Abstract
Purpose
To examine racial differences in smoking rates at the time of breast cancer diagnosis and subsequent survival among African-American and non-African-American women in the Carolina Breast Cancer Study (Phases I/II), a large population-based North Carolina study.
Methods
We interviewed 788 African-American and 1,020 Caucasian/non-African-American women diagnosed with invasive breast cancer from 1993–2000, to assess smoking history. After a median follow-up of 13.56 years, we identified 717 deaths using the National Death Index; 427 were breast cancer-related. We used Cox regression to examine associations between self-reported measures of smoking and breast cancer-specific survival within 5 years and up to 18 years after diagnosis conditional on 5-year survival. We examined race and estrogen receptor status as potential modifiers.
Results
Current (vs never) smoking was not associated with 5-year survival; however, risk of 13-year conditional breast cancer-specific mortality was elevated among women who were current smokers at diagnosis (HR=1.54, 95% CI=1.06–2.25), compared to never smokers. Although smoking rates were similar among African-American (22.0%) and non-African-American (22.1%) women, risk of breast cancer-specific mortality was elevated among African-American (HR=1.69, 95% CI=1.00–2.85), but only weakly elevated among non-African-American (HR=1.22, 95% CI=0.70–2.14) current (vs never) smokers (PInteraction=0.30). Risk of breast cancer-specific mortality was also elevated among current (vs never) smokers diagnosed with ER− (HR=2.58, 95% CI=1.35–4.93), but not ER+ (HR=1.11, 95% CI=0.69–1.78) tumors (PInteraction=0.17).
Conclusions
Smoking may negatively impact long-term survival following breast cancer. Racial differences in long-term survival, as related to smoking, may be driven by ER status, rather than by differences in smoking patterns.
Keywords: smoking, breast cancer, survival analysis, mortality
Introduction
In the United States (US), breast cancer is the most frequently diagnosed cancer and the second leading cause of cancer-related death among women of all races [1]. Since the early 1990s, there have been notable improvements in survival following breast cancer in both African-American and white women, largely attributed to improvements in early detection and advances in breast cancer treatment; however, mortality disparities persist by race [2]. African-American women have the highest breast cancer mortality rates of any racial or ethnic group; mortality rates from 2010–2014 are estimated at 29.2 per 100,000 African-American women compared with 20.6 per 100,000 Caucasian women [3].
Disparities in survival may be attributed to differences in access to care [4, 5] or tumor biology [6, 7], or both. Differences in the prevalences of risk factors such as cigarette smoking may also contribute to these disparities. In the general US population, white women have a slightly higher prevalence of smoking [8]; however, there is evidence that at older ages, the prevalence of smoking is higher in black than in white women [9]. To our knowledge, no studies have examined racial differences in smoking at the time of breast cancer diagnosis and subsequent survival. Despite the large, but inconsistent literature linking smoking and incident breast cancer [10], current smoking at diagnosis is consistently associated with increased risk of breast cancer-specific and all-cause mortality [11]. Several studies [12, 13], though not all [14, 15], also show that these associations may be stronger among women diagnosed with estrogen receptor (ER) negative tumors. The biological mechanisms underlying the associations between smoking and poorer breast cancer survival are not well understood; however, cigarettes are known to contain a vast number of carcinogenic and endocrine disrupting chemicals [16] that have the potential to increase the risk of treatment complications [17], recurrence [18], and second primary cancers [19].
In the present study, we examined the association between self-reported measures of smoking and survival among a population-based cohort of women diagnosed with breast cancer. Additionally, we examined whether these associations varied by race (African-American vs non-African-American) and ER status (ER+ vs ER−).
Methods
Study Population
This study uses data from the Carolina Breast Cancer Study (CBCS), a population-based study of 1,808 women diagnosed with invasive breast cancer from 1993–1996 (Phase I) and 1996–2000 (Phase II). Details on participant recruitment and eligibility have been previously published [20, 21]. In brief, women between the ages of 20 and 74 with a first diagnosis of invasive breast cancer in 24 North Carolina counties were identified from the North Carolina Central Cancer Registry using rapid case ascertainment. The CBCS oversampled young (<50 years of age) and African-American women so that sample sizes would be sufficient for analyses stratified by race. After signed informed consent and consent for release of medical records and pathology reports, on average within six months of breast cancer diagnosis, participants completed an interviewer-administered questionnaire. The questionnaire elicited information on known and suspected breast cancer risk and prognostic factors including family history of breast cancer, reproductive factors, exogenous hormone use, and lifestyle factors including smoking history, as well as demographic characteristics including self-reported race (see Supplemental Table 1 for participant characteristics). This study was approved by the Institutional Review Board of the University of North Carolina (UNC, Chapel Hill, NC).
Exposure Assessment
Smoking history was assessed by in-person interviewer-administered questionnaire [22]. Participants were asked about their current and past smoking, number of cigarettes smoked, and total number of years of smoking accounting for any periods the women did not smoke. We defined current smokers as women who had smoked at least 100 cigarettes during their lifetime and who reported smoking at the time of the interview, as well as women who smoked within one year of breast cancer diagnosis. Former smokers were women who had smoked at least 100 cigarettes during their lifetime, but quit smoking at least one year prior to breast cancer diagnosis. Never smokers were women who had smoked less than 100 cigarettes during their lifetime. Among former and current smokers, intensity of smoking (i.e., number of cigarettes smoked per day) was categorized as <20 cigarettes/day and >20 cigarettes/day, and duration of smoking (i.e., number of years smoked) was categorized as ≤20 years and >20 years. Among former smokers, recency of smoking cessation was categorized as ≥1–≤10 years and >10 years.
Covariates
Information on covariates were obtained by interviewer-administered questionnaire and by medical record review. Potential confounders of the association between smoking and survival were identified from prior literature on smoking and breast cancer survival [11, 23] and included: age at diagnosis (continuous, years), education (<High school, High school or GED, ≥College), marital status (unmarried vs married), menopausal status (pre- vs post-menopausal), body mass index (<25, 25–29, and ≥30 kg/m2), oral contraceptive use (never vs ever), hormone replacement therapy use (never vs ever), alcohol use (never vs ever), and recreational activity performed to keep physically fit three months before completion of the questionnaire (no vs yes). Disease and tumor characteristics abstracted from the medical records included stage (I/II vs III/IV), grade (I/II vs III) in Phase I only, tumor size (≤2.0 vs >2.0 cm), node status (negative vs positive), and ER status (ER+ vs ER−).
Outcome Assessment
Ascertainment of vital status and date/cause of death among deceased participants, was done through linkage with the National Death Index [24]. International Statistical Classification of Diseases codes 174.9 and C-50.9 listed as the underlying cause of death on the death certificate were used to identify breast cancer-related deaths. Follow-up for survival occurred from the date of diagnosis in 1993–2000 until December 31, 2011. By the end of follow-up at 18.66 years (median=13.56 years), we identified 717 deaths, of which 427 were breast cancer-related.
Statistical Analysis
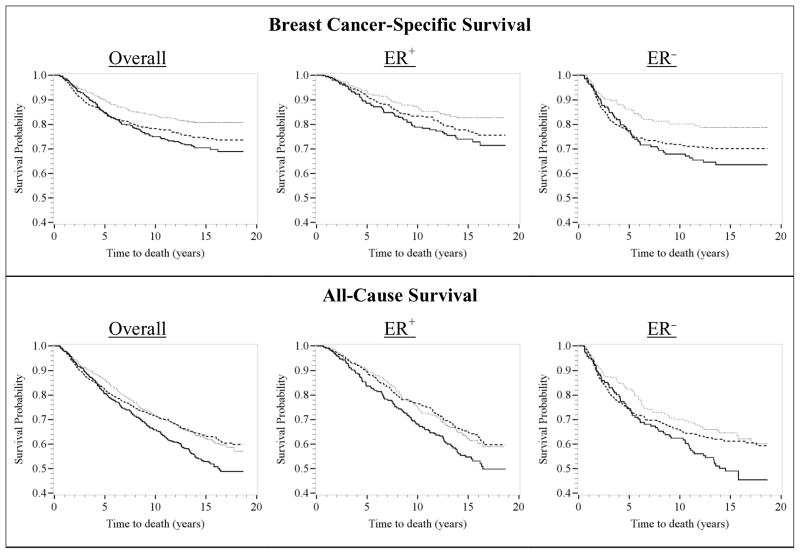
Kaplan-Meier survival curves for breast cancer-specific and all-cause survival, as related to smoking status, were used for preliminary examination of unadjusted data (Figure 1). The proportional hazards assumption was assessed by visual inspection of the Kaplan-Meier curves and by testing interaction terms of smoking with time/log-transformed time and by Schoenfeld residuals. A violation of the proportional hazards assumption was identified for smoking status; the survival curves for current versus never smokers appeared to diverge after approximately five years of follow-up (Figure 1). Therefore, all multivariable analyses included a smoking-by-time interaction to estimate associations with survival within five years of breast cancer diagnosis, as well as survival up to 18 years following diagnosis, conditional on surviving five years.
Figure 1.
Kaplan-Meier survival curves for at-diagnosis smoking status (never smokers – dashed line; former smokers – dotted line; and current smokers – solid line) and breast cancer-specific (top) and all-cause (bottom) mortality, overall and by estrogen receptor (ER) status. CBCS participants were diagnosed with invasive breast cancer from 1993–1996 (Phase I) and 1996–2000 (Phase II) and followed-up for vital status through December 31, 2011 (n=1,808). The x-axes show time to death in years; the y-axes show proportion of participants alive.
Multivariable Cox regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations between at-diagnosis smoking status, intensity, and duration and breast cancer-specific and all-cause survival within 5 years and up to 18 years following diagnosis. All initial models (i.e., Model 1) were adjusted for study design factors including age (continuous, years), race (African-American vs Non-African-American), and study phase (Phase I vs Phase II). Subsequent multivariable models also included adjustment for participant characteristics and behavioral factors (i.e., Model 2) and disease characteristics (i.e., Model 3). Because ER status was missing for ~7% of women, in sensitivity analyses, we restricted the models to women with non-missing ER status; however, estimates were not appreciably different. Therefore, we present the results from the complete-case analyses. Effect measure modification by race and ER status was assessed by including smoking-by-race and smoking-by-ER status interactions in the multivariable models and by conducting stratified analyses. Associations between smoking and all-cause survival are presented in Supplemental Tables 2 and 3). All analyses were done using the SAS version 9.4 (SAS Institute, Inc., Cary, NC) and used never smokers as the referent group.
Results
More than half (52.1%) of the women in the CBCS reported being never smokers, while one-quarter (25.9%) reported being former smokers, and approximately one-fifth (22.0%) reported being current smokers around the time of breast cancer diagnosis (see Supplemental Table 1). The proportions of current smokers were similar among African-American (22.0%) and non-African-American women (22.1%). Former smokers were more likely to be older (mean=54.1 yrs, SD=11.4 yrs) compared to never (mean=50.5 yrs, SD=11.8 yrs) smokers and were more likely to report ever use of HRT (35.5%) compared to never smokers (23.0%). Compared to never smokers, current smokers were less likely to have a college education or higher (34.2% vs 47.1%), more likely to be unmarried (50.8% vs 39.3%), and less likely to engage in regular physical activity (40.5% vs 53.2%). Higher proportions of current smokers (84.9%) and former smokers (82.0%) reported ever drinking alcohol regularly, compared to never smokers (55.3%). Proportions of ER+ breast tumors were similar among current (61.6%) and former (60.0%) smokers; 53.3% of never smokers had ER+ tumors. By race, a higher proportion of white women (63.9%) were diagnosed with ER+ tumors compared to black women (47.8%). Furthermore, as expected, compared to women who died within five years of diagnosis, long-term survivors were more likely to be diagnosed at stages I/II (91.7% vs 64.6%), grades I/II (60.6% vs 43.3%), with ≤2cm (58.4% vs 29.0%) ER+ (60.5% vs 39.3%) tumors, and were node negative at diagnosis (68.6% vs 40.0%).
Breast cancer-specific survival associations
In the Kaplan-Meier survival curves, breast cancer-specific survival rates were highest among former smokers, followed by never smokers, and then current smokers (Figure 1). Compared to never smokers, current smokers had similar breast cancer-specific survival rates among women with ER+ tumors; however, among women with ER− tumors, current smokers had slightly lower breast cancer-specific survival rates, five years after diagnosis.
Associations between breast cancer-specific survival five years after diagnosis and cigarette smoking status, intensity, and duration are presented in Table 1. At 5 years, there was no association between survival and current smoking (HR=1.05, 95% CI=0.75–1.47) and an inverse association between survival among former smokers (HR=0.69, 95% CI=0.47–1.00), compared to never smokers. The inverse association among former smokers was also evident among former smokers who smoked <20 cigarettes/day (HR=0.65, 95% CI=0.43–1.00) and among former smokers who quit within 10 years of breast cancer diagnosis (HR=0.51, 95% CI=0.29–0.91).
Table 1.
Association between at-diagnosis cigarette smoking and breast cancer-specific survival – CBCS Phases I and II (n=1,808).
| Deaths | Censored | 5-Year Survival (n deaths=248) | |||
|---|---|---|---|---|---|
|
| |||||
| Model 1a | Model 2b | Model 3c | |||
|
|
|
|
|||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Never smokers | 144 | 798 | 1.00 | 1.00 | 1.00 |
| Smoking Status | |||||
| Former smokers | 45 | 423 | 0.69 (0.49–0.96) | 0.75 (0.53–1.07) | 0.69 (0.47–1.00) |
| Current smokers | 59 | 339 | 0.93 (0.69–1.26) | 1.02 (0.74–1.40) | 1.05 (0.75–1.47) |
| Intensity of smoking | |||||
| Former smokers | |||||
| ≤20 cigarettes/day | 33 | 312 | 0.66 (0.45–0.97) | 0.72 (0.48–1.07) | 0.65 (0.43–1.00) |
| >20 cigarettes/day | 12 | 106 | 0.80 (0.44–1.44) | 0.91 (0.50–1.65) | 0.83 (0.43–1.60) |
| Current smokers | |||||
| ≤20 cigarettes/day | 46 | 221 | 1.04 (0.74–1.45) | 1.14 (0.81–1.62) | 1.19 (0.83–1.71) |
| >20 cigarettes/day | 13 | 118 | 0.68 (0.39–1.21) | 0.74 (0.41–1.32) | 0.71 (0.38–1.34) |
| Duration of smoking | |||||
| Former smokers | |||||
| ≤20 years | 35 | 276 | 0.75 (0.52–1.09) | 0.80 (0.54–1.17) | 0.71 (0.50–1.07) |
| >20 years | 10 | 144 | 0.53 (0.28–1.01) | 0.64 (0.33–1.24) | 0.63 (0.32–1.27) |
| Current smokers | |||||
| ≤20 years | 26 | 104 | 1.11 (0.72–1.69) | 1.18 (0.76–1.83) | 1.27 (0.80–2.04) |
| >20 years | 33 | 233 | 0.84 (0.57–1.22) | 0.92 (0.62–1.37) | 0.94 (0.63–1.41) |
| Recency of cessation | |||||
| ≥1–≤10 years | 16 | 186 | 0.53 (0.32–0.90) | 0.54 (0.32–0.93) | 0.51 (0.29–0.91) |
| >10 years | 29 | 230 | 0.86 (0.58–1.29) | 0.94 (0.62–1.43) | 0.88 (0.56–1.37) |
| Deaths | Censored | 13-Year Conditional Survival (n deaths=179) | |||
|
| |||||
| Model 1a | Model 2b | Model 3c | |||
|
|
|
|
|||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Never smokers | 88 | 686 | 1.00 | 1.00 | 1.00 |
| Smoking Status | |||||
| Former smokers | 39 | 365 | 0.97 (0.67–1.42) | 1.11 (0.76–1.64) | 1.06 (0.71–1.58) |
| Current smokers | 52 | 269 | 1.43 (1.02–2.02) | 1.54 (1.07–2.22) | 1.54 (1.06–2.25) |
| Intensity of smoking | |||||
| Former smokers | |||||
| ≤20 cigarettes/day | 25 | 274 | 0.81 (0.52–1.27) | 0.94 (0.60–1.48) | 0.85 (0.53–1.36) |
| >20 cigarettes/day | 14 | 86 | 1.59 (0.90–2.80) | 1.78 (1.00–3.16) | 1.89 (1.05–3.37) |
| Current smokers | |||||
| ≤20 cigarettes/day | 35 | 175 | 1.31 (0.95–2.09) | 1.57 (1.04–2.36) | 1.61 (1.05–2.46) |
| >20 cigarettes/day | 17 | 94 | 1.49 (0.88–2.51) | 1.50 (0.87–2.60) | 1.42 (0.79–2.54) |
| Duration of smoking | |||||
| Former smokers | |||||
| ≤20 years | 28 | 239 | 0.98 (0.64–1.50) | 1.11 (0.72–1.71) | 1.02 (0.65–1.59) |
| >20 years | 11 | 123 | 0.96 (0.51–1.80) | 1.14 (0.60–2.16) | 1.19 (0.62–2.27) |
| Current smokers | |||||
| ≤20 years | 19 | 85 | 1.41 (0.85–2.33) | 1.38 (0.81–2.35) | 1.23 (0.67–2.24) |
| >20 years | 33 | 182 | 1.47 (0.98–2.19) | 1.65 (1.09–2.50) | 1.73 (1.14–2.65) |
| Recency of cessation | |||||
| ≥1–≤10 years | 21 | 154 | 1.14 (0.71–1.84) | 1.25 (0.77–2.03) | 1.20 (0.73–1.97) |
| >10 years | 17 | 205 | 0.83 (0.49–1.39) | 0.92 (0.54–1.55) | 0.89 (0.52–1.55) |
Carolina Breast Cancer Study (CBCS) participants were diagnosed with invasive breast cancer from 1993–1996 (Phase I) and 1996–2000 (Phase II) and followed-up for vital status through December 31, 2011. Conditional analyses are among women who survived ≥5 years.
Adjusted for age at diagnosis (continuous), race (Non-African-American vs African-American), study phase (Phase I vs Phase II).
Adjusted for variables in Model 1 and education (<High school, High school/GED, and ≥College), marital status (unmarried vs married), menopausal status (pre- vs post-menopausal), body mass index (<25, 25–29, ≥30 kg/m2), HRT use (never vs ever), alcohol use (never vs ever), and physical activity (no vs yes).
Adjusted for variables in Model 2 and stage (I/II vs III/IV) and estrogen receptor status (ER+ vs ER−).
Also presented in Table 1 are breast cancer specific-survival associations, conditional on 5-year survival. Risk of breast cancer-specific mortality was elevated 54% (HR=1.54, 95% CI=1.06–2.25) among current smokers and elevated 73% (HR=1.73, 95% CI=1.14–2.65) among current smokers who smoked >20 years, compared to never smokers.
Breast cancer-specific survival by race
Associations between smoking status and breast cancer-specific survival at 5-years and 13-years (conditional on 5-year survival) after diagnosis, stratified by race are presented in Table 2. There was no association between current (vs never) smoking at 5-years by race. While the interaction was not statistically significant (PInteraction=0.30), risk of 13-year conditional survival was elevated among African-American current smokers (HR=1.69, 95% CI=1.00–2.85), but only weakly elevated among non-African-American current smokers (HR=1.22, 95% CI=0.70–2.14), compared to never smokers.
Table 2.
Association between at-diagnosis cigarette smoking status and breast cancer-specific survival, by race and estrogen receptor (ER) status – CBCS Phases I and II (n=1,808).
| Deaths | Censored | 5-Year Survival (n deaths=248) | |||
|---|---|---|---|---|---|
|
| |||||
| Model 1a | Model 2b | Model 3c | |||
|
|
|
|
|||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Race | |||||
| Non-African-American | |||||
| Never smokers | 61 | 445 | 1.00 | 1.00 | 1.00 |
| Former smokers | 18 | 271 | 0.55 (0.32–0.93) | 0.58 (0.34–1.01) | 0.54 (0.30–1.00) |
| Current smokers | 28 | 197 | 0.99 (0.64–1.56) | 1.03 (0.64–1.66) | 1.14 (0.70–1.86) |
| African-American | |||||
| Never smokers | 83 | 353 | 1.00 | 1.00 | 1.00 |
| Former smokers | 27 | 152 | 0.83 (0.54–1.28) | 0.92 (0.58–1.45) | 0.78 (0.48–1.26) |
| Current smokers | 31 | 142 | 0.88 (0.58–1.33) | 0.95 (0.61–1.47) | 0.89 (0.56–1.43) |
| ER Status | |||||
| ER+ | |||||
| Never smokers | 39 | 428 | 1.00 | 1.00 | 1.00 |
| Former smokers | 16 | 250 | 0.80 (0.44–1.43) | 0.79 (0.43–1.46) | 0.89 (0.48–1.65) |
| Current smokers | 25 | 201 | 1.29 (0.78–2.13) | 1.30 (0.77–2.20) | 1.24 (0.72–2.10) |
| ER− | |||||
| Never smokers | 94 | 315 | 1.00 | 1.00 | 1.00 |
| Former smokers | 24 | 153 | 0.60 (0.38–0.94) | 0.67 (0.42–1.08) | 0.62 (0.38–1.00) |
| Current smokers | 31 | 110 | 0.94 (0.62–1.41) | 1.06 (0.69–1.65) | 1.09 (0.70–1.71) |
| Deaths | Censored | 13-Year Conditional Survival (n deaths=179) | |||
|
| |||||
| Model 1a | Model 2b | Model 3c | |||
|
|
|
|
|||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Race | |||||
| Non-African-American | |||||
| Never smokers | 43 | 389 | 1.00 | 1.00 | 1.00 |
| Former smokers | 19 | 240 | 0.81 (0.47–1.40) | 0.92 (0.53–1.60) | 0.83 (0.46–1.48) |
| Current smokers | 26 | 161 | 1.37 (0.84–2.23) | 1.36 (0.81–2.31) | 1.22 (0.70–2.14) |
| African-American | |||||
| Never smokers | 45 | 297 | 1.00 | 1.00 | 1.00 |
| Former smokers | 20 | 125 | 1.16 (0.69–1.97) | 1.34 (0.78–2.31) | 1.31 (0.75–2.27) |
| Current smokers | 26 | 108 | 1.48 (0.91–2.40) | 1.60 (0.96–2.67) | 1.69 (1.00–2.85) |
| ER Status | |||||
| ER+ | |||||
| Never smokers | 62 | 355 | 1.00 | 1.00 | 1.00 |
| Former smokers | 26 | 215 | 0.82 (0.52–1.30) | 0.88 (0.55–1.41) | 1.03 (0.64–1.67) |
| Current smokers | 30 | 159 | 1.03 (0.66–1.59) | 1.05 (0.65–1.67) | 1.11 (0.69–1.78) |
| ER− | |||||
| Never smokers | 25 | 278 | 1.00 | 1.00 | 1.00 |
| Former smokers | 12 | 134 | 1.09 (0.55–2.17) | 1.32 (0.65–2.68) | 1.12 (0.54–2.31) |
| Current smokers | 18 | 87 | 2.16 (1.18–3.97) | 2.51 (1.32–4.78) | 2.58 (1.35–4.93) |
Carolina Breast Cancer Study (CBCS) participants were diagnosed with invasive breast cancer from 1993–1996 (Phase I) and 1996–2000 (Phase II) and followed-up for vital status through December 31, 2011. Conditional analyses are among women who survived ≥5 years.
Adjusted for age at diagnosis (continuous) and study phase (Phase I vs Phase II).
Adjusted for variables in Model 1 and education (<High school, High school/GED, and ≥College), marital status (unmarried vs married), menopausal status (pre- vs post-menopausal), body mass index (<25, 25–29, ≥30 kg/m2), HRT use (never vs ever), alcohol use (never vs ever), and physical activity (no vs yes).
Adjusted for variables in Model 2 and stage (I/II vs III/IV) and estrogen receptor status (ER+ vs ER−) as appropriate.
Breast cancer-specific survival by ER status
Associations between smoking status and breast cancer-specific survival at 5-years and 13-years (conditional on 5-year survival) after diagnosis, stratified by ER status are presented in Table 2. There were no associations between current smoking at 5-years by ER status. While the interaction was not statistically significant (PInteraction=0.17), risk of 13-year conditional breast cancer-specific mortality was elevated among women with ER− tumors (HR=2.58, 95% CI=1.35–4.93), but not among current smokers with ER+ tumors (HR=1.11, 95% CI=0.69–1.78), compared to never smokers.
Discussion
In our population based study of women diagnosed with breast cancer and followed for vital status for a median of 13 years, we observed increases in risk of breast cancer-specific mortality among current smokers, compared to never smokers, but only among women who survived at least 5 years after diagnosis. When stratified by race, we observed higher risks of breast cancer mortality, conditional on 5-year survival, among African-American smokers compared to non-African-American smokers and among smokers diagnosed with ER−, but not ER+ tumors. We also observed reduced risks of breast cancer-specific mortality at five years post-diagnosis among former smokers, former light smokers, and former smokers who quit smoking within 10 years of breast cancer diagnosis. Among all women, risk of 13-year conditional all-cause mortality was elevated 66% among current versus never smokers; however, stratified by race, risk of all-cause mortality was higher among non-African-American (92%) than among African-American (34%) smokers compared to never smokers (Supplemental Tables 2 and 3).
Our finding of no association between smoking and 5-year breast cancer survival is in agreement with prior studies with short duration of follow-up [15, 25–27]. One possible explanation for this is that short-term survival is more likely to be determined by tumor characteristics and treatment, than by behavioral factors [23]. Our finding of improved survival among former smokers is also consistent with at least one prior study [28]. The authors attributed this finding to observations that women who successfully quit smoking may be more likely to have children, live with a non-smoking partner, and be heavier. Another possible explanation is that successful quitters may also adopt healthier lifestyle behaviors, including an increase in the use of routine clinical preventive services such as mammographic screening [29]. Although the pattern of a divergence in the survival curves five years after diagnosis has not been previously reported, our finding of a 54% increase in risk of breast cancer-specific mortality among long-term breast cancer survivors, is in agreement with most studies of at-diagnosis smoking and survival following breast cancer published to date [12–14, 30–33], though, not all [15, 25–28]. Additionally, our results indicating that smoking may play a larger role in survival among women diagnosed with ER− tumors are also in agreement with two prior studies [12, 13], though inconsistent with two others [14, 15]. To our knowledge, ours is the first study to examine smoking in relation to breast cancer-specific survival among African-American women as compared to non-African-American women and raises the possibility that smoking at diagnosis contributes to the racial disparity in breast cancer survival.
Several biological mechanisms linking smoking and poor survival among women diagnosed with breast cancer have been proposed. There are numerous carcinogenic and endocrine disrupting chemicals found in cigarette smoke [16], which have the potential to increase the risk of comorbidities, treatment complications [17], recurrence [18], and second primary cancers [19]. Nicotine, one of the main constituents of cigarette smoke, has been shown in laboratory studies to suppress the immune system through loss of antibody responses and T-cell proliferation [34]. Nicotine, may also induce tumor growth and metastasis by promoting angiogenesis and epithelial-mesenchymal transition, and inhibiting apoptosis [35]. These mechanisms, however, do not directly explain the differences we observed by ER status, except for suppression of the immune system, for which there is accumulating evidence that over-expression of immune response genes may play a stronger role in ER− than ER+ breast cancers [36–38].
It is conceivable that smoking has differential effects on breast cancer survival depending on the genomic defects in breast tumors, which differ by ER status and intrinsic subtype. For example, the ER− basal-like intrinsic subtype is more prevalent among African-American and younger patients [7], carries a high frequency of p53 gene mutations [39, 40] and is relatively deficient in DNA repair and more genetically unstable [41]; it seems plausible that the insult of tobacco smoke chemicals could potentiate this instability, leading to more recurrences. The smoking-breast cancer mortality associations may also be mediated by smoking-induced alterations in methylation of breast tumor DNA; a recent study found that current smokers with hormone receptor negative breast tumors exhibited primarily CpG hypomethylation compared to never smokers [42]. From studies of head and neck and lung cancers, it has also been hypothesized that smoking may reduce treatment efficacy, in particular responses to radiation and chemotherapy treatments [43, 44]. In breast cancer, smoking may also have a more deleterious effect on disease-specific survival depending on the treatment modality; however, we did not have detailed treatment data and could not examine this potential interaction.
Although our study has several strengths, including the large population-based study design with follow-up of over 13 years, several limitations should be noted. First, we relied on self-reported measures of smoking; however, chemical verification of smoking can be costly and self-reported smoking has been shown to be reliably reported [45] and our estimates of at-diagnosis current smoking (20%) were similar to most studies conducted to date (15%–25%) and similar to smoking rates in the general adult US population (19%) [46]. Second, we did not have information on post-diagnosis changes in smoking. Approximately 30% of women diagnosed with breast cancer are estimated to quit smoking within two years of diagnosis [47] and as one research group has shown [48], post-diagnosis smoking cessation may improve survival; however, failure to account for post-diagnosis smoking cessation would attenuate results towards the null. Future studies should consider post-diagnosis changes in smoking among black and white women and their impacts on survival following breast cancer. Third, while the NDI provides high-quality ascertainment of vital status, there may be some misclassification of breast cancer-specific deaths. However, this misclassification is likely to be non-differential with respect to smoking status, which would attenuate effect estimates. Fourth, it is possible that our results may be confounded by other behaviors that correlate with smoking, such as mammographic screening use and delays in diagnosis [28]; however, in our study, the distributions of tumor size, stage, node positivity between smokers and non-smokers were similar suggesting that in our study, smokers were not diagnosed with larger tumors, which would be expected if they used mammography less and associations remained elevated even after adjustment for stage. Fifth, although we were interested in incorporating data on breast cancer molecular subtype defined by immunohistochemical markers [ER, progesterone receptor (PR), human epidermal growth factor receptor-1 (HER1), human epidermal growth factor receptor-2 (HER2) and cytokeratin 5/6 (CK5/6)][49], the high proportion of missing subtype data (36%), together with the low 5-year survival rates among women with basal-like breast cancers (ER−/PR−/HER2− and HER1+ or CK5/6+) precluded us from examining associations conditional on 5-year survival (data not shown). We, therefore, focused on ER status, for which data were more complete.
We hypothesized that disparities in survival among African-American women may be due in part to differences in rates of smoking; however, we observed similar rates of smoking among African-American (22.0%) and non-African-American (22.1%) women. We observed decreased rates of survival among African-American women as compared to non-African-American women; however, based on our findings stratified by ER status, it seems that racial differences in long-term survival, as related to smoking, may be driven by ER status, rather than by differences in smoking patterns. We suggest that the racial difference may be due to the larger proportion of ER− tumors, particularly basal-like tumors, among African-American women [7], or that there may be differential effects of smoking in ER− versus ER+ tumors, or both. Given reports that African-American women may be more likely to be diagnosed with ER− rather than ER+ breast cancers [50], if smoking decreases survival among ER− tumors, a subtype of breast cancer with already poor prognosis, attention should be given to smoking cessation among African-American women diagnosed with breast cancer.
Supplementary Material
Supplemental Table 1. Distribution of participant characteristics at baseline, overall at-diagnosis active smoking status – CBCS Phases I and II, (n=1,808).
Supplemental Table 2. Association between at-diagnosis cigarette smoking all-cause survival – CBCS Phases I and II (n=1,808).
Supplemental Table 3. Association between at-diagnosis cigarette smoking status and all-cause survival, by race and estrogen receptor (ER) status – CBCS Phases I and II (n=1,808).
Acknowledgments
Funding: This research was funded in part by the University Cancer Research Fund of North Carolina and the National Cancer Institute Specialized Program of Research Excellence (SPORE) in Breast Cancer (NIH/NCI P50-CA58223) and by the National Institute of Environmental Health Sciences (T32 ES007018).
Footnotes
Compliance with Ethical Standards
Conflict of interest: The authors declare they have no conflict of interest.
Ethical standards: All procedures performed in the Carolina Breast Cancer Study involving human participants were in accordance with the ethical standards of the Institutional Review Boards of the University of North Carolina at Chapel Hill and were in compliance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Ethical approval: Informed consent was obtained from all individual participants included in the study.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Smigal C, Jemal A, Ward E, et al. Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin. 2006;56:168–183. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute. [Accessed 19 Jun 2017];SEER Stat Fact Sheets: Breast Cancer. 2017 http://seer.cancer.gov/statfacts/html/breast.html.
- 4.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–57. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 5.Joslyn SA. Racial differences in treatment and survival from early-stage breast carcinoma. Cancer. 2002;95:1759–66. doi: 10.1002/cncr.10827. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien KM, Cole SR, Tse C-K, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16:6100–10. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 8.Jamal A, King BA, Neff LJ, et al. Current Cigarette Smoking Among Adults – United States, 2005–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1205–11. doi: 10.15585/mmwr.mm6544a2. [DOI] [PubMed] [Google Scholar]
- 9.Geronimus AT, Neidert LJ, Bound J. Age patterns of smoking in US black and white women of childbearing age. Am J Public Health. 1993;83:1258–64. doi: 10.2105/ajph.83.9.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaudet MM, Gapstur SM, Sun J, et al. Active smoking and breast cancer risk: original cohort data and meta-analysis. J Natl Cancer Inst. 2013;105:515–25. doi: 10.1093/jnci/djt023. [DOI] [PubMed] [Google Scholar]
- 11.Bérubé S, Lemieux J, Moore L, et al. Smoking at time of diagnosis and breast cancer-specific survival: new findings and systematic review with meta-analysis. Breast Cancer Res. 2014;16:R42. doi: 10.1186/bcr3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dal Maso L, Zucchetto A, Talamini R, et al. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008;123:2188–94. doi: 10.1002/ijc.23747. [DOI] [PubMed] [Google Scholar]
- 13.Braithwaite D, Izano M, Moore DH, et al. Smoking and survival after breast cancer diagnosis: a prospective observational study and systematic review. Breast Cancer Res Treat. 2012;136:521–533. doi: 10.1007/s10549-012-2276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boone SD, Baumgartner KB, Baumgartner RN, et al. Active and passive cigarette smoking and mortality among Hispanic and non-Hispanic white women diagnosed with invasive breast cancer. Ann Epidemiol. 2015;25:824–31. doi: 10.1016/j.annepidem.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes MD, Murin S, Chen WY, et al. Smoking and survival after breast cancer diagnosis. Int J cancer. 2007;120:2672–7. doi: 10.1002/ijc.22575. [DOI] [PubMed] [Google Scholar]
- 16.IARC. IARC Monographs on evaluation of carcinogenic risks to humans: Tobacco smoke and involuntary smoking. International Agency for Research on Cancer; Lyon, France: 2004. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhan M, Flaws JA, Gallicchio L, et al. Profiles of tamoxifen-related side effects by race and smoking status in women with breast cancer. Cancer Detect Prev. 2007;31:384–90. doi: 10.1016/j.cdp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Bishop JD, Killelea BK, Chagpar AB, et al. Smoking and breast cancer recurrence after breast conservation therapy. Int J Breast Cancer. 2014;2014:1–5. doi: 10.1155/2014/327081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neugut AI, Murray T, Santos J, et al. Increased risk of lung cancer after breast cancer radiation therapy in cigarette smokers. Cancer. 1994;73:1615–1620. doi: 10.1002/1097-0142(19940315)73:6<1615::aid-cncr2820730612>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Newman B, Moorman PG, Millikan R, et al. The Carolina Breast Cancer Study: integrating population-based epidemiology and molecular biology. Breast Cancer Res Treat. 1995;35:51–60. doi: 10.1007/BF00694745. [DOI] [PubMed] [Google Scholar]
- 21.Butler EN, Tse C-K, Bell ME, et al. Active smoking and risk of luminal and basal-like breast cancer subtypes in the Carolina Breast Cancer Study. Cancer Causes Control. 2016;27:775–86. doi: 10.1007/s10552-016-0754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millikan RC, Pittman GS, Newman B, et al. Cigarette smoking, N-acetyltransferases 1 and 2, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7:371–379. [PubMed] [Google Scholar]
- 23.Soerjomataram I, Louwman MWJ, Ribot JG, et al. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat. 2008;107:309–30. doi: 10.1007/s10549-007-9556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. [Accessed 29 Jun 2016];National Death Index. 2014 http://www.cdc.gov/nchs/ndi.htm.
- 25.Sagiv SK, Gaudet MM, Eng SM, et al. Active and passive cigarette smoke and breast cancer survival. Ann Epidemiol. 2007;17:385–93. doi: 10.1016/j.annepidem.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Hellmann SS, Thygesen LC, Tolstrup JS, Grønbæk M. Modifiable risk factors and survival in women diagnosed with primary breast cancer: results from a prospective cohort study. Eur J Cancer Prev. 2010;19:366–373. doi: 10.1097/CEJ.0b013e32833b4828. [DOI] [PubMed] [Google Scholar]
- 27.Kakugawa Y, Kawai M, Nishino Y, et al. Smoking and survival after breast cancer diagnosis in Japanese women: a prospective cohort study. Cancer Sci. 2015;106:1066–74. doi: 10.1111/cas.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fentiman IS, Allen DS, Hamed H. Smoking and prognosis in women with breast cancer. Int J Clin Pract. 2005;59:1051–1054. doi: 10.1111/j.1742-1241.2005.00581.x. [DOI] [PubMed] [Google Scholar]
- 29.Vander Weg MW, Howren MB, Cai X. Use of routine clinical preventive services among daily smokers, non-daily smokers, former smokers, and never-smokers. Nicotine Tob Res. 2012;14:123–130. doi: 10.1093/ntr/ntr141. [DOI] [PubMed] [Google Scholar]
- 30.Passarelli MN, Newcomb PA, Hampton JM, et al. Cigarette smoking before and after breast cancer diagnosis: mortality from breast cancer and smoking-related diseases. J Clin Oncol. 2016;34:1–8. doi: 10.1200/JCO.2015.63.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tominaga K, Andow J, Koyama Y, et al. Family environment, hobbies and habits as psychosocial predictors of survival for surgically treated patients with breast cancer. Jpn J Clin Oncol. 1998;28:36–41. doi: 10.1093/jjco/28.1.36. [DOI] [PubMed] [Google Scholar]
- 32.Manjer J. Survival of women with breast cancer in relation to smoking. Eur J Surg. 2000;166:852–858. doi: 10.1080/110241500447227. [DOI] [PubMed] [Google Scholar]
- 33.Warren GW, Kasza KA, Reid ME, et al. Smoking at diagnosis and survival in cancer patients. Int J cancer. 2012;132:401–10. doi: 10.1002/ijc.27617. [DOI] [PubMed] [Google Scholar]
- 34.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 35.Singh S, Pillai S, Chellappan S. Nicotinic acetylcholine receptor signaling in tumor growth and metastasis. J Oncol. 2011;2011:456743. doi: 10.1155/2011/456743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gingras I, HAA, Ignatiadis M. Immunology and breast cancer: toward a new way of understanding breast cancer and developing novel therapeutic strategies. Clin Adv Hematol Oncol. 2015;13:372–85. [PubMed] [Google Scholar]
- 37.Desmedt C, Haibe-Kains B, Wirapati P, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008;14:5158–65. doi: 10.1158/1078-0432.CCR-07-4756. [DOI] [PubMed] [Google Scholar]
- 38.Teschendorff AE, Miremadi A, Pinder SE, et al. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol. 2007;8:R157.2–15. doi: 10.1186/gb-2007-8-8-r157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfeifer GP, Denissenko MF, Olivier M, et al. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–7451. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- 40.Bertheau P, Lehmann-Che J, Varna M, et al. p53 in breast cancer subtypes and new insights into response to chemotherapy. The Breast. 2013;22:S27–S29. doi: 10.1016/j.breast.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol. 2008;26:2568–2581. doi: 10.1200/JCO.2007.13.1748. [DOI] [PubMed] [Google Scholar]
- 42.Conway K, Edmiston SN, Parrish E, et al. Breast tumor DNA methylation patterns associated with smoking in the Carolina Breast Cancer Study. Breast Cancer Res Treat. 2017 doi: 10.1007/s10549-017-4178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Browman GP, Wong G, Hodson I, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993;328:159–63. doi: 10.1056/NEJM199301213280302. [DOI] [PubMed] [Google Scholar]
- 44.Cooley ME, Sipples RL, Murphy M, Sarna L. Smoking cessation and lung cancer: oncology nurses can make a difference. Semin Oncol Nurs. 2008;24:16–26. doi: 10.1016/j.soncn.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krall EA, Valadian I, Dwyer JT, Gardner J. Accuracy of recalled smoking data. Am J Public Health. 1989;79:200–2. doi: 10.2105/ajph.79.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention (CDC) Vital signs: current cigarette smoking among adults aged ≥18 years--United States, 2005–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1207–12. [PubMed] [Google Scholar]
- 47.Westmaas JL, Newton CC, Stevens VL, et al. Does a recent cancer diagnosis predict smoking cessation? An analysis from a large prospective US cohort. J Clin Oncol. 2015;33:1647–52. doi: 10.1200/JCO.2014.58.3088. [DOI] [PubMed] [Google Scholar]
- 48.Newcomb P, Passarelli M, Hampton J, et al. Smoking history in relation to survival after a breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev. 2014;23:569–570. [Google Scholar]
- 49.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 50.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106:dju055. doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Distribution of participant characteristics at baseline, overall at-diagnosis active smoking status – CBCS Phases I and II, (n=1,808).
Supplemental Table 2. Association between at-diagnosis cigarette smoking all-cause survival – CBCS Phases I and II (n=1,808).
Supplemental Table 3. Association between at-diagnosis cigarette smoking status and all-cause survival, by race and estrogen receptor (ER) status – CBCS Phases I and II (n=1,808).