Abstract
Rationale
Ketamine is used by preadolescent and adolescent humans for licit and illicit purposes.
Objective
The goal of the present study was to determine the effects of acute and repeated ketamine treatment on the unconditioned behaviors and conditioned locomotor activity of preadolescent and adolescent rats.
Methods
To assess unconditioned behaviors, female and male rats were injected with ketamine (5–40 mg/kg) and distance traveled was measured on PD 21–25 or PD 41–45. To assess conditioned activity, male and female rats were injected with saline or ketamine in either a novel test chamber or the home cage on PD 21–24 or PD 41–44. One day later, rats were injected with saline and conditioned activity was assessed.
Results
Ketamine produced a dose-dependent increase in the locomotor activity of preadolescent and adolescent rats. Preadolescent rats did not exhibit sex differences, but ketamine-induced locomotor activity was substantially stronger in adolescent females than males. Repeated ketamine treatment neither caused a day-dependent increase in locomotor activity nor produced conditioned activity in preadolescent or adolescent rats.
Conclusions
The activity-enhancing effects of ketamine are consistent with the actions of an indirect dopamine agonist, while the inability of ketamine to induce conditioned activity is unlike what is observed after repeated cocaine or amphetamine treatment. This dichotomy could be due to ketamine’s ability to both enhance DA neurotransmission and antagonize NMDA receptors. Additional research will be necessary to parse out the relative contributions of DA and NMDA system functioning when assessing the behavioral effects of ketamine during early ontogeny.
Keywords: Ketamine, Cocaine, Locomotor activity, Conditioned Activity, Preadolescent rats, Adolescent rats
Introduction
Ketamine is a dissociative anesthetic used to induce anesthesia in children and adults (Kohrs and Durieux 1998; Bergman 1999), a quick-acting treatment for major depression (Zarate et al. 2006; aan het Rot et al. 2010), and an illicit drug commonly used at rave parties (Jansen 1993, 2000; Dillon et al. 2003). Recreational use of ketamine varies dramatically world-wide (Kalsi et al. 2011; Morgan and Curran 2012), with epidemiological studies reporting that ketamine is the most frequently used illicit drug in Hong Kong (Tan et al. 2012). In the United States, the annual (2014) prevalence rate of ketamine use among 12th graders was 1.5%, which was greater than for either methamphetamine or phencyclidine (Johnston et al. 2015).
Because of its many behavioral effects, it is perhaps understandable that the neural mechanisms by which ketamine produces its multiple actions are not fully understood. Ketamine is a non-competitive NMDA receptor antagonist (Anis et al. 1983; Duncan et al. 1999) that may produce some of its anesthetic, psychotropic, and anti-depressive effects via actions involving the dopamine (DA) system (Smith et al. 1981; Mantz et al. 1994; Belujon and Grace 2014). For example, ketamine and other NMDA receptor antagonists increase the firing rate and burst firing of DA neurons in the ventral tegmental area (VTA) (French and Ceci 1990; Belujon and Grace 2014; Witkin et al. 2016). These ketamine-induced effects are mediated by both descending and ascending fibers projecting to the VTA (Morikawa and Paladini 2011). There is also a large body of evidence, gained via both in vivo and in vitro techniques, showing that ketamine increases DA release and blocks DA reuptake in the dorsal striatum (Keita et al 1996; Tso et al. 2004; Usun et al. 2013), nucleus accumbens (Hancock and Stamford 1999; Witkin et al. 2016), and prefrontal cortex (Lindefors et al. 1997; Lorrain et al. 2003; see Smith et al. 1981). In some cases, the ability of ketamine to modulate DA release is attributed to actions involving afferent inhibitory interneurons in DA target areas (Verma and Moghaddam 1996; Lorrain et al. 2003; Usun et al. 2013; see also Can et al. 2016), while others suggest that ketamine acts as an indirect DA agonist (Irifune et al. 1991; Nishimura et al. 1998; Hancock and Stamford 1999).
Similar to indirect DA agonists (e.g., cocaine and amphetamine), ketamine causes a dose-dependent increase in the locomotor activity of adult male rats and mice (Irifune et al. 1991; Usun et al. 2013; Yamamoto et al. 2016). High subanesthetic doses of ketamine produce an initial period of hypoactivity, lasting for up to 30 min, and then a prolonged period of hyperactivity (Irifune et al. 1991). Female rats also exhibit elevated levels of locomotor activity after acute ketamine administration (Wiley et al. 2011), and there is some evidence that the locomotor activating effects of ketamine are more pronounced in females than in males. Specifically, Wilson et al. (2005; 2007) reported that a low dose of ketamine (10 mg/kg) increased the locomotion of both male and female rats on postnatal day (PD) 22, but stimulated the locomotor activity of only female rats during the early (PD 35) and late (PD 50) adolescent periods. To some extent the latter results are difficult to interpret because only a single low-dose of ketamine was used, and behavioral testing lasted for only 10 min. Repeatedly administering ketamine to adult male rats and mice causes a progressive increase in locomotor activity (i.e., behavioral sensitization) that can persist for at least 30 days (Uchihashi et al. 1993; Trujillo et al. 2008; Yamamoto et al. 2016). In contrast to male mice (Yamamoto et al. 2016), the sensitized responding of male rats is stronger if ketamine is administered intermittently (e.g., once every 7 days) rather than daily (Trujillo et al. 2008). Indeed, adult male rats may not exhibit a progressive increase in locomotor activity if ketamine is administered on five consecutive days (Becker et al. 2003). Female rats show ketamine-induced behavioral sensitization (Wiley et al. 2011), however the performance of male and female rats has not been directly compared when using these repeated treatment paradigms.
Although ketamine induces behavioral sensitization in rats and mice, it is uncertain whether repeated ketamine treatment is capable of producing the related phenomenon of conditioned activity. Conditioned activity is evident when drug-free animals exhibit increased locomotion in an environmental context in which they previously received a drug. This phenomenon is most frequently explained in terms of Pavlovian conditioning. Specifically, an excitatory Pavlovian association is hypothesized to form between the environmental context (conditioned stimulus, CS) and the test compound (unconditioned stimulus, US). As a consequence, the CS elicits enhanced locomotor activity (conditioned response, CR) when the rat is returned to the same environmental context in a drug-free state (Franklin and Druhan 2000; Michel and Tirelli 2002; Johnson et al. 2012). Under certain circumstances it is possible that “conditioned” activity could result from a failure to habituate rather than contextual conditioning (Damianopoulos and Carey 1992; 1994; Ahmed et al. 1995; Carey et al. 2008). In other words, the test compound might interfere with the normal habituation process, thus rats exhibit increased locomotor activity on the test day because of a hyperactive response to the seemingly novel environment. One way to distinguish between these possibilities (i.e., associative learning vs. a failure to habituate) is to include nonhabituated control groups that are pretreated with saline or the test compound, but are not exposed to the activity chamber until the test day (Damianopoulos and Carey 1992). With the proper controls in place, it is possible to determine whether repeated environment–ketamine (CS–US) pairings are capable of producing a Pavlovian conditioned locomotor response.
The purpose of this project was to examine the effects of a broad dose-range of ketamine (5–40 mg/kg) on the unconditioned and conditioned locomotor activity of male and female rats during early ontogeny. Because of the potential translational relevance to young humans (Spear 2000; Andersen 2003), we compared rats from the preadolescent (PD 21–25) and adolescent (PD 41–45) periods (for a discussion of ontogenetic epochs, see Smith 2003; Frantz et al. 2006). Based on previously cited adult ketamine studies, as well as ontogenetic data using psychostimulants (Becker et al. 2001; Parylak et al. 2008; McDougall et al. 2015), it was hypothesized that adolescent female rats would show stronger unconditioned and conditioned locomotor activity than male rats. Preadolescent rats were also hypothesized to show robust ketamine-induced unconditioned and conditioned activity, but no sex differences were predicted in this prepubertal age group.
Materials and methods
Subjects
Subjects were an equal number of male and female preadolescent (N = 192) and adolescent (N = 264) rats of Sprague-Dawley descent (Charles River, Hollister, CA) that were born and raised at California State University, San Bernardino (CSUSB). Litters were culled to 10 pups on PD 3. Preadolescent rats were kept with the dam and littermates, whereas adolescent rats were group housed with same-sex littermates. All rats were housed in large polycarbonate maternity cages (30.5 × 43 × 19 cm) on a ventilated rack. Food and water were freely available. The colony room was maintained at 22–23°C and kept under a 12:12 light/dark cycle. Testing was done in a separate experimental room and was conducted during the light phase of the cycle. Subjects were cared for according to the “Guide for the Care and Use of Laboratory Animals”(National Research Council 2010) under a research protocol approved by the Institutional Animal Care and Use Committee of CSUSB.
Apparatus
Behavioral testing was done in activity monitoring chambers that consisted of acrylic walls, a plastic floor, and an open top (Coulbourn Instruments, Whitehall, PA). Each chamber included an X–Y photobeam array, with 16 photocells and detectors, that was used to determine distance traveled (a measure of locomotor activity) in the margin and the center of the activity monitoring chambers. In order to equate for differences in body size (see also Campbell et al. 1969; Shalaby and Spear 1980), preadolescent rats were tested in smaller chambers (26 × 26 × 41 cm) than adolescent rats (41 × 41 × 41 cm).
Drugs
(±)-Ketamine hydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved in saline and injected intraperitoneally (IP) at a volume of 2.5 ml/kg (preadolescent rats) or 1 ml/kg (adolescent rats).
Procedure
Experiment 1: Effects of repeated ketamine treatment on the unlearned behaviors of male and female preadolescent and adolescent rats
During the habituation phase, which occurred on PD 20 or PD 40, rats were injected with saline and placed in activity chambers for 30 min. The testing phase occurred on the following fives days (i.e., PD 21–PD 25 or PD 41–PD 45), with male and female rats (n = 12 of each sex per group) receiving an injection of ketamine (0, 5, 10, 20, or 40 mg/kg, IP) immediately before being placed in the activity chambers for 120 min.
In addition to measuring distance traveled, discrete behaviors (wall climbing, rearing, and grooming) of a subset of rats (n = 8 per group) were quantified using the fixed interval momentary time sampling method described by Cameron et al. (1988). For a given rat, the presence or absence of a particular behavior was determined during a 15 s interval that occurred every 5 min. During the same 15 s interval, the behavior of each rat was coded using the following motoric capacity rating scale: 0 = asleep or inactive, 1 = normal forward locomotion (no balance disturbances), 2 = forward locomotion with minor balance problems (fully upright, but awkwardness when turning), 3 = forward locomotion with moderate balance problems (fully upright, but severe swaying during locomotion), 4 = forward locomotion with major balance problems I (fully upright, but occasional rolling), 5 = forward locomotion with major balance problems II (minor dragging with prominent rolling), 6 = predominate dragging (forward dragging with prominent rolling), 7 = circular dragging (forward dragging in a circular pattern), and 8 = splayed and immobile. In all cases, behavior was recorded via wall-mounted hard disk cameras (JVC, model GZ-MG670) and quantified by an observer blind to treatment conditions.
Experiment 2: Ability of repeated ketamine treatment to induce conditioned activity in male and female preadolescent and adolescent rats
On PD 20 or PD 40, male and female rats were injected with saline and habituated to the activity chambers for 30 min. Half as many preadolescent rats were tested as adolescent rats, because preadolescent rats did not exhibit sex differences in the first experiment. The pretreatment phase occurred on PD 21–PD 24 or PD 41–PD 44. In the Saline control group, preadolescent (n = 8 of each sex per group) and adolescent (n = 16 of each sex per group) rats were injected with saline and placed in the activity chambers, where distance traveled was measured for 120 min. These rats were given a second saline injection 30 min after being returned to the home cage. In the Nonhabituated control groups, preadolescent (n = 4 of each sex per group) and adolescent (n = 8 of each sex per group) rats were injected with saline or ketamine (10 or 40 mg/kg, IP) and immediately returned to the home cage (i.e., rats were not placed in activity chambers during the pretreatment phase). After 150 min, nonhabituated rats were given an injection of saline. In the ‘Unpaired’ groups, preadolescent (n = 4 of each sex per group) and adolescent (n = 8 of each sex per group) rats were injected with saline before being placed in activity chambers for 120 min, and then injected with ketamine (10 or 40 mg/kg, IP) 30 min after being returned to the home cage. In the ‘Paired’ groups, preadolescent (n = 4 of each sex per group) and adolescent (n = 8 of each sex per group) rats were injected with ketamine (10 or 40 mg/kg, IP) before being placed in activity chambers, and then injected with saline 30 min after being returned to the home cage. After 24 h (i.e., on PD 25 or PD 45), conditioned activity was assessed by injecting rats with saline and immediately placing them in activity chambers where distance traveled was measured for 120 min.
Data analysis
Multifactor analyses of variance (ANOVAs) were used to statistically analyze distance traveled data, percent margin distance [%MD = (margin distance/total distance) * 100], grooming, and vertical activity (wall climbing plus rearing). If ANOVAs included a repeated measures factor, the Greenhouse-Geisser epsilon statistic was used to adjust degrees of freedom when the assumption of sphericity was violated (Geisser and Greenhouse 1958). Corrected degrees of freedom were rounded to the nearest whole number and are indicated by a superscripted “a”. When further analyzing statistically significant higher order interactions, the mean square error terms (i.e., MSerror) used for the Tukey calculations were based on separate one-way ANOVAs at each day or time block. Ordinal data provided by the motoric capacity rating scale were analyzed using nonparametric statistics. Drug dose effects were analyzed using the Kruskal-Wallis (KW) test, with Dunn’s test (P<0.05) being used for post hoc comparisons (Dunn 1964). Sex effects at each dose were analyzed with Mann-Whitney U-tests (Siegel and Castellan 1988). To minimize litter effects, no more than one subject per litter was assigned to a given group (Zorrilla 1997).
Results
Experiment 1: Effects of repeated ketamine treatment on the behavior of male and female rats
Unlearned locomotor activity: Preadolescent rats
Among preadolescent rats, distance traveled scores did not vary according to sex (Fig. 1). Overall, ketamine caused a dose-dependent increase in locomotor activity (Fig. 1, right panels), with higher doses of ketamine (10, 20, and 40 mg/kg) stimulating significantly more distance traveled than saline [Dose main effect, F4,70 =73.81, P<0.001; and Tukey tests, P<0.05]. On each of the test days (PD 21–PD 25), preadolescent rats injected with 20 or 40 mg/kg ketamine exhibited significantly greater distance traveled scores than saline controls (Fig. 1, left panels) [aDose × Day interaction, F14,241 =38.64, P<0.001; and Tukey tests, P<0.05]. Among the two high-dose groups, ketamine (20 and 40 mg/kg) stimulated the most locomotor activity on PD 21 (the first day of drug exposure). Distance traveled scores declined on PD 22 and then stabilized across the testing phase (Tukey tests, P<0.05).
Fig. 1.
Mean distance traveled scores (±SEM) of ketamine (0–40 mg/kg) treated female (upper graph) and male (lower graph) preadolescent rats on PD 21–25. The right panels represent total distance traveled collapsed across the five test days. Asterisks = Significant difference between the saline and 40 mg/kg ketamine group; Daggers = Significant difference between the saline and 20 mg/kg ketamine group; Double daggers = Significant difference between the saline and 10 mg/kg ketamine group.
A finer grain analysis of locomotor activity on PD 21 and PD 25 shows how the various doses of ketamine differentially affected distance traveled scores across the individual testing sessions (Fig. 2). On the first test day (PD 21), the lower doses of ketamine (5–20 mg/kg) stimulated the most distance traveled on time block 1, after which distance traveled declined across the testing session [aDose × Time Block interaction, F14,398 =80.18, P<0.001; and Tukey tests, P<0.05]. In contrast, the higher dose of ketamine (40 mg/kg) stimulated little distance traveled on the first two 10-min time blocks and then a dramatically heightened locomotor response on time blocks 3–5. Distance traveled then declined across the remainder of the testing session. On PD 25, rats given 40 mg/kg ketamine exhibited a nonsignificant increase in locomotor activity from time block 1 to time block 2 (Fig. 2, lower graph). Except for this effect, distance traveled scores of all dose groups declined across the testing session until basal levels were reached [aDose × Time Block interaction, F20,373 =17.34, P<0.001; and Tukey tests, P<0.05].
Fig. 2.
Mean distance traveled scores (±SEM) of female and male preadolescent rats across the 12 time blocks on PD 21 (upper graph) and PD 25 (lower graph). Asterisks = Significant difference between the saline and 40 mg/kg ketamine group; Daggers = Significant difference between the saline and 20 mg/kg ketamine group; Double daggers = Significant difference between the saline and 10 mg/kg ketamine group.
On PD 21, preadolescent rats injected with saline exhibited substantially more locomotor activity in the margin (86.3%) of the testing chamber than in the center (13.7%) (Table 1). %MD scores did not vary according to sex; however, when preadolescent rats were injected with higher doses of ketamine (20 and 40 mg/kg) the %MD scores were significantly reduced relative to the saline group [Dose main effect, F4,110 =45.33, P<0.001; and Tukey tests, P<0.05].
Table 1.
Effects of ketamine on mean percent margin distance (%MD) and mean vertical activity counts of male and female preadolescent and adolescent rats
| Age – Treatment | % Margin Distance | Vertical Activity | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Male | Female | x̄ (M, F) | Male | Female | x̄ (M, F) | |
| Preadolescent Rats (PD 21) | ||||||
| 0 mg/kg Ketamine | 85.0% | 87.6% | 86.3% | 0.75 | 0.00 | 0.38 |
| 5 mg/kg Ketamine | 83.5 | 82.2 | 82.9 | 1.25 | 1.75 | 1.50 |
| 10 mg/kg Ketamine | 83.3 | 81.9 | 82.6 | 2.75 | 1.75 | 2.25 |
| 20 mg/kg Ketamine | 72.3 | 67.9 | 70.1a | 0.25 | 1.50 | 0.88 |
| 40 mg/kg Ketamine | 59.3 | 58.8 | 59.1a | 0.00 | 0.75 | 0.38 |
|
| ||||||
| Adolescent Rats (PD 41) | ||||||
| 0 mg/kg Ketamine | 84.8% | 87.4% | 86.1% | 1.50 | 0.25 | 0.88 |
| 5 mg/kg Ketamine | 77.7 | 86.3 | 82.0 | 1.25 | 0.25 | 0.75 |
| 10 mg/kg Ketamine | 85.4 | 85.8 | 85.6 | 0.00 | 0.00 | 0.00 |
| 20 mg/kg Ketamine | 76.7 | 78.5 | 77.6a | 0.50 | 0.00 | 0.25 |
| 40 mg/kg Ketamine | 73.9 | 64.4b | 69.1ac | 0.25 | 0.50 | 0.38 |
Note:
Significantly different from the 0 mg/kg ketamine group at the same age.
Significantly different from female rats in the 0 mg/kg ketamine group at the same age.
Significantly different from preadolescent rats in the 40 mg/kg ketamine group.
Unlearned locomotor activity: Adolescent rats
Overall, female adolescent rats injected with 20 or 40 mg/kg ketamine exhibited more locomotor activity than male adolescent rats (compare the right panels of Fig. 3) [Sex main effect, F1,70 =27.04, P<0.001; Sex × Dose interaction, F4,70 =6.27, P<0.001; and Tukey tests, P<0.05]. Among both male and female adolescent rats, only the two higher doses of ketamine (20 and 40 mg/kg) stimulated significantly greater distance traveled scores than saline (Tukey tests, P<0.05). The day-to-day effects of ketamine varied according to sex (compare the left panels of Fig. 3), as the distance traveled scores of female rats injected with 40 mg/kg ketamine declined from PD 41 to PD 43, while the locomotor activity of male rats was stable across test days [aSex × Dose × Day interaction, F14,240 =2.99, P<0.001; and Tukey tests, P<0.05]. In addition, 20 and 40 mg/kg ketamine significantly increased the distance traveled of female adolescent rats on all five test days; whereas, 20 mg/kg ketamine enhanced the distance traveled scores of male rats, relative to same-sex saline controls, on PD 42, PD 44, and PD 45.
Fig. 3.
Mean distance traveled scores (±SEM) of ketamine (0–40 mg/kg) treated female (upper graph) and male (lower graph) adolescent rats on PD 41–45. The right panels represent total distance traveled collapsed across the five test days. Asterisks = Significant difference between the saline and 40 mg/kg ketamine group; Daggers = Significant difference between the saline and 20 mg/kg ketamine group; Double daggers = Significant difference between female and male rats given the same dose of ketamine.
On PD 41 (the first test day), the pattern of ketamine-induced locomotor activity differed substantially between male and female rats (Fig. 4) [aSex × Dose × Time Block interaction, F16,426 =14.99, P<0.001]. Female adolescent rats responded similarly to preadolescent rats, as 40 mg/kg ketamine did not generate peak locomotor activity until time block 5, after which distance traveled scores declined across the remainder of the testing session [aDose × Time Block interaction, F14,191 =23.94, P<0.001; and Tukey tests, P<0.05]. For the other female ketamine groups, distance traveled scores decreased progressively from time block 1 to time block 7, and then stabilized at a low rate (Tukey tests, P<0.05). In contrast, male adolescent rats injected with 40 mg/kg ketamine exhibited peak distance traveled scores on time block 1, and differed significantly from saline-treated male rats on time blocks 2–5 [aDose × Time Block interaction, F14,189 =4.04, P<0.001; and Tukey tests, P<0.05]. On no time block did any of the other doses of ketamine significantly enhance distance traveled scores relative to the saline controls.
Fig. 4.
Mean distance traveled scores (±SEM) of ketamine (0–40 mg/kg) treated female (upper graph) and male (lower graph) adolescent rats across the 12 time blocks on PD 41. Asterisks = Significant difference between the saline and 40 mg/kg ketamine group; Daggers = Significant difference between the saline and 20 mg/kg ketamine group.
On PD 45, the distance traveled scores of female adolescent rats given 40 mg/kg ketamine increased from time block 1 to time block 3, and then declined across the remainder of the testing session (Fig. 5, upper graph) [aDose × Time Block interaction, F9,81 =7.61, P<0.001; and Tukey tests, P<0.05]. All other ketamine groups showed a progressive decline in distance traveled scores across the testing session. Female rats injected with 40 mg/kg ketamine exhibited elevated distance traveled scores, relative to the saline controls, on time blocks 2–8; whereas, 20 mg/kg ketamine increased locomotor activity on time blocks 1–4 (Tukey tests, P<0.05). Among male adolescent rats on PD 45, all doses of ketamine produced peak locomotor activity on time block 1, followed by a progressive decline across the first half of the testing session (Fig. 5, lower graph) [aDose × Time Block interaction, F10,91 =7.14, P<0.001; and Tukey tests, P<0.05]. The high dose of ketamine (40 mg/kg) increased distance traveled scores on time blocks 1–6, while 20 mg/kg ketamine only caused a significant increase on time blocks 1 and 2 (Tukey tests, P<0.05).
Fig. 5.
Mean distance traveled scores (±SEM) of ketamine (0–40 mg/kg) treated female (upper graph) and male (lower graph) adolescent rats across the 12 time blocks on PD 45. Asterisks = Significant difference between the saline and 40 mg/kg ketamine group; Daggers = Significant difference between the saline and 20 mg/kg ketamine group.
On PD 41, adolescent rats treated with 20 or 40 mg/kg ketamine traveled greater distances in the center of the testing apparatus, relative to the margin, when compared to saline-treated rats (Table 1) [Dose main effect, F4,106 =15.86, P<0.001; and Tukey tests, P<0.05]. Post hoc analysis of the significant Dose × Sex interaction showed that the greatest reduction in %MD scores occurred in female rats injected with 40 mg/kg ketamine [F4,106 =3.34, P<0.05; and Tukey tests, P<0.05]. The %MD scores of male and female adolescent rats did not differ from each other at any dose.
Unlearned locomotor activity: Age comparisons
Overall, the ketamine variable interacted with age to affect locomotor activity (compare Fig. 1 and Fig. 3) [Age main effect, F1,150 =5.42, P<0.05; Age × Dose interaction, F4,150 =2.78, P<0.05; aAge × Dose × Day interaction, F14,517 =15.50, P<0.001]. There were no significant age differences after saline or low-dose ketamine (5 or 10 mg/kg) treatment. When injected with 20 mg/kg ketamine, preadolescent rats had significantly greater distance traveled scores than adolescent rats on the first day of drug administration, whereas adolescent rats exhibited more locomotor activity than preadolescent rats on the final test day [aAge × Day interaction, F4,97 =12.58, P<0.001; and Tukey tests, P<0.05]. A similar pattern of effects was evident after treatment with 40 mg/kg ketamine, as preadolescent rats exhibited more distance traveled on the first test day, while adolescent rats had significantly greater distance traveled scores on all subsequent test days [aAge × Day interaction, F4,74 =33.25, P<0.001; and Tukey tests, P<0.05]. In terms of margin vs. center, adolescent rats had greater %MD scores than preadolescent rats [Age main effect, F1,216 =12.61, P<0.001], but this effect was only evident in preadolescent (59.1%) and adolescent rats (69.1%) injected with 40 mg/kg ketamine [Age × Dose interaction, F1,216 =3.78, P<0.01; and Tukey tests, P<0.05].
Vertical activity, grooming, and motoric capacity: Preadolescent rats
Among preadolescent rats, ketamine did not affect vertical activity (Table 1) or grooming (data not shown). Analysis of data provided by the motoric capacity scale (Table 2) showed that rats given 40 mg/kg ketamine had elevated scores relative to the saline control group on time blocks 1–11 (higher doses indicate greater motoric disturbances) [Dose effects, KW = 32.12 to 13.42, P<0.001 to P<0.05 and Dunn tests, P<0.05]. On time block 1, preadolescent rats given 40 mg/kg ketamine were immobile; on time blocks 2 and 3, rats had major balance problems; while on time blocks 4–11 rats exhibited some minor balance problems along with enhanced locomotor activity. Preadolescent rats injected with 20 mg/kg ketamine had balance problems on time block 1 followed by significantly enhanced locomotor activity, relative to the saline controls, on time blocks 2–4 (Dunn tests, P<0.05). No statistically significant sex effects were apparent at any dose.
Table 2.
Effects of ketamine on the median motoric capacity scores of preadolescent rats across the 120 min testing session
| Treatment | 10-Min Time Blocks | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| 0 mg/kg Ketamine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 mg/kg Ketamine | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 mg/kg Ketamine | 0.8 | 0 | 0.2 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 mg/kg Ketamine | 3.2a | 1.5a | 1.0a | 0.8a | 0.5 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 40 mg/kg Ketamine | 8.0a | 6.2a | 5.0a | 2.5a | 1.8a | 1.2a | 1.0a | 0.8a | 0.5a | 0.5a | 0.5a | 0 |
Note: Motoric capacity scale: 0 = asleep or inactive, 1 = normal forward locomotion (no balance disturbances), 2 = forward locomotion with minor balance problems (fully upright, but awkwardness when turning), 3 = forward locomotion with moderate balance problems (fully upright, but severe swaying during locomotion), 4 = forward locomotion with major balance problems I (fully upright, but occasional rolling), 5 = forward locomotion with major balance problems II (minor dragging with prominent rolling), 6 = predominate dragging (forward dragging with prominent rolling), 7 = circular dragging (forward dragging in a circular pattern), and 8 = splayed and immobile.
Significantly different from the 0 mg/kg ketamine group at the same time block.
Vertical activity, grooming, and motoric capacity: Adolescent rats
Ketamine did not affect either the vertical activity (Table 1) or grooming (data not shown) of adolescent rats. In terms of the motoric capacity scale, male and female adolescent rats exhibited different patterns of behavior (Table 3). On only time block 1 did male rats given 40 mg/kg ketamine score higher on the motoric capacity scale than saline controls [Dose effect, KW = 14.60, P<0.01 and Dunn tests, P<0.05]; whereas, female rats injected with the same dose of ketamine had significantly elevated motoric capacity scores on time blocks 1–7 [Dose effects, KW = 17.37 to 14.39, P<0.01 and Dunn tests, P<0.05]. Across the first 30 min of testing (time blocks 1–3), female adolescent rats were either immobile or exhibited severe motoric disturbances. Female rats given 20 mg/kg ketamine also exhibited motoric disturbances on time blocks 1 and 2 (Dunn tests, P<0.05). Among rats treated with 40 mg/kg ketamine, female rats had more severe motoric problems than male rats on time blocks 1–7 (Mann-Whitney U-tests, P<0.05).
Table 3.
Effects of ketamine on the median motoric capacity scores of male and female adolescent rats across the 120 min testing session
| Sex – Treatment | 10-Min Time Blocks | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Male Rats | ||||||||||||
| 0 mg/kg Ketamine | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 mg/kg Ketamine | 0.2 | 0.2 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 mg/kg Ketamine | 0.5 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 mg/kg Ketamine | 1.8 | 0.2 | 0 | 0c | 0c | 0c | 0 | 0 | 0 | 0 | 0 | 0 |
| 40 mg/kg Ketamine | 5.0ac | 1.5c | 0.2c | 0.2c | 0.2c | 0.5c | 0.2 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||||||
| Female Rats | ||||||||||||
| 0 mg/kg Ketamine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 mg/kg Ketamine | 0.5 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 | 0 | 0 |
| 10 mg/kg Ketamine | 0.5 | 0.5 | 0.5 | 0.5 | 0 | 0 | 0 | 0.2 | 0 | 0 | 0 | 0 |
| 20 mg/kg Ketamine | 4.8a | 2.0a | 1.2 | 0.2 | 0.2 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 40 mg/kg Ketamine | 8.0ab | 7.0ab | 5.5ab | 4.2abc | 3.0abc | 1.8ab | 1.0ab | 0.5 | 0 | 0 | 0 | 0 |
Note: Motoric capacity scale: 0 = asleep or inactive, 1 = normal forward locomotion (no balance disturbances), 2 = forward locomotion with minor balance problems (fully upright, but awkwardness when turning), 3 = forward locomotion with moderate balance problems (fully upright, but severe swaying during locomotion), 4 = forward locomotion with major balance problems I (fully upright, but occasional rolling), 5 = forward locomotion with major balance problems II (minor dragging with prominent rolling), 6 = predominate dragging (forward dragging with prominent rolling), 7 = circular dragging (forward dragging in a circular pattern), and 8 = splayed and immobile.
Significantly different from the same-sex 0 mg/kg ketamine group at the same time block.
Significantly different from male rats in the same ketamine group at the same time block.
Significantly different from same-sex preadolescent rats in the same ketamine group at the same time block.
Vertical activity, grooming, and motoric capacity: Age comparisons
Grooming did not vary according to age (data not shown), but preadolescent rats did have significantly more vertical activity counts than adolescent rats [Age main effect, F1,60 =4.25, P<0.05]. In terms of the motoric capacity scale, male preadolescent rats injected with 40 mg/kg ketamine had higher scores (i.e., exhibited more severe motoric disturbances) than male adolescent rats on time blocks 1–6 (Mann-Whitney U-tests, P<0.05). In contrast, female adolescent rats given 40 mg/kg ketamine had higher motoric capacity scores than female preadolescent rats on time blocks 4 and 5 (Mann-Whitney U-tests, P<0.05).
Experiment 2: Ability of repeated ketamine treatment to induce conditioned activity in male and female preadolescent and adolescent rats
Preadolescent rats
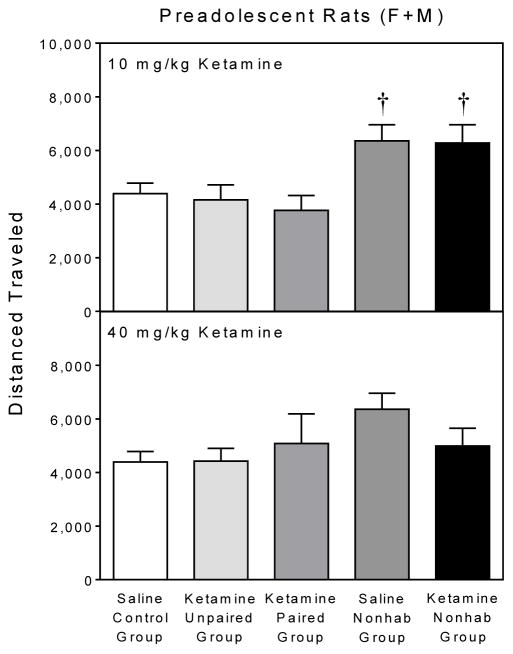
During the pretreatment phase, rats responded essentially the same as described in Experiment 1. On the test day, ketamine-pretreated male and female preadolescent rats did not show conditioned activity, since the paired groups did not have greater distance traveled scores than the various control groups (Fig. 6). Instead, the nonhabituated control groups receiving saline or 10 mg/kg ketamine exhibited significantly more locomotor activity than the paired group given 10 mg/kg ketamine [Group main effect, F4,38 =4.34, P<0.01; and Tukey tests, P<0.05]. The various groups treated with 40 mg/kg ketamine did not differ amongst themselves. Significant sex effects were not apparent during either the pretreatment phase or on the test day.
Fig. 6.
Mean test day (PD 25) distance traveled scores (±SEM) of preadolescent rats from the 10 mg/kg (upper graph) and 40 mg/kg (lower graph) ketamine groups. Asterisks = Significantly different from the saline control group in the same drug condition. Daggers = Significantly different from the paired group in the same drug condition.
Adolescent rats
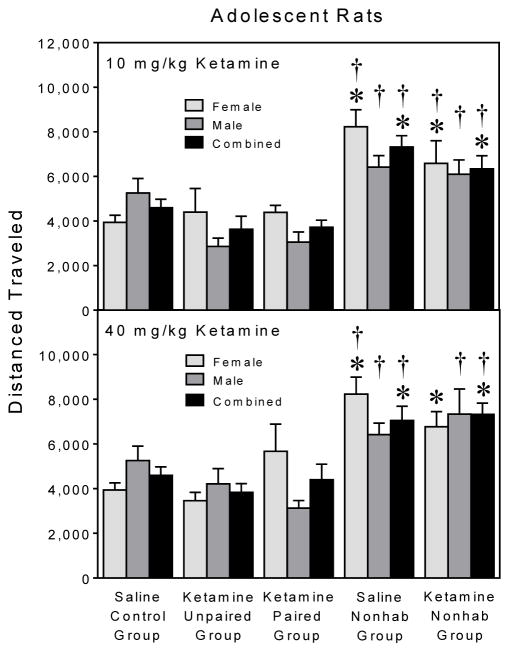
On the test day, adolescent rats in the saline and ketamine (10 or 40 mg/kg) nonhabituated groups exhibited greater distance traveled scores than the ketamine paired groups and the saline control group (Fig. 7) [Group main effects, F4,86 =11.07, P<0.001; F4,86 =9.69, P<0.001; and Tukey tests, P<0.05]. The sex variable did impact this result, since differences between the nonhabituated groups and saline control group were statistically significant in only female rats [Group × Sex interactions, F4,86 =2.52, P<0.05; F4,86 =3.06, P<0.05; and Tukey tests, P<0.05].
Fig. 7.
Mean test day (PD 45) distance traveled scores (±SEM) of female and male adolescent rats from the 10 mg/kg (upper graph) and 40 mg/kg (lower graph) ketamine groups. Asterisks = Significantly different from the saline control group in the same drug condition. Daggers = Significantly different from the paired group in the same drug condition.
Discussion
In the present study, the unconditioned and conditioned behavioral effects of ketamine were assessed in male and female preadolescent and adolescent rats. In rodents, preadolescence and adolescence are developmental stages that transition between the earlier preweanling period and adulthood. Puberty occurs during adolescence but the two terms are not interchangeable as puberty involves a series of biological changes that happen during the adolescent period (Spear 2000). Adult-like gonadotropin cycling is first evident on about PD 28 (Ojeda and Urbanski 1994); whereas, vaginal opening is typically observed by PD 35, and balanopreputial separation occurs in male rats at approximately PD 40 (for reviews, see McCormick and Mathews 2007; McCormick et al. 2017). With the aid of these pubertal guideposts, adolescence is usually defined as a time roughly between PD 28 and PD 55 or PD 59 (Smith 2003; Frantz et al. 2006; Caster et al. 2007; McCormick and Mathews 2007), while the preadolescent period occurs between weaning (PD 21) and adolescence (Romeo et al. 2016). In humans, adolescence extends from approximately 12 to 18 years of age (Spear 2000), with preadolescence corresponding to late childhood. Although no major multi-site studies have tracked ketamine consumption in the United States, the “Monitoring the Future” national survey indicates that the annual prevalence rate of ketamine use for 8th graders (≈ 13–14 year olds) ranges between 0.6–1.6% (2000–2011) and 1.3–2.6% for 12th graders (2000–2014) (Johnston et al. 2015). Because of these drug-use statistics, an ontogenetic examination of ketamine’s unconditioned and conditioned effects was warranted.
As hypothesized, sex was an important factor affecting ketamine-induced locomotor activity, but only in the older age group. In adolescent rats, ketamine (20 and 40 mg/kg) stimulated significantly more locomotor activity in females than males on PD 41. The pattern of ketamine-induced locomotor activity differed between sexes, as male adolescent rats injected with 40 mg/kg ketamine showed peak locomotor responding on time block 1 followed by a steady decline until basal levels were reached. The elevated locomotor activity evident on time block 1 coincided with moderate balance problems that quickly dissipated as the session progressed. In contrast, preadolescent rats and female adolescent rats injected with 40 mg/kg ketamine showed reduced forward movement across the first two time blocks (≈ 20 min), followed by intense hyperactivity that peaked on the fifth time block, and then a slow decline in locomotor activity across the remainder of the testing session (a similar pattern of locomotor activity was exhibited by adult male mice injected with 150 mg/kg ketamine; Irifune et al. 1991). In preadolescent rats and female adolescent rats, the initial 20-min period consisted of a sequential progression from immobility, to circular or forward dragging movements, and then forward locomotion with prominent balance problems. Peak locomotor activity coincided with a period of minor balance disturbances. When injected with 20 mg/kg ketamine, female adolescent rats showed peak locomotor activity on time block 1 along with balance problems like weaving and awkward turning. Therefore, based on overall activity levels and a careful analysis of behavior across time blocks, it is apparent that male adolescent rats were less sensitive to the motor-disrupting and locomotor-stimulating effects of ketamine than preadolescent rats or female adolescent rats.
Among female rats, age-dependent differences in ketamine responsivity were also evident. On the day of initial drug exposure, female preadolescent rats were more sensitive to the locomotor-stimulating effects of ketamine (20 or 40 mg/kg) than were female adolescent rats. On subsequent days, the reverse was the case as higher doses of ketamine tended to induce greater locomotor activity in adolescent female rats. In large part, this ontogenetic difference was a function of the substantial decline in locomotion shown by preadolescent rats from the first to the second test day. Because of this divergent pattern of effects across days, it is not possible to definitively state whether preadolescent or adolescent female rats are more sensitive to the locomotor stimulating effects of ketamine. It is clear, however, that the pattern of behavioral responsiveness to ketamine differs substantially between the preadolescent and adolescent periods.
Although ketamine did not affect vertical activity or grooming, ketamine did alter the topography of forward locomotion. In both preadolescent and adolescent rats, higher doses of ketamine (20 and 40 mg/kg) increased the relative amount of locomotion occurring in the center of the apparatus. Enhanced movement or time spent in the center of the testing arena, relative to the margin, is frequently interpreted as reflecting reduced anxiety (Treit and Fundytus 1989; Simon et al. 1994). In the present situation, however, much of the center movement occurred when rats were exhibiting major balance problems, thus making interpretation of margin/center effects difficult (e.g., see also Hodgson et al. 2010).
The neurobiological basis for ketamine-induced sex differences is uncertain, although it is possible that DA release characteristics and/or ketamine pharmacokinetics are involved. Consistent with the former possibility, indirect DA agonists stimulate more locomotor activity in adult female rats than male rats, an effect that has been linked to estrogen levels (Quiñones-Jenab et al. 1999; Sell et al. 2000). According to Becker et al. (2001) estrogen increases DA release by interacting with G-protein receptors located on the presynaptic wall of the DA neuron. If ketamine does, in fact, enhance locomotion by indirectly or directly activating a dopaminergic mechanism (Irifune et al. 1991; Hancock and Stamford 1999; Usun et al. 2013), then estrogen would also be expected to potentiate ketamine-induced locomotion. Alternatively, ketamine pharmacokinetics might be directly responsible for differences in the behavioral responsiveness of male and female rats. Other non-competitive NMDA receptor antagonists (e.g., phencyclidine and MK-801) cause sex-dependent behavioral effects that are often explained in terms of pharmacokinetic factors (Nabeshima 1984a; 1984b; Wessinger 1995; Turgeon et al. 2010). Indeed, adult female rats metabolize phencyclidine at a slower rate than males (Nabeshima et al. 1984a; 1984b; Shelnutt et al. 1999), probably as a result of estrogen (Nabeshima et al. 1984a). Whether ketamine metabolism also varies according to sex must await a pharmacokinetic analysis that includes both male and female rats. Regardless, because preadolescent female rats do not have an excess of circulating estrogen, either explanation would account for the lack of ketamine-induced sex differences in our younger age group.
Ketamine (10 or 40 mg/kg) did not produce conditioned activity in preadolescent or adolescent rats. Instead, restricting adolescent rats, and to a lesser extent preadolescent rats, to the home cage during the pretreatment phase caused an exaggerated locomotor response on the test day. Presumably, these “nonhabituated” rats showed a heightened exploratory response when placed in the activity chamber; whereas, rats in the “paired,” “unpaired,” and “saline control” groups exhibited a reduced locomotor response on the test day due to habituation (for a review, see Damianopoulos and Carey 1992). The inability of ketamine to induce conditioned activity was unexpected, since (a) both amphetamine and cocaine cause conditioned activity in adult rats and mice (Damianopoulos and Carey 1994; Ahmed et al. 1996; Michel and Tirelli 2002; Johnson et al. 2012), and (b) ketamine and its derivatives produce conditioned place preferences (CPP) in male and female adult rats (van der Kam et al. 2009; Botanas et al. 2015; Guo et al. 2016). The latter findings are relevant to the present study because both the CPP and conditioned activity paradigms rely on contextual conditioning [i.e., the formation of Pavlovian CS–US (environment–drug) associations].
Various explanations could account for the lack of conditioned activity in our preadolescent and adolescent rats. One possibility is that the inability of ketamine to induce conditioned activity is unique to early ontogeny. Indeed, preweanling rats (PD 10–PD 21) do not show cocaine-induced conditioned activity even after 10 daily environment-drug pairings (Zavala et al. 2000; see also Wood et al. 1998; McDougall et al. 1999; 2014). The reason for this age-dependent effect is unknown, but Spear and colleagues have shown that young rats perceive and process CSs differently than adults (for reviews, see Spear et al. 1988; Spear and McKenzie 1994). That being said, adolescent rats typically respond in a qualitatively similar manner to adult rats on learning tasks, so it is uncertain whether age is the critical factor responsible for the lack of ketamine-induced conditioned activity. Instead, the ability of ketamine to antagonize NMDA receptors may be responsible for the absence of conditioned activity, as it is well established that NMDA receptor blockade disrupts both associative learning processes and memory encoding (for a review, see Riedel et al. 2003). Additional research is needed to determine whether the lack of ketamine-induced conditioned activity is due to age factors, procedural issues (e.g., number of CS–US pairings), or mechanism of action.
In addition to not producing conditioned activity, repeated ketamine treatment did not induce a progressive day-dependent increase in locomotor activity (i.e., behavioral sensitization) at any of the doses tested. Indeed, the highest dose of ketamine (40 mg/kg) caused a tolerance-like decline in the locomotor activity of preadolescent rats and female adolescent rats. Although adult mice exhibit robust ketamine-induced behavioral sensitization (Uchihashi et al. 1993; Yamamoto et al. 2016), the present results are consistent with a study using adult male rats, which showed that five daily injections of 30 mg/kg ketamine did not increase locomotor activity from Day 1 to Day 5 (Becker et al. 2003). In contrast, Wiley et al. (2011) found that repeated daily injections of 10 mg/kg ketamine caused a modest increase in locomotor activity across an initial five-day span; however, in that study adolescent and adult female rats were tested for only 20 min. When we reanalyzed our data from the first 20 min of testing, we found that higher doses of ketamine (preadolescent, 40 mg/kg; adolescent, 20 and 40 mg/kg) stimulated more locomotor activity on Day 5 than Day 1. Rather than representing a true sensitized response, the day-dependent increase in locomotor activity was probably the result of rats habituating to the motor debilitating effects of high-dose ketamine treatment.
In conclusion, ketamine caused a dose-dependent increase in the locomotor activity of male and female rats, while neither producing conditioned activity nor causing a day-dependent increase in locomotion. This pattern of effects could be due to any number of factors, with the most obvious possibility being the ability of ketamine to both enhance DA neurotransmission (Hancock and Stamford 1999; Usun et al. 2013) and antagonize NMDA receptors (Anis et al. 1983; Duncan et al. 1999). Indeed, the locomotor activating effects of ketamine are consistent with the actions of an indirect DA agonist, while NMDA receptor antagonism could be responsible for motor disturbances (Irifune et al. 1991) and associative learning deficits (Riedel et al. 2003). Additional research will be necessary to parse out the relative contributions of DA and NMDA system functioning when assessing the behavioral effects of ketamine during early ontogeny.
Acknowledgments
Funding sources:
This research was supported by NIGMS training grant GM083883 (TJB and VR), and NIDA training grant DA033877 (AEM).
Footnotes
Conflict of interest:
All authors declare no conflict of interest.
References
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Cador M, Le Moal M, Stinus L. Amphetamine-induced conditioned activity in rats: comparison with novelty-induced activity and role of the basolateral amygdala. Behav Neurosci. 1995;109:723–733. doi: 10.1037//0735-7044.109.4.723. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Oberling P, Di Scala G, Sandner G. Amphetamine-induced conditioned activity does not result from a failure of rats to habituate to novelty. Psychopharmacology (Berl) 1996;123:325–332. doi: 10.1007/BF02246642. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity. Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Anis NA, Berry SC, Burton NR, Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983;79:565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A, Peters B, Schroeder H, Mann T, Huether G, Grecksch G. Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:687–700. doi: 10.1016/S0278-5846(03)00080-0. [DOI] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann NY Acad Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry. 2014;76:927–936. doi: 10.1016/j.biopsych.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman SA. Ketamine: review of its pharmacology and its use in pediatric anesthesia. Anesth Prog. 1999;46:10–20. [PMC free article] [PubMed] [Google Scholar]
- Botanas CJ, de la Peña JB, Dela Peña IJ, Tampus R, Yoon R, Kim HJ, Lee YS, Jang CG, Cheong JH. Methoxetamine, a ketamine derivative, produced conditioned place preference and was self-administered by rats: evidence of its abuse potential. Pharmacol Biochem Behav. 2015;133:31–36. doi: 10.1016/j.pbb.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Lytle LD, Fibiger HC. Ontogeny of adrenergic arousal and cholinergic inhibitory mechanisms in the rat. Science. 1969;166:635–637. doi: 10.1126/science.166.3905.635. [DOI] [PubMed] [Google Scholar]
- Can A, Zanos P, Moaddel R, Kang HJ, Dossou KS, Wainer IW, Cheer JF, Frost DO, Huang XP, Gould TD. Effects of ketamine and ketamine metabolites on evoked striatal dopamine release, dopamine receptors, and monoamine transporters. J Pharmacol Exp Ther. 2016;359:159–370. doi: 10.1124/jpet.116.235838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RJ, Damianopoulos EN, Shanahan AB. Cocaine conditioned behavior: a cocaine memory trace or an anti-habituation effect. Pharmacol Biochem Behav. 2006;90:625–631. doi: 10.1016/j.pbb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DL, Crosbie J, Crocker AD. A fixed interval momentary sampling method for assessing on-going behaviours induced by dopamine receptor agonists. Prog Neuro-Psychopharmacol Biol Psychi. 1988;12:595–606. doi: 10.1016/0278-5846(88)90005-x. [DOI] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. A single high dose of cocaine induces differential sensitization to specific behaviors across adolescence. Psychopharmacology (Berl) 2007;193:247–260. doi: 10.1007/s00213-007-0764-5. [DOI] [PubMed] [Google Scholar]
- Damianopoulos EN, Carey RJ. Pavlovian conditioning of CNS drug effects: a critical review and new experimental design. Rev Neurosci. 1992;3:65–77. doi: 10.1515/REVNEURO.1992.3.1.65. [DOI] [PubMed] [Google Scholar]
- Damianopoulos EN, Carey RJ. A new method to assess Pavlovian conditioning of psychostimulant drug effects. J Neurosci Methods. 1994;53:7–17. doi: 10.1016/0165-0270(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Dillon P, Copeland J, Jansen K. Patterns of use and harms associated with non-medical ketamine use. Drug Alcohol Depend. 2003;69:23–28. doi: 10.1016/s0376-8716(02)00243-0. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Miyamoto S, Leipzig JN, Lieberman JA. Comparison of brain metabolic activity patterns induced by ketamine, MK-801 and amphetamine in rats: support for NMDA receptor involvement in responses to subanesthetic dose of ketamine. Brain Res. 1999;843:171–183. doi: 10.1016/s0006-8993(99)01776-x. [DOI] [PubMed] [Google Scholar]
- Dunn OJ. Multiple comparisons using rank sums. Technometrics. 1964;5:241–252. [Google Scholar]
- Franklin TR, Druhan JP. Involvement of the nucleus accumbens and medial prefrontal cortex in the expression of conditioned hyperactivity to a cocaine-associated environment in rats. Neuropsychopharmacology. 2000;23:633–644. doi: 10.1016/S0893-133X(00)00162-7. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, O’Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- French ED, Ceci A. Non-competitive N-methyl-D-aspartate antagonists are potent activators of ventral tegmental A10 dopamine neurons. Neurosci Lett. 1990;119:159–162. doi: 10.1016/0304-3940(90)90823-r. [DOI] [PubMed] [Google Scholar]
- Geisser S, Greenhouse SW. An extension of Box’s results on the use of the F distribution in multivariate analysis. Ann Math Statist. 1958;29:885–891. [Google Scholar]
- Guo R, Tang Q, Ye Y, Lu X, Chen F, Dai X, Yan Y, Liao L. Effects of gender on ketamine-induced conditioned placed preference and urine metabonomics. Regul Toxicol Pharmacol. 2016;77:263–274. doi: 10.1016/j.yrtph.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Hancock PJ, Stamford JA. Stereospecific effects of ketamine on dopamine efflux and uptake in the rat nucleus accumbens. Br J Anaesth. 1999;82:603–608. doi: 10.1093/bja/82.4.603. [DOI] [PubMed] [Google Scholar]
- Hodgson SR, Hofford RS, Buckman SAG, Wellman PJ, Eitan S. Morphine-induced stereotyped thigmotaxis could appear as enhanced fear and anxiety in some behavioural tests. J Psychopharm. 2010;24:875–880. doi: 10.1177/0269881108100797. [DOI] [PubMed] [Google Scholar]
- Irifune M, Shimizu T, Nomoto M. Ketamine-induced hyperlocomotion associated with alteration of presynaptic components of dopamine neurons in the nucleus accumbens of mice. Pharmacol Biochem Behav. 1991;40:399–407. doi: 10.1016/0091-3057(91)90571-i. [DOI] [PubMed] [Google Scholar]
- Jansen KLR. Non-medical use of ketamine. Br Med J. 1993;306:601–602. doi: 10.1136/bmj.306.6878.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen KLR. A review of the nonmedical use of ketamine: use, users and consequences. J Psychoactive Drugs. 2000;32:419–433. doi: 10.1080/02791072.2000.10400244. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Sediqzadah S, Erb S. Expression and resilience of a cocaine-conditioned locomotor response after brief and extended drug-free periods. Behav Brain Res. 2012;230:69–77. doi: 10.1016/j.bbr.2012.01.049. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use: 1975–2014: Overview, Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, The University of Michigan; 2015. [Google Scholar]
- Kalsi SS, Wood DM, Dargan PI. The epidemiology and patterns of acute and chronic toxicity associated with recreational ketamine use. Emerg Health Threats J. 2011;4:7107. doi: 10.3402/ehtj.v4i0.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keita H, Lecharny JB, Henzel D, Desmonts JM, Mantz J. Is inhibition of dopamine uptake relevant to the hypnotic action of i.v. anaesthetics? Br J Anaesth. 1996;77:254–256. doi: 10.1093/bja/77.2.254. [DOI] [PubMed] [Google Scholar]
- Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998;87:1186–1193. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- Lindefors N, Barati S, O’Connor WT. Differential effects of single and repeated ketamine administration on dopamine, serotonin and GABA transmission in rat medial prefrontal cortex. Brain Res. 1997;759:205–212. doi: 10.1016/s0006-8993(97)00255-2. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003;117:697–706. doi: 10.1016/s0306-4522(02)00652-8. [DOI] [PubMed] [Google Scholar]
- Mantz J, Varlet C, Lecharny JB, Henzel D, Lenot P, Desmonts JM. Effects of volatile anesthetics, thiopental, and ketamine on spontaneous and depolarization-evoked dopamine release from striatal synaptosomes in the rat. Anesthesiology. 1994;80:352–363. doi: 10.1097/00000542-199402000-00015. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Green MR, Simone JJ. Translational relevance of rodent models of hypothalamic-pituitary-adrenal function and stressors in adolescence. Neurobiol Stress. 2017;6:31–43. doi: 10.1016/j.ynstr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86:220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Collins RL, Karper PE, Watson JB, Crawford CA. Effects of repeated methylphenidate treatment in the young rat: sensitization of both locomotor activity and stereotyped sniffing. Exp Clin Psychopharm. 1999;7:208–218. doi: 10.1037//1064-1297.7.3.208. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Pipkin JA, Der-Ghazarian T, Cortez AM, Gutierrez A, Lee RJ, Carbajal S, Mohd-Yusof A. Age-dependent differences in the strength and persistence of psychostimulant-induced conditioned activity in rats: effects of a single environment-cocaine pairing. Behav Pharmacol. 2014;25:695–704. doi: 10.1097/FBP.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SA, Eaton SE, Mohd-Yusof A, Crawford CA. Age-dependent changes in cocaine sensitivity across early ontogeny in male and female rats: possible role of dorsal striatal D2High receptors. Psychopharmacology (Berl) 2015;232:2287–2301. doi: 10.1007/s00213-014-3860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel A, Tirelli E. Post-sensitisation conditioned hyperlocomotion induced by cocaine is augmented as a function of dose in C57BL/6J mice. Behav Brain Res. 2002;132:179–186. doi: 10.1016/s0166-4328(01)00400-4. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Curran HV. Ketamine use: a review. Addiction. 2012;107:27–38. doi: 10.1111/j.1360-0443.2011.03576.x. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Paladini CA. Dynamic regulation of midbrain dopamine neuron activity: intrinsic, synaptic, and plasticity mechanisms. Neuroscience. 2011;198:95–111. doi: 10.1016/j.neuroscience.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima T, Yamaguchi K, Furukawa H, Kameyama T. Role of sex hormones in sex-dependent differences in phencyclidine-induced stereotyped behaviors in rats. Eur J Pharmacol. 1984a;105:197–206. doi: 10.1016/0014-2999(84)90610-1. [DOI] [PubMed] [Google Scholar]
- Nabeshima T, Yamaguchi K, Yamada K, Hiramatsu M, Kuwabara Y, Furukawa H, Kameyama T. Sex-dependent differences in the pharmacological actions and pharmacokinetics of phencyclidine in rats. Eur J Pharmacol. 1984b;97:217–227. doi: 10.1016/0014-2999(84)90453-9. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. 8. Washington, DC: National Academies Press; 2010. [Google Scholar]
- Nishimura M, Sato K, Okada T, Yoshiya I, Schloss P, Shimada S, Tohyama M. Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells. Anesthesiology. 1998;88:768–774. doi: 10.1097/00000542-199803000-00029. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF. Puberty in the rat. In: Knobil R, Neill JD, editors. The physiology of reproduction. 2. Vol. 2. Raven Press; New York: 1994. pp. 363–409. [Google Scholar]
- Parylak SL, Caster JM, Walker QD, Kuhn CM. Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacol Biochem Behav. 2008;89:314–323. doi: 10.1016/j.pbb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ. Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats. Behav Brain Res. 1999;101:15–20. doi: 10.1016/s0166-4328(98)00073-4. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Patel R, Pham L, So VM. Adolescence and the ontogeny of the hormonal stress response in male and female rats and mice. Neurosci Biobehav Rev. 2016;70:206–216. doi: 10.1016/j.neubiorev.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther. 2000;293:879–886. [PubMed] [Google Scholar]
- Shalaby IA, Spear LP. Psychopharmacological effects of low and high doses of apomorphine during ontogeny. Eur J Pharmacol. 1980;67:451–459. doi: 10.1016/0014-2999(80)90186-7. [DOI] [PubMed] [Google Scholar]
- Shelnutt SR, Gunnell M, Owens SM. Sexual dimorphism in phencyclidine in vitro metabolism and pharmacokinetics in rats. J Pharmacol Exp Ther. 1999;290:1292–1298. [PubMed] [Google Scholar]
- Siegel S, Castellan NJ., Jr . Nonparametric statistics for the behavioral sciences. 2. Boston: McGraw-Hill; 1988. [Google Scholar]
- Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Azzaro AJ, Zaldivar SB, Palmer S, Lee HS. Properties of the optical isomers and metabolites of ketamine on the high affinity transport and catabolism of monoamines. Neuropharmacology. 1981;20:391–396. doi: 10.1016/0028-3908(81)90015-0. [DOI] [PubMed] [Google Scholar]
- Smith RF. Animal models of periadolescent substance abuse. Neurotoxicol Teratol. 2003;25:291–301. doi: 10.1016/s0892-0362(02)00349-5. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear NE, Kraemer PJ, Molina JC, Smoller DE. Developmental change in learning and memory: infantile disposition for unitization. In: Delacour J, Levy JCS, editors. Systems with learning and memory abilities. Elsevier; New York: 1988. pp. 27–52. [Google Scholar]
- Spear NE, McKenzie DL. Intersensory integration in the infant rat. In: Lewkowicz DJ, Terrace H, editors. The development of intersensory perception: comparative perspectives. Erlbaum; Hillsdale: 1994. pp. 133–161. [Google Scholar]
- Tan S, Lam WP, Wai MS, Yu WH, Yew DT. Chronic ketamine administration modulates midbrain dopamine system in mice. PLoS One. 2012;7:e43947. doi: 10.1371/journal.pone.0043947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1989;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Zamora JJ, Warmoth KP. Increased response to ketamine following treatment at long intervals: implications for intermittent use. Biol Psychiatry. 2008;63:178–183. doi: 10.1016/j.biopsych.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Tso MM, Blatchford KL, Callado LF, McLaughlin DP, Stamford JA. Stereoselective effects of ketamine on dopamine, serotonin and noradrenaline release and uptake in rat brain slices. Neurochem Int. 2004;44:1–7. doi: 10.1016/s0197-0186(03)00104-9. [DOI] [PubMed] [Google Scholar]
- Turgeon SM, Anderson N, O’Loughlin K. Phencyclidine (PCP) produces sexually dimorphic effects on voluntary sucrose consumption and elevated plus maze behavior. Pharmacol Biochem Behav. 2010;95:173–178. doi: 10.1016/j.pbb.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Uchihashi Y, Kuribara H, Morita T, Fujita T. The repeated administration of ketamine induces an enhancement of its stimulant action in mice. Japanese J Pharmacol. 1993;61:149–151. doi: 10.1254/jjp.61.149. [DOI] [PubMed] [Google Scholar]
- Usun Y, Eybrard S, Meyer F, Louilot A. Ketamine increases striatal dopamine release and hyperlocomotion in adult rats after postnatal functional blockade of the prefrontal cortex. Behav Brain Res. 2013;256:229–237. doi: 10.1016/j.bbr.2013.08.017. [DOI] [PubMed] [Google Scholar]
- van der Kam EL, De Vry J, Tzschentke TM. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP) potentiates ketamine and heroin reward as assessed by acquisition, extinction, and reinstatement of conditioned place preference in the rat. Eur J Pharmacol. 2009;606:94–101. doi: 10.1016/j.ejphar.2008.12.042. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessinger WD. Sexual dimorphic effects of chronic phencyclidine in rats. Eur J Pharmacol. 1995;277:107–112. doi: 10.1016/0014-2999(95)00107-v. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Evans RL, Grainger DB, Nicholson KL. Locomotor activity changes in female adolescent and adult rats during repeated treatment with a cannabinoid or club drug. Pharmacol Rep. 2011;63:1085–1092. doi: 10.1016/s1734-1140(11)70627-2. [DOI] [PubMed] [Google Scholar]
- Wilson C, Cone K, Kercher M, Hibbitts J, Fischer J, Van Lake A, Sumner J. Naloxone increases ketamine-induced hyperactivity in the open field in female rats. Pharmacol Biochem Behav. 2005;81:530–534. doi: 10.1016/j.pbb.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Wilson C, Kercher M, Quinn B, Murphy A, Fiegel C, McLaurin A. Effects of age and sex on ketamine-induced hyperactivity in rats. Physiology and Behavior. 2007;91:202–207. doi: 10.1016/j.physbeh.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Monn JA, Schoepp DD, Li X, Overshiner C, Mitchell SN, Carter G, Johnson B, Rasmussen K, Rorick-Kehn LM. The rapidly acting antidepressant ketamine and the mGlu2/3 receptor antagonist LY341495 rapidly engage dopaminergic mood circuits. J Pharmacol Exp Ther. 2016;358:71–82. doi: 10.1124/jpet.116.233627. [DOI] [PubMed] [Google Scholar]
- Wood RD, Tirelli E, Snyder KJ, Heyser CJ, LaRocca TM, Spear LP. Evidence for behavioral sensitization to cocaine in preweanling rat pups. Psychopharmacology (Berl) 1998;138:114–123. doi: 10.1007/s002130050653. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nakayama T, Yamaguchi J, Matsuzawa M, Mishina M, Ikeda K, Yamamoto H. Role of the NMDA receptor GluN2D subunit in the expression of ketamine-induced behavioral sensitization and region-specific activation of neuronal nitric oxide synthase. Neurosci Lett. 2016;610:48–53. doi: 10.1016/j.neulet.2015.10.049. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Nazarian A, Crawford CA, McDougall SA. Cocaine-induced behavioral sensitization in the young rat. Psychopharmacology (Berl) 2000;151:291–298. doi: 10.1007/s002130000377. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]