Abstract
Background
After traumatic spinal cord injury (SCI), there is increased risk of venous thromboembolism (VTE), but chemoprophylaxis (PPX) may cause expansion of intraspinal hematoma (ISH).
Methods
Single-center retrospective study of adult trauma patients from 2012–2015 with SCI. Exclusion criteria: VTE diagnosis, death, or discharge within 48 hours. Patients were dichotomized based on early (≤48 hours) heparinoid and/or aspirin PPX. ISH expansion was diagnosed intraoperatively or by follow-up radiology. We used multivariable Cox proportional hazards to estimate the effect of PPX on risk of VTE and ISH expansion controlling for age, ISS, complete SCI, and mechanism as static covariates and operative spine procedure as a time-varying covariate.
Results
501 patients with SCI were dichotomized into early PPX (n=260, 52%) and no early PPX (n=241, 48%). Early PPX patients were less likely blunt-injured (91% vs 97%) and had fewer operative spine interventions (65% vs 80%), but age (median 43 vs 49 years), ISS (median 24 vs 21), admission ISH (47% vs 44%), and VTE (5% vs 9%) were similar.
Cox analysis found that early heparinoids was associated with reduced VTE (HR 0.37, 95% CI 0.16–0.84) and reduced pulmonary embolism (PE) (HR 0.20, 95% CI 0.06–0.69). The estimated number needed to treat with heparinoids was 10 to prevent one VTE and 13 to prevent one PE at 30 days. Early aspirin was not associated with reduced VTE or PE. Seven patients (1%) had ISH expansion, of which 4 were on PPX at time of expansion. Using heparinoid and aspirin as time-varying covariates, neither heparinoids (HR 1.90, 95% CI 0.32–11.41) nor aspirin (HR 3.67, 95% CI 0.64–20.88) was associated with ISH expansion.
Conclusion
Early heparinoid therapy was associated with decreased VTE and PE risk in SCI patients without concomitant increase in ISH expansion.
Level of Evidence
Level IV (Therapeutic)
Keywords: spinal cord injury, spinal cord hematoma, venous thromboembolism, pulmonary embolism
Introduction
Venous thromboembolism (VTE), comprising of deep vein thrombosis (DVT) and pulmonary embolism (PE), is a significant source of morbidity and mortality. In the United States, the overall incidence of VTE is estimated at 0.1–0.2% of the population per year, resulting in 300,000 PE-related deaths annually.1 For those who survive their initial injury, trauma patients are at increased risk of developing VTE. In 1961, Sevitt and Gallagher found that 65% of deceased trauma patients had DVT and 16.5% had PE in an autopsy-based study.2 Geert et al in 1994 reported that 58% of trauma patients without VTE prophylaxis developed DVT, the vast majority of which were asymptomatic at time of diagnosis.3 Currently, chemoprophylaxis with subcutaneous administration of heparinoids (unfractionated heparin [UFH] and low molecular weight heparin [LMWH]) is recommended for VTE prophylaxis after major trauma.4 Aspirin may further decrease the VTE risk in non-trauma5–7 and trauma patients8 when used in conjunction with heparinoids. However, VTE continues to represent a substantial disease burden in trauma patients despite initiation of chemoprophylaxis.9
Spinal cord injury (SCI) is independently associated with increased VTE risk among trauma patients,10 and this group would a priori seem to benefit from early and aggressive VTE prophylaxis. However, one important but unresolved issue in this setting is timing. Since these medications are anticoagulants, there is a theoretical increased risk of intraspinal (ISH) hematoma expansion (including epidural hematoma [EDH], subdural hematoma [SDH], subarachnoid hemorrhage [SAH], and intrameduallary hemorrhage) upon their initiation. Although the actual volume of bleeding which complicates VTE prophylaxis is likely small,11 even relatively small amounts of hemorrhage can cause spinal cord compression with catastrophic neurologic consequences. An expert opinion-based consensus statement from the North American Spine Society recommends that LMWH may be used postoperatively in patients at high risk for VTE (including multiple trauma), but should be decided carefully and on a case-by-case basis.12 However, the specific time interval after spine surgery to start LMWH was not defined. The objective of the present study was to estimate the risks of VTE versus ISH expansion after initiation of early (≤48 hours) heparinoid and/or aspirin prophylaxis in trauma patients with SCI.
Methods
Study Setting and Selection of Participants
Approval was obtained from the Institutional Review Board of the McGovern Medical School, a part of the University of Texas Health Science Center at Houston. We queried our trauma registry to perform a single-institution, retrospective study of consecutive adult (≥16 years) highest-level (based on triage criteria) trauma patients admitted between January 2012 to April 2015 and included patients with SCI based on Abbreviated Injury Scale (AIS) for analysis. We excluded patients with length of stay <48 hours, VTE diagnosed within the first 48 hours, and patients transitioned to comfort care within the first 48 hours. The length of follow-up was until discharge or 30 hospital days, whichever came first.
VTE prophylaxis
Our institutional guideline is to initiate VTE chemoprophylaxis in patients with spine fractures and SCI after the consulting spine surgeon has determined there is no emergent need for spinal cord decompression or stabilization. For those requiring operative spine intervention, chemoprophylaxis is held the night prior to surgery and for 24 hours post-operatively. For all patients, LMWH is preferred, while UFH is typically used only in cases of renal insufficiency. All patients are prescribed sequential compression devices (SCDs) unless contraindicated by bilateral lower extremity fractures. Aspirin is prescribed for a variety of indications including history of cardiovascular disease or pre-admission medication, blunt cerebrovascular injury, and at the discretion of the rounding physician.
Definitions
To account for stoppages and missed doses, we defined initiation of VTE prophylaxis as continuous therapy for >24 hours, and the time of initiation was defined as the time of the first such dose. Patients were dichotomized based on early (≤48 hours) versus no early initiation of heparinoids and/or aspirin for VTE prophylaxis. The timing and indication of therapeutic anticoagulation (by continuous heparin infusion) was also recorded.
Imaging reports by the attending radiologist were reviewed for presence of ISH (EDH, SDH, SAH, and intrameduallary hemorrhage). Almost all were magnetic resonance imaging (MRI) reports; rarely, CT reports were reviewed because MRI was contraindicated. Expansion of ISH was defined as 1) increased hematoma size on subsequent imaging, 2) new hematoma on subsequent imaging, or 3) worsening clinical exam with operative evacuation of ISH. Subsequent spine imaging was ordered in response to a change in physical exam or after operative spine intervention at the discretion of the consulting spine surgeon. If an emergent operation for spinal cord decompression was performed based on the initial clinical exam and admission spine imaging, this was not counted as ISH expansion.
DVT was defined as occurring in the popliteal vein or above and diagnosed by venous duplex ultrasonography. Any pulmonary emboli detected on computed tomography angiogram (CTA) including at the subsegmental level was defined as PE for this study. Of note, no routine screening for DVT or PE was performed at our institution. Instead, diagnostic imaging was performed on patients exhibiting symptoms suggestive of VTE; therefore, all DVT and PE reported in this study were symptomatic.
Statistical analysis
Stata 14.1 (Stata Corporation, College Station, TX) was used for all statistical calculations. Continuous data are expressed as median with interquartile range. Non-parametric comparisons of continuous data were performed by Wilcoxon rank-sum test. Comparisons of categorical data were performed by Chi-squared test or Fisher’s exact test for categories of ≤5. We used multivariable Cox regression (controlling for age, ISS, presence of complete SCI, and mechanism as static covariates and operative spine intervention as a time-varying covariate) to estimate the effect of early (≤48 hours) heparinoids and early aspirin on the risk of VTE and ISH expansion. We calculated the number needed to treat by the method described by Altman and Andersen for static covariates after Cox regression.13 UFH and LMWH were grouped into one category (“heparinoids”). Time of event was defined as the time of radiological imaging diagnosing VTE or ISH expansion, or the time of initiation of operation diagnosing ISH expansion. For the VTE analysis, patients were censored when therapeutic anticoagulation was started for any condition other than VTE (e.g. stroke, acute coronary syndrome, etc.) since these patients were no longer at risk for VTE. A two-tailed significance level of α=0.05 was used for all statistical tests.
Results
Demographics
During the 40-month study period, there were 17,583 highest-level trauma admissions, of which 566 (3.2%) had SCI. We excluded 65 patients for the following reasons: 37 patients who died within 48 hours (16 dead on arrival, 7 by exsanguination, 11 by TBI, 1 by acute coronary syndrome, 2 unknown), 5 patients who had care withdrawn within 48 hours, 19 patients who were discharged within 48 hours, and 4 patients with VTE diagnosed within 48 hours. The remaining 501 patients were dichotomized into early prophylaxis (PPX) (n=260, 52%) and no early PPX (n=241, 48%) groups based on initiation of prophylactic heparinoids and/or aspirin within 48 hours after hospital presentation.
The early PPX group had decreased incidence of blunt mechanism, but there were no differences in age, sex, injury severity, presence of bilateral lower extremity fractures, obesity (body mass index ≥30), any rapid thromboelastography (r-TEG) parameter including maximum amplitude (mA), SCI level, incidence of complete SCI, or presence of ISH on admission imaging (47% vs 44%) (Table 1). Most patients had cervical injuries. Early PPX patients were less likely to have undergone an operative spine intervention (65% vs 80%), although the time to operation was not different.
Table 1.
Demographics
| Variable | Early PPX (n=260, 52%) |
No Early PPX (n=241, 48%) |
p |
|---|---|---|---|
|
| |||
| Age (years) | 43 (28, 61) | 49 (30, 62) | 0.08 |
|
| |||
| Male (n, %) | 195 (75%) | 169 (70%) | 0.28 |
|
| |||
| Blunt (n, %) | 237 (91%) | 232 (97%) | <0.01 |
|
| |||
| AIS head ≥3 (n, %) | 174 (67%) | 171 (71%) | 0.27 |
|
| |||
| AIS chest ≥3 (n, %) | 136 (52%) | 102 (42%) | 0.03 |
|
| |||
| AIS abd ≥3 (n, %) | 46 (18%) | 60 (25%) | <0.05 |
|
| |||
| AIS extremity ≥3 (n, %) | 36 (14%) | 27 (11%) | 0.38 |
|
| |||
| Bilateral lower extremity fractures | 8 (3%) | 3 (1%) | 0.23 |
|
| |||
| ISS | 24 (17, 30) | 21 (16, 30) | 0.16 |
|
| |||
| Obese (BMI ≥30) (n, %) | 73 (28%) | 73 (30%) | 0.59 |
|
| |||
| Hemoglobin (g/dL) | 12.9 (11.6, 14.7) | 13.3 (11.7, 14.3) | 0.73 |
|
| |||
| Base excess (mEq/L) | −4 (−6, −1) | −3 (−6, 0) | 0.21 |
|
| |||
| Platelet count (x109/L) | 201 (161, 256) | 204 (162, 249) | 0.96 |
|
| |||
| rTEG ACT (sec) | 113 (105, 121) | 113 (105, 121) | 0.60 |
|
| |||
| rTEG K-time (min) | 1.4 (1.2, 1.8) | 1.4 (1.1, 1.8) | 0.56 |
|
| |||
| rTEG alpha angle (deg) | 73 (69, 76) | 73 (69, 77) | 0.50 |
|
| |||
| rTEG max amplitude (mm) | 63 (58, 68) | 64 (59, 68) | 0.26 |
|
| |||
| rTEG LY30 (%) | 0.9 (0.2, 2.4) | 1.1 (0.2, 2.6) | 0.69 |
|
| |||
| Spine cord injury level (n, %) | 0.19 | ||
| Cervical spine | 166 (63%) | 157 (66%) | |
| Thoracic spine | 70 (27%) | 51 (21%) | |
| Lumbar spine | 25 (10%) | 32 (13%) | |
|
| |||
| Complete injury (n, %) | 69 (27%) | 60 (25%) | 0.67 |
|
| |||
| ISH on admission (n, %) | 123 (47%) | 107 (44%) | 0.51 |
|
| |||
| Epidural hematoma | 113 (92%) | 96 (90%) | 0.94 |
|
| |||
| Subdural hematoma | 5 (4%) | 6 (5%) | |
|
| |||
| Other* | 5 (4%) | 5 (5%) | |
|
| |||
| Operative spine intervention | 169 (65%) | 193 (80%) | <0.001 |
|
| |||
| Time to operation (days) | 1.48 (0.63, 3.46) | 1.24 (0.70, 2.18) | 0.18 |
including subarachnoid and intraparenchymal hemorrhage
AIS, abbreviated injury scale; ISS, injury severity score; rTEG: rapid thromboelastography; ACT, activated clotting time; LY30, lysis at 30 minutes; ISH, intraspinal hematoma
Heparinoid and aspirin therapy
A greater number of patients in the early PPX group received heparinoids or aspirin at any time during their hospitalization (Table 2). Early PPX patients were relatively more likely to have received LMWH rather than UFH and relatively more likely to have received 325mg per day of aspirin rather than 81mg compared to no early ppx patients. The time to heparinoid and aspirin therapy are summarized in Table 2. The indication for aspirin therapy in 55 patients receiving early aspirin were blunt cerebrovascular injury (73%), pre-admission medication (18%), other blunt arterial injury (5%), and acute coronary syndrome (4%).
Table 2.
Heparinoid and aspirin usage
| Variable | Early PPX (n=260, 52%) |
No Early PPX (n=241, 48%) |
P |
|---|---|---|---|
| Any heparinoid* (n, %) | 254 (97%) | 205 (85%) | <0.001 |
| LMWH (n, %)† | 198 (78%) | 136 (66%) | <0.01 |
| UFH (n, %)† | 56 (22%) | 69 (34%) | |
| Time to heparinoid (days) | 1.12 (0.58, 1.65) | 2.94 (2.42, 3.83) | <0.001 |
| Any aspirin* (n, %) | 81 (31%) | 59 (24%) | 0.02 |
| Max dose 81mg (n, %)‡ | 17 (21%) | 27 (47%) | 0.001 |
| Max dose 325mg (n, %)‡ | 65 (79%) | 31 (53%) | |
| Time to aspirin (days) | 1.56 (0.67, 2.67) | 4.82 (3.30, 9.56) | <0.001 |
during hospital stay
proportion with LMWH versus UFH among patients receiving heparinoids
proportion with 81mg versus 325mg aspirin among patients receiving aspirin
LMWH, low molecular weight heparin; UFH, unfractionated heparin.
Estimated effect of early heparinoids and aspirin on VTE risk
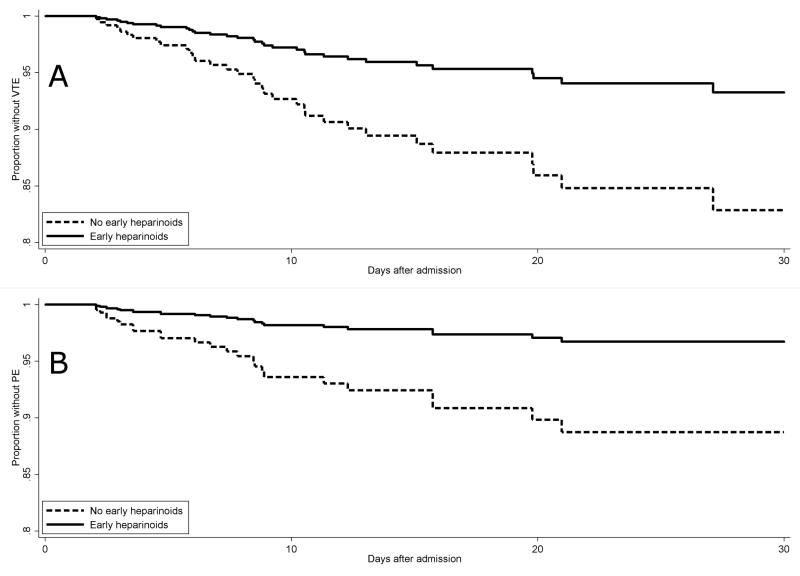
There was a trend toward decreased incidence of VTE in the early PPX group compared to the no early PPX group (5% vs 9%, p=0.06) (Table 3). In both groups, symptomatic PE was more common than symptomatic DVT. One PE was at the subsegmental level in a patient with hypoxemic respiratory failure and tachycardia without an alternative explanation for these symptoms. All other PEs were at the segmental level or above. One patient in the early PPX group died of PE. The time to VTE diagnosis was not different. On multivariable Cox analysis using age, ISS, and complete SCI as static covariates and operative spine intervention as a time-varying covariate, early heparinoids (hazard ratio 0.37, 95% confidence interval 0.16 – 0.84) but not early aspirin (HR 1.06, 95% CI 0.36 – 3.13) was associated with reduced VTE risk. Using the same covariates, early heparinoids (HR 0.20, 95% CI 0.06 – 0.69) but not early aspirin (HR 2.63, 95% CI 0.83 – 8.33) was associated with reduced PE risk. Visual inspection of the log-log plots (Supplement) as well as formal testing of Schoenfeld residuals for both models above found no evidence that the proportional hazards assumption was violated. The survival curves for the estimated impact of early heparinoid therapy on VTE and PE risk are shown in Figure 1a and 1b respectively. The estimated number needed to treat with early heparinoids is 10 patients to prevent one VTE by 30 days and 13 patients to prevent one PE by 30 days. A Cox model could not be successfully generated to estimate the effect of early heparinoids and early aspirin on DVT risk, likely due to the small number of DVT cases (n=8).
Table 3.
Outcomes
| Variable | Early PPX (n=260, 52%) |
No Early PPX (n=241, 48%) |
P |
|---|---|---|---|
| Venous thromboembolism (n, %) | 12 (5%) | 21 (9%) | 0.06 |
| Deep vein thrombosis (n, %) | 2 (1%) | 6 (3%) | 0.16 |
| Pulmonary embolism (n, %) | 10 (4%) | 15 (6%) | 0.21 |
| Time to venous thromboembolism (days) | 7.26 (5.19, 11.41) | 8.81 (4.70, 11.32) | 0.63 |
| Length of stay | 10 (6, 19) | 11 (6, 20) | 0.14 |
| Ventilator Days | 0 (0, 7) | 0 (0, 9) | 0.27 |
| Death (n, %) | 9 (3%) | 11 (5%) | 0.52 |
Figure 1.
a and b. Estimated effect of early heparinoid prophylaxis on risk of VTE and PE. Early (≤48 hours) heparinoid prophylaxis was associated with reduced risk of VTE with estimated number needed to treat of 10 to prevent one VTE at 30 days (a). Early heparinoid prophylaxis was associated with reduced risk of PE with estimated number needed to treat of 13 to prevent one PE at 30 days (b).
Estimated effect of heparinoids and aspirin on ISH expansion risk
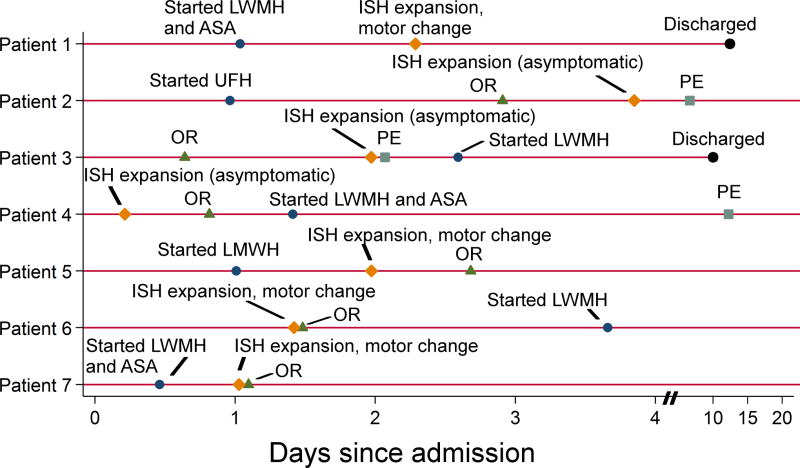
Seven patients had ISH expansion, giving an overall incidence of 1.4% in patients with traumatic SCI (n=501) and 3.0% in patients with ISH on admission (n=230). At the time of expansion diagnosis, two patients were on heparinoid therapy, two were on heparinoid and aspirin therapy, and the remaining three were on neither (Table 4). In two patients, asymptomatic ISH expansion was observed in follow-up imaging after an operative spine procedure. In two patients, head and spine imaging were obtained due to a change in motor exam, which was attributed to progression of brain injury rather than ISH expansion, and no spine procedure was performed. In three patients, operative decompression of ISH was performed due to a change in motor exam from potential ISH expansion. One patient who underwent urgent decompression remained quadriplegic at discharge; his symptoms were attributed to spinal cord edema rather than ISH. The remaining two patients underwent emergent decompression; one had recovered fully by the time of discharge, while the other suffered a devastating neurologic complication. The timing of ISH expansion relative to initiation of heparinoid and aspirin therapy for all seven patients are summarized in Figure 2. An additional 15 patients with ISH on admission underwent incidental hematoma evacuation during a spine stabilization procedure; these patients were not counted as having ISH expansion because the decision for operative intervention was made based on the admission physical and MRI findings, and there were no changes in clinical exam or additional imaging performed prior to OR. Prior to OR, only one of these patients was on heparinoid therapy, and none were on aspirin therapy.
Table 4.
Characteristics of patients with expansion of intraspinal hematoma
| Patient | Heparinoids or aspirin received prior to expansion? |
Method of expansion diagnosis | Clinical outcome |
|---|---|---|---|
| Patient 1 | Heparinoids and aspirin | Change in motor exam led to repeat MRI, which detected increased EDH extending C2-T2. | EDH was not cord-compressing and no intervention performed. Change in motor exam was attributed to progression of brain injury. |
| Patient 2 | Heparinoids only | Increased EDH at C4-C5 on MRI after operative spine stabilization. | Asymptomatic and no intervention performed. |
| Patient 3 | Neither | Increased SDH at C1-C3 on MRI after operative spine procedure. | Asymptomatic and no intervention performed. |
| Patient 4 | Neither | Repeat spine CT* to reevaluate unstable spine fracture and known EDH at C2-C3 detected new EDH at C6-C7. | Patient underwent urgent spine stabilization and evacuation of EDH at C2-C3 (no specific treatment of C6-C7 EDH). She remained quadriplegic at discharge. |
| Patient 5 | Heparinoids only | Change in motor exam led to repeat MRI, which detected increased EDH extending from C2 to T10. | Patient underwent urgent EDH evacuation and spinal fixation at C2 level. He remained quadriplegic at discharge, which was attributed to cord edema. |
| Patient 6 | Neither | Change in motor exam prompted intraoperative evacuation of C2-C6 EDH seen on admission imaging. | Moving all extremities at discharge. |
| Patient 7 | Heparinoids and aspirin | Change in motor exam led to repeat MRI, which detected increased C3-C7 EDH with cord compression. | Patient underwent emergent operative EDH evacuation, but was quadriplegic at discharge (prior to EDH expansion, he was moving three of four extremities). |
MRI contraindicated due to intracranial pressure monitor.
EDH, epidural hematoma; SDH, subdural hematoma.
Figure 2.
Timing of VTE chemoprophylaxis and ISH expansion in seven patients. The circumstances of intraspinal hematoma expansion including diagnosis, treatment, and clinical outcome are summarized in Table 4. LWMH, low molecular weight heparin; ASA, aspirin; ISH, intraspinal hematoma; PE, pulmonary embolism.
On multivariable Cox analysis using age, ISS, and complete SCI as static covariates and operative spine intervention, heparinoid therapy, and aspirin therapy as time-varying covariates, neither heparinoids (HR 1.90, 95% CI 0.32–11.41) nor aspirin (HR 3.67, 95% CI 0.64–20.88) was significantly associated with ISH expansion. A sensitivity analysis was performed for patients with ISH on admission (n=230): results were unchanged (heparinoids, HR 1.53, 95% CI 0.15–15.80; aspirin, HR 5.06, 95% CI 0.42–61.32). Visual inspection of the log-log plots (Supplement) as well as formal testing of Schoenfeld residuals for both models above found no evidence that the proportional hazards assumption was violated.
Discussion
Despite deliberate and escalating efforts to combat this disease, the incidence of VTE has doubled over the last ten years.14 Major trauma augments all components of Virchow’s triad, thereby significantly increasing the risk of VTE. Trauma patients have profoundly upregulated procoagulant mechanisms such as thrombin generating potential15 (hypercoagulability); they often have limited mobility due to critical illness, fractures, or pain (stasis); and endothelial injury (“endotheliopathy”16) occurs through a variety of mechanisms including direct tissue injury, catecholamines, and hypoperfusion.17 SCI increases this risk even further, possibly as a result of increased stasis due to paraplegia and quadriplegia. However, a difficult treatment conundrum is the theoretical increased risk of bleeding and ISH expansion upon initiating chemical VTE prophylaxis in patients with SCI.
Traumatic ISH is poorly described in the literature with a reported incidence of 0.5% to 7.5% from two case series.18, 19 In our study, we detected traumatic ISH in 220 patients out of 17,583 highest-level trauma activations during the 40-month time period, or an incidence of 1.3%. However, since we restricted our inclusion criteria to patients with SCI, we may have missed some patients who presented with ISH in the absence of SCI. To our knowledge, the natural history of traumatic ISH including its risk for expansion has not been described in the literature. Treatment guidelines are limited to expert opinion and anecdotal reports with suggestion of surgical decompression for patients demonstrating severe neurologic deficit or symptom progression, and conservative management suggested for patients with no symptom progression or early neurologic recovery.20 Of the 220 patients with ISH on admission in the present study, 7 patients (3.2%) had any ISH expansion as described above, 15 patients (6.8%) had documented incidental evacuation of ISH at time of surgery for spine stabilization, and no specific treatment was documented in the vast majority (90%) who had ISH on admission. Only one patient arguably suffered a neurologic complication as a direct result of ISH expansion, albeit a devastating one.
Our objective was to estimate the effect of chemical VTE prophylaxis on risk of developing VTE versus ISH expansion in patients with SCI. We found that early (≤48 hours) initiation of heparinoid prophylaxis was associated with decreased risk of VTE and PE in SCI patients, with a clinically-significant estimated number needed to treat of 10 for VTE and 13 for PE. This is largely consistent with a recent study by Tracy et al who found that earlier initiation of prophylactic LMWH was associated with decreased risk of VTE in patients with TBI and SCI.21 We did not find evidence that aspirin was associated with any independent benefit, likely because of the relatively small number of patients who received early aspirin (n=52, 10%), and our previous study estimated that the treatment effect for aspirin was a number needed to treat of 55 to prevent one VTE at 60 days when used in conjunction with standard (heparinoid-based) prophylaxis in trauma patients.8 We did not find any convincing evidence that early heparinoids or early aspirin therapy was associated with ISH expansion.
Nevertheless, given the magnitude of potential harm associated with ISH expansion, we advise caution when interpreting these results. One important caveat is that patients without early PPX had a higher incidence of operative spine interventions, and the timing of perioperative VTE prophylaxis with regard to spine surgeries continues to be controversial.12 In the orthopedic literature, dosing of VTE prophylaxis close to the time of surgery was associated with increased post-operative bleeding. A systematic review of 1,926 patients undergoing total hip replacement found that chemical VTE prophylaxis given up to 2 hours before or ≤4 hours after surgery resulted in increased bleeding risk (5–7%) compared to at least 12 hours before or ≥12 hours after surgery (1–3%) without a difference in DVT risk diagnosed by routine screening.22 However, such a study has not been performed in the spine surgery population. Finally, consideration of patient values and preferences in addition to scientific evidence was introduced in the American College of Chest Physicians VTE guidelines in 2004.23 Although we agree that patients should be aware of the magnitude of harm resulting from ISH expansion, the lack of scientific evidence regarding the baseline rate of ISH expansion and the effect of VTE prophylaxis on this risk was one of the primary motivators for this study.
Our study has several limitations. First, as a retrospective study, it is subject to the biases and limitations inherent in such a design. Specifically, the differing rates of operative spine intervention was a form of indication bias, but we attempted to mitigate this by including operative spine intervention as a time-dependent covariate in our analyses. However, further studies are needed to define minimum safe time windows in which to use chemical VTE prophylaxis for patients undergoing spine surgery. Second, although we found no association between early VTE prophylaxis and risk of ISH expansion, our study was likely underpowered to detect a difference given the small number of cases. Since follow-up imaging was not routinely performed, the true incidence of ISH expansion is likely underestimated, such as those with asymptomatic ISH expansion distal to a complete SCI. Additionally, we used imaging reports from the attending radiologist without independent adjudication of ISH findings. Nevertheless, we present evidence that the risk of ISH expansion is small compared to the magnitude of VTE risk reduction with early heparinoid prophylaxis. Third, we combined patients with UFH and LMWH into one group for analysis. Although studies in the past have suggested that LMWH was inferior to UFH as prophylaxis,24 the UFH dosing used in previous studies was less than that of current recommendations and was likely inadequate. A more recent study reported that UFH every 8 hours was potentially non-inferior compared to enoxaparin every 12 hours in 436 randomized trauma patients.25 When we analyzed LMWH and UFH separately, there was no longer a significant association between early prophylaxis and decreased VTE risk, likely due to lack of power. Fourth, no patients were followed up after hospital discharge, and the risk of VTE in SCI patients may continue to be elevated months after injury.26 Finally, we did not account for held or missed doses of heparinoids or aspirin after patients met the criteria for chemoprophylaxis initiation (>24 hours of continuous therapy). Since we dichotomized patients into groups based on initiation of prophylaxis within 48 hours, missed doses may have had an effect on the primary analysis in early prophylaxis patients who had a pause in their therapy. However, this would have increased the risk of VTE in the early prophylaxis group and decreased the likelihood of detecting a difference between groups in terms of VTE risk. These limitations were balanced by the primary strengths of this study, which were our time-to-event analyses and transparency in reporting the results of diagnostic tests after all Cox regression models. Additionally, to our knowledge we are the first in the literature to report the incidence of ISH expansion after trauma.
In conclusion, we found that the risk of ISH expansion was 1.4% in patients with traumatic SCI and 3.2% in patients with admission ISH. Early heparinoid, but not aspirin, prophylaxis was associated with reduced symptomatic VTE and PE risk without a concomitant increase in risk of ISH expansion. This supports early heparinoid prophylaxis for SCI patients not undergoing spine surgery within the first 48 hours. However, further studies to determine the minimum safe time window for heparinoid prophylaxis in SCI patients undergoing spine surgery are needed.
Supplementary Material
Acknowledgments
Funding: RC and MHS are supported by a T32 fellowship (grant no. 5T32GM008792) from NIGMS.
Footnotes
Conflicts of interest: All authors report no conflicts of interest.
This study was accepted for presentation at WTA as part of the Earl Young Competition on 3/6/17.
Author contributions: Study inception and design by RC, MHS, CEW, and JBH. Data acquisition by SDA and TJC. Data analysis by RC. All authors participated in manuscript preparation.
References
- 1.Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 Suppl):S495–501. doi: 10.1016/j.amepre.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Sevitt S, Gallagher N. Venous thrombosis and pulmonary embolism. A clinico-pathological study in injured and burned patients. Br J Surg. 1961;48:475–89. doi: 10.1002/bjs.18004821103. [DOI] [PubMed] [Google Scholar]
- 3.Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331(24):1601–6. doi: 10.1056/NEJM199412153312401. [DOI] [PubMed] [Google Scholar]
- 4.Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, Samama CM American College of Chest Physicians. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e227S–77S. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becattini C, Agnelli G, Schenone A, Eichinger S, Bucherini E, Silingardi M, Bianchi M, Moia M, Ageno W, Vandelli MR, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366(21):1959–67. doi: 10.1056/NEJMoa1114238. [DOI] [PubMed] [Google Scholar]
- 6.Brighton TA, Eikelboom JW, Mann K, Mister R, Gallus A, Ockelford P, Gibbs H, Hague W, Xavier D, Diaz R, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367(21):1979–87. doi: 10.1056/NEJMoa1210384. [DOI] [PubMed] [Google Scholar]
- 7.Pulmonary Embolism Prevention (PEP) trial Collaborative Group. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet. 2000;355(9212):1295–302. [PubMed] [Google Scholar]
- 8.Scerbo MH, Macaluso A, Pommerening MJ, Tomasek JS, Guervil DK, Wade CE, Cardenas JC, Cotton BA, Miller CC, Holcomb JB. Aspirin chemoprophylaxis decreases venous thromboembolism in 13,221 patients. American Association for the Surgery of Trauma 75th Annual Meeting; 2016 September 14–17; Waikoloa, HI. Chicago, IL. American Association for the Surgery of Trauma; 2016. Quickshot #16. [Google Scholar]
- 9.Paffrath T, Wafaisade A, Lefering R, Simanski C, Bouillon B, Spanholtz T, Wutzler S, Maegele M Trauma Registry of DGU. Venous thromboembolism after severe trauma: incidence, risk factors and outcome. Injury. 2010;41(1):97–101. doi: 10.1016/j.injury.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Toker S, Hak DJ, Morgan SJ. Deep vein thrombosis prophylaxis in trauma patients. Thrombosis. 2011;2011:505373. doi: 10.1155/2011/505373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leonardi MJ, McGory ML, Ko CY. The rate of bleeding complications after pharmacologic deep venous thrombosis prophylaxis: a systematic review of 33 randomized controlled trials. Arch Surg. 2006;141(8):790–7. doi: 10.1001/archsurg.141.8.790. [DOI] [PubMed] [Google Scholar]
- 12.Bono CM, Watters WC, Heggeness MH, Resnick DK, Shaffer WO, Baisden J, Ben-Galim P, Easa JE, Fernand R, Lamer T, et al. An evidence-based clinical guideline for the use of antithrombotic therapies in spine surgery. Spine J. 2009;9(12):1046–51. doi: 10.1016/j.spinee.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319(7223):1492–5. doi: 10.1136/bmj.319.7223.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knudson MM, Gomez D, Haas B, Cohen MJ, Nathens AB. Three thousand seven hundred thirty-eight posttraumatic pulmonary emboli: a new look at an old disease. Ann Surg. 2011;254(4):625–32. doi: 10.1097/SLA.0b013e3182300209. [DOI] [PubMed] [Google Scholar]
- 15.Cardenas JC, Rahbar E, Pommerening MJ, Baer LA, Matijevic N, Cotton BA, Holcomb JB, Wade CE. Measuring thrombin generation as a tool for predicting hemostatic potential and transfusion requirements following trauma. J Trauma Acute Care Surg. 2014;77(6):839–45. doi: 10.1097/TA.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 16.Johansson PI, Henriksen HH, Stensballe J, Gybel-Brask M, Cardenas JC, Baer LA, Cotton BA, Holcomb JB, Wade CE, Ostrowski SR. Traumatic Endotheliopathy: A Prospective Observational Study of 424 Severely Injured Patients. Ann Surg. doi: 10.1097/SLA.0000000000001751. Epub 2016 May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang R, Cardenas JC, Wade CE, Holcomb JB. Advances in the understanding of trauma-induced coagulopathy. Blood. 2016;128(8):1043–9. doi: 10.1182/blood-2016-01-636423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessel G, Bocher-Schwarz HG, Ringel K, Perneczky A. The role of endoscopy in the treatment of acute traumatic anterior epidural hematoma of the cervical spine: case report. Neurosurgery. 1997;41(3):688–90. doi: 10.1097/00006123-199709000-00039. [DOI] [PubMed] [Google Scholar]
- 19.Foo D, Rossier AB. Post-traumatic spinal epidural hematoma. Neurosurgery. 1982;11(1 Pt 1):25–32. doi: 10.1227/00006123-198207010-00006. [DOI] [PubMed] [Google Scholar]
- 20.Pillai A, Crane E, Chappell A, Buchan M. Traumatic cervical hematomyelia: report of a rare spinal cord injury without radiographic abnormality. J Trauma. 2008;65(4):938–41. doi: 10.1097/01.ta.0000197909.10358.0f. [DOI] [PubMed] [Google Scholar]
- 21.Tracy BM, Dunne JR, O'Neal CM, Clayton E. Venous thromboembolism prophylaxis in neurosurgical trauma patients. J Surg Res. 2016;205(1):221–7. doi: 10.1016/j.jss.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 22.Strebel N, Prins M, Agnelli G, Büller HR. Preoperative or postoperative start of prophylaxis for venous thromboembolism with low-molecular-weight heparin in elective hip surgery? Arch Intern Med. 2002;162(13):1451–1456. doi: 10.1001/archinte.162.13.1451. [DOI] [PubMed] [Google Scholar]
- 23.Schünemann HJ, Cook D, Grimshaw J, Liberati A, Heffner J, Tapson V, Guyatt G. Antithrombotic and thrombolytic therapy: from evidence to application: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004 Sep;126(3 Suppl):688S–696S. doi: 10.1378/chest.126.3_suppl.688S. [DOI] [PubMed] [Google Scholar]
- 24.Geerts WH, Jay RM, Code KI, Chen E, Szalai JP, Saibil EA, Hamilton PA. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med. 1996;335(10):701–7. doi: 10.1056/NEJM199609053351003. [DOI] [PubMed] [Google Scholar]
- 25.Olson EJ, Bandle J, Calvo RY, Shackford SR, Dunne CE, Van Gent JM, Zander AL, Sikand H, Bongiovanni MS, Sise MJ, Sise CB. Heparin versus enoxaparin for prevention of venous thromboembolism after trauma: A randomized noninferiority trial. J Trauma Acute Care Surg. 2015;79(6):961–8. doi: 10.1097/TA.0000000000000750. [DOI] [PubMed] [Google Scholar]
- 26.Alabed S, Belci M, Van Middendorp JJ, Al Halabi A, Meagher TM. Thromboembolism in the Sub-Acute Phase of Spinal Cord Injury: A Systematic Review of the Literature. Asian Spine J. 2016;10(5):972–981. doi: 10.4184/asj.2016.10.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.