Abstract
Background
Mitochondrial damage-associated molecular patterns (mtDAMPs), such as mitochondrial DNA and N-formylated peptides, are endogenous molecules released from tissue after traumatic injury. mtDAMPs are potent activators of the innate immune system. They have similarities with bacteria, which allow mtDAMPs to interact with the same pattern recognition receptors and mediate the development of Systemic Inflammatory Response Syndrome (SIRS). Current recommendations for management of an open abdomen include returning to the operating room every 48 hours for peritoneal cavity lavage until definitive procedure. These patients are often critically ill and develop SIRS. We hypothesized that mitochondrial DAMPs are present in the peritoneal cavity fluid in this setting, and that they accumulate in the interval between washouts.
Methods
We conducted a prospective pilot study of critically ill adult patients undergoing open abdomen management in the Surgical and Trauma ICUs. Peritoneal fluid was collected daily from 10 open abdomen patients. Specimens were analyzed via qPCR for mitochondrial DNA (mtDNA), via enzyme immunoassay for DNAse activity and via Western blot analysis for the ND6 subunit of the NADH: ubiquinone oxidoreductase, an N-formylated peptide.
Results
We observed a reduction in the expression of ND6 the day following lavage of the peritoneal cavity, that was statistically different from the days with no lavage (% change in ND6 expression, Post-Op from washout: −50±11 vs. No Washout day: 42±9, p<0.05). Contrary to expectation, the mtDNA levels remained relatively constant from sample to sample. We then hypothesized that DNAse present in the effluent may be degrading mtDNA.
Conclusions
These results indicate the peritoneal cavity irrigation reduces the presence of mitochondrial DAMPs in the open abdomen. It is possible that, increased frequency of peritoneal cavity lavage may lead to decreased systemic absorption of mtDAMPs, and thereby reducing the risk of SIRS.
Level of Evidence
Prospective study, Case Series, level V
Study Type
Prognostic
Keywords: trauma, open abdomen, mtDAMPs, sepsis
Background
Despite advances in critical care, patients with multi-system trauma and abdominal catastrophes continue to have substantial rates of morbidity and mortality (1). Patients with abdominal trauma or peritonitis who require damage control laparotomy and are unable to be closed immediately undergo open abdomen management with temporary abdominal closure (2). These patients often develop sepsis and multi-system organ dysfunction (MSOD). The current guidelines for open abdomen management are based on level II and level III evidence, and call for serial abdominal re-exploration with irrigation of the peritoneal cavity every 48-hours until the abdominal fascia is approximated (3). Serial re-explorations and irrigations may be postponed due to the patient’s clinical status.
Recent studies have implicated mitochondrial damage associated molecular patterns (mtDAMPs) as an inciting factor for microorganism-free sepsis (MFS) in critically ill patients (4–6). These mtDAMPs, such as mitochondrial DNA (mtDNA) and N-formylated peptides, are endogenous molecules released from damaged mitochondria after tissue injury caused by trauma, ischemia, hypoxia, etc. Mitochondrial DAMPs share biochemical similarities with bacterial pathogen associated molecular patterns (PAMPs) such as bacterial DNA and N-formylated peptides. For instance, mitochondria contain unmethylated CpG repeats which resemble those of bacterial DNA. MtDNA and mitochondrial cellular derivatives’ similarities with PAMPs can be explained by the Endosymbiotic Theory (7, 8).
In the same way that microbial PAMPs elicit a response from the innate immune system by interacting with pattern recognition receptors (PRRs), mitochondrial DAMPs also express immune-stimulatory activity by binding to PRRs. PRRs are found on various immune cells (e.g. dendritic cells, neutrophils, mast cells) and include Toll-like receptors and Formyl-peptide receptors (FPR) among others. Toll-like receptor 9 (TLR9) binding by a mtDAMP or PAMP activates cell signaling pathways leading to the transcription of inflammatory cytokines. Similarly, FPR-1 activation leads to a neutrophil-mediated inflammatory reaction that can result in organ injury (5, 7).
Zhao et al. introduced mtDAMPs derived from rat liver into the peritoneum of Sprague-Dawley rats and noticed an influx of neutrophils into the peritoneal cavity as a response (9). These researchers also saw that the inflammatory reaction elicited by sterile mtDAMPs was similar to that elicited by the cecal ligation and puncture model of intraabdominal sepsis. Iyer et al. also injected mitochondrial fractions intraperitoneally into mice and induced an inflammatory reaction (10). Furthermore, the researchers inhibited the Nlrp3 inflammasome, a receptor of the innate immune system acted on by mtDAMPs, which mediates pro-inflammatory cytokine release. The inhibition of Nlrp3 inflammasome decreased neutrophil influx, renal dysfunction and acute inflammation, thereby protected the mice against mortality. Based on this evidence we hypothesized that mtDAMPs are present in the peritoneal cavity fluid of open abdomen patients, and that their concentration accumulates during the interval between serial re-exploration and irrigation.
Methods
Study Design
We conducted a prospective observational pilot study approved by the Institutional Review Board at the Medical College of Georgia-Augusta University Medical Center (MCG-AUMC). Ten consecutive critically ill adult patients who were admitted to Shock Trauma Intensive Care Unit or Surgical Intensive Care Unit at MCG-AUMC undergoing open abdomen management were enrolled from October 2015 to February 2016. Patients less than 18 years of age and pregnant females were excluded, as were patients with known autoimmune disease, malignancy, and immunodeficiency disorders. At the time of specimen analysis one patient was excluded from the study due to insufficient number of specimens for analysis.
Specimen Acquisition and Analysis
Peritoneal fluid samples were collected daily from enrolled patients for 7 days or until their fascia was approximated. A fresh aliquot of 5ml of peritoneal fluid was collected every morning from the negative pressure wound closure system. Specimen was obtained under waiver of consent for discarded materials. The specimen was placed into a BD Vacutainer CPT tube with sodium citrate (Becton, Dickinson & Company, NJ). Samples were centrifuged within one hour of collection at 1500RCF for 15 minutes at 21 °C, the supernatant was then decanted into at least 2 collection vials. The samples were then stored at −80°C until analyzed. Due to the observational nature of this pilot study, no other patient clinical variables were recorded other than noting if the sample was obtained on an operative day (i.e. morning of abdominal irrigation/washout) or a non-operative day (i.e. no abdominal irrigation/washout).
Peritoneal fluid samples were first diluted 1:10 and then 25 µl of the diluted sample was mixed with 25 µl of reducing agent dithiothreitol (DTT). This 50 µl mixture was then loaded into sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (12%) for Western blotting. Polyvinylidene difluoride membranes were probed for the expression of the NH2-terminus of mitochondria NADPH dehydrogenase subunit 6 (ND6), which is structurally different from bacterial formylated peptides, to prove that mitochondrial DAMPs are present in peritoneal fluid and no bacterial contamination was present in our sample analysis. ND6 contains N-formyl methyl-methionine residues and is a chemoattractant to neutrophils (11). The specimen analysis process has previously been explained in detail by our group (12). Densitometric analysis was performed by Un-Scan-It software (Version 6.1) (Silk Scientific, Orem, UT, USA). DNA from peritoneal samples was isolated using the QIAamp DNA Blood Mini Kit (Qiagen, Germantown, MD, USA). Isolated DNA was amplified and quantified using real time (RT)-PCR (MyiQ Single-Color Real-Time PCR Detection System). The primers (Invitrogen, Grand Island, NY, USA) that were used to amplify mtDNA were cytochrome B (Cyt B) (fwd 5’-ATGACCCCAATACGCAAAAT-3’ and rev 5’-CGAAGTTTCATCATGCGGAG-3’) and ND6 (fwd 5’- CCAAGACCTCAACCCCTGAC-3’ and rev 5’- ATGGGGTTTGTGGGGTTTTCT-3’). Bacterial 16S ribosomal RNA (forward 5’-CGTCAGCTCGTGTTGTGAAA-3’ and reverse 5’-GGCAGTCTCCTTGAGTTCC-3’) was used to control for bacterial contamination of the samples. Primer sequences have no significant homology with DNA found in any bacterial species published on Basic Local Alignment Search Tool (BLAST) (13). RT-PCR results are presented as the inverse of cycle threshold (CT) for gene amplification.
All study data analyses were performed using data analysis software GraphPad Prism 5.0. Statistical significance was accepted at a p <0.05.
Results
Mitochondrial ND6 decreases after abdominal irrigations
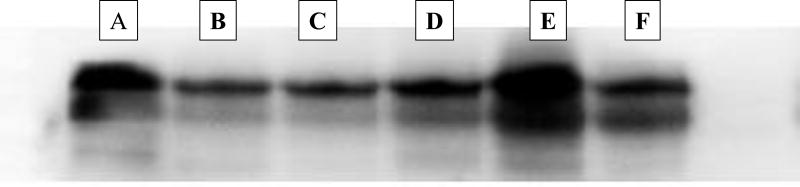
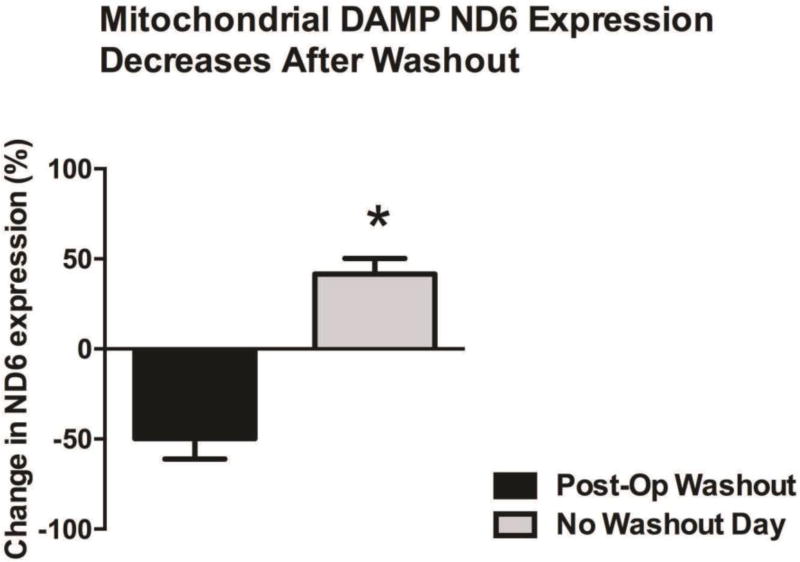
Mitochondrial ND6 was detected in all peritoneal fluid samples (Fig. 1). On the densitometric analysis of ND6 expression in the peritoneal fluid samples, we observed a reduction in the expression of ND6 on the days following abdominal cavity re-exploration and irrigation. The reduction observed, measured as percent change in ND6 expression, was statistically significant (Fig. 2) with a 50% reduction after abdominal cavity irrigation was performed. These results indicate that peritoneal cavity lavage (i.e. abdominal cavity irrigation) reduces the presence of mitochondrial DAMPs in the open abdomen.
Figure 1.
Western Blotting of ND6 subunit of the NADH: ubiquinone oxidoreductase (complex I of the mitochondrial respiratory chain). Representative immunoblotting from one of our patients. A, Day 1: abdominal catastrophe. B, Day 2: preop sample re-exploration and irrigation. C, Day 3: post-op day (POD) 1 from irrigation. D, Day 4: POD 2 from irrigation. E, Day 5: preop sample re-exploration and irrigation. F, Day 6: pre-op sample, fascial closure day.
Figure 2. Mitochondrial DAMP ND6 Expression.
We observed a reduction in the expression of ND6 the day following lavage of the peritoneal cavity, that was statistically different from the days with no lavage (% change in ND6 expression, Post-Op from washout: −50±11 vs. No Washout day: 42±9, p<0.05).
Mitochondrial DNA
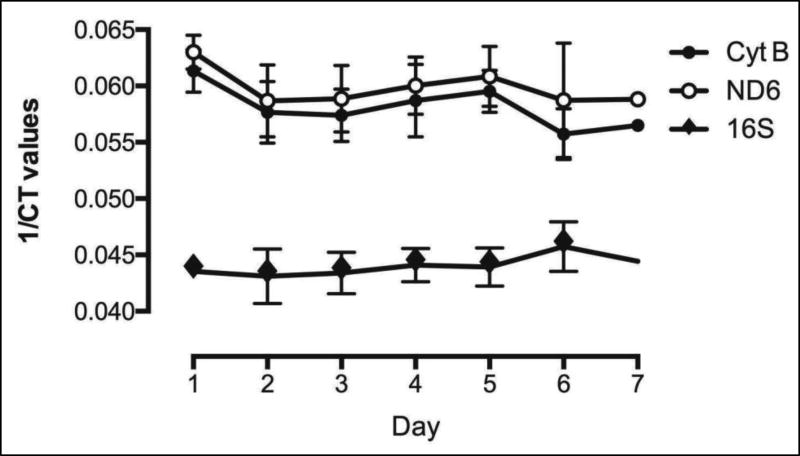
Upon PCR amplification of the peritoneal fluid samples mitochondrial DNA was detected in all peritoneal fluid samples and this was significantly elevated compared to bacterial 16S ribosomal RNA across all days of sample collection (p<0.05) (Fig. 3). However, we did not observe a change in expression of any representative mitochondrial DNA gene on the days of abdominal irrigation when compared to the days of no abdominal irrigation. The levels of mitochondrial DNA remained constant across all samples.
Figure 3. mtDNA levels in peritoneal fluid.
rtPCR amplification of the peritoneal fluid samples. mtDNA was detected in all peritoneal fluid samples and this was significantly elevated compared to bacterial 16S ribosomal RNA across all days of sample collection.
Discussion
This observational study demonstrated the presence of mitochondrial DAMPs in the peritoneal fluid of open abdomen patients. We were able to show a decrease in ND6 concentration following abdominal cavity irrigation, supporting our hypothesis. Contrary to our expectations the level of mitochondrial DNA remained relatively constant throughout our samples. We are uncertain of the cause of this observation, but we suspect that the cleavage rate of mtDNA by mitochondrial DNase may be high enough that the result of creation versus destruction of mitochondrial DNA is balanced, resulting in near constant levels. In a recent study, Simmons et al. (14) administered intratracheal DNase to isolated rat lungs before or after intratracheal challenge with Pseudomonas aeruginosa strain 103 (PA103). The animals treated with DNase were protected from increases in mtDAMPs and the pulmonary endothelial dysfunction evoked after initial challenge with PA103 was reversed.
The mitochondrial DAMP ND6 results we obtained raise the question whether increased frequency of peritoneal cavity lavage may lead to decreased systemic absorption of mtDAMPs, which could reduce the risk of sterile systemic-inflammatory response syndrome (SIRS) or sepsis-like symptoms in open abdomen patients. Additionally, it may be possible to add antagonists of mtDAMPs activity, such as formyl peptide receptor antagonists and mtDNase, directly to the irrigation fluid thereby reducing their inflammatory action.
Though we analyzed our samples through immunoblotting, unlike other studies we reference, such as quantitative real-time PCR (4), this method has been successfully employed by our lab and others (11, 15). Our predictions parallel those of Simmons et al. (4) and Nkahira et al. (16) where a reduction in mtDAMPs is associated in time with clinical improvement (3) and increased mtDNA levels are associated with increased ICU mortality (11). Moreover, a pilot study involving orthopedic trauma patients demonstrated a correlation between preoperative mtDNA levels and time from injury to surgery indicating that mtDNA could serve as a marker for optimal surgical timing (17). This study also showed a correlation between the timing and the magnitude of surgical intervention with mtDNA concentration, presenting the possibility of mtDNA as a risk indicator for development of post-injury complications and the need for secondary interventions. The aforementioned studies, along with ours, suggest the possibility that mtDAMPs could be used as a biomarker related to clinical outcomes in critically ill surgical patients. We are particularly interested in whether it can serve as a risk indicator for the development of SIRS and MSOD, specifically in the setting of the open abdomen.
In conclusion, our pilot study demonstrates a decrease in mtDAMPs concentration, specifically ND6, with abdominal irrigation. Further work is required to verify the findings we present and determine the clinical significance of mtDAMPs in the peritoneal cavity fluid. This feasibility study served as justification for a larger randomized study, currently under way, to expand upon this initial work and elucidate on the questions raised by our results.
Limitations
Due to the nature of this observational pilot study no patient clinical data variables were collected, obviating the possibility of establishing any relationship between mtDAMPs in peritoneal fluid and the patient’s clinical status. We viewed this as a pilot study which served to demonstrate the feasibility of carrying out a larger and more definitive prospective randomized study.
Acknowledgments
Disclosures of funding: No funding was received for this work.
Footnotes
Conflicts of Interest: No conflicts of interest are declared by the authors involved in this study.
Meetings associated with manuscript: 47th Annual Meeting of Western Trauma Association, March-05-10-2017
Author Contributions
PMQ: specimen collection, literature search, writing; CMc: study design, data collection, data analysis, data interpretation, writing; CJM: study design, literature review, critical review of manuscript; CFW: data collection, data analysis, data interpretation; SBH: review of manuscript; RCW: study design, data interpretation, critical review of manuscript; KOM: study design, specimen collection, data interpretation, writing, critical review of manuscript
Contributor Information
Patricia Angellice Martinez-Quinones, Medical College of Georgia at Augusta University, Department of Surgery.
Cameron Grant McCarthy, Medical College of Georgia at Augusta University, Department of Physiology.
Caleb J Mentzer, Division of Trauma and Surgical Critical Care, Miller School of Medicine, University of Miami.
Camilla Ferreira Wenceslau, Medical College of Georgia at Augusta University, Department of Physiology.
Steven Barry Holsten, Medical College of Georgia at Augusta University, Department of Surgery.
R Clinton Webb, Medical College of Georgia at Augusta University, Department of Physiology; American Heart Association; NIH.
Keith O’Malley, Medical College of Georgia at Augusta University, Department of Surgery.
References
- 1.Cristaudo AT, Jennings SB, Hitos K, Gunnarsson R, DeCosta A. Treatments and other prognostic factors in the management of the open abdomen: A systematic review. J Trauma Acute Care Surg. 2017 Feb;82(2):407–418. doi: 10.1097/TA.0000000000001314. [DOI] [PubMed] [Google Scholar]
- 2.Diaz JJ, Jr, Dutton WD, Ott MM, Cullinane DC, Alouidor R, Armen SB, Bilanuik JW, Collier BR, Gunter OL, Jawa R, et al. Eastern Association for the Surgery of Trauma: a review of the management of the open abdomen--part 2 "Management of the open abdomen". J Trauma. 2011;71(2):502–12. doi: 10.1097/TA.0b013e318227220c. [DOI] [PubMed] [Google Scholar]
- 3.Diaz JJ, Jr, Cullinane DC, Dutton WD, Jerome R, Bagdonas R, Bilaniuk JW, Collier BR, Como JJ, Cumming J, Griffen M, et al. The management of the open abdomen in trauma and emergency general surgery: part 1-damage control. J Trauma. 2010;68(6):1425–38. doi: 10.1097/TA.0b013e3181da0da5. [DOI] [PubMed] [Google Scholar]
- 4.Simmons JD, Lee YL, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, Gillespie MN, Richards WO, et al. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg. 2013;258(4):591–6. doi: 10.1097/SLA.0b013e3182a4ea46. discussion 6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wenceslau CF, McCarthy CG, Goulopoulou S, Szasz T, NeSmith EG, Webb RC. Mitochondrial-derived N-formyl peptides: novel links between trauma, vascular collapse and sepsis. Med Hypotheses. 2013;81(4):532–5. doi: 10.1016/j.mehy.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamanouchi S, Kudo D, Yamada M, Miyagawa N, Furukawa H, Kushimoto S. Plasma mitochondrial DNA levels in patients with trauma and severe sepsis: time course and the association with clinical status. J Crit Care. 2013;28(6):1027–31. doi: 10.1016/j.jcrc.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Margulis L. Symbiotic theory of the origin of eukaryotic organelles; criteria for proof. (0081-1386 (Print)) [PubMed] [Google Scholar]
- 9.Zhao C, Itagaki K, Gupta A, Odom S, Sandler N, Hauser CJ. Mitochondrial damage-associated molecular patterns released by abdominal trauma suppress pulmonary immune responses. J Trauma Acute Care Surg. 2014;76(5):1222–7. doi: 10.1097/TA.0000000000000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Candy B, Jones L, Vickerstaff V, Tookman A, King M. Interventions for sexual dysfunction following treatments for cancer in women. Cochrane Database Syst Rev. 2016;2:CD005540. doi: 10.1002/14651858.CD005540.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wenceslau CF, Szasz T, McCarthy CG, Baban B, NeSmith E, Webb RC. Mitochondrial N-formyl peptides cause airway contraction and lung neutrophil infiltration via formyl peptide receptor activation. Pulm Pharmacol Ther. 2016;37:49–56. doi: 10.1016/j.pupt.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenceslau CF, McCarthy CG, Szasz T, Goulopoulou S, Webb RC. Mitochondrial N-formyl peptides induce cardiovascular collapse and sepsis-like syndrome. Am J Physiol Heart Circ Physiol. 2015;308(7):H768–77. doi: 10.1152/ajpheart.00779.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215(3):403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 14.Simmons JD, Freno DR, Muscat CA, Obiako B, Lee YL, Pastukh VM, Brevard SB, Gillespie MN. Mitochondrial DNA damage associated molecular patterns in ventilator-associated pneumonia: Prevention and reversal by intratracheal DNase I. J Trauma Acute Care Surg. 2017;82(1):120–5. doi: 10.1097/TA.0000000000001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabiet MJ, Huet E, Boulay F. Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. European Journal of Immunology. 2005;35:2486–95. doi: 10.1002/eji.200526338. [DOI] [PubMed] [Google Scholar]
- 16.Nakahira K, Kyung SY, Rogers AJ, Gazourian L, Youn S, Massaro AF, Quintana C, Osorio JC, Wang Z, Zhao Y, et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 2013;10(12):e1001577. doi: 10.1371/journal.pmed.1001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIlroy DJ, Bigland M, White AE, Hardy BM, Lott N, Smith DW, et al. Cell necrosis-independent sustained mitochondrial and nuclear DNA release following trauma surgery. J Trauma Acute Care Surg. 2015;78(2):282–8. doi: 10.1097/TA.0000000000000519. [DOI] [PMC free article] [PubMed] [Google Scholar]