Abstract
Background
A history of brief intermittent social defeat stress can escalate cocaine self-administration and induce long-term adaptations in the mesolimbic dopamine system. Extra-hypothalamic corticotrophin releasing factor (CRF) has been shown to be closely associated with stress-induced escalation of drug use. How repeated stress modulates CRF release in the ventral tegmental area (VTA) and the roles of CRF receptors during different phases of stress-induced cocaine self-administration remain to be defined.
Objective
The current study examines the roles of CRF and CRF receptor 1 (CRFR1) in escalated intravenous cocaine self-administration after exposure to social defeat stress in mice.
Methods and Results
First, CRFR1 antagonist (CP 376,395, 15mg/kg, i.p.) given 30 min prior to each social defeat episode prevented later escalated cocaine self-administration. When CP 376,395 (5 and 15 mg/kg, i.p.) was administered 10 days after the last episode of social stress, the escalation of cocaine intake was dose-dependently reversed. Moreover, socially defeated mice showed increased CRF release in the VTA compared to controls. To further explore the role of CRFR1, CP 376,395 (0.5 and 1 μg/0.2 μl) was infused directly into the VTA before the cocaine self-administration session. Intra-VTA antagonism of CRFR1 was sufficient to reverse social defeat stress-escalated cocaine self-administration.
Conclusion
These findings suggest that CRF and CRFR1 exert multiple roles in the response to social stress that are relevant to escalated cocaine self-administration.
Keywords: social defeat stress, CRF microdialysis, CRFR1, intravenous cocaine self-administration, microinjection, VTA, Mice
Introduction
Exposure to stressful life events can profoundly increase the vulnerability to drug use in humans (Sinha 2001; Sinha 2008). Evidence from preclinical studies also confirms that stressful events, such as mild foot shock, social defeat, and social isolation, lead to increased drug self-administration and drug seeking behavior (Bossert et al. 2013; Holly et al. 2016; Piazza and Le Moal 1998). Studies investigating the neurobiological mechanisms by which stress influences drug self-administration have mainly focused on the hypothalamic-pituitary-adrenal axis, particularly the glucocorticoid receptors and corticotropin releasing factor (CRF) (Koob and Zorrilla 2010; Piazza and Le Moal 1998), yet how stress modulates CRF release and the roles of CRF during different phases of stress-escalated drug self-administration remain to be identified.
Stress stimulates CRF release from the hypothalamus, particularly from a subset of neurosecretory neurons with cell bodies in the paraventricular nucleus (PVN), and activates the hypothalamic-pituitary-adrenal axis, eventually increasing glucocorticoid secretion (Sarnyai et al. 2001). In addition, CRF is produced by neurons in extra-hypothalamic brain regions, such as the basolateral amygdala, the locus coeruleus and the dorsal raphe nucleus (Cummings et al. 1983; Curtis et al. 1995; Snyder et al. 2012). CRF release into the ventral tegmental area (VTA) after repeated stress and its long-lasting effects have received only sparse attention (Holly et al. 2016).
The VTA has been implicated as a critical site of action for modulation of drug-taking by social stress (Miczek et al. 2008; Miczek et al. 2011). Intact dopaminergic cellular activity in the VTA is necessary for many motivated behaviors, including drug self-administration and drug seeking (Wise and Rompre 1989; Wise 1996). The VTA is rich in dopamine-containing neurons that are a key site for direct and indirect CRF-dopamine interactions (Borgland et al. 2010; Boyson et al. 2014; Korotkova et al. 2006; Wanat et al. 2008). The release of CRF in the VTA is increased both during acute social stress and after repeated social defeat stress in rats (Holly et al. 2016). Furthermore, the blockades of CRF receptor 1 (CRFR1) or receptor 2 (CRFR2) in the VTA have been found to prevent behavioral sensitization and escalated cocaine “binge” taking produced by social stress in rats (Boyson et al. 2011). The evidence suggests that the VTA may be a critical brain region at which we can target to treat stress-escalated cocaine taking.
CRF exerts its cellular effects by activating G-protein-coupled R1 and R2 (Hauger et al. 2006), and these receptors are the targets for CRF antagonists. CRF has a much higher affinity for CRFR1 than CRFR2 (Vaughan et al. 1995). Preclinical studies have shown that CRFR1 antagonists can also effectively block the escalation of drug self-administration and aversive symptoms of drug withdrawal (Koob 2010), as well as stress-induced reinstatement of drug seeking (Mantsch et al. 2016; Shaham et al. 1997). The role of CRF in stress-escalated drug self-administration and seeking has been studied extensively (Holly et al. 2016; Shaham et al. 1997; Wang et al. 2005); nonetheless, how repeated social defeat stress influences VTA CRF release in mice and whether or not antagonism of CRF receptors can prevent and reverse the stress effects needs to be further characterized.
To characterize the neurobiological mechanisms through which psychosocial experiences alter cocaine use, we adopted a social defeat procedure that mimics the stressful experience in humans (Björkqvist 2001; Miczek et al. 2008). We focus on a type of social stress that consists of intermittent episode of social defeat in brief confrontations with an aggressive resident mouse (Miczek et al. 1982; Yap and Miczek 2007). To address the CRF mechanisms through which experience of social stress alters cocaine use, we first investigated whether pretreatment with a CRFR1 antagonist can block the social stress effects on cocaine self-administration in mice. After demonstrating prevention of stress-escalated drug intake, we then gave the drug prior to the cocaine self-administration to reverse the stress effects. Moreover, we added a CRF microdialysis experiment to directly measure CRF release in the VTA after repeated social defeat stress. Finally, we focused on the VTA as a key site of CRF action after repeated social defeat experience. Specifically, we microinjected a CRFR1 antagonist into the VTA and investigated the role of CRFR1 in the escalated cocaine self-administration in socially defeated mice.
Methods
Animals
Male Swiss Webster (CFW) mice (Charles River Laboratories; Wilmington, MA) weighing 23–25 g were singly housed under a reverse light/dark cycle (lights on from 6:00 p.m. to 6 a.m.) with ad libitum access to food and water. A separate group of adult male CFW mice was housed in pairs with females and served as aggressive stimulus residents. Before being used in these experiments, residents were screened to ensure the display of reliable aggressive behavior. All experimental procedures were approved by the Institutional Care and Use Committee (IACUC) of Tufts University and carried out in accordance with the NIH Guide for Care and Use of Laboratory Animals (National Research Council 2011).
Experimental design
The experimental design is depicted in Fig. 1. In experiment 1 (Fig. 1A), mice (n=32) were randomly assigned to receive CRFR1 antagonist (CP 376,395) at a previously determined dose (15 mg/kg, i.p.) or saline 30 min before either intermittent social defeat stress or handling from day 1 to day 10. Six days later, mice were fitted with permanently indwelling jugular catheters for later cocaine self-administration. In experiment 2, the procedure was similar, but mice (n=26) received CP 376,395 pretreatment (5 and 15 mg/kg, i.p.) 10 min prior to each of two cocaine self-administration sessions (see Fig. 1B). In addition, experiment 3 measured CRF in the VTA (n=14) ten days after the last episode of social defeat stress (see Fig. 1C). Finally, experiment 4 evaluated the role of VTA CRFR1 after repeated social defeat stress on cocaine self-administration. As is shown in Fig. 1D, mice (n=30) exposed for 10 days of social defeat stress or handling, subsequently were pretreated with intra-VTA CP 376,395 (0.5 and 1 μg/0.2 μl) prior to each of two cocaine self-administration sessions.
Fig. 1.
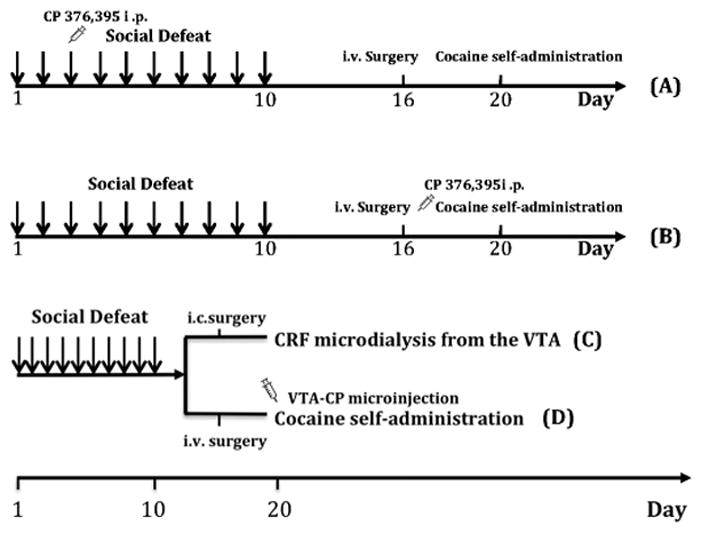
Timeline for experiments. Social defeats (indicated by arrows) were conducted daily for 10 days. All animals were catheterized six days after the last episode of defeat and allow to self-administer cocaine on Day 20 (except experiment 3). In experiment 1(Fig 1A), mice were pretreated with CP 376,395 (15 mg/kg, i.p) 30 min prior to each defeat. In experiment 2 (Fig 1B), mice were administered CP 376,395 (5 and 15 mg/kg) prior to each cocaine self-administration test after acquisition. A separate cohort in Experiment 3 was implanted with intracranial cannulae in the VTA on Day 16 and underwent in vivo microdialysis to determine CRF on Day 20 (Fig 1C). In experiment 4 (Fig 1D), mice were pretreated with intra-VTA CP 376,395 (0.5 and 1 μg/0.2 μl) 10 min prior to each cocaine self-administration test.
Social defeat procedure
As previously described (Han et al. 2015; Miczek and O’Donnell 1978), we used a resident-intruder paradigm for social defeat. Mice in the stressed groups were exposed to ten daily brief episodes of social defeats. Residents were used once daily and rotated through intruders so that intruders did not encounter the same resident more than once to avoid familiarity. The social defeat consisted of three phases. The first phase was termed instigation, in which the experimental animal was placed in a protective plastic cage inside the resident’s home cage for 5 min, allowing unrestricted auditory, olfactory, and visual contact. The second phase was termed defeat, in which the intruder was removed from the perforated protective cage and was placed back into the resident’s home cage for another 5 min or until the intruder received 30 attack bites. The third phase was termed threat, in which the intruder was then returned to the protective cage for another 5 min in the resident’s home cage. During this last phase, intruders were still kept in a stressful environment, but without receiving further attack bites.
Intracranial surgery
Mice were anesthetized with a combination of ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.). For the microdialysis experiment (experiment 3), a unilateral microdialysis cannula (5 mm length, Synaptech Inc, Marquette, MI) aimed at the VTA (AP, −3.20; ML, − 0.70; DV, −3.50 mm; Paxinos and Franklin 2001) was implanted. For the microinjection experiment (experiment 4), mice were surgically implanted with 26-gauge bilateral cannulae (Plastics One, Roanoke, VA, USA) aimed at the VTA (AP, −3.20; ML, ± 0.75; DV, −4.50 mm; Albrechet-Souza et al. 2015).
Microinjection
On test days, mice were given intra-VTA injections of vehicle (aCSF) or CRFR1 antagonist CP 376,395 (0.5–1.0 μg). Bilateral injections were delivered simultaneously in a volume of 0.2 μl per side at a flow rate of 0.1 μl/min into the VTA controlled by an automatic CMA/100 microinjection pump (CMA Microdialysis, Sweden). The injector needles were extended by 0.1 mm beyond the end of the guide cannulae (Plastics One, Roanoke, VA) and they were left in place for 1 min after the end of the infusion to allow diffusion and to prevent backflow. Microinjections occurred 10 min prior to the cocaine self-administration test.
Intravenous (i.v.) cocaine self-administration
Surgery
Mice were implanted with permanently indwelling catheters (Silastic laboratory silicone tubing, Dow Corning, Midland, MI; ID 0.30 mm, OD 0.64 mm) into the right jugular vein under a combination of ketamine hydrochloride (100 mg/mg i.p.) and xylazine (10 mg/mg i.p.) anesthesia. The catheter was tied to the vein with surgical silk and was passed subcutaneously to the back of the mouse where the catheter was affixed to a small plastic pedestal (Plastics One, Roanoke, VA). After the surgery, the catheters were flushed daily with heparin (30 IU/ml) to avoid clotting.
Acquisition
Mice were allowed to freely self-administer cocaine (1.0 mg/kg/infusion). The custom apparatus used for cocaine self-administration was as described previously (Han et al. 2015; Yap and Miczek 2007). A panel equipped with cue lights and two nose-poke operanda was inserted vertically into the home cage of the animal. Mice were reinforced for nose-poking the cue-paired (active) operandum by delivery of intravenous cocaine. A timeout period of 30 s followed each drug injection to avoid overdose. All responses were recorded automatically using a computer interface and software from Med Associates (St. Albans, VT, USA). Mice were trained to self-administer cocaine one session daily according a fixed ratio 1 (FR1) schedule. Each session lasted for a maximum of 2-h or until the mouse received 20 cocaine infusions. The acquisition was conducted for 5 days.
Cocaine self-administration
After the acquisition, mice were subsequently tested under an FR1 schedule with 0.3 mg/kg/infusion unit dose of cocaine, which was indicated as an optimal dosage to identify the stress effects on escalated cocaine intake in mice (Han et al. 2015). In experiment 2, mice were pretreated with CP 376,395 (0, 5 and 15 mg/kg, i.p.) 10 min prior to cocaine self-administration test. In experiment 4, mice received intra-VTA CRFR1 antagonist (0, 0.5 and 1 μg/0.2 μl) prior to the mice being given access to cocaine. The drug doses were injected in a counterbalanced within-subjects design prior to each 2-h cocaine self-administration daily session.
In vivo CRF microdialysis
In vivo microdialysis occurred 10 days after the last episode of the social defeat as described above. On the night before the microdialysis experiment, the guide cannula cap was removed and replaced with a microdialysis probe (1 mm active membrane, Synaptech Inc, Marquette, MI), which was perfused with an artificial cerebrospinal fluid (aCSF, 147 mM NaCl, 2.8 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2) at a flow rate of 0.5 μl/min overnight. The following morning, aCSF was replaced with aCSF containing 0.2% bovine serum albumin (BSA) and the flow rate increased to 2.0 μl/min two hours prior to sample collection. Samples were collected by hand into Eppendorf Protein LoBind tubes every 15 min and immediately stored at −80°C. Tonic levels of CRF were measured in five baseline samples before experimental manipulations. Then, seven more samples were collected to evaluate the time course of CRF changes after a cocaine challenge (10 mg/kg, i.p.).
CRF was quantified by a commercially available enzyme immunoassay (EIA kit, Peninsula Laboratories, San Carlos, CA). Standards were diluted with aCSF 0.2% BSA rather than the provided standard diluent buffer to make sure standards and samples were treated equally. All other steps were identical to the recommended EIA protocol.
Histology
After the completion of the self-administration or dialysis experiment, mice were deeply anesthetized with an overdose of ketamine and xylazine combination and underwent transcardial perfusion with 0.9% saline and 4% paraformaldehyde (PFA). Brain tissue was preserved in 4% PFA and then sectioned in 50 μm slices using a cryostat (Leica CM1900, Bannockburn, IL). The brain sections were mounted on gelatin-coated slides and stained with cresyl violet, and the cannula placements were verified by light microscopy, according to the mouse brain atlas (Paxinos and Franklin 2001).
Drugs
Cocaine hydrochloride was obtained from the National Institute on Drug Abuse through the drug supply program (Rockville, MD). It was dissolved in sterile 0.9% saline solution (0.6 and 2.0 mg/ml). Cocaine was self-administered intravenously based on each animal’s body weight. CP 376, 395 is a selective CRFR1 antagonist (Ki values are 12 and >10000 nM for CRFR1 and CRFR2 respectively) and it can dissolve easily in an aqueous solution. For systemic injection, CP-376, 395 was dissolved in sterile 0.9% saline (5 and 15 mg/kg i.p.) at a volume of 0.1 ml/kg body weight. For microinjection, it was freshly suspended in aCSF immediately before microinjections (0.5 μg/0.2 μl and 1 μg/0.2 μl).
Statistical analysis
Data analyses were performed using Sigma Stat Version 13.0 (Systat Software Inc., San Jose, CA, USA). To assess the effects of pretreatment with CRFR1 antagonist (systemic injection) prior to social stress on cocaine self-administration and the effects of CRFR1 (systemic injection) after social stress on cocaine self-administration, data were analyzed with a two-way ANOVA. To analyze the effects of microinjection of the CRFR1 antagonist into the VTA after repeated stress, the numbers of cocaine infusions were assessed with a two-way repeated ANOVA with group (stressed or non-stressed control) as the between-subject factor and the drug dosage as the within-subject factor. For CRF microdialysis, data were also analyzed with a two-way repeated ANOVA. All post hoc comparisons were performed with Bonferroni tests. Data are presented as mean ± SEM. The criterion for statistical significance was p<0.05.
Results
Experiment 1: Pharmacological blockade of CRFR1 prevents the effects of stress on cocaine intake
Of the original 32 mice, two mice died during surgery. Two mice were removed due to catheter failure. CRFR1 antagonist (15 mg/kg, i.p.) given before each episode of social defeat stress did not change the animals’ reactions during social defeat stress. Neither stress nor CRFR1 antagonist influenced nose-poking responses (see Fig. 2) or the average number of infusions (data were not shown) during acquisition phase.
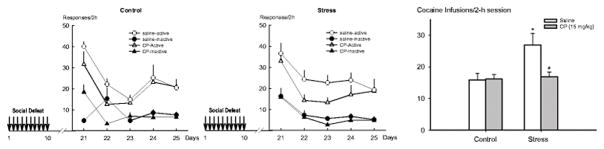
Fig. 2.
Pretreatment of CRFR1 antagonist prior to each episode of social defeat stress blocked stress-escalated cocaine self-administration. Left panel: Neither repeated social defeat stress nor CRFR1 antagonist altered the pattern of cocaine self-administration in the acquisition phase. Right panel: CRFR1 pretreatment blocked stress-induced escalated cocaine self-administration. Nonstressed + saline (n=7); nonstressed + CP 376,395 (n=7); stressed + saline (n=6), stressed + CP 376,395 group (n=8). Data are represented as mean ± SEM; * p < 0.05 vs. nonstressed+saline group. # p < 0.05 vs. Defeated-saline group.
CRFR1 antagonism (CP 376,395, 15mg/kg, i.p.) given 30 min prior to each social defeat episode prevented subsequent escalation of cocaine self-administration. Overall, two-way ANOVA revealed a significant stress effect (F1, 27 = 7.30; p < 0.05), a significant drug treatment effect (F1, 27 = 4.99; p < 0.05), and a significant interaction effect between stress and drug treatment (F1, 27 = 2.58; p < 0.05). As shown in Fig. 2, post hoc analysis revealed that repeated social defeat stress escalated cocaine self-administration relative to nonstressed controls under saline conditions (t12 =3.46; p<0.05). Pretreatment with the CRFR1 antagonist CP 376,395 (15 mg/kg, i.p.) prior to each episode of social defeat stress prevented the effects of social defeat on later cocaine self-administration (t13 =3.24; p<0.05). In addition, CRFR1 antagonist did not alter cocaine self-administration in control groups. Although several studies have demonstrated that CRFR1 antagonists can decrease cocaine intake in rats (Goeders and Guerin 2000), the lack of effects in the current experiment could be due to the difference in species.
Experiment 2: Pharmacological blockade of CRFR1 reverses stress-escalated cocaine self-administration
Three mice were excluded from the results because of catheter patency issues. Repeated social defeat stress did not alter the pattern of cocaine self-administration during acquisition phase (data are not shown).
CRFR1 antagonist (CP 376,395) given immediately before each self-administration session dose-dependently reversed the long-term effects of social defeat stress on cocaine taking. Overall, there was a significant drug treatment effect (F3, 73 = 5.82; p < 0.01), and a significant interaction effect between stress and drug treatment (F3, 73 = 4.10; p < 0.05), but no significant stress effect (F1, 73 = 3.03; p = 0.096). As shown in Fig. 3, post hoc analysis revealed that repeated social defeat stress escalated cocaine self-administration relative to nonstressed saline treated controls (t22 = 2.85; p<0.05). High- but not low-dose CP 376,395 significantly reduced cocaine self-administration in mice that underwent prior social defeat but not on non-defeated mice (t11 =3.89; p<0.05).
Fig. 3.
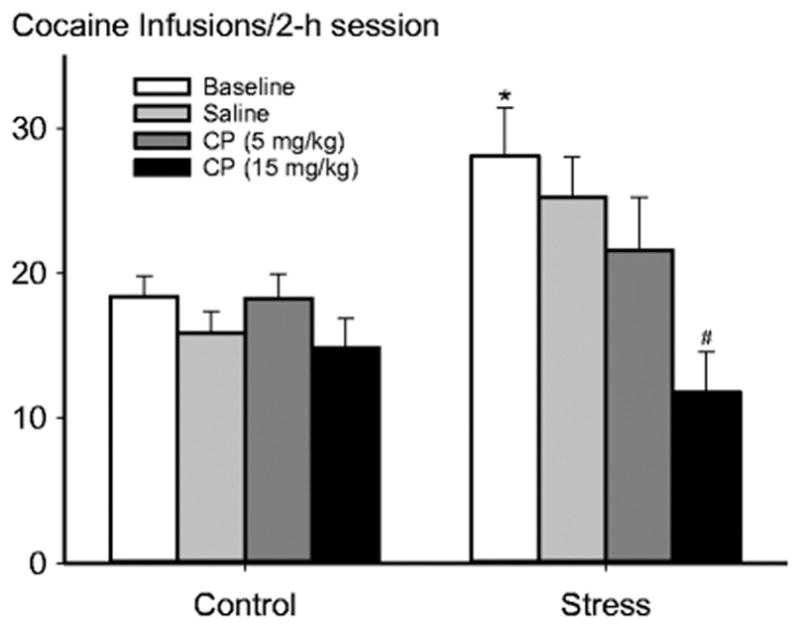
CRFR1 antagonism (CP 376,395; 5 mg/kg and 15 mg/kg, i.p.) given immediately before each cocaine self-administration session dose dependently reversed stress-escalated cocaine intake. Mean ± SEM total infusions of cocaine self-administration after the social defeat phase with or without prior defeat stress is portrayed. Left bars represent nonstressed control group (n=11). Right bars represent stressed group (n=12). * p < 0.05 vs. Nonstressed + saline group. # p < 0.05 vs. Stressed + saline group.
Experiment 3: Repeated social defeat stress increased CRF release in the VTA
Schematic representations of probe placements in the VTA and a representative Nissl-stained section showing the location of the probe are presented in Fig. 4 (two mice with incorrect placement and two mice that chewed the tubing during sample collections were excluded from the data analysis).
Fig. 4.
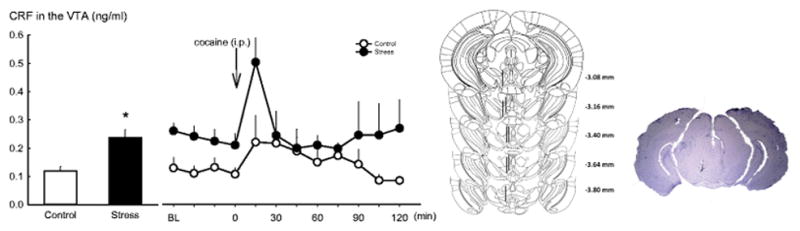
Microdialysis for CRF in the VTA after repeated intermittent social defeat stress. Left panel:
Repeated social defeat stress elevated CRF release in the VTA (data are shown in the first four baseline samples). A cocaine (10 mg/kg, i.p.) challenge increased VTA CRF in both stressed (n=5) and nonstresse d group (n=5). Data are represented as mean ± SEM; * p < 0.05 versus nonstressed control. Right panel: Placements of intra-VTA unilateral probes for the in vivo microdialysis experiment. Left, each figure corresponds to a coronal section of the mouse brain from −3.08 to −3.88 mm from bregma (Paxinos and Franklin 2001). The dark bars represent the location of unilateral probes. Right, a photomicrograph of correct placement after Nissl staining.
Overall, repeated social defeat stress elevated the basal level of CRF in the VTA and also resulted in greater increases in CRF after cocaine injection. The averages of first four samples in each mouse served as a CRF baseline measurement. A t-test revealed a significant increase compared to the control group in mice that underwent social defeat (t9 = −3.65; p < 0.05 see Fig. 4). Thereafter, the stress-experienced and the nonstressed controls were challenged with a 10 mg/kg cocaine injection. A two-way repeated ANOVA analysis revealed a significant time effect (F8, 89 = 2.91; p < 0.01), however, neither stress (F1, 89 = 2.68; p = 0.14) nor the interaction between stress and time (F 8, 89 = 0.97; p = 0.47) showed a significant role in VTA CRF release. Interestingly, mice with a history of defeat showed a higher CRF release in the VTA immediately after the cocaine challenge (t9 = 2.49; p < 0.05; see Fig. 4), but CRF levels returned to baseline within 30 min. This evidence indicates that social defeat stress may perhaps change the vulnerability to cocaine challenge.
Experiment 4: Intra-VTA CRFR1 antagonism blocked stress-escalated cocaine intake
Schematic representations of probe placements in the VTA and a representative Nissl-stained section showing the location of the probe are presented in Fig. 5 (three mice with incorrect placements were excluded from data analysis). Another two mice with catheter failure were also excluded from the results.
Fig. 5.
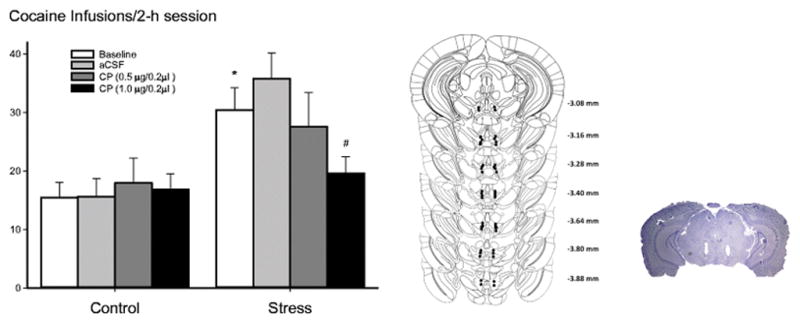
VTA CRFR1 antagonism dose dependently reversed stress-escalated cocaine intake. Left Panel: VTA CRFR1 antagonism (CP 376,395; 0.5 and 1 μg/0.2μl/side) given prior to each cocaine self-administration session dose dependently blocked stress-escalated cocaine intake. Mean ± SEM total infusions of cocaine self-administration after the social defeat phase with or without prior defeat stress is portrayed. Nonstressed controls (n=13); stressed group (n=12). Right panel: Schematic portrayal of accurately placed intra-VTA sites. Left, each figure corresponds to coronal sections of the mouse brain from −3.08 to −3.88 mm from bregma (Paxinos and Franklin 2001). Filled circles represent the location of each bilateral cannula. Right, a photomicrograph of correct placement after Nissl staining.
To further explore the role of CRFR1, CP 376,395 (0.5 and 1 μg/0.2 μl) was infused directly into the VTA before the cocaine self-administration session. Intra-VTA CP 376,395 dose dependently reversed the long-term effects of stress-escalated cocaine self-administration. A two-way repeated ANOVA revealed a significant effect of stress on cocaine taking (F1, 99 = 7.63; p < 0.05), a significant drug treatment effect (F3, 99 = 2.83; p < 0.05), and a significant interaction between stress and drug treatment (F3, 99 = 4.11; p < 0.05). As shown in Fig. 5, post hoc analysis revealed that under aCSF conditions, repeated social defeat stress increased cocaine self-administration compared to nonstressed controls (t24 =2.81; p<0.05). Pretreatment with the higher dose of CP 376,395 (1 μg/0.2μl/side), but not the lower dose (0.5 μg/0.2μl/side), reversed the stress-escalated cocaine intake (t11 =4.31; p<0.05).
Discussion
The current experiments demonstrate that repeated episodes of social defeat stress can result in long-lasting increased CRF release in the VTA. Moreover, the results suggest that CRFR1 exerts multiple roles during the initial reaction to social stress and during long-term neuroadaptations that are relevant to escalated cocaine taking. Antagonism of CRFR1 has proven effective in preventing and reversing social stress effects on cocaine self-administration and reinstatement. Our study shows reversal of stress-escalated cocaine intake long after the stress experience; both systemic and intra-VTA CP 376,395 dose-dependently block social stress effects when given prior to cocaine self-administration.
In agreement with previous studies (Boyson et al. 2011; Han et al. 2015; Miczek et al. 2011), the current findings demonstrate that social defeat stress escalates cocaine self-administration in mice. Despite substantial differences in social defeat stress protocols, durations of cocaine self-administration access, strains and species, most studies indicate that brief episodes of intermittent social defeat stress reliably escalate cocaine self-administration and seeking (Covington, III and Miczek 2001; Manvich et al. 2016; Quadros and Miczek 2009).
Different kinds of stressors result in CRF release and CRF receptor activation in extra-hypothalamic pathways (Holmes et al. 2003). CRF-like immunoreactivity has been identified in the neocortex, extended amygdala, medial septum, hypothalamus, thalamus, cerebellum, and autonomic midbrain and hindbrain nuclei (Charlton et al. 1987; Swanson et al. 1983). Recent studies have drawn more attention to CRF signaling in the VTA after stress exposure. For example, Wang et al. (2005) demonstrated that acute foot shock stress (0.3–0.6 mA) increases CRF release within the VTA in rats. However, the nature of CRF release in the VTA during acute and repeated social defeat stress, as well as its role in enduring neuroadaptations driving later drug taking and seeking, are still poorly understood. The present results show that repeated social defeat stress results in escalated CRF basal levels in the VTA in mice, suggesting that long-lasting changes in VTA CRF after repeated social defeat stress may be critical for stress escalated cocaine self-administration.
A recent study from our laboratory has shown that CRF release during social defeat stress may contribute to later escalated cocaine taking, and that persistently elevated CRF tone in the VTA may drive later cocaine seeking in rats (Holly et al. 2016). The current study confirms and extends the findings that repeated social defeat stress elevates CRF tone in the VTA, which may contribute to later stress-escalated cocaine self-administration in mice. Together, these findings suggest that CRF in the VTA may underlie the fundamental link between social stress and escalated cocaine self-administration in rodents. In addition, the present study demonstrates that repeated social defeat stress results in a sensitized CRF overflow in the VTA after a cocaine challenge in mice, which is consistent with previous findings (Richter et al. 1995) that CRF is increased in the central amygdaloid nucleus after a cocaine challenge.
The roles of CRFR1 and CRFR2 in stress-induced drug self-administration and seeking are not consistent in literature. Some studies have demonstrated that CRFR1 is more relevant than CRFR2 for foot shock-induced reinstatement of cocaine seeking (Blacktop et al. 2011; Koob 2010), while others suggests that both CRFR1 and CRFR2 are involved in social stress-escalated cocaine self-administration (Boyson et al. 2014). The current study confirms and extends the role of CRFR1 in social defeat stress escalated cocaine self-administration in mice. Although previous studies have explored the roles of CRF receptor antagonists in preventing on social defeat stress-induced cocaine self-administration in rats (Boyson et al. 2011; Boyson et al. 2014), the current work focuses on how to reverse escalated cocaine self-administration after repeated social defeat stress experience. To our knowledge, this is the first study showing that several weeks after exposure to repeated social defeat stress, systemic injection or VTA microinjection of a CRFR1 antagonist prior to cocaine self-administration reversed stress-escalated cocaine self-administration in mice. Whether CRFR2 also plays a role in social defeat stress escalated cocaine self-administration in mice remains to be determined.
Our findings suggest that brain penetrant CRFR1 antagonists may have therapeutic potential for stress-escalated cocaine taking. Although a substantial number of preclinical studies have demonstrated consistent effects of CRFR1 blockade on drug self-administration and drug seeking particularly in dependent individuals (Heilig and Koob 2007; Mantsch et al. 2016; Shaham et al. 1997; Zorrilla et al. 2013), two recent clinical studies show negative outcomes with CRFR1 antagonists treatment in alcoholics (Kwako et al. 2015; Schwandt et al. 2016). Why do the CRFR1 antagonists fail in the translation from preclinical to clinical research? One potential reason is that preclinical research has not yet captured essential features of human addiction. In addition, the human laboratory model and the laboratory animal model may differ in terms of evaluating efficacy of the drugs. Lastly, the two clinical studies with negative findings deals with alcoholic patients. So far, there are still no clinical studies evaluating the effects of CRFR1 antagonists in psychostimulant or opiate use disorders (Shaham and de Wit 2016). Particularly, whether CRFR1 antagonists can block stress-escalated drug taking or seeking requires further verifications in clinical research.
In summary, the present experiments confirm and extend the roles of CRFR1 in stress-escalated cocaine self-administration, indicating that CRFR1 can be a target for either preventing or reversing stress effects. Importantly, we demonstrate that CRF within the VTA is increased after repeated social defeat stress, which may be responsible for the subsequent escalated cocaine self-administration. The current study starts to explore CRF-dopamine interactions by targeting CRF receptors in one of the key sources of dopamine, namely the VTA. For future studies, the precise interactions of CRF and dopamine systems need to be further investigated by targeting the specific CRF and dopaminergic neurons, using optogenetic or chemogenetic approaches.
Acknowledgments
This research was supported by National Institute on Drug Abuse Grant DA031734, KAM, PI.
Footnotes
The authors declare no competing financial interests.
References
- Albrechet-Souza L, Hwa LS, Han X, Zhang EY, DeBold JF, Miczek KA. Corticotropin releasing factor binding protein and CRF2 receptors in the ventral tegmental area: modulation of ethanol binge drinking in C57BL/6J mice. Alcohol Clin Exp Res. 2015;39:1609–1618. doi: 10.1111/acer.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf EN, Mantsch JR. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J Neurosci. 2011;31:11396–11403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Ungless MA, Bonci A. Convergent actions of orexin/hypocretin and CRF on dopamine neurons: emerging players in addiction. Brain Res. 2010;1314:139–144. doi: 10.1016/j.brainres.2009.10.068. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson CO, Holly EN, Shimamoto A, Albrechet-Souza L, Weiner LA, DeBold JF, Miczek KA. Social stress and CRF-dopamine interactions in the VTA: role in long-term escalation of cocaine self-administration. J Neurosci. 2014;34:6659–6667. doi: 10.1523/JNEUROSCI.3942-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson CO, Miguel TT, Quadros IM, DeBold JF, Miczek KA. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology (Berl) 2011;218:257–269. doi: 10.1007/s00213-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton BG, Ferrier IN, Perry RH. Distribution of corticotropin-releasing factor-like immunoreactivity in human brain. Neuropeptides. 1987;10:329–334. doi: 10.1016/s0143-4179(87)90083-7. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Cummings S, Elde R, Ells J, Lindall A. Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. J Neurosci. 1983;3:1355–1368. doi: 10.1523/JNEUROSCI.03-07-01355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Pavcovich LA, Grigoriadis DE, Valentino RJ. Previous stress alters corticotropin-releasing factor neurotransmission in the locus coeruleus. Neuroscience. 1995;65:541–550. doi: 10.1016/0306-4522(94)00496-r. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Effects of the CRH receptor antagonist CP-154,526 on intravenous cocaine self-administration in rats. Neuropsychopharmacology. 2000;23:577–586. doi: 10.1016/S0893-133X(00)00148-2. [DOI] [PubMed] [Google Scholar]
- Han X, Albrechet-Souza L, Doyle MR, Shimamoto A, DeBold JF, Miczek KA. Social stress and escalated drug self-administration in mice II. Cocaine and dopamine in the nucleus accumbens. Psychopharmacology (Berl) 2015;232:1003–1010. doi: 10.1007/s00213-014-3734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly EN, Boyson CO, Montagud-Romero S, Stein DJ, Gobrogge KL, DeBold JF, Miczek KA. Episodic Social Stress-Escalated Cocaine Self-Administration: Role of Phasic and Tonic Corticotropin Releasing Factor in the Anterior and Posterior Ventral Tegmental Area. J Neurosci. 2016;36:4093–4105. doi: 10.1523/JNEUROSCI.2232-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Heilig M, Rupniak NM, Steckler T, Griebel G. Neuropeptide systems as novel therapeutic targets for depression and anxiety disorders. Trends Pharmacol Sci. 2003;24:580–588. doi: 10.1016/j.tips.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP. Neurobiological mechanisms of addiction: focus on corticotropin-releasing factor. Curr Opin Investig Drugs. 2010;11:63–71. [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- Kwako LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan R, et al. The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology. 2015;40:1053–1063. doi: 10.1038/npp.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y. Stress- induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology. 2016;41:335–356. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvich DF, Stowe TA, Godfrey JR, Weinshenker D. A Method for Psychosocial Stress-Induced Reinstatement of Cocaine Seeking in Rats. Biol Psychiatry. 2016;79:940–946. doi: 10.1016/j.biopsych.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE., III Escalated or suppressed cocaine reward, tegmental BDNF and accumbal dopamine due to episodic vs. continuous social stress in rats. J Neurosci. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, O’Donnell JM. Intruder-evoked aggression in isolated and nonisolated mice: Effects of psychomotor stimulants and l-dopa. Psychopharmacology. 1978;57:47–55. doi: 10.1007/BF00426957. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Shuster L. Opioid-like analgesia in defeated mice. Science. 1982;215:1520–1522. doi: 10.1126/science.7199758. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., III Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals: Eighth Edition. National Academy Press; Washington DC: 2011. [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2. Academic Press; San Diego: 2001. [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends in Pharmacological Sciences. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Quadros IM, Miczek KA. Two modes of intense cocaine bingeing: increased persistence after social defeat stress and increased rate of intake due to extended access conditions in rats. Psychopharmacology (Berl) 2009;206:109–121. doi: 10.1007/s00213-009-1584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter RM, Pich EM, Koob GF, Weiss F. Sensitization of cocaine-stimulated increase in extracellular levels of corticotropin-releasing factor from the rat amygdala after repeated administration as determined by intracranial microdialysis. Neurosci Lett. 1995;187:169–172. doi: 10.1016/0304-3940(95)11365-4. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacological Reviews. 2001;53:209–243. [PubMed] [Google Scholar]
- Schwandt ML, Cortes CR, Kwako LE, George DT, Momenan R, Sinha R, et al. The CRF1 Antagonist Verucerfont in Anxious Alcohol-Dependent Women: Translation of Neuroendocrine, But not of Anti-Craving Effects. Neuropsychopharmacology. 2016;41:2818–2829. doi: 10.1038/npp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, de Wit H. Lost in Translation: CRF1 Receptor Antagonists and Addiction Treatment. Neuropsychopharmacology. 2016;41(12):2795–2797. doi: 10.1038/npp.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder K, Wang WW, Han R, McFadden K, Valentino RJ. Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology. 2012;37:520–530. doi: 10.1038/npp.2011.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6(2):243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Miczek KA. Social defeat stress, sensitization, and intravenous cocaine self-administration in mice. Psychopharmacology (Berl) 2007;192:261–273. doi: 10.1007/s00213-007-0712-4. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Heilig M, de Wit H, Shaham Y. Behavioral, biological, and chemical perspectives on targeting CRF(1) receptor antagonists to treat alcoholism. Drug Alcohol Depend. 2013;128:175–186. doi: 10.1016/j.drugalcdep.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]