samples were kept at −80 °C until delivery in dry ice and then stored at this temperature until measurement of cytokine levels. Human TGF-β1 ELISA kits were purchased from R&D Systems (Quantikine®, Human TGF-β1 Immunoassay. R&D Systems, Inc. Minneapolis, MN, USA). All plasma and serum samples were activated prior to detection using 2.5 N acetic acid/10M urea and were neutralized with 2.7 N NaOH/1M HEPES before TGF-β1 detection. Since the plasma or serum TGF-β1 was activated before detection, this procedure cannot distinguish between the active and latent forms of TGF-β1. Therefore, unless otherwise specified, the term “TGF-β1 level” refers to total TGF-β1.
PF4 levels were determined using ELISA kits from American Diagnostica Inc. (Stamford, CT). Cytokine profiles were measured in PF4-negative plasma samples. PF4-positive plasma samples were not further tested for cytokine measurement. Microsphere-based sandwich immunoassays for flow cytometry were used. This system uses highly sensitive and selective multiplexed assay platforms to simultaneously measure levels of many cytokines in low volume samples, e.g., 29 human cytokines/chemokines in a 25-µL sample. The technology uses multiple sets of microspheres (5.5-µm outer diameter from Luminex, Fisher Scientific) that are embedded with varying concentration ratios of two fluorophores. One cytokine-specific capture antibody is coated on one set of microspheres with an identical fluorophore concentration ratio and serves as the capture bead for a specific cytokine. These different microsphere sets are pooled together to form the assay array. Cytokines bound to the capture antibodies are then subjected to detection antibodies that have a third fluorophore (or fluorochrome). The entire sample set is then assayed by the Luminex Instrument and a plot of internal bead fluorescent intensity (denoting each cytokine) vs. the concentration of the detection antibody fluorophore is obtained. The Luminex Instrument was available in a Core Facility at University of Michigan Medical School. A human cytokine/chemokine Lincoplex kit (Linco Research, Inc., St. Charles, MO, USA) was used to detect the levels of interleukin (IL)-1α, IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-15, IL-17, epidermal growth factor (EGF), eotaxin, fractalkine, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon (IFN)-γ, IP-10, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, MIP-1β, sCD40L, TGFα, tumor necrosis factor (TNF)-α, vascular endothelial growth factor (VEGF), and RANTES (Regulated on Activation, Normal T Cell Expressed and Secreted). This kit was selected as it included most of the important inflammatory and immunomodulating cytokines of our interest.
The proteomic analysis was performed using a previously described method (19,20). Processed samples were sent to the University of Michigan (US Centers) or Peking Union Medical College (Chinese Centers) for assessment of platelet degradation based on PF4 measurement. The testing centers were blinded to the sample processing method.
Statistical analysis
We hypothesized that plasma cytokine levels differ significantly depending on the technique used to prepare samples for analysis. Our preliminary results showed that plasma PF4 and TGF-β1 levels were 12.5±3.8 and 1.9±0.4 ng/mL for well-prepared plasma samples versus 64.8±60.2 and 2.7±0.9 ng/mL for suboptimally prepared plasma samples, respectively. Well-prepared plasma were those with plasma samples prepared according to Kong’s laboratory manual, i.e., blood collected in EDTA tube, with 19–21 gage needle, with blood samples setting in ice less than 2 hours and spun down using 2,500–3,000 ×g 30 min (2-6,9,21,22). Based on these preliminary data, a sample size of 12 from each center would provide at least a 90% study power at a 1% significant level. Linear regression correlation and Student paired t-test were used to test the significance of differences. P<0.05 was considered to be statistically significant.
Results
Study population
A total of 74 healthy volunteers from six centers, three centers from the US and three centers from China, were enrolled in this study. The mean plasma level of TGF-β1 samples from the Chinese centers was not significantly different from that of samples from the US centers; neither the levels of PF4 differed.
PF4 and TGF-β1 measurement
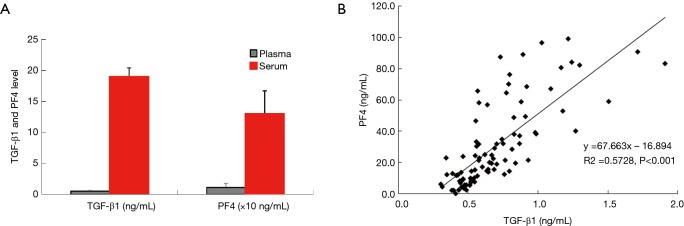
Serum levels of PF4 and TGF-β1 were about 10–29 times higher than those of plasma samples (Figure 2A), and the PF4 level was highly correlated with the TGF-β1 level (P<0.01, Figure 2B). These results were not significantly different across all the participating centers.
Figure 2.
Relationship between TGF-β1 and PF4 levels. There are significant difference in levels of both transforming growth factor-beta1 (TGF-β1) and platelet factor-4 (PF4) between serum and plasma (A), and there is a significant correlation between plasma TGF-β1 and PF4 levels (B).
PF4 levels with different blood sat times and temperatures
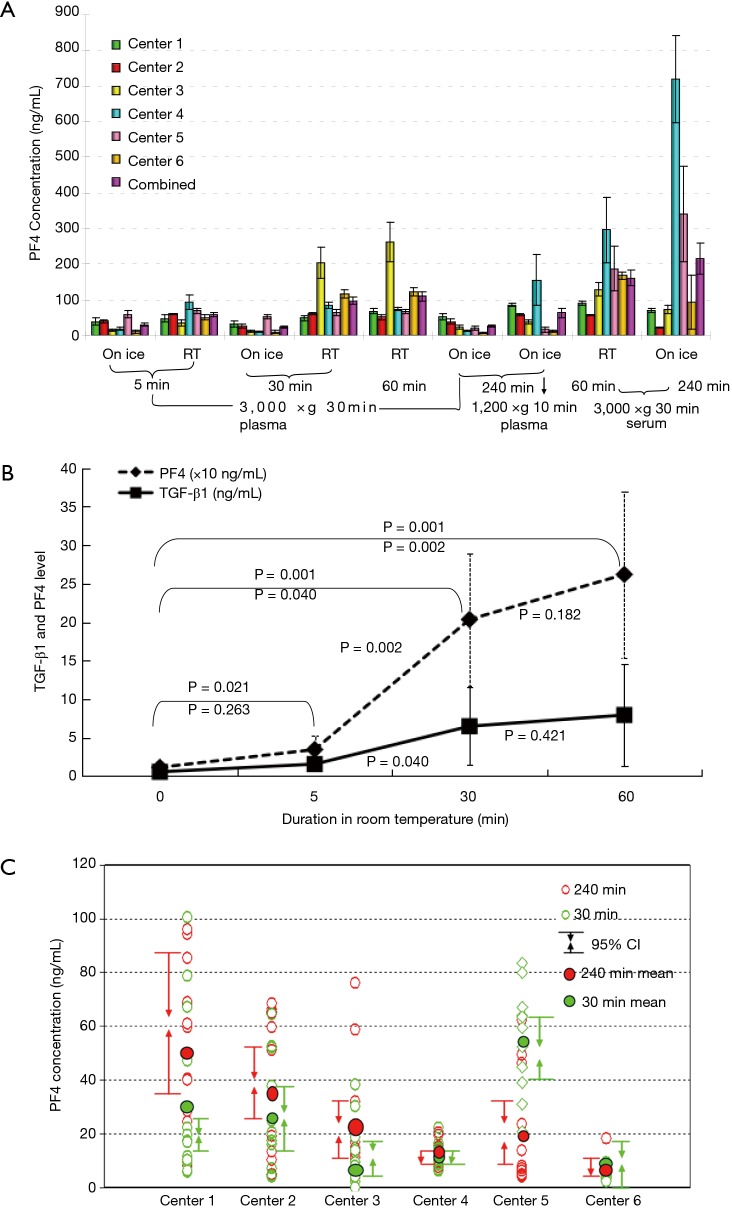
Table 1 shows the results for PF4 levels measured in plasma and serum samples prepared using nine different methods. PF4 levels did not differ significantly in samples set on ice for 5 or 30 min and then centrifuged of 2,500–3,000 ×g for 30 min. However, in samples that sat on ice for 240 min, artificial increases in the levels of PF4 and TGF-β1 were seen in samples from some centers, but there were no significant differences in the levels of the other cytokines among samples from all centers (Figure 3A). In blood samples that sat at room temperature, the levels of TGF-β1 and PF4 increased significantly, even after only 5 min (Figure 3B). Inadequate centrifugation (1,200 ×g for 15 min) and sitting on ice at 240 min also led to a significant increase in the plasma PF4 level. Interestingly, in samples from one of the centers, the level of PF4 was significantly greater than levels in samples from other centers (Figure 3C).
Table 1. Levels of PF4 and TGF-β1 under various blood handling process.
| Samples | Temperature | Setting time (min) | RCF and duration | TGF-β1* | 95% CI for mean of TGF-β1 | PF4* | 95% CI for mean of PF4 | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | Lower bound | Upper bound | ||||||
| Plasma | On ice | 5 | 2,500 ×g for 30 min | 0.6±0.2 | 0.5 | 0.6 | 14.8±13.3 | 7.6 | 22.1 |
| On ice | 30 | 2,500 ×g for 30 min | 0.5±0.2 | 0.5 | 0.6 | 11.5±11.3 | 5.4 | 17.6 | |
| On ice | 180 | 1,200 ×g for 10 min | 0.9±0.4 | 0.7 | 1.1 | 39.2±21.1 | 27.8 | 50.7 | |
| On ice | 180 | Re-centrifuge at 10,000 ×g for 15 min | 0.6±0.2 | 0.5 | 0.7 | 25.3±22.3 | 13.1 | 37.4 | |
| On ice | 240 | 2,500 ×g for 30 min | 0.7±0.3 | 0.5 | 0.9 | 23.1±21.6 | 11.4 | 34.9 | |
| RT | 5 | 2,500 ×g for 30 min | 1.0±1.4 | 0.3 | 1.8 | 34.6±34.7 | 15.8 | 53.5 | |
| RT | 30 | 2,500 ×g for 30 min | 2.2±2.8 | 0.7 | 3.8 | 203.5±158.4 | 117.4 | 289.6 | |
| RT | 60 | 2,500 ×g for 30 min | 2.8±2.1 | 1.6 | 3.9 | 262.0±198.9 | 153.9 | 370.1 | |
| Serum | One ice | 240 | 2,500 ×g for 30 min | 1.3±1.0 | 0.7 | 1.8 | 71.8±43.2 | 48.4 | 95.3 |
| RT | 60 | 2,500 ×g for 30 min | 19.0±2.5 | 17.7 | 20.4 | 129.6±69.2 | 92.0 | 167.2 | |
*, mean ± standard deviation (ng/mL). PF4, platelet factor 4; RT, room temperature; RCF, relative centrifugal field; TGF-β1, transforming growth factor-beta.
Figure 3.
PF4 levels in samples prepared using different procedures. (A) PF4 levels in samples prepared according to the different methods from each center; (B) changes in PF4 levels over time as plasma samples were kept at room temperature; (C) PF4 levels measured after 30 min and 4 hours in individual samples from each center.
Cytokine levels in platelet-poor plasma samples
The levels of 29 cytokines were measured in 38 samples with lowest PF4 concentration, i.e., platelet-poor samples. The results are summarized in Table 2.
Table 2. Cytokine levels in platelet-poor samples.
| Cytokine | n | Mean | Std. error | 95% CI for mean | P value | Minimum | Maximum | |
|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||||
| EGF | 38 | 289.2 | 49.0 | 189.9 | 388.5 | 0.7 | 101.3 | 1,627.3 |
| Eotaxin | 38 | 54.8 | 10.3 | 34.0 | 75.6 | 0.6 | 2.8 | 200.5 |
| Fractal | 38 | 109.9 | 14.2 | 81.2 | 138.6 | 0.3 | 20.1 | 437.6 |
| G-CSF | 38 | 611.2 | 221.9 | 161.1 | 1,061.3 | 0.9 | 3.0 | 5,756.9 |
| GM-CSF | 38 | 39.8 | 11.0 | 17.6 | 62.1 | 0.3 | 2.0 | 413.2 |
| IFN-γ | 38 | 865.2 | 134.4 | 592.8 | 1,137.6 | 0.7 | 3.0 | 2,749.2 |
| IL-10 | 38 | 69.8 | 14.7 | 40.0 | 99.6 | 0.2 | 1.6 | 351.2 |
| IL-12p40 | 38 | 24.9 | 10.6 | 3.4 | 46.4 | 0.5 | 5.0 | 349.5 |
| IL-12p70 | 38 | 366.5 | 177.8 | 6.2 | 726.7 | 0.4 | 3.0 | 6,599.6 |
| IL-13 | 38 | 93.6 | 37.4 | 17.8 | 169.4 | 0.4 | 1.4 | 1,013.7 |
| IL-15 | 38 | 48.3 | 21.8 | 4.1 | 92.4 | 0.3 | 2.9 | 769.4 |
| IL-17 | 38 | 12.9 | 6.3 | 0.2 | 25.6 | 0.6 | 0.2 | 232.1 |
| IL-1α | 38 | 33.7 | 9.1 | 15.3 | 52.0 | 0.3 | 1.6 | 222.8 |
| IL-1β | 38 | 399.9 | 98.5 | 200.4 | 599.5 | 0.6 | 3.0 | 2,975.8 |
| IL-1Rα | 38 | 12.4 | 5.0 | 2.3 | 22.5 | 0.7 | 0.4 | 156.3 |
| IL-2 | 38 | 31.1 | 16.9 | -3.1 | 65.4 | 0.6 | 0.8 | 639.5 |
| IL-4 | 38 | 17.0 | 5.2 | 6.4 | 27.6 | 0.5 | 0.4 | 150.9 |
| IL-5 | 38 | 30.9 | 16.2 | -1.9 | 63.7 | 0.2 | 0.1 | 457.9 |
| IL-6 | 38 | 4.3 | 1.4 | 1.5 | 7.1 | 0.7 | 1.8 | 48.5 |
| IL-7 | 38 | 80.2 | 20.7 | 38.2 | 122.2 | 0.8 | 0.8 | 520.5 |
| IL-8 | 38 | 15.9 | 4.0 | 7.8 | 23.9 | 0.4 | 3.0 | 92.6 |
| IP-10 | 38 | 16.4 | 4.1 | 8.1 | 24.7 | 0.2 | 0.5 | 116.0 |
| MCP-1 | 38 | 1,021.3 | 72.7 | 873.9 | 1,168.6 | 0.5 | 267.9 | 2,122.5 |
| MCP-1α | 38 | 220.9 | 12.3 | 195.9 | 245.8 | 0.6 | 81.8 | 393.6 |
| MCP-1β | 38 | 193.3 | 29.9 | 132.7 | 253.9 | 0.2 | 3.0 | 695.3 |
| sCD40L | 38 | 126.9 | 20.5 | 85.3 | 168.4 | 0.4 | 16.5 | 530.8 |
| TGF-α | 38 | 15.8 | 3.8 | 8.2 | 23.5 | 0.1 | 3.0 | 72.9 |
| TNF-α | 38 | 8.0 | 2.1 | 3.7 | 12.3 | 0.6 | 1.0 | 59.1 |
| VEGF | 38 | 260.6 | 63.4 | 132.3 | 389.0 | 0.5 | 3.0 | 1,724.1 |
EGF, epidermal growth factor; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; MCP, monocyte chemoattractant protein; TGF, transforming growth factor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Sample preparation and proteomic profiles
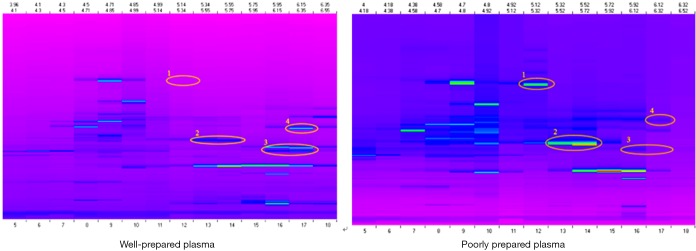
It is known that there are remarkable differences between serum and plasma proteomes [17–21]. In the present study, poorly prepared plasma samples (as described above with blood samples either sitting at room temperature or inadequately centrifuged) had a different protein profile from that of good plasma samples (Figure 4). Thus, the results of our proteomic analysis indicated that proteomic analyses are also confounded by “malpreparation” of plasma samples.
Figure 4.
Proteomic profiles of well and poorly prepared plasma samples from the same subject. Well-prepared samples were prepared using the optimal method described above (30 min on ice), and poorly prepared samples were allowed to sit at room temperature for 60 min before blood samples were spun down.
Discussion
This multicenter study including centers from China and United States demonstrated that: (I) the PF4 levels was highly correlated with the TGF-β1 levels in both serum and plasma; (II) serum levels of PF4 and TGF-β1 were approximately 10-fold greater than those in plasma; (III) the level of PF4 in samples remained lower, and no significant difference was seen after 30 min kept on ice, and (IV) many conditions increased the levels of PF4 and TGF-β1, with the variation resulting from various blood processing methods reached over 10 times of the original level (from 1.9 to 20.0 ng/mL). The procedures and steps shown to increase PF4 and TGF-β1 levels included: (I) delayed blood processing with blood samples left at room temperature (1.4-fold increase after 30 min, and 4- to 10-fold increase after 4 h); (II) extended delay in blood processing when samples were kept on ice for 4 h; and (III) inappropriate centrifugal force and duration of centrifugation.
Our results showing a significant correlation between PF4 and TGF-β1 levels are consistent with previous findings (17,18). This confirmed that the source of elevated TGF-β1 was platelet degradation or contamination. PF4 can thus be used as a reliable surrogate marker for platelet contamination/degradation. This is particularly important as kits for PF4 measurement are much less expensive than that of TGF-β1, and the testing procedure for PF4 is simpler.
It is important to note that serum samples had significantly higher levels of PF4 and TGF-β1 than plasma samples. The reason for this may be simple, as the blood clotting process alters the physiological condition, and TGF-β1 and other cytokines may be released from platelets and other blood cells. Nevertheless, it is important to avoid the use of serum for measurement of any biomarkers that may be contained in platelets, such as TGF-β1 and various other cytokines.
The significance of the temperature at which blood samples are kept before plasma preparations as well as the time, even on ice, is extremely important for clinical trials, and the majority of blood biomarker studies have not commented on this important matter and may have obtained misleading results. For example, among studies on the ability of TGF-β1 levels to predict radiation lung toxicity (4,5,8,23,24), two reporting negative results used insufficient gravitational force during the plasma preparation (8,24). These results validated our previous finding from one single Institute that blood sample handing process has significantly impact on the measure level of TGF-β1 in blood (11). This is also in agreement with findings from. Findeisen et al. (25) and Qundos et al. (26) In the former study, they found that the concentration of proteolytic fragments changed in a time-dependent manner. And Qundos’s investigation found that temperature the samples were kept after collection and time intervals between sample collection and centrifugation can significantly influence the proteins profiles. However, Mateos et al. (27) reported that time delay for the first centrifugation of the original blood sample (4 or 24 h) had no significant impact on the distribution of proteins in all samples. But it should be noted that 4 hour’s delayed maybe is enough to cause a complete change of the protein distribution and any time longer has no more effect on it. Of another important note, our results also showed that insufficient centrifugation also increased PF4 levels, suggesting possibilities of platelet contamination.
A variety of pre-analytic factors may affect biospecimen quality and thus biomarker results (28-30). This study was limited in that only sample selection, collection, and processing were studied, without consideration of variations in the physiological conditions of the participants, biospecimen delivery, freezing, storage, and thawing. Systematic studies in Biospecimen Science have been sponsored and conducted by programs such as the US National Cancer Institute’s Biospecimen Research Network [https://biospecimens.cancer.gov/default.asp], and the Standardization and improvement of generic Pre-analytical tools and procedures for In-vitro DIAgnostics project (SPIDIA) [http://www.spidia.eu], a consortium that was funded by the European Union and coordinated by QIAGEN in Germany. We advise researchers particularly cooperative group for clinical trials to follow the best practice proposed by NCI (NCI Best practice for biospecimens https://biospecimens.cancer.gov/bestpractices/2016-NCIBestPractices.pdf), to develop evidence-based practices to guide biospecimen methodology to minimize confounding effect of pre-analytic factors, and mitigate pre-analytic effects when collecting and utilizing stored biospecimens.
In summary, the present study validated an optimal plasma processing method and demonstrated the challenges and feasibility of a multicenter biomarker study. Factors to be considered include collecting blood and preparing plasma samples onsite, delivering specimens for analysis, and having standard assays performed in designated centers for reproducible results. The optimal method should be used for plasma sample preparation. Specifically, to generate platelet-poor plasma for biomarker testing, blood samples should always be stored on ice before centrifugation and should never be left at room temperature. Blood samples should be spun at least 2,500–3,000 ×g for 30 min for plasma preparation. Due to confounding effect of platelets, serum samples are not recommended for testing circulating biomarkers unless it is indicated for a special reason.
Acknowledgements
This study was supported in parts by RTOG translational research program and NIH/NCI R01 CA142840 (FM Kong).
Ethical Statement: All centers received approval from their respective Institutional Review Boards prior to subject enrollment. All subjects signed a study-specific, written, informed consent form.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Greco V, Piras C, Pieroni L, et al. Direct Assessment of Plasma/Serum Sample Quality for Proteomics Biomarker Investigation. Methods Mol Biol 2017;1619:3-21. 10.1007/978-1-4939-7057-5_1 [DOI] [PubMed] [Google Scholar]
- 2.Kong FM, Anscher MS, Jirtle RL. Transforming Growth Factor Beta: A Plasma Tumor Marker In: Hanausek M, Walaszek Z. editors. Methods in Molecular Biology: Tumor Marker Protocols. New Jersey: Human Press Inc., 1997:417-30. [Google Scholar]
- 3.Anscher MS, Kong FM, Marks LB, et al. Changes in plasma transforming growth factor beta during radiotherapy and the risk of symptomatic radiation-induced pneumonitis. Int J Radiat Oncol Biol Phys 1997;37:253-8. 10.1016/S0360-3016(96)00529-9 [DOI] [PubMed] [Google Scholar]
- 4.Anscher MS, Kong FM, Andrews K, et al. Plasma transforming growth factor beta1 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys 1998;41:1029-35. 10.1016/S0360-3016(98)00154-0 [DOI] [PubMed] [Google Scholar]
- 5.Fu XL, Huang H, Bentel G, et al. Predicting the risk of symptomatic radiation-induced lung injury using both the physical and biologic parameters V(30) and transforming growth factor beta. Int J Radiat Oncol Biol Phys 2001;50:899-908. 10.1016/S0360-3016(01)01524-3 [DOI] [PubMed] [Google Scholar]
- 6.Rabbani ZN, Anscher MS, Zhang X, et al. Soluble TGFbeta type II receptor gene therapy ameliorates acute radiation-induced pulmonary injury in rats. Int J Radiat Oncol Biol Phys 2003;57:563-72. 10.1016/S0360-3016(03)00639-4 [DOI] [PubMed] [Google Scholar]
- 7.Nishioka A, Ogawa Y, Mima T, et al. Histopathologic amelioration of fibroproliferative change in rat irradiated lung using soluble transforming growth factor-beta (TGF-beta) receptor mediated by adenoviral vector. Int J Radiat Oncol Biol Phys 2004;58:1235-41. 10.1016/j.ijrobp.2003.11.006 [DOI] [PubMed] [Google Scholar]
- 8.De Jaeger K, Seppenwoolde Y, Kampinga HH, et al. Significance of plasma transforming growth factor-beta levels in radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004;58:1378-87. 10.1016/j.ijrobp.2003.09.078 [DOI] [PubMed] [Google Scholar]
- 9.Anscher MS, Kong FM. In regard to De Jaeger et al.: significance of plasma transforming growth factor-beta levels in radiotherapy for non-small-cell lung cancer (Int J Radiat Oncol Biol Phys 2004;58:1378-1387). Int J Radiat Oncol Biol Phys 2005;61:1276-7. 10.1016/j.ijrobp.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 10.De Jaeger K, Seppenwoolde Y, Lebesque JV, et al. In response to Drs. Anscher and Kong. Int J Radiat Oncol Biol Phys 2005;63:308. 10.1016/j.ijrobp.2005.04.054 [DOI] [PubMed] [Google Scholar]
- 11.Zhao L, Wang L, Ji W, et al. The influence of the blood handling process on the measurement of circulating TGF-beta1. Eur Cytokine Netw 2012;23:1-6. [DOI] [PubMed] [Google Scholar]
- 12.Grainger DJ, Mosedale DE, Metcalfe JC. TGF-beta in blood: a complex problem. Cytokine Growth Factor Rev 2000;11:133-45. 10.1016/S1359-6101(99)00037-4 [DOI] [PubMed] [Google Scholar]
- 13.Assoian RK, Komoriya A, Meyers CA, et al. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem 1983;258:7155-60. [PubMed] [Google Scholar]
- 14.Alessandro R, Fontana S, Kohn E, et al. Proteomic strategies and their application in cancer research. Tumori 2005;91:447-55. [DOI] [PubMed] [Google Scholar]
- 15.Banks RE, Stanley AJ, Cairns DA, et al. Influences of blood sample processing on low-molecular-weight proteome identified by surface-enhanced laser desorption/ionization mass spectrometry. Clin Chem 2005;51:1637-49. 10.1373/clinchem.2005.051417 [DOI] [PubMed] [Google Scholar]
- 16.Tammen H, Schulte I, Hess R, et al. Peptidomic analysis of human blood specimens: comparison between plasma specimens and serum by differential peptide display. Proteomics 2005;5:3414-22. 10.1002/pmic.200401219 [DOI] [PubMed] [Google Scholar]
- 17.Wakefield LM, Letterio JJ, Chen T, et al. Transforming growth factor-beta1 circulates in normal human plasma and is unchanged in advanced metastatic breast cancer. Clin Cancer Res 1995;1:129-36. [PubMed] [Google Scholar]
- 18.Jeon JH, Kim YS, Choi EJ, et al. Implication of co-measured platelet factor 4 in the reliability of the results of the plasma transforming growth factor-beta 1 measurement. Cytokine 2001;16:102-5. 10.1006/cyto.2001.0895 [DOI] [PubMed] [Google Scholar]
- 19.Ao X, Lubman DM, Davis MA, et al. Comparative proteomic analysis of radiation-induced changes in mouse lung: fibrosis-sensitive and -resistant strains. Radiat Res 2008;169:417-25. 10.1667/RR1173.1 [DOI] [PubMed] [Google Scholar]
- 20.Cai XW, Shedden K, Ao X, et al. Plasma proteomic analysis may identify new markers for radiation-induced lung toxicity in patients with non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2010;77:867-76. 10.1016/j.ijrobp.2010.01.038 [DOI] [PubMed] [Google Scholar]
- 21.Wang S, Campbell J, Stenmark MH, et al. Plasma Levels of IL-8 and TGF-beta1 Predict Radiation-Induced Lung Toxicity in Non-Small Cell Lung Cancer: A Validation Study. Int J Radiat Oncol Biol Phys 2017;98:615-21. 10.1016/j.ijrobp.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong FM, Wang S. Nondosimetric risk factors for radiation-induced lung toxicity. Semin Radiat Oncol 2015;25:100-9. 10.1016/j.semradonc.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao L, Wang L, Ji W, et al. Elevation of plasma TGF-beta1 during radiation therapy predicts radiation-induced lung toxicity in patients with non-small-cell lung cancer: a combined analysis from Beijing and Michigan. Int J Radiat Oncol Biol Phys 2009;74:1385-90. 10.1016/j.ijrobp.2008.10.065 [DOI] [PubMed] [Google Scholar]
- 24.Novakova-Jiresova A, Van Gameren MM, Coppes RP, et al. Transforming growth factor-beta plasma dynamics and post-irradiation lung injury in lung cancer patients. Radiother Oncol 2004;71:183-9. 10.1016/j.radonc.2004.01.019 [DOI] [PubMed] [Google Scholar]
- 25.Findeisen P, Thumfart JO, Costina V, et al. MS-based monitoring of proteolytic decay of synthetic reporter peptides for quality control of plasma and serum specimens. Am J Clin Pathol 2013;140:314-23. 10.1309/AJCPOS9Z5KVZSFSC [DOI] [PubMed] [Google Scholar]
- 26.Qundos U, Hong MG, Tybring G, et al. Profiling post-centrifugation delay of serum and plasma with antibody bead arrays. J Proteomics 2013;95:46-54. 10.1016/j.jprot.2013.04.020 [DOI] [PubMed] [Google Scholar]
- 27.Mateos J, Carneiro I, Corrales F, et al. Multicentric study of the effect of pre-analytical variables in the quality of plasma samples stored in biobanks using different complementary proteomic methods. J Proteomics 2017;150:109-20. 10.1016/j.jprot.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 28.Tuck MK, Chan DW, Chia D, et al. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res 2009;8:113-7. 10.1021/pr800545q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rai AJ, Vitzthum F. Effects of preanalytical variables on peptide and protein measurements in human serum and plasma: implications for clinical proteomics. Expert Rev Proteomics 2006;3:409-26. 10.1586/14789450.3.4.409 [DOI] [PubMed] [Google Scholar]
- 30.Shabihkhani M, Lucey GM, Wei B, et al. The procurement, storage, and quality assurance of frozen blood and tissue biospecimens in pathology, biorepository, and biobank settings. Clin Biochem 2014;47:258-66. 10.1016/j.clinbiochem.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]