Abstract
Background
Topical corticosteroids or six-food elimination diet are recommended as initial therapy for eosinophilic esophagitis (EoE).
Aims
We aimed to summarize published manuscripts that report outcomes of these therapies for EoE.
Methods
We performed a systematic review in MEDLINE, Web of Science, and Embase of published manuscripts describing topical fluticasone, topical budesonide, and six-food elimination diet as therapies for EoE. We conducted meta-analysis of symptom improvement and the change in peak mucosal eosinophil count, with heterogeneity between studies examined with meta-regression analysis.
Results
Systematic review yielded 51 articles that met inclusion criteria. Summary histologic response rates were 68.3% (95% prediction limits [PL] 16.2 to 96.0%) for fluticasone, 76.8% (95% PL 36.1 to 95.1%) for budesonide, and 69.0% (95% PL 31.9 to 91.4%) for six-food elimination diet. Corresponding decreases in eosinophil counts were 37.8 (95% PL 19.0 to 56.7), 62.5 (95% PL 125.6 to −0.67, and 44.6 (95% PL 26.5 to 62.7), respectively. Symptom response rates were 82.3% (95% PL 68.1 to 91.1%), 87.9% (95% PL 42.7 to 98.6%), and 87.3% (95% PL 64.5 to 96.3%), respectively. Meta-regression analyses decreased the initially large estimate of residual heterogeneity and suggested differences in histologic response rate associated with study populations’ baseline eosinophil count and age.
Conclusions
The literature describing topical corticosteroids and six-food elimination diet consists of small studies with diverse methods and population characteristics. Meta-analysis with meta-regression shows initial histologic and symptomatic response rates on the same order of magnitude for topical corticosteroids and six-food elimination diet, but heterogeneity of study designs prevent direct comparison of modalities.
Keywords: eosinophilic esophagitis, meta-analysis, corticosteroids, dietary elimination therapy, outcomes
Introduction
Eosinophilic esophagitis (EoE) is a clinicopathologic disease with a substantial healthcare burden [1,2]. Clinical guidelines suggest patients diagnosed with EoE, defined as the failure of esophageal eosinophilia to resolve after effective acid suppression, should receive a therapy with the aim of decreasing mucosal eosinophil counts, and recommend either topical corticosteroids (tCS) or the six-food elimination diet (SFED; removal of dairy, wheat, egg, soy, nuts, and seafood) as initial therapy [1,3]. However, there are no studies that directly compare these two treatment modalities.
In addition to SFED, other dietary approaches have been examined for therapy of EoE [4]. A four-food elimination diet, elemental diet, and allergy-test targeted elimination diets have been studied. Swallowed topical ciclesonide has also been studied as an alternative tCS for EoE [5]. These treatments are not addressed in this manuscript because the meta-regression techniques used here require a large number of studies for each modality.
Previously published systematic reviews and/or meta-analyses have considered tCS and SFED in isolation but have included studies that have been heterogeneous in design and outcomes [6–13]. Novel meta-regression techniques are particularly useful in such cases, as they can explore the high residual heterogeneity reported as a limitation in prior meta-analyses describing dietary and steroid therapies [6–13]. Such analysis can also give insight into differences in outcomes associated with study design or baseline population characteristics, but this has never been done for EoE. The most useful estimate for clinicians in describing a treatment to patients is its average effectiveness in a population of similar patients. The overall mean reported in classical fixed and random effects meta-analysis can produce biased estimates and erroneously narrow confidence intervals if there are systematic differences in populations or the treatments [14], and this limitation can be addressed by meta-regression.
Aims
The aims of this study were: 1) To perform a systematic review of studies that describe SFED and tCS as therapies for EoE; 2) To describe design features, outcomes reported, and characteristics of study populations for all included studies; and 3) To perform stratified meta-analysis and meta-regression analysis on groups of included studies with similarly specified outcomes.
Methods
We performed a systematic review of published manuscripts describing topical fluticasone, topical budesonide, and SFED as therapies for EoE, in accordance with the PRISMA guidelines [15]. Our search strategy was developed in consultation with a dedicated research librarian with expertise in systematic review methods. We searched MEDLINE, Web of Science, and Embase databases using search settings and filters as described in Table 1. The search was independently performed by two investigators (CCC and SE) who reviewed all titles and abstracts, and selected articles for detailed review and data extraction. For meta-analysis, we selected articles from those included in the systematic review that reported the mean and standard deviation of eosinophil counts (eosinophils per high-powered field; eos/hpf) before and after treatment, or the proportion of patients with histologic response defined as a threshold of eos/hpf. We also performed meta-analyses of studies that reported the proportion of patients with improvement in symptoms or reported symptoms in a way that could be simplified into a dichotomous improvement variable (yes or no).
Table 1.
Search Text, Filters, and Settings for MEDLINE, Embase, and Web of Science Searches.
| Search Text | Filters and Settings | |
|---|---|---|
| MEDLINE search: | Eosinophili*[tw] AND Esophagitis[tw] AND (dilat*[tw] OR fluticasone[tw] OR budesonide[tw] OR ciclesonide[tw] OR steroid*[tw] OR diet[tw] OR diets[tw] OR dietary[tw] OR food*[tw]) | Default |
| Embase search: | Eosinophili*:de,ti,ab AND Esophagitis:de,ti,ab AND (dilat*:de,ti,ab OR fluticasone:de,ti,ab OR budesonide:de,ti,ab OR ciclesonide:de,ti,ab OR steroid*:de,ti,ab OR diet:de,ti,ab OR diets:de,ti,ab OR dietary:de,ti,ab OR food*:de,ti,ab) | Embase, Embase and Medline; Reviews, Articles, and Conference Reviews. |
| Web of science search: | Eosinophili* AND Esophagitis AND (dilat* OR fluticasone OR budesonide OR ciclesonide OR steroid* OR diet OR diets OR dietary OR food*) | Articles, Reviews. |
|
| ||
| Criteria for study selection: | Journal articles that report measures of the effect of topical fluticasone, topical budesonide, or six-food elimination diet on eosinophil counts, adverse events, or symptoms. | |
Heterogeneity between studies was examined in meta-regression analysis. Potential meta-regression moderators were selected a priori based on potential importance in describing differences in study populations or methods, as well as their availability in published manuscripts. Moderators included were study design as trial or cohort, proportion of male subjects, mean age, proportion with a PPI trial or pH/impedance testing to diagnose EoE, proportion of subjects with prior therapy, whether maximum eosinophil counts were reported overall or in the distal esophagus, and baseline mean maximum eosinophil count. Quantifying studies’ risk of bias was not attempted to be quantified because studies could be subject to bias in different directions, while adjustment for risk of bias would assume all bias was in the same direction. Moderators were significance-tested using a bootstrap permutation method to estimate p [16]. Moderators with individual two-sided p value less than 0.2 were included for iterative reverse selection by maximum p value with a criterion for retention of p less than 0.05.
Meta-analyses were presented in forest plots. The summary estimate of the mean with 95% confidence limits was presented as a diamond and the 95% prediction limits (PL) were presented as brackets with a dotted line. Prediction limits include estimated residual heterogeneity between studies in addition to sampling error of the overall mean [17]. For analyses with one or more statistically significant meta-regression moderators, the predicted mean at common values of the moderator was presented beneath the overall random effects estimate and the studies were sorted in forest plots by descending value of the most statistically significant moderator. If no moderator was statistically significant, the studies were ordered by publication date.
Data for meta-analysis were abstracted from study text, tables, and figures. Parameters of interest were obtained from figures when possible and when estimates of interest were not present in text or tables. The outcome of histologic response was specified in several different ways: as a threshold, as a difference, and as a ratio. For meta-analysis of the difference and ratio, studies that did not provide the eosinophil counts at baseline and follow-up were excluded. Meta-analysis and meta-regression models were fit using mixed-effects models with the Hodges estimator of between-study variance. Statistical analysis was performed in R version 3.3.0 using the metafor package version 1.9 [18,19].
Results
The systematic review search in MEDLINE, Web of Science, and Embase databases yielded 1533 unique records (Figure 1). Of these, 193 full articles were considered pertinent based on the title or abstract, and 51 were selected for inclusion: 33 articles describing therapy with topical fluticasone [20–52], 17 articles describing therapy with topical budesonide [28,32–34,36,44,51,53–62], and 9 articles describing therapy with SFED [60,63–70]. Manuscripts started in year of publication in 2003 among studies of topical fluticasone, 2007 among studies of topical budesonide, and 2006 among studies of SFED. Four randomized trials described topical fluticasone therapy and five described topical budesonide therapy, and none described SFED.
Figure 1.
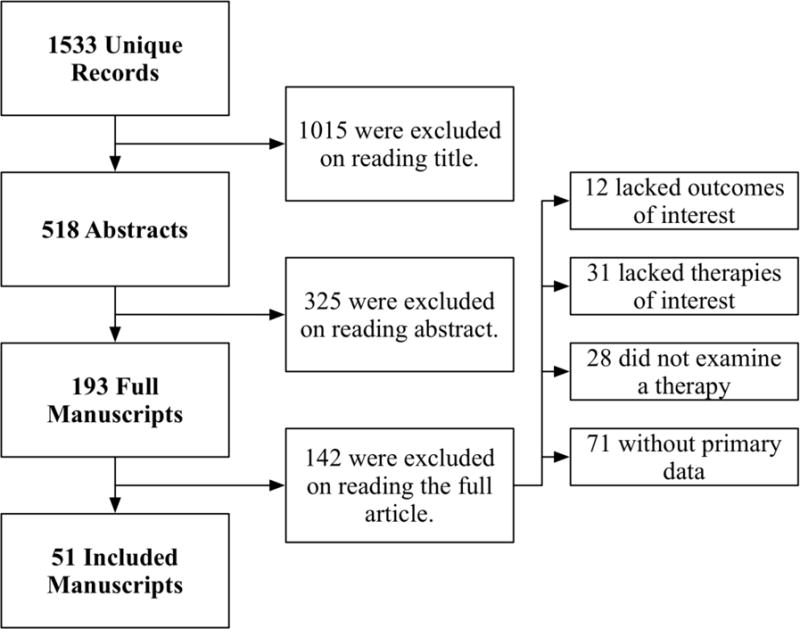
Inclusion of 36 Articles for Meta-analysis and 51 Articles for Systematic Review from the 1533 Unique Articles Retrieved May 12, 2016 from MEDLINE, Web of Science, and Embase Search.
Histologic response was reported as a proportion under a threshold of eos/hpf in the largest subset of studies included in systematic review [21,25,30,31,37,43,45–48,50,52–57,59–63,65–70]. The most common threshold was 15 eos/hpf, though two studies used thresholds of near but not 15 eos/hpf and were included with an adjustment term for the difference of reported threshold from 15 [46,52,57,60,62,71]. Without meta-regression moderators, the predicted overall mean proportion of patients with a threshold histologic response to topical fluticasone was 68.3% (95% PL 16.2 to 96.0%). Studies of topical fluticasone where diagnosis of EoE was confirmed for all subjects with a PPI trial or pH/Impedance testing had significantly higher proportions with histologic response (Figure 2a). The estimated proportion of patients with histologic response to topical budesonide was 76.8% (95% PL 36.1 to 95.1%) (Figure 2b). The estimated proportion of patients with histologic response to SFED was 69.0% (95% PL 31.9 to 91.4%). A significant meta-regression moderator was not identified in studies of threshold histologic response for SFED (Figure 2c).
Figure 2.
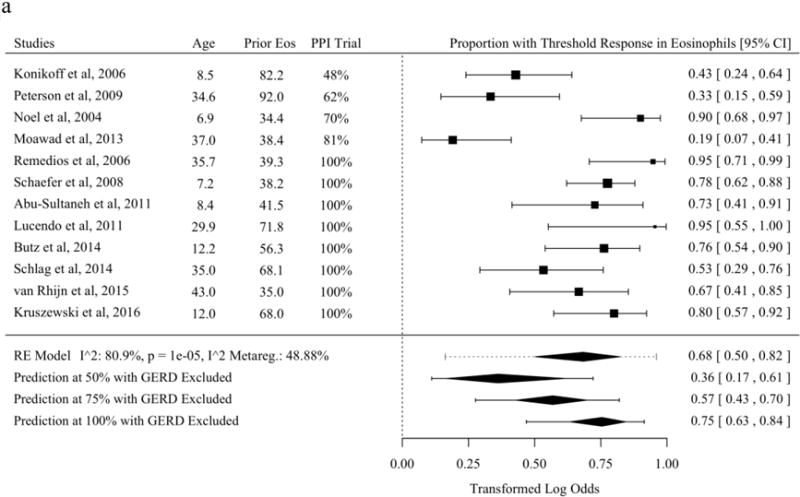
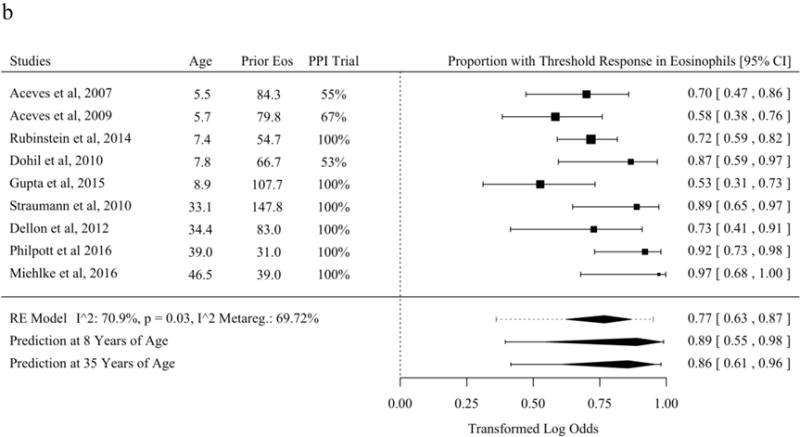
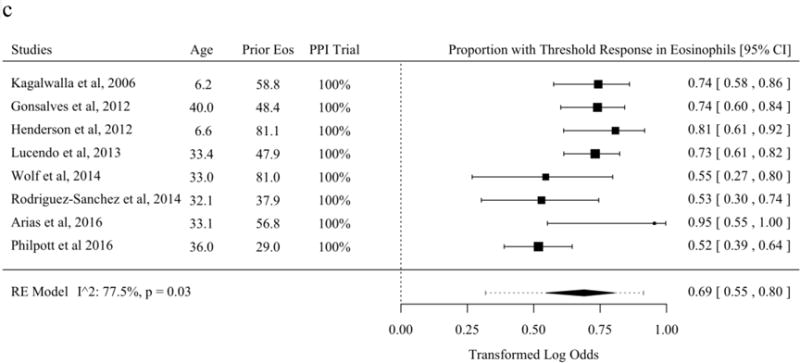
Proportion of Subjects with Histologic Response Defined as a Threshold of Eosinophils per High-Power Field of or Near Fifteen After Therapy in Studies of Topical Fluticasone (A), Topical Budesonide (B), and Six-Food Elimination Diet (C) With Additional Study Details and Summary Estimates with 95% Prediction Limits. Studies are sorted in order of the statistically significant meta-regression moderator or by year if no moderator was significant at a threshold p < 0.05. Studies from systematic review that included the proportion of patients with response at a threshold of or near 15 eosinophils per high-power field without missing values for meta-regression variables were included.
A subset of studies that reported histologic response as the mean and standard deviations of eosinophils per HPF before and after treatment allowed meta-analysis of the mean difference [21,30,31,37,43,45,47,48,50,53–56,59,60,62,63,65–68,70]. The mean difference before and after therapy with topical fluticasone was a decrease of 37.8 eosinophils per HPF (95% PL 19.0 to 56.7). No meta-regression moderator was statistically significant for studies of mean difference of fluticasone (Figure 3a). The mean difference before and after therapy with topical budesonide was a decrease of 62.5 (95% PL 125.6 to −0.7). Studies of topical budesonide with a higher baseline peak eosinophil count per HPF had a significantly greater decrease in eosinophil count after treatment (Figure 3b). The mean difference before and after therapy with SFED was a decrease of 44.6 eosinophils per HPF (95% PL 26.5 to 62.7). As with studies of budesonide, studies of SFED with a higher baseline peak eosinophil count per HPF had a significantly greater decrease in eosinophil count before and after treatment (Figure 3c).
Figure 3.
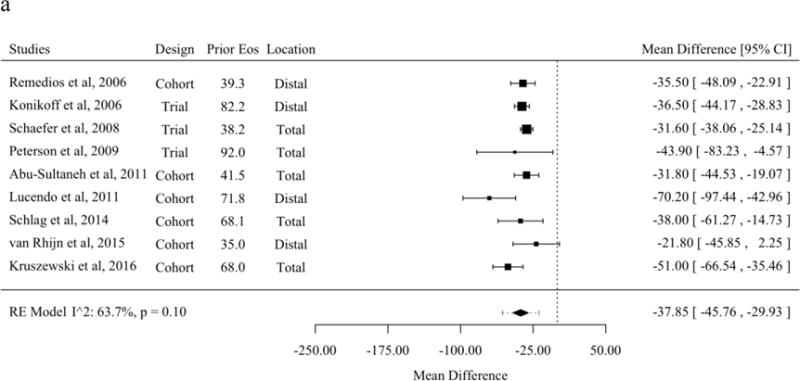
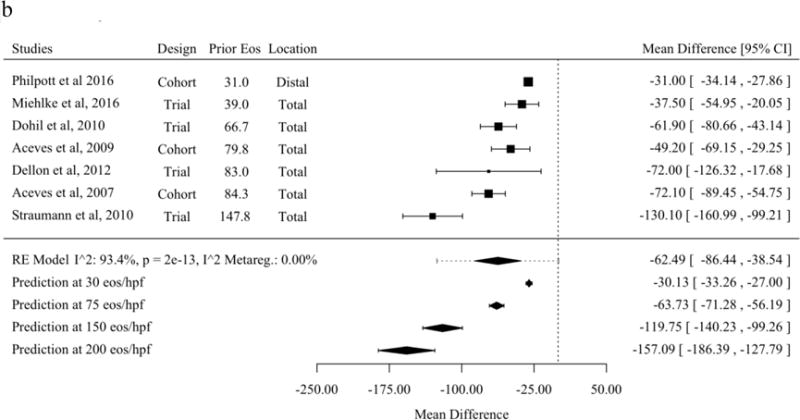
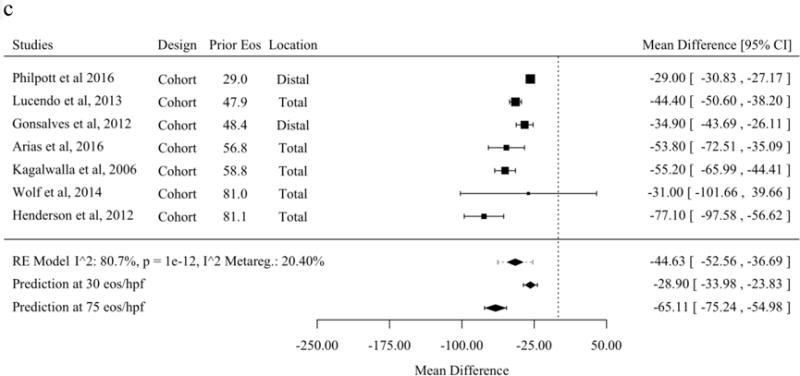
Mean Difference for Eosinophils Before and After Therapy in Studies of Topical Fluticasone (A), Topical Budesonide (B), and Six-Food Elimination Diet (C) in Order of Publication Date or, if Present, Selected Meta-Regression Moderator. Studies are sorted in order of the statistically significant meta-regression moderator or by year if no moderator was significant at a threshold p < 0.05. Studies from systematic review that included the mean and standard deviation of eosinophils per high-power field before and after therapy without missing values for meta-regression variables were included.
The subset of studies that reported individual peak eosinophil counts before and after treatment or the Pearson correlation coefficient between counts before and after treatment were included in a meta-analysis of the ratio of maximum esophageal eosinophils before treatment to the maximum after treatment [21,30,31,37,45,47,48,50,53,54,56,59,63,65–67]. The mean ratio before and after therapy with topical fluticasone was 0.20 (95% PL 0.03 to 1.49), with topical budesonide was 0.11 (95% PL 0.01 to 0.87), and with SFED was 0.10 (95% PL 0.02 to 0.39). No meta-regression moderators were statistically significant in analyses of the mean ratio (Supplemental Figures 1a–c).
The literature describing symptom response from tCS and SFED partially overlaps with the literature describing histologic response [21–23,27,37,39,40,45,47,54,56,62,63,65–68,70]. The proportion of patients with symptom improvement was 82.3% (95% PL 68.1 to 91.1%) among studies of topical fluticasone. The proportion of patients with symptom improvement was 87.9% (95% PL 42.7 to 98.6%) among studies of topical budesonide. The proportion of patients with symptom improvement was 87.3% (95% PL 64.5 to 96.3%) among studies of SFED. No meta-regression moderators were statistically significant in analyses of the mean ratio (Figures 4a–c).
Figure 4.
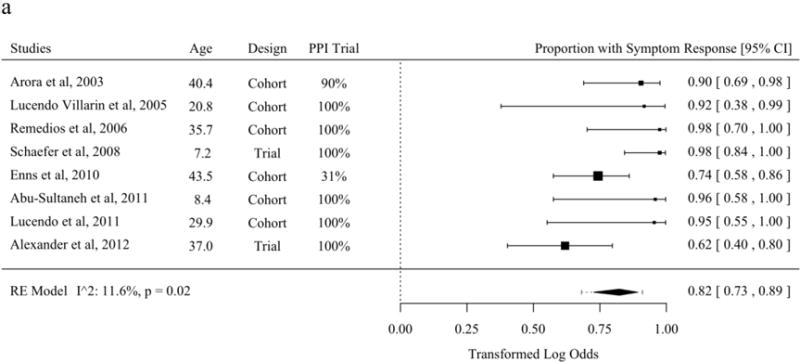
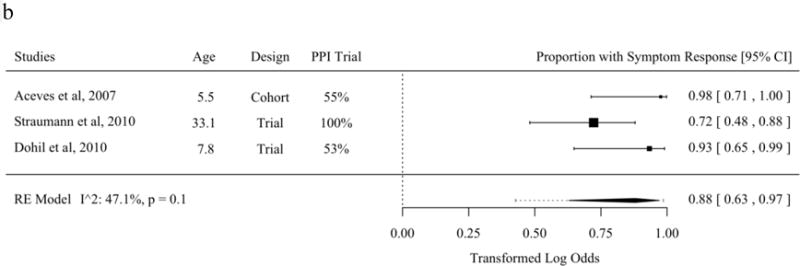
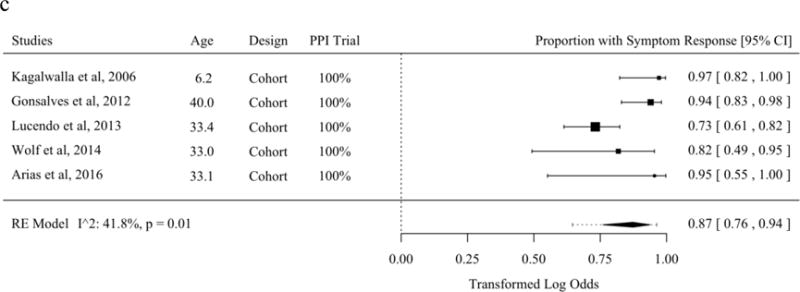
Proportion of Patients with Symptom Response in Studies of Topical Fluticasone (A), Topical Budesonide (B), and Six-Food Elimination Diet (C) in Order of Publication Date or, if Present, Selected Meta-Regression Moderator. Studies are sorted in order of the statistically significant meta-regression moderator or by year if no moderator was significant at a threshold p < 0.05. Studies from systematic review that included the proportion of patients with symptom response without missing values for meta-regression variables were included.
Discussion
Current guidelines recommend that either tCS or dietary elimination, typically with SFED, are acceptable first line treatments for EoE [1,3]. There have been no clinical studies directly comparing these modalities, and meta-analyses to date have been performed for each of the treatments in isolation without meta-regression. We therefore performed a meta-analysis with meta-regression of studies that examined the effects of topical fluticasone, topical budesonide, and SFED on peak mucosal eosinophil count and symptom response, based on data derived from the most inclusive systematic review of studies of tCS and SFED for EoE to date. We found that patients treated with all three therapies generally had improvement in symptoms and reduction in mean eosinophil count, but substantial uncertainty remains about which is most effective. Meta-regression analysis techniques showed studies’ methods and characteristics of study populations could explain some of the hetereogeneity between studies and suggested differences in histologic response rate associated with study populations’ baseline peak eosinophil counts and age.
The clinical implications of this work are that topical fluticasone, topical budesonide, and SFED are generally effective treatments for eosinophilic esophagitis, and response rates are roughly of the same order of magnitude. It is premature to conclude, however, that one of these therapies is most effective for eosinophil counts or symptom improvement given the currently available literature, and given the overlap of wide probability limits between the different treatment modalities. Prior meta-analysis with classical methods and stringent criteria for study inclusion (primarily with RCTs) were well designed to show that tCS or SFED were effective for initial therapy of EoE in highly selected clinical trial populations [8,11–13]. Our findings support these studies’ conclusions, but caution the clinician that generalization of their estimates to particular populations that differ demographically or histologically from the included studies may not be appropriate.
We also found that there were substantial gaps in current knowledge describing EoE treatments. Only a few studies examined the effectiveness of maintenance therapy with a mucosal agent [25,72,73]. Very little is known about the longitudinal course of EoE patients under treatment, especially among patients on SFED, and what is known suggests relapse is common if treatments are stopped. Only two retrospective studies examined the effectiveness of second-line therapies, though at least a fifth to a third of patients fail to respond to first line therapies [34,51].
Our study is limited in that we did not directly compare the results of our analyses between tCS and dietary elimination. Our findings show that such an analysis would be problematic because study outcomes seem to vary non-randomly with factors other than the choice of therapy. For example, study results were correlated with subject age, baseline maximal eosinophil count, and the rigor of studies’ diagnosis of EoE. In a direct comparison it would be unclear whether outcomes differed because of effectiveness of the interventions or variability in these features. This study is also limited in that it provides no new primary data but rather summarizes and interprets existing literature. Additionally, the definition of symptom improvement varied widely between included studies, and this outcome is limited in that a standard symptom score is not reported in most of the studies. Conclusions about the effectiveness of SFED should be tempered by the absence of a randomized study and potential challenges of adherence to an elimination diet [74,75].
The strengths of the study include the rigorous and comprehensive systematic review, analysis of both threshold eosinophil count responses as well as absolute and relative change in counts, stratifying analysis by tCS type (fluticasone vs budesonide), and incorporating novel meta-regression methods. Moreover, we have included more studies and therefore more patients under treatment than other recent meta-analyses of EoE treatment.
In summary, this systematic review with meta-analysis using meta-regression techniques shows histologic response rates for tCS and SFED ranging from 68–77%, decreasing in eosinophil counts ranging from 38–63 eos/hpf, and symptom responses of 82–88%. Future studies should compare therapies to one another in a randomized study design, report symptoms and endoscopic findings using validated instruments, and report mucosal response as the proportion of subjects with a total esophageal peak eosinophil count under 15 using a standard area of high-power field [76]. For this purpose, validated symptom scores are available for adults and children [77,78], and a standardized endoscopic findings score has emerged [79]. In addition, future studies should examine the durability of each therapy in maintaining remission, and consider analyses stratified by baseline age and maximum eosinophil count. Until these data are available, the recommendation in guidelines that either dietary elimination or topical steroids could be considered as a first line treatment for EoE after non-response to PPIs, remains reasonable.
Supplementary Material
S-1 Mean Ratio for Eosinophils Before and After Therapy in Studies of Topical Fluticasone (A), Topical Budesonide (B), and Six-Food Elimination Diet (C) in Order of Publication Date or, if Present, Selected Meta-Regression Moderator. Studies are sorted in order of the statistically significant meta-regression moderator or by year if no moderator was significant at a threshold p < 0.05. Studies from systematic review that included the mean and standard deviation of eosinophils per high-power field before and after therapy and the Pearson correlation coefficient without missing values for meta-regression variables were included.
Table 2.
The 51 Included Studies in Systematic Review and Their Study Design, Mean Characteristics, Therapies, Outcomes, and Adverse Events Reported.
| Author and Year | Study Design | Age, Male, N | Therapies Described | Outcomes Reported | Adverse Events |
|---|---|---|---|---|---|
| Studies of Topical Fluticasone | |||||
|
| |||||
| Teitelbaum et al, 2002* | Prospective cohort | 8, 74%, 15 | Topical fluticasone, specific elimination | Eos per HPF, other immune cells | 13% candida |
| Arora et al, 2003 | Retrospective cohort | 40.4, 81%, 21 | Topical fluticasone | Symptoms (binary†) | Not reported |
| Noel et al, 2004 | Retrospective cohort | 6.9, 75%, 20 | Topical fluticasone | Symptoms (3 level§) | 20% candida |
| Lucendo Villarín et al, 2005 | Prospective cohort | 20.8, 80% 5 | Topical fluticasone | Symptoms (binary†), eos per HPF | Not reported |
| Konikoff et al, 2006 | Randomized trial | 8.5, 81%, 21 | Topical fluticasone | Symptoms (specific‡), eos per HPF | 5% candida |
| Remedios et al, 2006 | Prospective cohort | 35.7, 32%, 19 | Topical fluticasone | Eos per HPF | 16% candida |
| Assa’ad et al, 2007* | Retrospective cohort | 6.2, 78.6%, 89 | Topical fluticasone, specific elimination | EoE resolution | Not reported |
| Lucendo et al, 2007* | Prospective cohort | 36.2, 90%, 30 | Topical fluticasone | Endoscopic findings | Not reported |
| Pasha et al, 2007* | Retrospective cohort | 44, 74%, 42 | Topical fluticasone | Symptoms (binary†) | Not reported |
| Helou et al, 2008* | Cross-sectional | 34.9, 59%, 32 | Topical fluticasone | Symptoms (MDQ) | Not reported |
| Schaefer et al, 2008 | Randomized trial | 7.2, 70%, 40 | Topical fluticasone | Symptoms (specific‡), eos per HPF | 15% candida |
| Pentiuk et al, 2009* | Cross-sectional | 10.6, 89%, 14 | Topical fluticasone | Symptoms (score°), eos per HPF | Not reported |
| Sayej et al, 2009 | Prospective cohort | 10.4, 59%, 15 | Topical fluticasone | Eos per HPF | Not reported |
| Peterson et al, 2009 | Randomized trial | 34.6, 73%, 15 | Topical fluticasone | Symptoms (score°), eos per HPF, endoscopic findings | Not reported |
| Enns et al, 2010 | Retrospective cohort | 43.5, 76%, 35 | Topical fluticasone | Symptoms (binary†), endoscopic findings | Not reported |
| Abe et al, 2010* | Retrospective cohort | 38.5, 100%, 2 | Topical fluticasone | Eos per HPF, endoscopic findings | Not reported |
| Abu-Sultaneh et al, 2011 | Retrospective cohort | 8.4, 45%, 11 | Topical fluticasone | Symptoms (specific‡), eos per HPF | Not reported |
| Lucendo et al, 2011 | Prospective cohort | 29.9, 90%, 10 | Topical fluticasone | Symptoms (binary†), eos per HPF | Not reported |
| Alexander et al, 2012 | Randomized trial | 37.0, 86, 21 | Topical fluticasone | Symptoms (binary†), eos per HPF | 26% candida |
| Czaja-Bulsa et al, 2012 | Retrospective cohort | 9.2, 79%, 2 | Topical fluticasone | Symptoms (binary†) | Not reported |
| Li et al, 2012* | Prospective cohort | 35.0, 88%, 8 | Topical fluticasone + monteleukast | Symptoms (binary†) | Not reported |
| Moawad et al, 2013 | Randomized trial | 37.0, 90%, 21 | Topical fluticasone | Symptoms (MDQ), eos per HPF, endoscopic findings | 5% candida |
| Butz et al, 2014 | Retrospective cohort | 12.2, 79%, 21 | Topical fluticasone | Eos per HPF, durability | 0% AI |
| Schlag et al, 2014 | Prospective cohort | 35.0, 93%, 15 | Topical fluticasone | Eos per HPF, serum ECP, symptoms (score°) | Not reported |
| van Rhijn et al, 2015 | Prospective cohort | 43.0, 67%, 15 | Topical fluticasone | Eos per HPF, mucosal integrity | Not reported |
| Kruszewski et al, 2016 | Prospective cohort | 12.0, 63%, 20 | Topical fluticasone | Symptoms (PedsQL), eos per HPF, endoscopic findings | Not reported |
|
| |||||
| Studies of Topical Budesonide | |||||
|
| |||||
| Aceves et al, 2007 | Retrospective cohort | 5.5, 80%, 20 | Topical budesonide | Symptoms (score°), eos per HPF | 5% candida |
| Aceves et al, 2009 | Retrospective cohort | 5.7, 74%, 24 | Topical budesonide | Eos per HPF | Not reported |
| Dohil et al, 2010 | Randomized trial | 7.8, 80%, 15 | Topical budesonide | Symptoms (score°), eos per HPF, endoscopic findings | 7% candida, 0% AI |
| Straumann et al, 2010 | Randomized trial | 33.1, 94%, 18 | Topical budesonide | Symptoms (score°), eos per HPF, endoscopic findings | 17% candida |
| Dellon et al, 2012 | Randomized trial | 34.4, 58%, 11 | Topical budesonide | Symptoms (MDQ), eos per HPF, endoscopic findings | 18% candida, 0% AI |
| Rubinstein et al, 2014 | Retrospective cohort | 7.4, 83%, 60 | Topical budesonide | Eos per HPF | Not reported |
| Gupta et al, 2015 | Randomized trial | 8.5, 70%, 71 | Topical budesonide | Symptoms (EoE CSS), eos per HPF | 3% candida |
| Harel et al, 2015* | Prospective cohort | 10.6, 93%, 14 | Topical budesonide | Adrenocorticotropin stimulation testing | 43% AI |
| Miehlke et al, 2016 | Randomized trial | 46.5, 74%, 19 | Topical budesonide | Symptoms (score°), eos per HPF | 11% candida, 5% AI |
|
| |||||
| Studies of Six Food Elimination Diet | |||||
|
| |||||
| Kagalwalla et al, 2006 | Retrospective cohort | 6.2, 74%, 35 | Six food elimination, specific elimination | Symptoms (binary†), eos per HPF | Not reported |
| Gonsalves et al, 2012 | Prospective cohort | 40.0, 50%, 50 | Six food elimination | Symptoms (SF-36), endoscopic findings, eos per HPF | Not reported |
| Henderson et al, 2012 | Retrospective cohort | 6.6, 77%, 26 | Six food elimination, specific elimination | Symptoms (binary†), eos per HPF | Not reported |
| Lucendo et al, 2013 | Prospective cohort | 33.4, 82%, 67 | Six food elimination | Symptoms (specific‡), eos per HPF | Not reported |
| Colson et al, 2014* | Retrospective cohort | 6.5, 63%, 59 | Six food + specific elimination | Symptoms (specific‡), eos per HPF | Not reported |
| Rodriguez-Sanchez et al, 2014 | Prospective cohort | 32.1, 77%, 17 | Six food elimination, specific elimination | Symptoms (VAS-EoE), endoscopic findings, eos per HPF | Not reported |
| Wolf et al, 2014 | Retrospective cohort | 33.0, 56%, 11 | Six food elimination | Symptoms (binary†), eos per HPF, endoscopic findings | Not reported |
| Arias et al, 2016 | Prospective cohort | 33.1, 80%, 10 | Six food elimination | Symptoms (score°), eos per HPF, gene expression | Not reported |
|
| |||||
| Studies of Multiple Included Therapies | |||||
|
| |||||
| Lee et al, 2012* | Prospective cohort | 40, 27%, 11 | Topical budesonide, topical fluticasone | Symptoms (MDQ), esophageal diameter | Not reported |
| Lieberman et al, 2012* | Retrospective cohort | 14.6, 78%, 9 | Topical budesonide, topical fluticasone, specific elimination | Symptoms (binary†), eos per HPF | Not reported |
| Kuchen et al, 2014* | Retrospective cohort | 31, 76%, 206 | Topical budesonide or topical fluticasone | Long-lasting bolus impactions | Not reported |
| Golekoh et al, 2015* | Prospective cohort | 12.9, 81%, 58 | Topical fluticasone, topical budesonide | Adrenocorticotropin stimulation testing | 10% AI |
| Wolf et al, 2015* | Retrospective cohort | 25.6, 70%, 221 | Topical budesonide or topical fluticasone | Symptoms (binary†), eos per HPF, endoscopic findings | 5% candida |
| Leung et al, 2015* | Retrospective cohort | 13.0, 78%, 100 | Topical budesonide, topical fluticasone, multiple dietary | Eos per HPF, second-line therapies | Not reported |
| Philla et al, 2015 | Prospective cohort | 10.1, 71%, 14 | Topical fluticasone, topical budesonide | Morning serum cortisol | 0% AI |
| Philpott et al 2016 | Prospective cohort | 34, 84%, 82 | Topical budesonide, six food elimination | Symptoms (binary†), eos per HPF | Not reported |
Study not included in meta-analysis because necessary estimates for outcome measures could not be taken from review of the published manuscript.
, improvement or no improvement.
, no improvement, partial improvement, complete improvement.
, individual symptoms such as dysphagia or abdominal pain.
, the study reported an internally defined symptom score.
N, number; HPF, high-power field; MDQ, Mayo Dysphagia Questionnaire; AI, adrenal insufficiency; ECP, Eosinophil Cationic Protein.
Acknowledgments
Grant Support: This research was funded by NIH Awards T32 DK007634 (CCC, SE, WAW), and R01DK101856 (ESD)
Dr. Dellon is consultant for Adare, Alivio, Banner, Receptos/Celgene, Regeneron, and Shire, has received grant funding from Meritage, Miraca, Nutricia, Receptos/Celegene, Regeneron, and Shire, and has received an educational grant: Banner.
Footnotes
Disclosures and potential conflicts of interest: The other authors have no potential conflicts related to this manuscript to report.
References
- 1.Dellon ES, Gonsalves N, Hirano I, Furuta GT, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE) Am J Gastroenterol. 2013;108:679–692. doi: 10.1038/ajg.2013.71. quiz 693. [DOI] [PubMed] [Google Scholar]
- 2.Jensen ET, Kappelman MD, Martin CF, Dellon ES. Health-care utilization, costs, and the burden of disease related to eosinophilic esophagitis in the United States. Am J Gastroenterol. 2015;110:626–632. doi: 10.1038/ajg.2014.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liacouras CA, Furuta GT, Hirano I, Atkins D, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. e26. doi: 10.1016/j.jaci.2011.02.040. quiz 21–22. [DOI] [PubMed] [Google Scholar]
- 4.Shah NA, Albert DM, Hall NM, Moawad FJ. Managing eosinophilic esophagitis: challenges and solutions. Clinical and experimental gastroenterology. 2016;9:281–290. doi: 10.2147/CEG.S78428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroeder S, Fleischer DM, Masterson JC, Gelfand E, et al. Successful treatment of eosinophilic esophagitis with ciclesonide. J Allergy Clin Immunol. 2012;129:1419–1421. doi: 10.1016/j.jaci.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arias A, Gonzalez-Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology. 2014;146:1639–1648. doi: 10.1053/j.gastro.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Bahgat M, Dawe N, Flood L. Eosinophilic oesophagitis: a systematic review for otolaryngologists. J Laryngol Otol. 2015;129:1156–1166. doi: 10.1017/S0022215115002777. [DOI] [PubMed] [Google Scholar]
- 8.Chuang MY, Chinnaratha MA, Hancock DG, Woodman R, et al. Topical Steroid Therapy for the Treatment of Eosinophilic Esophagitis (EoE): A Systematic Review and Meta-Analysis. Clin Transl Gastroenterol. 2015;6:e82. doi: 10.1038/ctg.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipka S, Kumar A, Miladinovic B, Richter JE. Systematic review with network meta-analysis: comparative effectiveness of topical steroids vs. PPIs for the treatment of the spectrum of eosinophilic oesophagitis Aliment Pharmacol Ther. 2016;43:663–673. doi: 10.1111/apt.13537. [DOI] [PubMed] [Google Scholar]
- 10.Lucendo AJ. Meta-Analysis-Based Guidance for Dietary Management in Eosinophilic Esophagitis. Curr Gastroenterol Rep. 2015;17:464. doi: 10.1007/s11894-015-0464-y. [DOI] [PubMed] [Google Scholar]
- 11.Murali AR, Gupta A, Attar BM, Ravi V, Koduru P. Topical steroids in Eosinophilic Esophagitis: Systematic Review and Meta-analysis of Placebo Controlled Randomized Clinical Trials. J Gastroenterol Hepatol. 2015 doi: 10.1111/jgh.13281. [DOI] [PubMed] [Google Scholar]
- 12.Sawas T, Dhalla S, Sayyar M, Pasricha PJ, Hernaez R. Systematic review with meta-analysis: pharmacological interventions for eosinophilic oesophagitis. Aliment Pharmacol Ther. 2015;41:797–806. doi: 10.1111/apt.13147. [DOI] [PubMed] [Google Scholar]
- 13.Tan ND, Xiao YL, Chen MH. Steroids therapy for eosinophilic esophagitis: Systematic review and meta-analysis. J Dig Dis. 2015;16:431–442. doi: 10.1111/1751-2980.12265. [DOI] [PubMed] [Google Scholar]
- 14.Poole C, Greenland S. Random-effects meta-analyses are not always conservative. Am J Epidemiol. 1999;150:469–475. doi: 10.1093/oxfordjournals.aje.a010035. [DOI] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, Mulrow C, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23:1663–1682. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- 17.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 18.R Core Team. R: A Language and Environment for Statistical Computing City. R Foundation for Statistical Computing. 2015 [Google Scholar]
- 19.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 20.Abe Y, Iijima K, Ohara S, Koike T, et al. A Japanese case series of 12 patients with esophageal eosinophilia. J Gastroenterol. 2011;46:25–30. doi: 10.1007/s00535-010-0295-4. [DOI] [PubMed] [Google Scholar]
- 21.Abu-Sultaneh SM, Durst P, Maynard V, Elitsur Y. Fluticasone and food allergen elimination reverse sub-epithelial fibrosis in children with eosinophilic esophagitis. Dig Dis Sci. 2011;56:97–102. doi: 10.1007/s10620-010-1259-5. [DOI] [PubMed] [Google Scholar]
- 22.Alexander JA, Jung KW, Arora AS, Enders F, et al. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2012;10:742–749. e741. doi: 10.1016/j.cgh.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Arora AS, Perrault J, Smyrk TC. Topical corticosteroid treatment of dysphagia due to eosinophilic esophagitis in adults. Mayo Clinic proceedings. 2003;78:830–835. doi: 10.4065/78.7.830. [DOI] [PubMed] [Google Scholar]
- 24.Assa’ad AH, Putnam PE, Collins MH, Akers RM, et al. Pediatric patients with eosinophilic esophagitis: an 8-year follow-up. J Allergy Clin Immunol. 2007;119:731–738. doi: 10.1016/j.jaci.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 25.Butz BK, Wen T, Gleich GJ, Furuta GT, et al. Efficacy, dose reduction, and resistance to high-dose fluticasone in patients with eosinophilic esophagitis. Gastroenterology. 2014;147:324–333. e325. doi: 10.1053/j.gastro.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czaja-Bulsa G, Jakubik M. Primary eosinophilic esophagitis in children of West Pomerania. Pediatria Wspolczesna. 2012;14:5–9. [Google Scholar]
- 27.Enns R, Kazemi P, Chung W, Lee M. Eosinophilic esophagitis: Clinical features, endoscopic findings and response to treatment. Canadian Journal of Gastroenterology. 2010;24:547–551. doi: 10.1155/2010/341925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golekoh MC, Hornung LN, Mukkada VA, Khoury JC, et al. Adrenal Insufficiency after Chronic Swallowed Glucocorticoid Therapy for Eosinophilic Esophagitis. J Pediatr. 2016;170:240–245. doi: 10.1016/j.jpeds.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 29.Helou EF, Simonson J, Arora AS. 3-yr-follow-up of topical corticosteroid treatment for eosinophilic esophagitis in adults. Am J Gastroenterol. 2008;103:2194–2199. doi: 10.1111/j.1572-0241.2008.01989.x. [DOI] [PubMed] [Google Scholar]
- 30.Konikoff MR, Noel RJ, Blanchard C, Kirby C, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131:1381–1391. doi: 10.1053/j.gastro.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 31.Kruszewski PG, Russo JM, Franciosi JP, Varni JW, et al. Prospective, comparative effectiveness trial of cow’s milk elimination and swallowed fluticasone for pediatric eosinophilic esophagitis. Dis Esophagus. 2016;29:377–384. doi: 10.1111/dote.12339. [DOI] [PubMed] [Google Scholar]
- 32.Kuchen T, Straumann A, Safroneeva E, Romero Y, et al. Swallowed topical corticosteroids reduce the risk for long-lasting bolus impactions in eosinophilic esophagitis. Allergy. 2014;69:1248–1254. doi: 10.1111/all.12455. [DOI] [PubMed] [Google Scholar]
- 33.Lee J, Huprich J, Kujath C, Ravi K, et al. Esophageal diameter is decreased in some patients with eosinophilic esophagitis and might increase with topical corticosteroid therapy. Clin Gastroenterol Hepatol. 2012;10:481–486. doi: 10.1016/j.cgh.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 34.Leung J, Mehrzad R, Hundal NV, Alejos A, et al. Longitudinal Perspective on Managing Refractory Eosinophilic Esophagitis. J Allergy Clin Immunol Pract. 2015;3:951–956. doi: 10.1016/j.jaip.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li N, Green B, Sideridis K. Efficacy of combined treatment on eosinophilic esophagitis in adults: Case study. Journal of Gastroenterology and Hepatology Research. 2012;1:153–156. [Google Scholar]
- 36.Lieberman JA, Morotti RA, Konstantinou GN, Yershov O, Chehade M. Dietary therapy can reverse esophageal subepithelial fibrosis in patients with eosinophilic esophagitis: a historical cohort. Allergy. 2012;67:1299–1307. doi: 10.1111/j.1398-9995.2012.02881.x. [DOI] [PubMed] [Google Scholar]
- 37.Lucendo AJ, Arias A, De Rezende LC, Yague-Compadre JL, et al. Subepithelial collagen deposition, profibrogenic cytokine gene expression, and changes after prolonged fluticasone propionate treatment in adult eosinophilic esophagitis: a prospective study. J Allergy Clin Immunol. 2011;128:1037–1046. doi: 10.1016/j.jaci.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Lucendo AJ, Pascual-Turrion JM, Navarro M, Comas C, et al. Endoscopic, bioptic, and manometric findings in eosinophilic esophagitis before and after steroid therapy: a case series. Endoscopy. 2007;39:765–771. doi: 10.1055/s-2007-966738. [DOI] [PubMed] [Google Scholar]
- 39.Lucendo Villarín AJ, Carrión Alonso G, Navarro Sánchez M, Martín Chavarri S, et al. Eosinophilic esophagitis in adults, an emerging cause of dysphagia. Description of 9 cases Revista Espanola de Enfermedades Digestivas. 2005;97:229–239. doi: 10.4321/s1130-01082005000400003. [DOI] [PubMed] [Google Scholar]
- 40.Noel RJ, Putnam PE, Collins MH, Assa’ad AH, et al. Clinical and immunopathologic effects of swallowed fluticasone for eosinophilic esophagitis. Clinical Gastroenterology and Hepatology. 2004;2:568–575. doi: 10.1016/s1542-3565(04)00240-x. [DOI] [PubMed] [Google Scholar]
- 41.Pasha SF, DiBaise JK, Kim HJ, De Petris G, et al. Patient characteristics, clinical, endoscopic, and histologic findings in adult eosinophilic esophagitis: a case series and systematic review of the medical literature. Dis Esophagus. 2007;20:311–319. doi: 10.1111/j.1442-2050.2007.00721.x. [DOI] [PubMed] [Google Scholar]
- 42.Pentiuk S, Putnam PE, Collins MH, Rothenberg ME. Dissociation between symptoms and histological severity in pediatric eosinophilic esophagitis. Journal of Pediatric Gastroenterology and Nutrition. 2009;48:152–160. doi: 10.1097/MPG.0b013e31817f0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterson KA, Thomas KL, Hilden K, Emerson LL, et al. Comparison of esomeprazole to aerosolized, swallowed fluticasone for eosinophilic esophagitis. Dig Dis Sci. 2010;55:1313–1319. doi: 10.1007/s10620-009-0859-4. [DOI] [PubMed] [Google Scholar]
- 44.Philla KQ, Min SB, Hefner JN, Howard RS, et al. Swallowed glucocorticoid therapy for eosinophilic esophagitis in children does not suppress adrenal function. J Pediatr Endocrinol Metab. 2015;28:1101–1106. doi: 10.1515/jpem-2014-0260. [DOI] [PubMed] [Google Scholar]
- 45.Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63:3–12. doi: 10.1016/j.gie.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 46.Sayej WN, Patel R, Baker RD, Tron E, Baker SS. Treatment with high-dose proton pump inhibitors helps distinguish eosinophilic esophagitis from noneosinophilic esophagitis. Journal of Pediatric Gastroenterology and Nutrition. 2009;49:393–399. doi: 10.1097/MPG.0b013e31819c4b3e. [DOI] [PubMed] [Google Scholar]
- 47.Schaefer ET, Fitzgerald JF, Molleston JP, Croffie JM, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol. 2008;6:165–173. doi: 10.1016/j.cgh.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 48.Schlag C, Pfefferkorn S, Brockow K, Haller B, et al. Serum eosinophil cationic protein is superior to mast cell Tryptase as marker for response to topical corticosteroid therapy in Eosinophilic Esophagitis. Journal of Clinical Gastroenterology. 2014;48:600–606. doi: 10.1097/01.mcg.0000436439.67768.8d. [DOI] [PubMed] [Google Scholar]
- 49.Teitelbaum JE, Fox VL, Twarog FJ, Nurko S, et al. Eosinophilic esophagitis in children: Immunopathological analysis and response to fluticasone propionate. Gastroenterology. 2002;122:1216–1225. doi: 10.1053/gast.2002.32998. [DOI] [PubMed] [Google Scholar]
- 50.van Rhijn BD, Verheij J, van den Bergh Weerman MA, Verseijden C, et al. Histological Response to Fluticasone Propionate in Patients With Eosinophilic Esophagitis Is Associated With Improved Functional Esophageal Mucosal Integrity. Am J Gastroenterol. 2015;110:1289–1297. doi: 10.1038/ajg.2015.247. [DOI] [PubMed] [Google Scholar]
- 51.Wolf WA, Cotton CC, Green DJ, Hughes JT, et al. Predictors of response to steroid therapy for eosinophilic esophagitis and treatment of steroid-refractory patients. Clin Gastroenterol Hepatol. 2015;13:452–458. doi: 10.1016/j.cgh.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moawad FJ, Veerappan GR, Dias JA, Baker TP, et al. Randomized controlled trial comparing aerosolized swallowed fluticasone to esomeprazole for esophageal eosinophilia. Am J Gastroenterol. 2013;108:366–372. doi: 10.1038/ajg.2012.443. [DOI] [PubMed] [Google Scholar]
- 53.Aceves SS, Bastian JF, Newbury RO, Dohil R. Oral viscous budesonide: a potential new therapy for eosinophilic esophagitis in children. Am J Gastroenterol. 2007;102:2271–2279. doi: 10.1111/j.1572-0241.2007.01379.x. quiz 2280. [DOI] [PubMed] [Google Scholar]
- 54.Aceves SS, Newbury RO, Chen D, Mueller J, et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy. 2010;65:109–116. doi: 10.1111/j.1398-9995.2009.02142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dellon ES, Sheikh A, Speck O, Woodward K, et al. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology. 2012;143:321–324. e321. doi: 10.1053/j.gastro.2012.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dohil R, Newbury R, Fox L, Bastian J, Aceves S. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010;139:418–429. doi: 10.1053/j.gastro.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Gupta SK, Vitanza JM, Collins MH. Efficacy and safety of oral budesonide suspension in pediatric patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2015;13:66–76. e63. doi: 10.1016/j.cgh.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 58.Harel S, Hursh BE, Chan ES, Avinashi V, Panagiotopoulos C. Adrenal Suppression in Children Treated With Oral Viscous Budesonide for Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr. 2015;61:190–193. doi: 10.1097/MPG.0000000000000848. [DOI] [PubMed] [Google Scholar]
- 59.Miehlke S, Hruz P, Vieth M, Bussmann C, et al. A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis. Gut. 2016;65:390–399. doi: 10.1136/gutjnl-2014-308815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Philpott H, Nandurkar S, Royce SG, Thien F, Gibson PR. A prospective open clinical trial of a proton pump inhibitor, elimination diet and/or budesonide for eosinophilic oesophagitis. Aliment Pharmacol Ther. 2016;43:985–993. doi: 10.1111/apt.13576. [DOI] [PubMed] [Google Scholar]
- 61.Rubinstein E, Lee JJ, Fried A, Logvinenko T, et al. Comparison of 2 delivery vehicles for viscous budesonide to treat eosinophilic esophagitis in children. J Pediatr Gastroenterol Nutr. 2014;59:317–320. doi: 10.1097/MPG.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 62.Straumann A, Conus S, Degen L, Felder S, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139:1526–1537. doi: 10.1053/j.gastro.2010.07.048. 1537 e1521. [DOI] [PubMed] [Google Scholar]
- 63.Arias A, Lucendo AJ, Martinez-Fernandez P, Gonzalez-Castro AM, et al. Dietary treatment modulates mast cell phenotype, density, and activity in adult eosinophilic oesophagitis. Clin Exp Allergy. 2016;46:78–91. doi: 10.1111/cea.12504. [DOI] [PubMed] [Google Scholar]
- 64.Colson D, Kalach N, Soulaines P, Vannerom Y, et al. The impact of dietary therapy on clinical and biologic parameters of pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2014;2:587–593. doi: 10.1016/j.jaip.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 65.Gonsalves N, Yang GY, Doerfler B, Ritz S, et al. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology. 2012;142:1451–1459. e1451. doi: 10.1053/j.gastro.2012.03.001. quiz e1414–1455. [DOI] [PubMed] [Google Scholar]
- 66.Henderson CJ, Abonia JP, King EC, Putnam PE, et al. Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2012;129:1570–1578. doi: 10.1016/j.jaci.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kagalwalla AF, Sentongo TA, Ritz S, Hess T, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1097–1102. doi: 10.1016/j.cgh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 68.Lucendo AJ, Arias A, Gonzalez-Cervera J, Yague-Compadre JL, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol. 2013;131:797–804. doi: 10.1016/j.jaci.2012.12.664. [DOI] [PubMed] [Google Scholar]
- 69.Rodriguez-Sanchez J, Gomez Torrijos E, Lopez Viedma B, de la Santa Belda E, et al. Efficacy of IgE-targeted vs empiric six-food elimination diets for adult eosinophilic oesophagitis. Allergy. 2014;69:936–942. doi: 10.1111/all.12420. [DOI] [PubMed] [Google Scholar]
- 70.Wolf WA, Jerath MR, Sperry SL, Shaheen NJ, Dellon ES. Dietary elimination therapy is an effective option for adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2014;12:1272–1279. doi: 10.1016/j.cgh.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Noel RJ, Putnam PE, Collins MH, Assa’ad AH, et al. Clinical and immunopathologic effects of swallowed fluticasone for eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2004;2:568–575. doi: 10.1016/s1542-3565(04)00240-x. [DOI] [PubMed] [Google Scholar]
- 72.Rajan J, Newbury RO, Anilkumar A, Dohil R, et al. Long-term assessment of esophageal remodeling in patients with pediatric eosinophilic esophagitis treated with topical corticosteroids. J Allergy Clin Immunol. 2016;137:147–156. e148. doi: 10.1016/j.jaci.2015.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Andreae DA, Hanna MG, Magid MS, Malerba S, et al. Swallowed Fluticasone Propionate Is an Effective Long-Term Maintenance Therapy for Children With Eosinophilic Esophagitis. Am J Gastroenterol. 2016;111:1187–1197. doi: 10.1038/ajg.2016.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dellon ES, Liacouras CA. Advances in Clinical Management of Eosinophilic Esophagitis. Gastroenterology. 2014;147:1238–1254. doi: 10.1053/j.gastro.2014.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asher Wolf W, Huang KZ, Durban R, Iqbal ZJ, et al. The Six-Food Elimination Diet for Eosinophilic Esophagitis Increases Grocery Shopping Cost and Complexity. Dysphagia. 2016 doi: 10.1007/s00455-016-9739-1. [DOI] [PubMed] [Google Scholar]
- 76.Wolf WA, Cotton CC, Green DJ, Hughes JT, et al. Evaluation of Histologic Cutpoints for Treatment Response in Eosinophilic Esophagitis. J Gastroenterol Hepatol Res. 2015;4:1780–1787. doi: 10.17554/j.issn.2224-3992.2015.04.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Franciosi JP, Hommel KA, Bendo CB, King EC, et al. PedsQL eosinophilic esophagitis module: feasibility, reliability, and validity. J Pediatr Gastroenterol Nutr. 2013;57:57–66. doi: 10.1097/MPG.0b013e31828f1fd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taft TH, Kern E, Kwiatek MA, Hirano I, et al. The adult eosinophilic oesophagitis quality of life questionnaire: a new measure of health-related quality of life. Alimentary pharmacology & therapeutics. 2011;34:790–798. doi: 10.1111/j.1365-2036.2011.04791.x. [DOI] [PubMed] [Google Scholar]
- 79.Dellon ES, Cotton CC, Gebhart JH, Higgins LL, et al. Accuracy of the Eosinophilic Esophagitis Endoscopic Reference Score in Diagnosis and Determining Response to Treatment. Clin Gastroenterol Hepatol. 2016;14:31–39. doi: 10.1016/j.cgh.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S-1 Mean Ratio for Eosinophils Before and After Therapy in Studies of Topical Fluticasone (A), Topical Budesonide (B), and Six-Food Elimination Diet (C) in Order of Publication Date or, if Present, Selected Meta-Regression Moderator. Studies are sorted in order of the statistically significant meta-regression moderator or by year if no moderator was significant at a threshold p < 0.05. Studies from systematic review that included the mean and standard deviation of eosinophils per high-power field before and after therapy and the Pearson correlation coefficient without missing values for meta-regression variables were included.


