Abstract
Rationale
Accumulating evidence shows that the serotonergic system plays a major role in psychostimulant abuse through its interactions with the dopaminergic system. Studies indicate that serotonin 5-HT2C receptors are one of the main classes of receptors involved in mediating the influence of serotonin in drug abuse.
Objective
The aim of the present study was to evaluate the effects of the selective serotonin 5-HT2C receptor agonist WAY163909 on the behavioral neuropharmacology of cocaine and methamphetamine in adult rhesus macaques.
Methods
Cocaine or methamphetamine self-administration and reinstatement were evaluated under second-order and fixed-ratio schedules of reinforcement, respectively. Cocaine- and methamphetamine-induced increases in dopamine were assessed through in vivo microdialysis targeting the nucleus accumbens.
Results
Pretreatment with WAY163909 dose-dependently attenuated cocaine and methamphetamine self-administration and drug-induced reinstatement of extinguished behavior previously maintained by cocaine or methamphetamine delivery. In an additional experiment, WAY163909 induced a dose-dependent attenuation of cocaine- or methamphetamine-induced dopamine overflow in the nucleus accumbens.
Conclusions
Our data indicate that selective 5-HT2C receptor activation decreases drug intake and drug-seeking behavior in nonhuman primate models of psychostimulant abuse through neurochemical mechanisms involved in the modulation of mesolimbic dopamine.
Keywords: cocaine, methamphetamine, self-administration, reinstatement, in vivo microdialysis, rhesus monkeys
Introduction
Psychostimulant abuse is a chronic relapsing disorder characterized by loss of control over drug intake despite harmful consequences (American Psychiatric Association 2013). Although psychostimulants differ in their pharmacodynamic properties, stimulant drugs exhibit abuse liability as a common feature and exert their reinforcing properties by increasing dopamine levels in the mesolimbic dopaminergic system (Koob and LeMoal 2006). For instance, the effectiveness of psychostimulants to increase extracellular dopamine levels in the nucleus accumbens has been shown to parallel their stimulant, reinforcing and reinstatement effects in nonhuman primates (for review see Howell and Negus 2014). Thus, dopaminergic neurotransmission has been a primary target for medications development for psychostimulant abuse.
Despite decades of research, there are no approved dopamine-based medications for the treatment of stimulant abuse. This has prompted researchers to investigate other possible neural systems that contribute to the development and maintenance of drug abuse. In this context, the serotonergic system has gained attention for playing an important role in the addictive cycle, especially through its direct and indirect interactions with the mesolimbic dopaminergic system (Howell and Cunningham 2015). Several serotonin receptors have been implicated in mediating the effects of serotonin on dopamine neurotransmission. However, studies indicate that the serotonin 5-HT2A and 5-HT2C receptors play an important role in mediating the influence of serotonin in drug abuse (Bubar and Cunningham 2006, 2008; Cunningham and Anastasio 2014).
Serotonin 5-HT2A receptors are highly expressed on dopaminergic neurons in the ventral tegmental area and on glutamatergic neurons in the prefrontal cortex, whereas GABAergic interneurons in those brain regions predominantly express 5-HT2C receptors (Howell and Cunningham 2015), suggesting that these receptors may directly modulate dopamine neurotransmission and dopamine levels in the nucleus accumbens. In vivo microdialysis studies provide convincing evidence that the reinforcing properties of psychostimulants are mediated primarily by dopamine release in the nucleus accumbens (Schultz et al. 1998; Martin-Soelch et al. 2001; Watanabe et al. 2001; Czoty et al. 2002). Both the self-administration and the reinstatement models provide a measure of drug-seeking behavior and have been used increasingly as laboratory models to evaluate the reinforcing properties of drugs of abuse in nonhuman primates (Howell and Wilcox 2002; Katz and Higgins 2003; Epstein et al. 2006). Because of the regional distribution of serotonin 5-HT2A and 5-HT2C receptors in the brain, those receptors have been shown to exert opposing effects on cocaine self-administration and cocaine-induced reinstatement in nonhuman primates (Murnane et al. 2013; Manvich et al. 2012a). Particularly, activation of 5-HT2C receptors has been shown to decrease cocaine self-administration, cocaine-induced reinstatement and dopamine overflow in the nucleus accumbens in both rodents (Grottick et al. 2000; Neisewander and Acosta 2007; Burbassi and Cervo 2008; Fletcher et al. 2008; Navailles et al. 2008) and squirrel monkeys (Manvich et al. 2012b; Rüedi-Bettschen et al. 2015). However, the effects of receptor subtype-selective compounds and the extent of those findings to other stimulant drugs have yet to be systematically evaluated.
The aim of the present study, therefore, was to evaluate the effects of the highly selective serotonin 5-HT2C receptor agonist WAY163909 (Dunlop et al. 2005; Neelamegam et al. 2014) on the behavioral neuropharmacology of cocaine and methamphetamine in rhesus monkeys. Studies have shown that WAY163909 exhibits excellent binding affinity (Ki of ∼10 nM) and functional selectivity for the human 5-HT2C receptor (Dunlop et al. 2005), being one of the most selective agents identified to date compared with other compounds reported in the literature, including Ro 60-0175 (Martin et al., 1998) and novel compounds such as lorcaserin (Thomsen et al., 2008) and vabicaserin (SCA-136) (Dunlop et al., 2011). WAY163909 presents low intrinsic activity as a partial agonist at 5-HT2B receptors and lack of functional activity at 5-HT2A receptors (Dunlop et al. 2005). The effects of WAY163909 were evaluated on cocaine and methamphetamine self-administration and reinstatement, as well as on cocaine- and methamphetamine- induced increases in dopamine in the nucleus accumbens. The present study adds to the literature by evaluating the effects of a highly selective 5-HT2C receptor agonist on self-administration, reinstatement and neurochemistry induced by distinct psychostimulants in a unique translational primate model.
Material and Methods
Subjects
Five adult female and one adult male rhesus monkeys (Macaca mulatta) weighing 9–16 kg served as subjects in the cocaine behavioral studies. Two adult female and three adult male rhesus monkeys weighing 10–16 kg served as subjects in the methamphetamine behavioral studies. In vivo microdialysis experiments were conducted in a group of four adult female rhesus monkeys weighing 7–9 kg. Animals were fitted with collars (Primate Products) prior to the initiation of the studies. Each subject was individually housed in stainless steel home cages and fed Purina monkey chow (Ralston Purina, St. Louis, MO), supplemented with fruit and vegetables daily. Water was continuously available in the colony. Environmental enrichment was provided on a regular basis. The colony was maintained at an ambient temperature of 22±2°C at 45–50% humidity, and the lights were set to a 12-h light/dark cycle (lights on at 7h; lights off at 19h). All subjects had a history of exposure to psychostimulants. All protocols and animal care and handling strictly followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition, revised 2011) and the recommendations of the American Association for Accreditation of Laboratory Animal Care, and were approved by the Institutional Animal Care and Use Committee of Emory University.
Drugs
Cocaine hydrochloride and methamphetamine hydrochloride (National Institute on Drug Abuse, Bethesda, MD, USA) were dissolved in 0.9% saline and administered intravenously. The 5-HT2C agonist WAY163909 (WAY) hydrochloride [(7b-R,10a-R)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta[b][1,4] diazepino [6,7,1hi] indole] (provided as a generous gift from Pfizer Inc®, New York, NY, USA), was dissolved in 10mg/mL beta-cyclodextrin and administered intramuscularly.
Surgery
All surgeries were conducted under aseptic conditions. Animals were initially anesthetized with Telazol (tiletamine HCl and zolazepam HCl, 2.0 mg i.m.) and ketamine HCl (20 mg i.m.), and anesthesia was maintained throughout the procedure with inhaled isoflurane (0.5–1.5%). A major vein (femoral or jugular) was implanted with a chronic indwelling catheter attached to a subcutaneous vascular access port, as previously described (Howell and Wilcox 2001). The subjects of the microdialysis experiments were also implanted with bilateral CMA/11 guide cannulae (CMA Microdialysis, Holliston, MA, USA) as previously described (Murnane et al. 2010). To target the area directly above the nucleus accumbens (NAc), the guides were placed 23mm anterior to the interaural midpoint in each subject and placed bilaterally at 4mm off of the midline.
Self-administration
The animals were positioned in a primate chair (Primate Products) and placed in a sound-attenuating experimental chamber for the duration of the self-administration (SA) sessions. Five animals (RYj8, RLa10, RDn8, RRh7, RYt8) responded for i.v. infusions of cocaine under a second-order schedule of reinforcement. The apparatus and SA schedule of reinforcement were as previously described (Wilcox et al. 2005), except that the limited hold was 60 s. Briefly, in the beginning of the session, the behavioral chamber was illuminated with a red light positioned over the lever in the operant panel which served as a discriminative stimulus. The second-order schedule was an [FI 10(FR 20:S)] where the first fixed ratio (FR) 20 completed after 10 min (fixed interval, FI 10) resulted in the illumination of a white stimulus light, the conditioned stimulus (CS), for 15 s and a cocaine infusion (1 ml infused over 6s). Following each drug infusion, there was a 1-min timeout during which responding on the lever had no programmed consequences. Completion of an FR20 during the FI 10-min component resulted in the illumination of the CS for 2 s. There were five [FI 10(FR 20:S)] components during the operant session, so that a total of five infusions could be delivered over a daily session.
For the methamphetamine experiments, 5 animals (ROf8, RVm8, RLk4, RZs9, RJl8) were trained to respond under a FR 20 schedule of drug delivery. The apparatus and SA procedure were previously described in detail by Howell and Wilcox (2001). Subjects had the opportunity to self-administer methamphetamine during 60-min daily sessions. During the test session, the behavioral chamber was illuminated with a white light positioned over the lever in the operant panel that served as a discriminative stimulus. Completion of the FR 20 response requirement resulted in a change in the stimulus light from white to red (CS) for 15s and a methamphetamine infusion (0.5 ml infused over 3s). This infusion was followed by a 60-s timeout during which responding on the lever had no programmed consequences. At the end of the timeout, the white light was presented again to signal the opportunity to complete another FR.
Response rates were calculated as the total number of lever presses during the active period divided by the active time throughout the session. Before the beginning of drug-interaction studies, the maximally effective behavioral-stimulant dose of methamphetamine or cocaine (EDMax), i.e., the dose that maintained the highest rates of responding during self-administration maintenance, was identified for each individual subject. The unit dose of cocaine (0.01, 0.03, 0.1 or 0.3 mg/kg/infusion) or methamphetamine (0.001, 0.003, 0.01 or 0.03 mg/kg/infusion) was altered until the EDMax was identified. The order of doses was randomized and counterbalanced across subjects within an experiment. The EDMax dose of cocaine was 0.03 mg/kg/infusion in one subject and 0.1 mg/kg/infusion in the remaining subject. The EDMax dose of methamphetamine was 0.003 mg/kg/infusion in two subjects and 0.01 mg/kg/infusion in the remaining subjects.
For drug-interaction studies, the effects of pretreatment with WAY were tested in combination with two doses of cocaine or methamphetamine: EDMax and one-half log-step unit dose below the EDMax dose (−0.5 EDMax). Stable SA behavior was defined as response rates that varied by <30% over 3 days. Once responding stabilized at a given dose (EDMax or −0.5 EDMax) for each of the animals in each group (cocaine or methamphetamine), vehicle or WAY (0.1, 0.3 or 1.0 mg/kg) were administered intramuscularly in the home-cage 45 min before the initiation of the SA session. WAY pretreatments (vehicle, 0.3 or 1.0 mg/kg) were then repeated with the other dose (EDMax or −0.5 EDMax) of cocaine or methamphetamine. Between different pretreatments, animals were returned to normal cocaine or methamphetamine SA conditions until stability criteria were met again, then a new pretreatment was administered on the following day. The order of self-administration doses (EDMax or −0.5 EDMax) and the order of pretreatments (vehicle or WAY doses) were randomized and counterbalanced across subjects.
Reinstatement
For the reinstatement experiments, behavior was maintained by the unit doses of 0.1 mg/kg/infusion for cocaine (n = 5; RYj8, RLa10, RIt8, RRh7, RYt8) and 0.03 mg/kg/infusion for methamphetamine (n = 4; ROf8, RLk4, RZs9, RJl8). Behavior was extinguished by substituting saline for cocaine or methamphetamine, and was operationally defined as a rate of responding over 2 sequential sessions that was <20% of the 3-day mean rate of responding during the maintenance of cocaine or methamphetamine SA. During the extinction sessions, the CS (white light for cocaine, red light for methamphetamine) was never illuminated. Otherwise, the extinction sessions were identical to the SA maintenance sessions. Starting on the day after subjects reached extinction criteria, reinstatement test days consisted of an experimenter-administered prime of cocaine or methamphetamine given through the vascular access port 5 min before the start of the session. During these sessions, saline was substituted for cocaine or methamphetamine, and the CS was illuminated again throughout the session. Therefore, we describe the sessions as drug- and cue-induced reinstatement tests. Firstly, the dose of cocaine (0.03, 0.1 or 0.3 mg/kg) or methamphetamine (0.01, 0.03, 0.1, 0.3 or 1.0 mg/kg) was altered until the EDMax dose of cocaine or methamphetamine priming was identified for each individual subject. The order of doses was randomized and counterbalanced across subjects within an experiment. The EDMax for all of the subjects in the cocaine group was 0.1 mg/kg. The EDMax for subjects in the methamphetamine group varied from 0.03 to 0.3 mg/kg. The subsequent reinstatement experiments were conducted with the EDMax doses of cocaine or methamphetamine prime.
WAY (0.1, 0.3 or 1.0 mg/kg, i.m.) or its vehicle were administered in the home-cage 45 min before the drug prime. Reinstatement sessions with vehicle pretreatment alternated with WAY pretreatment sessions to ensure a reliable reinstatement response. If the reinstatement effect dissipated, animals were returned to cocaine or methamphetamine SA conditions until stability criteria were met again, then a new block of extinction and reinstatement began. The order of treatments or doses was randomized and counterbalanced across subjects within an experiment.
In vivo microdialysis
Microdialysis samples were collected and analyzed as previously described (Murnane et al. 2010, 2012). Briefly, all procedures were performed in fully conscious subjects while they sat in a primate chair (Primate Products) within a sound-attenuating testing chamber. After the subject was placed in the chamber, 28mm stainless steel microdialysis probes with 4mm membranes (CMA Microdialysis, Holliston, MA, USA) were inserted into the surgically implanted guide cannulae. For the cocaine studies, three subjects (RDn8, RHp8, RNb7) underwent the microdialysis protocol six times, such that each subject received vehicle and WAY (0.1 and 1.0 mg/kg, i.m.) pretreatments combined with subsequent saline or cocaine (1.0 mg/kg, i.v.) treatments. For the methamphetamine (1.0 mg/kg, i.v.) studies, the same protocol was conducted in four subjects (RDn8, RHp8, RNb7, RJs6). Experiments consisted of a 1h equilibrium period after which samples were collected every 10 min. Vehicle and WAY were administered 30 min after the sampling began and 30 min before saline, cocaine or methamphetamine administration and samples were collected over the next 2h. The viability of the sampling site was verified through retrodialysis of a potassium-enriched (100mm) solution otherwise ionically matched to artificial cerebrospinal fluid (aCSF). Dopamine concentrations within the dialysate were quantified using high-pressure liquid chromatography with electrochemical detection, as previously described (Murnane et al. 2010, 2012). The data were analyzed by comparison with standard concentration curves using Chromeleon 6.8 Chromatography Data System (Thermo Fisher Scientific, Waltham, MA, USA). The doses of cocaine and methamphetamine used in the microdialysis experiments were based on previous studies from our group (Kirkland Henry et al. 2009; Murnane et al. 2013; Berro et al. 2017). In vivo microdialysis sessions were performed no more frequently than every two weeks for each subject. A microdialysis session with either cocaine or methamphetamine following saline pretreatment was conducted between blocks of experiments to rule out the effects of non-specific site damage on the results, and data on different sessions were averaged for each animal. The order of vehicle and WAY doses was randomized and counterbalanced across subjects.
Data analysis
Data for the self-administration experiments are presented and were analyzed as normalized data (percentage of average response rates during self-administration maintenance) or as raw data (drug intake/session). Data for the reinstatement experiments are presented as normalized data (percentage of average response rates/saline infusions during reinstatement induced by the EDMax dose of cocaine/methamphetamine prime). Behavioral data were analyzed by one- or two-way repeated-measures (RM) analysis of variance (ANOVA). Microdialysis data were analyzed by two-way RM ANOVA. The three data points immediately preceding the pretreatment administration were averaged to create the baseline. All post hoc comparisons were performed using Dunnett’s test. All graphical data presentations were created using Prism 5 (GraphPad Software), all statistical tests were performed using PASW Statistics 18 (SPSS Statistics Software), and significance was arbitrated at a probability of p<0.05.
Results
Self-administration
The effects of pretreatment with WAY on cocaine SA are shown in Fig. 1A. The mean response rate (± S.E.M.) during cocaine SA maintenance was 0.88 ± 0.16 responses/s for the EDMax dose of methamphetamine, and 0.69 ± 0.2 for the −0.5 EDMax dose. Two-way RM ANOVA corrected for multiple comparisons using Dunnett’s post hoc test revealed a significant effect of treatment (vehicle vs WAY) [F(2,12)=120.1, p<0.0001]. Pretreatment with WAY dose-dependently decreased response rates during cocaine SA regardless of cocaine dose. Specifically, pretreatment with the middle (p<0.05) and the high (p<0.001) doses of WAY significantly decreased response rates compared to vehicle at the EDMax dose of cocaine. Pretreatment with the high dose of WAY also significantly decreased response rates at the −0.5 EDMax dose of cocaine (p<0.001). Analysis of cocaine intake during SA sessions (Fig. 1B) revealed a significant interaction effect between treatment (vehicle vs WAY) and cocaine dose (EDMax vs −0.5 EDMax) (two-way RM ANOVA [F(2,12)=8.15, p<0.01]), showing that WAY dose-dependently decreased drug intake, with the high dose of WAY significantly decreasing cocaine intake compared to vehicle at the EDMax (p<0.001) and the −0.5 EDMax (p<0.001) doses of cocaine.
Figure 1.
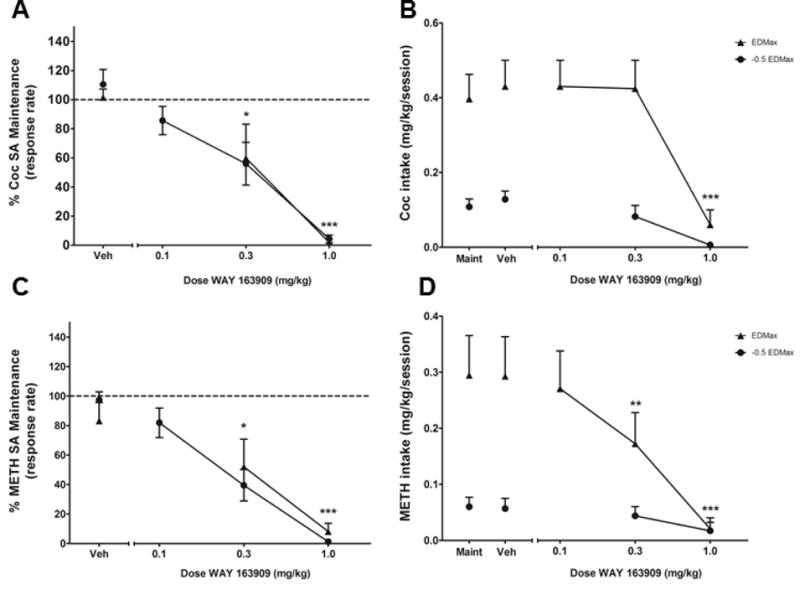
Effects of pretreatment with the serotonin 5-HT2C receptor agonist WAY163909 on cocaine (Coc, N = 5) or methamphetamine (METH, N = 5) self-administration (SA). Response rates (responses/s) during cocaine (A, second-order schedule, 0.1 mg/kg/infusion, i.v.) or methamphetamine (C, FR 20 schedule, 0.01 mg/kg/infusion, i.v.) self-administration (SA) and drug intake (mg/kg/session) during cocaine (B) or methamphetamine (D) maintenance (Maint) or after pretreatment with vehicle (Veh) or WAY 163909 (0.1, 0.3 and 1.0 mg/kg, i.m.). Data for response rates are presented as normalized data (percentage of average response rates during self-administration maintenance). Dotted lines represent response rates during self-administration maintenance (100%). Data are expressed as mean ± SEM. *p<0.05 and **p<0.01 for the EDMax dose compared with vehicle pretreatment; ***p<0.0001 for both EDMax and -0.5EDMax doses compared with vehicle pretreatment.
Fig. 1C illustrates the effects of pretreatment with WAY on methamphetamine SA. The mean response rate (± S.E.M.) during SA maintenance was 1.03 ± 0.11 responses/s for the EDMax dose of methamphetamine, and 0.44 ± 0.13 for the −0.5 EDMax dose. Two-way RM ANOVA corrected for multiple comparisons using Dunnett’s post hoc test revealed a significant effect of treatment (vehicle vs WAY) [F(2,12)=63.37, p<0.0001]. Pretreatment with WAY dose-dependently decreased response rates during methamphetamine SA regardless of methamphetamine dose. Pretreatment with the middle (p<0.01) and the high (p<0.001) doses of WAY significantly decreased response rates compared to vehicle at the EDMax dose of methamphetamine. Pretreatment with the high dose of WAY also significantly decreased response rates at the −0.5 EDMax dose of methamphetamine (p<0.001). Analysis of methamphetamine intake during SA sessions (Fig. 1D) also revealed a significant interaction effect between treatment (vehicle vs WAY) and methamphetamine dose (EDMax vs −0.5 EDMax) (two-way RM ANOVA [F(2,12)=7.307, p<0.01]), showing that WAY dose-dependently decreased drug intake, with the middle (p<0.01) and the high (p<0.001) doses of WAY significantly decreasing methamphetamine intake compared to vehicle at the EDMax dose of methamphetamine. The high dose of WAY also significantly decreased drug intake at the −0.5 EDMax dose of methamphetamine (p<0.001).
Reinstatement
For cocaine experiments, the mean cocaine SA response rate (± S.E.M.) typically declined within two to three sessions during extinction, from 0.99 ± 0.32 responses/s during SA maintenance to 0.1 ± 0.05 responses/s during effective extinction conditions. The mean response rate (± S.E.M.) engendered by the priming dose of 0.1 mg/kg was 1.0 ± 0.35 responses/s (approximately 101% of the rates typically observed during cocaine SA). The effects of pretreatment with a range of doses of WAY on drug- and cue-induced reinstatement are shown in Fig. 2A. One-way RM ANOVA [F(4,19)=8.35, p<0.01] corrected for multiple comparisons using Dunnett’s post hoc test showed that pretreatment with WAY dose-dependently decreased response rates during reinstatement. Pretreatment with the middle (p<0.05) and the high (p<0.001) doses significantly decreased response rates compared to vehicle. Analysis of the number of saline infusions during reinstatement sessions (Fig. 2B) also showed that WAY dose-dependently decreased the number of infusions (one-way RM ANOVA [F(4,19)=28.22, p<0.0001]), with the high (p<0.001) dose of WAY significantly decreasing the number of saline infusions compared to vehicle.
Figure 2.
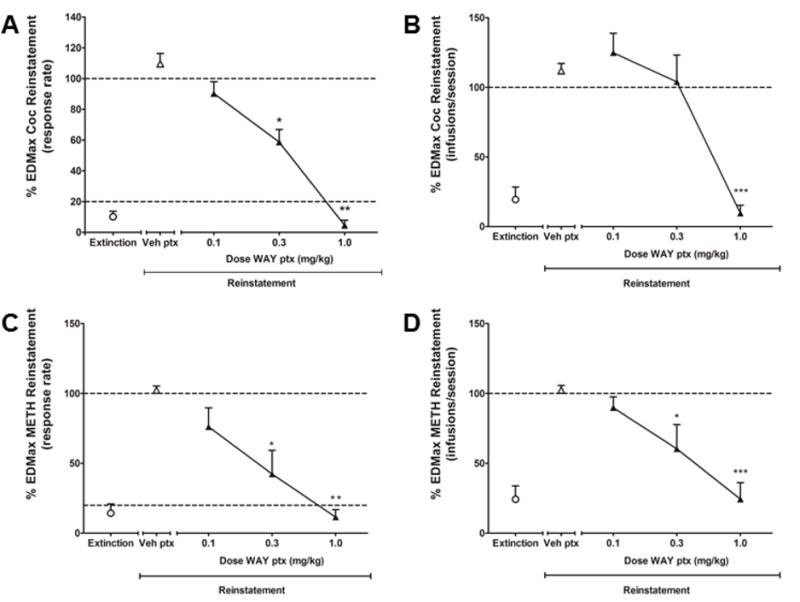
Effects of pretreatment with the serotonin 5-HT2C receptor agonist WAY163909 on cocaine (Coc, N = 5) or methamphetamine (METH, N = 4) reinstatement. Response rates (responses/s) during the reinstatement of extinguished behavior previously maintained by cocaine (A, 2nd order schedule, 0.1 mg/kg/infusion, i.v.) or methamphetamine (C, fixed-ratio 20 schedule of reinforcement, 0.03 mg/kg/infusion, i.v.) self-administration (SA) and number of saline infusions/session during cocaine (B) or methamphetamine (D) reinstatement after pretreatment (ptx) with vehicle (Veh) or WAY163909 (0.1, 0.3 and 1.0 mg/kg, i.m.). Data for response rates are presented as normalized data (percentage of average response rates during self-administration maintenance). A, C: dotted lines represent extinction criteria (20%) and response rates during self-administration maintenance (100%); B, D: dotted lines represent average number of infusions during self-administration maintenance (~4.8 for cocaine; ~26.1 for methamphetamine). Data are expressed as mean ± SEM. *p<0.05, **p<0.01 and ***p<0.001 compared with vehicle pretreatment.
For methamphetamine experiments, the mean SA response rate (± S.E.M.) typically declined within two-three sessions during extinction, from 0.32 ± 0.03 during SA maintenance to 0.04 ± 0.01 responses/s during effective extinction conditions. The mean response rate (± S.E.M.) engendered by the EDMax prime dose of methamphetamine was 0.59 ± 0.17 responses/s (approximately 187% of the rates typically observed during methamphetamine SA). The effects of pretreatment with a range of doses of WAY on drug- and cue-induced reinstatement are shown in Fig. 2C. One-way RM ANOVA [F(3,15)=8.99, p<0.01] corrected for multiple comparisons using Dunnett’s post hoc test showed that pretreatment with WAY dose-dependently decreased response rates during reinstatement. Pretreatment with the middle (p<0.05) and the high (p<0.01) doses significantly decreased response rates compared to vehicle. Analysis of the number of saline infusions during reinstatement sessions (Fig. 2D) also showed that WAY dose-dependently decreased the number of infusions (one-way RM ANOVA [F(3,15)=12.11, p<0.01]), with the middle (p<0.05) and the high (p<0.001) doses of WAY significantly decreasing the number of saline infusions compared to vehicle.
In vivo microdialysis
For cocaine experiments, the mean baseline dopamine levels (± S.E.M.) varied from 3.29 ± 2.03 nM for sessions conducted with vehicle pretreatments to 4.56 ± 0.93 nM and 3.57 ± 1.21 nM for sessions conducted with 0.1 and 1.0 mg/kg WAY pretreatments, respectively. Two-way RM ANOVA revealed a significant main effect of time [F(12,48)=52.85, p<0.0001], but not pretreatment or interaction for pretreatment with 0.1 mg/kg WAY (Fig. 3A). Dunnett’s post hoc test showed that, following vehicle pretreatment, cocaine significantly elevated extracellular dopamine levels from min 20 through 40 after injection compared with baseline, an effect that was not altered by pretreatment with the lowest dose of WAY. For pretreatment with 1.0 mg/kg WAY, two-way RM ANOVA revealed a significant interaction effect between time and pretreatment [F(12,48)=8.82, p=0.01] (Fig. 3B). Dunnett’s post hoc test showed that cocaine was significantly less effective at increasing extracellular dopamine following 1.0 mg/kg WAY pretreatment at times 20 through 40 compared to vehicle. An additional two-way ANOVA conducted with the 3 pretreatment conditions (vehicle, 0.1 mg/kg WAY or 1.0 mg/kg WAY) for times 0 through 60 revealed a significant interaction effect between time and pretreatment [F(12,36)=2.09, p<0.05].
Figure 3.
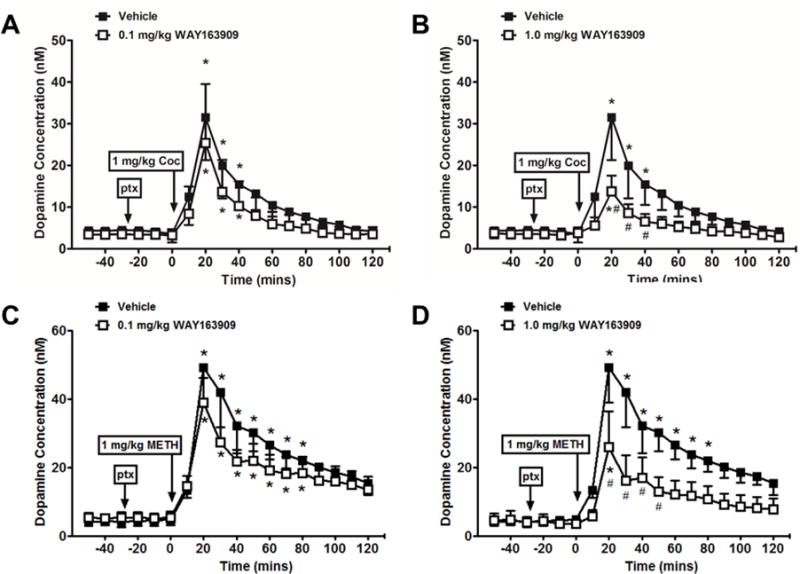
Effects of pretreatment with the serotonin 5-HT2C receptor agonist WAY163909 on dopamine overflow induced by cocaine (Coc, N = 3) or methamphetamine (METH, N = 4) in the nucleus accumbens. Increases in extracellular dopamine levels induced by cocaine (1 mg/kg, i.v.) or methamphetamine (1 mg/kg, i.v.) in the nucleus accumbens following pretreatment (ptx) with vehicle, (A,C) 0.1 mg/kg or (B,D) 1.0 mg/kg WAY163909 (i.m). All data points represent the mean ± SEM. *p<0.05 compared with baseline; #p<0.05 compared with vehicle pretreatment.
For methamphetamine experiments, the mean baseline dopamine levels (± S.E.M.) varied from 4.35 ± 1.38 nM for sessions preceded by vehicle pretreatments to 5.28 ± 1.51 nM and 4.08 ± 1.83 nM for sessions preceded by 0.1 and 1.0 mg/kg WAY pretreatments, respectively. Two-way RM ANOVA revealed a significant main effect of time [F(17,102)=65.55, p<0.0001], but not pretreatment or interaction for pretreatment with 0.1 mg/kg WAY (Fig. 4A). Dunnett’s post hoc test showed that following vehicle pretreatment, methamphetamine significantly elevated extracellular dopamine levels from min 20 through 80 after injection compared with baseline, an effect that was not altered by pretreatment with the lowest dose of WAY. For pretreatment with 1.0 mg/kg WAY, two-way RM ANOVA revealed a significant interaction effect between time and pretreatment [F(17,102)=8.26, p<0.01] (Fig. 4B). Dunnett’s post hoc test showed that methamphetamine was significantly less effective at increasing extracellular dopamine following 1.0 mg/kg WAY pretreatment at times 20 through 80 compared to vehicle. Importantly, 1.0 mg/kg WAY had no effects on baseline dopamine levels per se (data not shown). An additional two-way ANOVA conducted with the 3 pretreatment conditions (vehicle, 0.1 mg/kg WAY or 1.0 mg/kg WAY) for times 0 through 60 revealed a significant interaction effect between time and pretreatment [F(12,54)=1.95, p<0.05].
Discussion
The major findings of the present study were that a highly selective 5-HT2C receptor agonist dose-dependently attenuated cocaine and methamphetamine self-administration and the reinstatement of extinguished behavior previously maintained by cocaine or methamphetamine delivery in rhesus monkeys. Importantly, in an additional experiment, WAY163909 induced a dose-dependent attenuation of cocaine- or methamphetamine-induced increases in dopamine in the nucleus accumbens, indicating that selective 5-HT2C receptor activation attenuates the behavioral and neurochemical effects of stimulant drugs in rhesus monkeys.
The present findings are consistent with previous studies showing that the 5-HT2C receptor agonist Ro 60-0175 attenuated the behavioral and neurochemical effects of cocaine in squirrel monkeys (Manvich et al. 2012b). Pretreatment with Ro 60-0175 dose-dependently attenuated cocaine self-administration and cocaine-induced reinstatement, also decreasing extracellular dopamine levels induced by cocaine in the nucleus accumbens. In a recent study, Rüedi-Battschen and colleagues (2015) also demonstrated attenuation of reinstatement of drug seeking induced by cocaine priming after pretreatment with Ro 60-0175 even in the absence of the cocaine-paired stimulus. Accordingly, antagonism at the serotonin 5-HT2C receptor has been shown to exhibit abuse-related effects typical of stimulants in squirrel monkeys, also interacting with cocaine in an apparently additive manner (Manvich et al. 2012a). Pretreatment with the selective 5-HT2C receptor antagonist SB 242084 in subjects trained to lever-press on a fixed-interval schedule of stimulus termination produced behavioral-stimulant effects per se and potentiated the self-administration, drug-induced reinstatement and dopaminergic effects of low doses of cocaine in squirrel monkeys. The potentiating effects of SB 242084 on cocaine-induced reinstatement, however, were not confirmed in a recent study by others (Rüedi-Battschen et al. 2015).
Importantly, in the present study WAY was effective in decreasing self-administration and drug- and cue-induced reinstatement for both cocaine and methamphetamine under different schedules of reinforcement. An important feature of FR schedules is the direct relationship between rate of responding and frequency of drug injection, while the reinforcing effects of the drug, but not to drug intake, can be directly related to response rates under second-order schedules (for review see Spealman and Goldberg 1978). Second-order schedules are particularly useful for the study of the ways in which drug-associated stimuli can control drug-seeking behavior that results in a limited number of drug presentations, minimizing possible interference of drug effects with operant responding (for review see Howell and Fantegrossi 2009). In addition to the different schedules of reinforcement, the reinstatement procedure also provides a measure of drug-seeking behavior and has been used increasingly as a laboratory model of relapse to drug abuse, although the validity of the model to drug relapse in humans remains to be documented (Katz and Higgins 2003; Epstein et al. 2006). Our results show that selective 5-HT2C receptor agonist WAY dose-dependently decreased not only drug intake (methamphetamine self-administration, FR schedule), but also drug-seeking behavior that was maintained by both cue and drug presentations (cocaine self-administration, second-order schedule) and drug-seeking behavior induced by a drug prime and maintained by the presentation of drug-associated cue (methamphetamine and cocaine, reinstatement). Thus, our data indicate that selective 5-HT2C receptor activation has a broad action on the abuse-related effects of different types of stimulant drugs, inhibiting both drug intake and drug-seeking behavior in nonhuman primate models of drug abuse.
The mechanism of action of 5-HT2C receptors on the behavioral-stimulant effects of cocaine and methamphetamine has been proposed to be related to increased GABAergic activity, both in the ventral tegmental area and in the prefrontal cortex (Howell and Cunningham 2015). Serotonin 5-HT2C receptors are highly expressed on GABAergic interneurons in those brain areas, and activation of 5-HT2C receptors activates those neurons, increasing GABA release. Increased GABAergic activity in the ventral tegmental area and in the prefrontal cortex inhibits dopaminergic and glutamatergic neurons, respectively, ultimately decreasing dopamine levels in the nucleus accumbens. In our studies, although the high dose of WAY did not affect baseline dopamine levels in the nucleus accumbens, it was effective in attenuating the dopaminergic effects of both cocaine and methamphetamine.
Our results also shed light on the neurochemical and neuroanatomical mechanisms by which serotonin receptors differentially regulate the abuse-related behavioral effects of psychostimulants. We have previously demonstrated that the selective serotonin 5-HT2A receptor antagonist M100907 blocks cocaine-induced reinstatement, but does not attenuate cocaine self-administration in rhesus monkeys, an effect that was accompanied by a decrease in cocaine-induced dopamine overflow in the caudate nucleus, but not in the nucleus accumbens (Murnane et al. 2013). Those results suggest that 5-HT2A receptors modulate the nigrostriatal and mesocortical dopamine pathways, and that those pathways are mainly involved in drug-induced reinstatement, but not self-administration, of psychostimulant drugs (Murnane et al. 2013). The present findings, on the other hand, indicate that the influence of 5-HT2C receptors is broader, with 5-HT2C activation blocking both cocaine- and methamphetamine-induced reinstatement and attenuating drug self-administration. Although we did not evaluate the effects of WAY on psychostimulant-induced dopamine overflow in the caudate nucleus, in a previous study conducted in squirrel monkeys, selective 5-HT2C activation attenuated the dopaminergic effects of cocaine in the nucleus accumbens, but not in the caudate nucleus (Manvich et al. 2012b). Thus, 5-HT2C receptors selectively modulate the mesolimbic dopamine pathway, and this pathway appears to be important for several abuse-related effects of psychostimulants, including drug intake and drug-seeking behavior.
In this context, 5-HT2A antagonists and 5-HT2C agonists have been shown to exert complementary effects in the context of psychostimulant abuse, indicating that combined therapies with action at both receptor subtypes may be a promising avenue for developing medications that reduce the abuse-related effects of psychostimulants. In fact, combined administration of subthreshold doses of the selective 5-HT2A receptor antagonist M100907 and the 5-HT2C agonist WAY163909 have been shown to synergistically suppress the behavioral effects of cocaine in rodents (Cunningham et al. 2013) and the sleep-disrupting effects of methamphetamine self-administration in rhesus monkeys (Perez Diaz et al. 2017). Importantly, combination of low doses of 5-HT2A receptor antagonists and 5-HT2C receptor agonists might help minimize possible side effects of administration of high doses of serotonergic compounds. For instance, daytime administration of the high dose of WAY used in the present study has been shown to significantly decrease general home-cage daytime activity (Perez Diaz et al, 2017). However, the intermediate dose of the 5-HT2C compound had no effects on baseline daytime activity while exerting a marked attenuation on cocaine and methamphetamine self-administration and reinstatement. Although we did not evaluate the effects of WAY on non-drug control conditions, previous studies in rodents have shown that WAY dose-dependently decreased reinstatement induced by the presentation of cocaine-, but not sucrose-, associated cues, suggesting that WAY differentially altered the incentive-salience value of cocaine- vs. sucrose-associated cues (Cunningham et al. 2011). The same has been observed for the serotonin 2C receptor agonist Ro 60-0175 (Burbassi and Cervo 2008). In addition, in the study by Manvich and colleagues (2013), Ro 60-0175 exhibited behavioral specificity, decreasing the reinforcing effects of cocaine without significantly altering non-drug-maintained responding in squirrel monkeys (Manvich et al. 2013).
In summary, our results support previous studies indicating that serotonin 5-HT2C receptors play a major role in the behavioral and neurochemical effects of psychostimulants. Unlike 5-HT2A receptor antagonists, which are not expected to suppress drug intake, the current data show that activation of 5-HT2C receptors may have a broad effect on the abuse-related behavioral effects of both cocaine and methamphetamine, inhibiting drug intake and drug-seeking behavior in nonhuman primate models through mechanisms involved in the modulation of the mesolimbic dopamine pathway. Modulation of 5-HT2C receptors, or combined therapies with serotonin 5-HT2A and 5-HT2C receptor agents, may represent promising avenues for addiction therapies.
Acknowledgments
The authors thank Lisa Neidert, Juliet Brown, Marisa Olsen, Cydney Eisenberg, Melis Odabas-Geldiay, Amanda Spielman and Hannah Shields for their excellent assistance with the experiments. This research was supported by USPHS Grants DA10344 (LLH), DA031246 (LLH) and ODP51OD11132 (Yerkes National Primate Research Center), AFIP (LFB) and FAPESP Grants 2015/16109-7 (LFB) and 2015/25482-3 (LFB).
Footnotes
The authors declare no conflict of interest.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Washington, DC: 2013. [Google Scholar]
- Berro LF, Andersen ML, Tufik S, Howell LL. Effects of GABAA receptor positive allosteric modulators on the behavioral and neurochemical effects of methamphetamine in rhesus monkeys. Neuropharmacology. 2017;2017 doi: 10.1016/j.neuropharm.2017.05.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog Brain Res. 2008;172:319–346. doi: 10.1016/S0079-6123(08)00916-3. [DOI] [PubMed] [Google Scholar]
- Burbassi S, Cervo L. Stimulation of serotonin2C receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology (Berl) 2008;196:15–27. doi: 10.1007/s00213-007-0916-7. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Fox RG, Anastasio NC, Bubar MJ, Stutz SJ, Moeller FG, Gilbertson SR, Rosenzweig-Lipson S. Selective serotonin 5-HT(2C) receptor activation suppresses the reinforcing efficacy of cocaine and sucrose but differentially affects the incentive-salience value of cocaine- vs. sucrose-associated cues. Neuropharmacology. 2011;61:513–23. doi: 10.1016/j.neuropharm.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC, Fox RG, Stutz SJ, Bubar MJ, Swinford SE, et al. Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chem Neurosci. 2013;4:110–21. doi: 10.1021/cn300072u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2002;300:831–837. doi: 10.1124/jpet.300.3.831. [DOI] [PubMed] [Google Scholar]
- Dunlop J, Sabb AL, Mazandarani H, Zhang J, Kalgaonker S, Shukhina E, et al. WAY-163909 [(7bR, 10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi]indole], a novel 5-hydroxytryptamine 2C receptor-selective agonist with anorectic activity. J Pharmacol Exp Ther. 2008;313:862–9. doi: 10.1124/jpet.104.075382. [DOI] [PubMed] [Google Scholar]
- Dunlop J, Watts SW, Barrett JE, Coupet J, Harrison B, Mazandarani H, Nawoschik S, Pangalos MN, Ramamoorthy S, Schechter L, Smith D, Stack G, Zhang J, Zhang G, Rosenzweig-Lipson S. Characterization of vabicaserin (SCA-136), a selective 5-hydroxytryptamine 2C receptor agonist. J Pharmacol Exp Ther. 2011;337:673–80. doi: 10.1124/jpet.111.179572. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. The 5-HT2C receptor agonist Ro60-0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology. 2008;33:1402–1412. doi: 10.1038/sj.npp.1301509. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA. Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 2007;195:223–34. doi: 10.1007/s00213-007-0891-z. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Fletcher PJ, Higgins GA. Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. J Pharmacol Exp Ther. 2000;295:1183–1191. [PubMed] [Google Scholar]
- Howell LL, Cunningham KA. Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev. 2015;67:176–97. doi: 10.1124/pr.114.009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Fantegrossi WE. Intravenous Drug Self-Administration in Nonhuman Primates. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 9. [PubMed] [Google Scholar]
- Howell LL, Negus SS. Monoamine transporter inhibitors and substrates as treatments for stimulant abuse. Adv Pharmacol. 2014;69:129–76. doi: 10.1016/B978-0-12-420118-7.00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Wilcox KM. Intravenous drug self-administration in nonhuman primates. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. CRC Press; Boca Raton, FL: 2001. pp. 91–110. [Google Scholar]
- Howell LL, Wilcox KM. Functional imaging and neurochemical correlates of stimulant self-administration in primates. Psychopharmacology (Berl) 2002;163:352–61. doi: 10.1007/s00213-002-1207-y. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl) 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Kirkland Henry P, Davis M, Howell LL. Effects of cocaine self-administration history under limited and extended access conditions on in vivo striatal dopamine neurochemistry and acoustic startle in rhesus monkeys. Psychopharmacology (Berl) 2009;205:237–47. doi: 10.1007/s00213-009-1534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Neurobiology of Addiction. London: Elsevier; 2006. [Google Scholar]
- Manvich DF, Kimmel HL, Cooper DA, Howell LL. The serotonin 2C receptor antagonist SB 242084 exhibits abuse-related effects typical of stimulants in squirrel monkeys. J Pharmacol Exp Ther. 2012;342:761–9. doi: 10.1124/jpet.112.195156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvich DF, Kimmel HL, Howell LL. Effects of serotonin 2C receptor agonists on the behavioral and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2012;341:424–34. doi: 10.1124/jpet.111.186981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Soelch C, Leenders KL, Chevalley A-F, Missimer J, Kunig G, Magyar S, Mino A, Schultz W. Reward mechanisms in the brain and their role in dependence: evidence from neurophysiological and neuroimaging studies. Brain Res Rev. 2001;36:139–149. doi: 10.1016/s0165-0173(01)00089-3. [DOI] [PubMed] [Google Scholar]
- Murnane KS, Fantegrossi WE, Godfrey JR, Banks ML, Howell LL. Endocrine and neurochemical effects of 3,4-methylenedioxymethamphetamine and its stereoisomers in rhesus monkeys. J Pharmacol Exp Ther. 2010;334:642–650. doi: 10.1124/jpet.110.166595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Kimmel HL, Rice KC, Howell LL. The neuropharmacology of prolactin secretion elicited by 3,4-methylenedioxymethamphetamine (“ecstasy”): a concurrent microdialysis and plasma analysis study. Horm Behav. 2012;61:181–190. doi: 10.1016/j.yhbeh.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Winschel J, Schmidt KT, Stewart LM, Rose SJ, Cheng K, et al. Serotonin 2A receptors differentially contribute to abuse-related effects of cocaine and cocaine-induced nigrostriatal and mesolimbic dopamine overflow in nonhuman primates. J Neurosci. 2013;33:13367–74. doi: 10.1523/JNEUROSCI.1437-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navailles S, Moison D, Cunningham KA, Spampinato U. Differential regulation of the mesoaccumbens dopamine circuit by serotonin2C receptors in the ventral tegmental area and the nucleus accumbens: an in vivo microdialysis study with cocaine. Neuropsychopharmacology. 2008;33:237–246. doi: 10.1038/sj.npp.1301414. [DOI] [PubMed] [Google Scholar]
- Neelamegam R, Hellenbrand T, Schroeder FA, Wang C, Hooker JM. Imaging evaluation of 5HT2C agonists, [(11)C]WAY-163909 and [(11)C]vabicaserin, formed by Pictet-Spengler cyclization. J Med Chem. 2014;57:1488–94. doi: 10.1021/jm401802f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Acosta JI. Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behav Pharmacol. 2007;18:791–800. doi: 10.1097/FBP.0b013e3282f1c94b. [DOI] [PubMed] [Google Scholar]
- Perez Diaz M, Andersen ML, Rice KC, Howell LL. Combined serotonin 5-HT2CR agonist and 5-HT2AR antagonist administration attenuates the effects of methamphetamine self-administration on actigraphy-based sleep parameters in rhesus monkeys. Submitted. [Google Scholar]
- Rüedi-Bettschen D, Spealman RD, Platt DM. Attenuation of cocaine-induced reinstatement of drug seeking in squirrel monkeys by direct and indirect activation of 5-HT2C receptors. Psychopharmacology (Berl) 2015;232:2959–2968. doi: 10.1007/s00213-015-3932-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Goldberg SR. Drug self-administration by laboratory animals: control by schedules of reinforcement. Annu Rev Pharmacol Toxicol. 1978;18:313–39. doi: 10.1146/annurev.pa.18.040178.001525. [DOI] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, Al-Shamma H, Smith B, Chalmers D, Behan D. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther. 2008;325:577–87. doi: 10.1124/jpet.107.133348. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Cromwell HC, Tremblay L, Hollerman JR, Hikosaka K, Schultz W. Behavioral reactions reflecting differential reward expectations in monkeys. Exp Brain Res. 2001;140:511–518. doi: 10.1007/s002210100856. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Kimmel HL, Lindsey KP, Votaw JR, Goodman MM, Howell LL. In vivo comparison of the reinforcing and dopamine transporter effects of local anesthetics in rhesus monkeys. Synapse. 2005;58:220–8. doi: 10.1002/syn.20199. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 2004;176:376–85. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]


