Abstract
Rationale
Studies have demonstrated that brain dopamine D2/D3 receptors (D2/D3R) and the reinforcing effects of cocaine can be influenced by a monkey's position in the social dominance hierarchy.
Objective
In this study, we manipulated the social ranks of monkeys by reorganizing social groups and assessed effects on D2/D3R availability and cocaine self-administration.
Methods
Male cynomolgus monkeys (N=12) had been trained to self-administer cocaine under a concurrent cocaine-food reinforcement schedule. Previously, PET measures of D2/D3R availability in the caudate nucleus and putamen had been obtained with [18F]fluoroclebopride during cocaine abstinence, while living in stable social groups of four monkeys/pen. For this study, monkeys were reorganized into groups that consisted of (1) four previously dominant, (2) four previously subordinate and (3) a mix of previously dominant and subordinate monkeys. After 3 months, D2/D3R availability was redetermined and cocaine self-administration was re-examined.
Results
D2/D3R availability significantly increased after reorganization in monkeys who were formerly subordinate, with the greatest increases observed in those that became dominant. No consistent changes in D2/D3R availability were observed in formerly dominant monkeys. Cocaine self-administration did not vary according to rank after reorganization of social groups. However, when compared to their previous cocaine self-administration data, cocaine potency as a reinforcer decreased in 9 of 11 monkeys.
Conclusions
These results indicate that changing the social conditions can alter D2/D3R availability in subordinate monkeys in a manner suggestive of environmental enrichment. In most monkeys, social reorganization shifted the cocaine dose-response curve to the right in a manner consistent with environmental enrichment.
Keywords: Social rank, PET imaging, Drug choice, Drug self-administration, Dopamine receptors, Cynomolgus monkey
Introduction
Drug abuse continues to be a major public health problem worldwide (Degenhardt et al. 2014), including an estimated 1.6 million Americans confirming current stimulant abuse (SAMHSA 2013). At present there are no FDA-approved treatments for cocaine use disorder (e.g. Haile et al. 2012; Heidbreder 2013). The development of drug addiction is influenced by interactions between the pharmacological effects of a drug, biological characteristics of the individual and environmental factors that render some individuals vulnerable (see Nader et al. 2008). Acute exposure to environmental stressors and enrichment can influence brain dopamine (DA) systems and the behavioral effects of drugs that target these systems. For example, Miczek and colleagues have demonstrated that the ability of social-defeat stress to increase DA levels in the nucleus accumbens is closely related to its enhancement of cocaine self-administration and to the discriminative stimulus effects of cocaine or amphetamine (Tidey and Miczek 1997; Miczek et al. 1999). Conversely, rodents housed in social groups or in environments that feature novel foods and toys (i.e. “enriched” environments) are less sensitive to the locomotor-stimulant, discriminative stimulus and reinforcing effects of cocaine and amphetamine compared to those reared in isolation or in “impoverished” environments devoid of such stimuli (e.g. Stairs and Bardo 2009; Smith et al. 2012; Lynch et al. 2013; Zlebnik and Carroll 2015).
We have extended the study of environmental variables and DA to include social hierarchy in male (e.g. Morgan et al. 2002; Czoty et al. 2009, 2010) and female (e.g. Riddick et al. 2009; Nader et al. 2012b; Kromrey et al. 2016) cynomolgus monkeys. We have conceptualized the linear social dominance hierarchy as representing chronic environmental enrichment at one end (dominance) and chronic social stress (subordination) at the other (Nader et al. 2012a). For example, studies in male monkeys using the noninvasive brain imaging technique positron emission tomography (PET) demonstrated that DA D2/D3 receptor (D2/D3R) availability did not differ across monkeys while they were individually housed and did not predict eventual social rank. However, after 3 months of social housing, D2/D3R binding increased significantly in monkeys who became dominant but was unchanged in monkeys that became subordinate (Morgan et al. 2002); these findings have been extended to humans (Martinez et al. 2010). We have hypothesized that these differential effects on D2/D3R availability between dominant and subordinate monkeys result from chronic exposure to environmental enrichment vs. social stress, respectively (Nader et al. 2012a). This view is consistent with findings of differences in DA neuroanatomy and physiology between animals reared in enriched versus impoverished environments, which have been proposed to mediate differential sensitivity to the abuse-related effects of cocaine and amphetamine in rodent models (see Bardo et al. 2013). In this regard, environmental effects on D2/D3R availability may be particularly important. Brain imaging studies in rats, monkeys and humans have documented an inverse relationship between D2/D3R availability and sensitivity to the abuse-related effects of cocaine and other stimulants (Volkow et al. 1993, 1999; Morgan et al. 2002; Nader et al. 2006; Dalley et al. 2007; Martinez et al. 2010).
Whereas social rank significantly influenced cocaine reinforcement during initial exposure, after several years of cocaine self-administration, social rank-related differences in response rates under a 50-response fixed-ratio (FR 50) schedule of reinforcement and in D2/D3R availability had dissipated (Czoty et al. 2004). Thus, it appeared that, with prolonged cocaine exposure, the impact of environmental variables (in this case social status) on certain measures of cocaine reinforcement was attenuated. This view is supported by the observation that rank-related differences in D2/D3R availability re-emerged after approximately eight months of abstinence from cocaine while monkeys remained in their social groups (Czoty et al. 2010). These data provided evidence for neuroplasticity in brain DA systems in response to environmental enrichment despite an extensive history of cocaine self-administration.
The purpose of the present experiment was to further examine the interactions between social rank, brain D2/D3R and sensitivity to cocaine reinforcement by assessing whether reorganization of social groups would lead to predictable changes in D2/D3R availability and sensitivity to the reinforcing effects of cocaine based on the social ranks attained by the monkeys. Using PET imaging, D2/D3R availability was measured in 12 socially housed male cynomolgus monkeys during abstinence from cocaine (as reported previously, Czoty et al. 2010) and following formation of new social groups of four monkeys per pen. To mitigate the effects of social reorganization per se, which has been shown to be acutely stressful for all monkeys under various experimental conditions (Sapolsky 1983, 1992; Czoty et al. 2009), at least 3 months elapsed after placement in new social groups before self-administration experiments were begun. For this study, monkeys were exposed to ascending doses of cocaine as the alternative to food under a cocaine-food choice procedure. It was hypothesized that, regardless of previous social experience, D2/D3R availability in dominant monkeys would be significantly higher than those of subordinates, in parallel with previous observations (Morgan et al. 2002; Czoty et al. 2010) and that preference for cocaine versus food would be greater and occur at lower cocaine doses in subordinate monkeys, reflecting a greater sensitivity to the reinforcing effects of cocaine (Czoty et al. 2005).
Materials and Methods
Subjects
At the start of this study, twelve male cynomolgus monkeys (Macaca fascicularis), aged 10-14 years old, had lived in stable social groups of 3-4 monkeys/pen for over three years, occupying either a dominant or a subordinate rank in the pen. Each monkey had received an [18F]fluoroclebopride ([18F]FCP) PET scan (see below) while housed in his former group (Czoty et al. 2010). At the time of that PET scan (see Table 1), 7.8 ± 0.8 months had passed since the monkeys had last self-administered cocaine. After those PET scans had been conducted and in an effort to alleviate excessive aggression and injury during the formation of new social groups, monkeys were housed individually for approximately five months by placing them into a cage (Allentown Caging, Allentown, New Jersey) along with future pen-mates, but leaving the monkeys separated into 0.71 × 0.84 × 0.84 m quadrants of the cage by insertion of removable partitions. Thus, during this individual-housing phase, monkeys had olfactory, auditory, visual and limited tactile contact with each other. One social group in the present study consisted of four formerly dominant (all previously #1-ranked) monkeys, one consisted of four formerly subordinate monkeys and a third group consisted of two formerly dominant and two formerly subordinate monkeys. Monkeys were weighed weekly and fed enough food daily (Purina Monkey Chow and fresh fruit) to maintain body weights at approximately 95% of free-feeding weights. Each quadrant was equipped with a spout from which water was available ad libitum. Each monkey was fitted with a collar (Primate Products, Redwood City, CA) and trained over several weeks to sit calmly in a standard primate chair (Primate Products) using a specially designed stainless steel pole that attached to the collar. All manipulations were performed in accordance with the 2011 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research and were approved by the Animal Care and Use Committee of Wake Forest University.
Table 1. D2/D3 Receptor Availability in the Caudate Nucleus and Putamen Following Social Reorganization.
| CAUDATE NUCLEUS | PUTAMEN | |||||
|---|---|---|---|---|---|---|
| Initial | After Reorganization | Initial | After Reorganization | |||
| Dominant | Dom | Sub | Dom | Sub | ||
| C-5386 | 2.20 | 2.17 (-1.4%) | - | 2.56 | 2.58 (0.8%) | - |
| C-6626 | 2.99 | 2.87 (-4%) | - | 3.18 | 2.80 (-11.9%) | - |
| C-6628 | 2.78 | 2.61 (-6.1%) | - | 2.86 | 2.65 (-7.3%) | - |
| C-6216 | 2.35 | - | 1.88 (-20%) | 2.78 | - | 2.48 (-10.8%) |
| C-6526 | 2.72 | - | 2.61 (-4.0) | 3.11 | - | 3.15 (1.3%) |
| C-6528 | 2.40 | - | 2.69 (12.1%) | 2.43 | - | 2.66 (9.5%) |
|
| ||||||
| Mean ± SEM | 2.57±0.14 | 2.55±0.20 | 2.39±0.26 | 2.82±0.13 | 2.68±0.06 | 2.76±0.20 |
| (% change) | (-3.8±1.7%) | (-4.0±11.3%) | (-6.2±4.6%) | (0.0±7.2%) | ||
| Subordinate | Dom | Sub | Dom | Sub | ||
| C-6625 | 2.00 | 2.17 (8.5%) | - | 2.27 | 2.48 (9.3%) | - |
| C-6529 | 2.20 | 2.39 (8.6%) | - | 2.83 | 2.93 (3.5%) | - |
| C-6629 | 1.60 | 1.92 (20%) | - | 1.75 | 2.05 (17.1%) | - |
| C-6530 | 2.16 | - | 2.39 (10.6%) | 2.61 | - | 2.48 (-5.0%) |
| C-6527 | 1.81 | - | 1.85 (2.2%) | 2.38 | - | 2.38 (0.0%) |
| C-6214 | 2.36 | - | 2.56 (8.5%) | 2.65 | - | 2.79 (5.3%) |
|
| ||||||
| Mean ± SEM | 2.02±0.12 | 2.16±0.14 | 2.27±0.21 | 2.42±0.17 | 2.49±0.25 | 2.55±0.12 |
| (% change) | (+12.4±4.7%) | (+7.1±3.1%) | (+10.0±4.8%) | (+0.1±3.6%) | ||
| Mean ± SEM | New Ranks | 2.36±0.15 | 2.33±0.17 | 2.58±0.14 | 2.66±0.13 | |
Social Reorganization
The methods used for social reorganization have been published previously (Czoty et al. 2009). Briefly, at approximately 0900 on the first day of social housing, all animals in the pen received a low dose of ketamine (< 3.0 mg/kg, i.m.) and partitions were removed so that four monkeys occupied the entire cage (0.71 × 1.73 × 1.83 m). For the next 5 days, to prevent injury occurring when laboratory personnel were not present, partitions were replaced at approximately 1600 and monkeys remained separated overnight. After approximately 1 week, monkeys were socially housed overnight. During the first two weeks of social housing, the two pens that consisted of monkeys that were previously all dominant and all subordinate displayed a high frequency of aggressive behaviors. To facilitate establishment of the hierarchy, these monkeys were transferred to the Wake Forest University Primate Center where they were housed for 2.5 months in larger enclosures (1.5 × 2.5 × 3.0 m). After 2.5 months both groups returned to the laboratory.
Social status was determined for each monkey according to the outcomes of agonistic encounters as described and reported previously (Czoty et al. 2009). Aggressive, submissive and affiliative behaviors were recorded for individual monkeys in each pen during several 15-min observation sessions according to an ethogram described previously (Kaplan et al. 1982) using Noldus Observer software (Noldus Information Technology; Wageningen, The Netherlands). Both initiators and recipients of behaviors were recorded. In each pen, the animal that aggressed toward, and elicited submissive behaviors from, all others was designated the most dominant (#1-ranked) monkey. The monkey aggressing at all except the #1-ranked monkey and submitting only to the #1-ranked monkey was ranked #2, etc. The monkey designated #4 displayed a low frequency of aggressive behaviors and submitted to all other monkeys in the pen. Thus, a transitive, linear hierarchy was established in each pen. For the present studies, the #1- and #2-ranked monkeys were considered dominant and the #3- and #4-ranked monkeys were considered subordinate.
Imaging procedures
As described previously (Czoty et al. 2010), 3D MRI brain images were acquired (TE 5, TR 45, flip angle 45, RBW 15.6 kHz, FOV 18 cm, 256 × 192 matrix, slice thickness 2 mm, NEX 3) with a 1.5-Tesla GE Signa NR scanner (GE Medical Systems). T1-weighted whole brain images were used to anatomically define spherical regions of interest (ROIs), including the right and left caudate nucleus, putamen (2.5-mm radius) and cerebellum (4.0-mm radius), for later co-registration with PET images.
During cocaine abstinence and at least 3 months after social reorganization, PET scans were conducted in each monkey using the D2/D3R radioligand [18F]fluoroclebopride (FCP; Mach et al. 1996). Prior to each scan, monkeys were anesthetized with 10 mg/kg ketamine and transported to the PET Center. This dose of ketamine does not affect D2/D3R availability as measured with [18F]FCP (Nader et al. 1999). Details regarding [18F]FCP synthesis, PET data acquisition protocol, blood sampling procedure, and metabolite analysis have been fully described previously (Nader et al. 1999). Briefly, an arterial and a venous catheter were inserted by percutaneous stick for blood sampling and tracer injection, respectively. A paralytic agent (0.07 mg/kg vecuronium Br, i.v.) was administered and ventilation was maintained by a respirator throughout the 3-hr PET scan. Supplemental doses of vecuronium (0.1 mg/h) were administered throughout the study. Body temperature was maintained at 40°C and vital signs (heart rate, blood pressure, respiration rate and temperature) were monitored throughout the scanning procedure.
Images were acquired on a General Electric Advance NXi PET scanner. In a single scan, the Advance NXi provided 35 transverse slices with a 4.25-mm center-to-center spacing over a 15.2-cm axial field of view. The transaxial resolution of the scanner ranges from 3.8-mm at the center of the FOV to 7.3 mm radial and 5.0 tangential at a radius of 20 cm when reconstructed with a ramp filter. Its axial resolution ranges from 4.0 mm at the center to 6.6 mm at a radius of 20 cm when reconstructed with a ramp filter. At the start of the scan, approximately 5 mCi of [18F]FCP was injected, followed by 3 mL of heparinized saline. Scans were conducted and images were registered to each subject's MRI (see Czoty et al. 2010). Tissue-time-activity curves were generated and distribution volumes were obtained for each ROI using the linear portion of the Logan plot (Logan et al. 1990). Distribution volume ratios (DVRs) for the caudate nucleus and putamen were calculated using the cerebellum as the reference region. DVR thus served as an index of specific [18F]FCP binding in each ROI. There were no differences between left and right DVRs for the caudate nucleus and the putamen, so means from both sides are presented.
Catheter Implantation
Each monkey had previously been prepared with a chronic indwelling venous catheter and subcutaneous vascular access port (Cat. No. CP4AC-5H; Access Technologies, Skokie, IL) under sterile surgical conditions. Anesthesia was induced with ketamine (15 mg/kg) and maintained with ketamine supplements. A catheter was inserted into a major vein (femoral, internal or external jugular, brachial) to the level of the vena cava. The distal end of the catheter was passed subcutaneously to a point slightly off the midline of the back, where an incision was made. The end of the catheter was attached to the vascular access port which was placed in a pocket formed by blunt dissection. To prolong patency, each port and catheter was filled with a solution of 10% heparinized saline (100 U/mL) at the end of each experimental session.
Cocaine self-administration procedures
While PET scans were being completed and for several weeks thereafter, monkeys participated in operant behavioral sessions five days per week in which responding was maintained by food delivery under an FR 50 schedule of reinforcement. Each day, monkeys were separated by partitioning the living space into quadrants. Next, each monkey was seated in a primate chair and placed into a ventilated, sound-attenuating chamber (1.5 × 0.74 × 0.76 m; Med Associates, East Fairfield, VT). Two retractable response levers (5 cm wide) were located on one side of the chamber with a horizontal row of three stimulus lights 14 cm above each lever. Levers were positioned to be easily within reach of the monkey seated in the primate chair. A food receptacle located between the levers was connected with a tygon tube to a pellet dispenser (Gerbrands Corp., Arlington, MA) located on the top of the chamber for delivery of 1-g banana-flavored food pellets (P.J. Noyes Co., Lancaster, NH).
Cocaine self-administration was examined under a concurrent FR 50 schedule (see Czoty et al. 2005). Monkeys were placed into a primate chair and the back of the animal was cleaned with 95% betadine and EtOH and the port was connected to an infusion pump (Cole-Parmer, Inc. Chicago, IL) located outside the chamber via a 20-gauge Huber Point Needle (Access Technologies). The pump was operated for approximately 3 sec to fill the port and catheter with the dose of cocaine available for the session. During behavioral sessions, responding on one of two levers was reinforced by delivery of a 1.0-g banana-flavored food pellet (P.J. Noyes Co., Lancaster, NH) under an FR 50 schedule of reinforcement. A 10-s timeout period (TO) followed each reinforcer; sessions ended after 30 total reinforcers had been earned or 60 min had elapsed. Next, saline and several doses of (-)cocaine HCl (National Institute on Drug Abuse, Bethesda, MD; 0.003-0.56 mg/kg per injection) were examined as an alternative to food. Different doses of cocaine were studied by changing the drug concentration prepared in sterile saline. Injections consisted of approximately 1.5 mL of drug solution over 10 sec. Availability of each dose began with three days of “forced choice” on which responding on the food-associated lever was not reinforced and had no scheduled consequences. Next, response-contingent food was again made available under choice conditions. Both levers were presented simultaneously and both associated sets of stimulus lights were illuminated. Fifty consecutive responses on either lever produced the appropriate reinforcer; responses on the alternate lever reset the response requirement. The session ended after 30 total reinforcers had been earned or 60 min had elapsed. Doses were presented in ascending order, with each dose available for at least 3 consecutive sessions and until responding was deemed stable (% injection-lever responding ±15% of the mean of the last 3 sessions with no trend; if criteria were not met within 15 sessions, the mean of the last 5 days was used). The cocaine dose was increased until a dose was reached for which monkeys allocated >90% of responses to the cocaine-associated lever (i.e., >90% cocaine choice). Of the 12 monkeys in the study, one monkey (the lowest-ranked monkey in the pen of former dominant monkeys) was unable to complete self-administration experiments due to a lack of veins available for catheterization. For each available dose in each monkey, percent cocaine choice was calculated as the number of responses emitted on the injection-associated lever divided the total number of responses emitted on both levers, multiplied by 100.
Data analysis
PET Imaging Data: DVRs were calculated for two ROIs, the caudate nucleus and putamen (left hemisphere, right hemisphere, and average), by using PMOD Biomedical Image Quantification Software (version 3.1; PMOD Technologies, Zurich, Switzerland) using the cerebellum as the reference region (Logan et al. 1990). There were no differences between left and right regions, so mean data were used. Initially, for each region, DVRs in dominant (#1- and #2-ranked) versus subordinate (#3- and #4-ranked) monkeys were compared after reorganization using t-tests (N=6/group). After reorganization, there were four distinct groups of monkeys whose ranks (Pre-Post reorganization) were dominant-dominant, dominant-subordinate, subordinate-dominant, subordinate-subordinate (N=3/group); these small numbers precluded statistical analyses. However, linear regression analyses were conducted comparing changes in previously dominant (N=6) and previously subordinate (N=6) monkeys. Cocaine-Food Choice: The primary dependent variable was percent cocaine choice (i.e. the number of reinforcers earned on the cocaine-associated lever divided by the total completed choices on both levers, multiplied by 100). Data were analyzed using repeated-measures two-way analyses of variance (ANOVA), with cocaine dose and social rank as factors using Prism 6 for Mac OS X software (Graphpad Software, Inc.). In all cases, differences were considered significant when p<0.05. For comparison, the lowest dose in which cocaine was preferred (defined as > 70% cocaine choice) for each monkey prior to social reorganization (from Czoty et al. 2005) has been re-plotted in Figure 1.
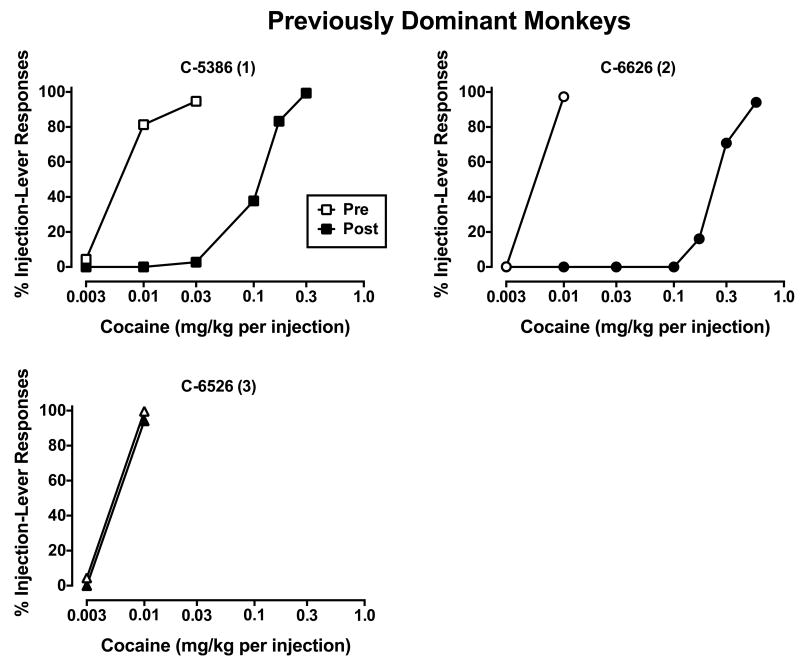
Figure 1.
Cocaine choice in individual monkeys who had been dominant in former social groups. Numbers in parenthesis represent final social rank following reorganization. Closed symbols represent data following social reorganization and open symbols represent pre-reorganization data from Czoty et al. (2005). Ordinates: percent of responses emitted on the cocaine-associated lever. Abscissae: cocaine dose available as an alternative to food. Each point is the mean of the last three sessions.
Results
Effect of social reorganization on D2/D3 receptor availability
As reported previously (Czoty et al. 2010), measures of D2/D3R availability determined before social reorganization were higher in dominant versus subordinate monkeys in both the caudate nucleus and putamen (Table 1). Within three months following placement in new social groups, linear dominance hierarchies developed in all three pens. Monkeys could be split into four distinct groups (N=3/group) with respect to their ranks before and after reorganization. Three monkeys who were previously subordinate attained a dominant rank (#1- or #2-ranked) in the new group and three that were previously dominant became subordinate (#3 or #4-ranked). Three monkeys who were formerly dominant became dominant in the new social group and three that were formerly subordinate were also subordinate in their new groups. After reorganization, absolute [18F]FCP DVRs in the caudate nucleus and putamen did not differ between dominant and subordinate monkeys (Table 1, last row). However, when compared to monkeys' most recent PET scan performed prior to social reorganization, the largest effect was a 12.4 ± 4.7% increase in D2/D3R availability in the caudate nucleus and a 10 ± 4.8% increase in the putamen, on average, in the three monkeys who increased their social rank after reorganization (i.e. from previously subordinate to dominant; C-6625, C-6529, C-6629). Former subordinate monkeys who were also subordinate in the reorganized groups also showed an increase (7.1 ± 3.1%) in [18F]FCP DVR in the caudate nucleus. On average, the DVRs of formerly dominant monkeys decreased regardless of the rank they attained in their new social groups (range of 0 to -6.2% in the caudate nucleus and putamen; see Table 1). One dominant monkey who became subordinate (C-6528) did show an ∼ 10% increase in [18F]FCP DVRs in the caudate nucleus and putamen. A regression analysis confirmed that the changes in previously subordinate monkeys were significantly greater than previously dominant monkeys, irrespective of their current rank in the caudate nucleus (p=0.002) and trended in the putamen (p=0.054).
Cocaine self-administration in reorganized social groups
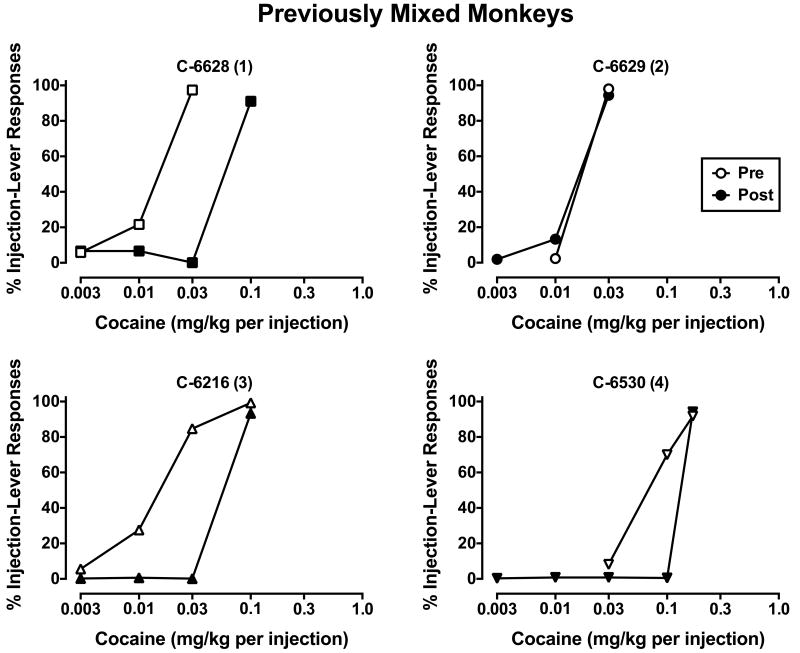
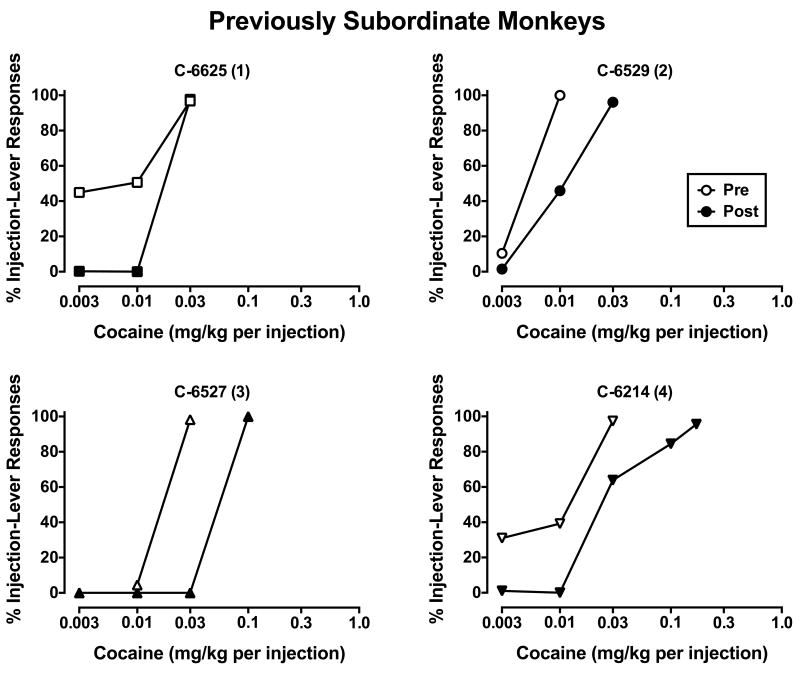
When ascending doses of cocaine were made available as an alternative to food pellets, dose-related increases in cocaine choice were observed in all monkeys up to a dose that was chosen almost exclusively over food in each subject (Figs. 1-3). In the pen of monkeys who had held the most dominant (#1) rank in their previous social groups (Fig. 1), cocaine was substantially less potent in two of the three monkeys; in the monkey (C-6526) who became subordinate (#3-ranked), the cocaine dose-response curve did not change. Unfortunately, cocaine choice could not be assessed in the other monkey (C-6528) who became subordinate in the pen due to unavailability of veins for catheterization. C-6528 was the only dominant monkey who showed increases in D2/D3R availability after reorganization and he became subordinate (#4-ranked). In the pen of monkeys who were formerly subordinate (Fig. 2), the cocaine dose-response curve shifted down and to the right in all four monkeys, irrespective of their new social rank. Finally, in the pen of monkeys with mixed former ranks (Fig. 3), the cocaine dose-response curve shifted to the right in three of the four monkeys. As it relates to changes in D2/D3R availability, contrary to our hypothesis, C-6629 had the largest increases, but his cocaine dose-response curve did not change, while C-6216 had the largest decreases in receptor availability and his cocaine dose-response curve shifted right.
Figure 3.
Cocaine choice in individual monkeys who had been dominant or subordinate in former social groups. Numbers in parenthesis represent final social rank following reorganization. All other details are as described in Figure 1.
Figure 2.
Cocaine choice in individual monkeys who had been subordinate in former social groups. Numbers in parenthesis represent final social rank following reorganization. All other details are as described in Figure 1.
Discussion
The objective of these experiments was to extend our examination of the influence of position in the social dominance hierarchy on brain DA D2/D3R availability and sensitivity to the reinforcing effects of cocaine. Specifically, we manipulated monkeys' social ranks by placing them into new social groups, and assessed whether the new ranks attained by the monkeys were associated with D2/D3R availability and/or sensitivity to cocaine. Prior to social reorganization, monkeys' baseline measures of D2/D3R availability differed considerably as a result of having held different social ranks (see Table 1); D2/D3R availability was higher in monkeys that were dominant at baseline compared to subordinates (Czoty et al. 2010). After social groups had been reorganized, absolute measures of D2/D3R availability did not differ according to the monkeys' new social rank. This finding is important in that it indicates that D2/D3R availability is not simply and solely determined by present social rank. One factor that may have influenced this result in the present study is that each of the subjects had extensive experience self-administering cocaine. In addition, the monkeys' history of being socially housed in other groups may have resulted in a lack of difference in absolute measures of [18F]FCP DVRs. For these reasons, in the present study, it was more informative to examine alterations in D2/D3R availability with respect to the monkey's position in the dominance hierarchy at baseline and whether social rank changed or stayed the same after reorganization.
Previously subordinate monkeys showed significant increases in caudate nucleus D2/D3R availability after being placed in new social groups; the largest changes were seen in monkeys whose social rank increased after reorganization. In the three monkeys who had been subordinate but became dominant in their new social groups, [18F]FCP DVRs in both brain regions increased 10-12% on average. A moderate (7.1%) increase in the caudate nucleus was observed in monkeys that had been subordinate but did not increase their rank in the new social groups, indicating that, for these chronically stressed monkeys, simply changing the social environment was enough to increase D2/D3R availability. Considering that the reported test-retest variability for [18F]FCP is approximately 2% (Nader et al. 1999), these effects are likely to be biologically significant. It is noteworthy that the magnitude of this change was less than the 20% average increase in D2/D3R availability that was observed when initially individually housed monkeys attained dominance (Morgan et al. 2002). In monkeys who were formerly dominant, D2/D3R availability decreased slightly (less than 4% on average) after reorganization regardless of the rank they attained in their new social groups. As described above, it may be that the monkeys' history of social housing and/or cocaine self-administration interacted with environmental variables to dampen the effect of attaining social dominance on brain D2/D3R availability after reorganization (versus initial placement in to social groups).
From a clinical perspective, the observation of a significant increase in D2/D3R availability when monkeys moved from conditions of chronic stress (i.e., social subordination) to a new social group suggests that such an environmental alteration may contribute to a successful therapeutic approach to cocaine addiction. In male cocaine-dependent and non drug-abusing humans, as well as rodent and nonhuman primate models of cocaine abuse, lower D2/D3R availability has been associated with increased sensitivity to cocaine and other monoamine transporter ligands, whereas individuals with relatively higher D2/D3R availability are less sensitive to the abuse-related behavioral effects of these drugs (Volkow et al. 1993, 1999; Morgan et al. 2002; Nader et al. 2006; Dalley et al. 2007). Moreover, the lack of a substantial decrease in D2/D3R DVRs in monkeys who transitioned from dominant to subordinate suggests that the effects of being dominant do not diminish when that position in the social hierarchy is lost. While it appears clear that D2/D3R availability influences vulnerability during early cocaine use (Morgan et al. 2002; Dalley et al. 2007), the present study addressed whether D2/D3R availability influenced maintenance of cocaine reinforcement.
To assess the relationship between monkeys' new social ranks and sensitivity to cocaine reinforcement, we examined the relative reinforcing strength of cocaine in the reorganized social groups. Because these monkeys had not self-administered cocaine in more than one year, we assessed sensitivity to cocaine by presenting increasing doses of cocaine in ascending order as an alternative to food reinforcement. We hypothesized that, in each pen, the reinforcing strength of cocaine relative to food would be inversely associated with social rank as observed previously under a similar food-drug choice procedure (Czoty et al. 2005). That is, we expected that subordinate monkeys would choose lower doses of cocaine over food than would dominant monkeys. In general, this hypothesis was not borne out. The only example to support this was noted in the group of previously dominant monkeys such that the one monkey who became subordinate (C-6526) was substantially more sensitive to cocaine compared to the two monkeys who remained dominant. In fact, the lowest dose of cocaine that this monkey preferred exclusively to food (0.01 mg/kg per injection) was 1.25-1.75-log units lower than that for the two dominant monkeys in the pen. Unfortunately cocaine choice could not be examined in the other formerly dominant monkey who became subordinate in this pen. This formerly dominant monkey (C-6528) showed increases in D2/D3R receptor availability after becoming subordinate in the new social group, which would have provided additional insight into whether social rank or D2/D3R availability influences cocaine self-administration more prominently.
No clear relationship between current social rank and cocaine choice was observed for any group (formally dominant, subordinate or mixed). However, reorganization of social groups did result in decreased potency for cocaine to function as a reinforcer in 9 of the 11 monkeys; we have not noted shifts over time in self-administered cocaine dose-effect functions (unpublished observation). This suggests that manipulating social context can have profound effects on cocaine self-administration even when there are modest to no changes in D2/D3R availability. We have previously hypothesized that rightward shifts in the cocaine dose-response curve represent consequences of environmental enrichment (Nader et al. 2012a). The present findings suggest that allowing reorganized groups to stabilize may be enriching, while acute social reorganization is most likely stressful (Sapolsky 1983, 1992). While the cocaine self-administration dose-response curves shifted in the majority of the monkeys, when one examines “current” cocaine-maintained responding, there were no social rank-related differences. Despite these lack of differences, we showed in subsequent experiments using several of these same monkeys that the behavioral effects of dopaminergic drugs and environmental manipulations could be influenced by current social ranks (Czoty and Nader 2012, 2013, 2015; Gould et al. 2017). Thus, it is possible that while D2/D3R availability influences vulnerability to drug abuse, maintenance of cocaine self-administration may be mediated by different mechanisms. The mechanisms mediating these rank-related differences remain to be determined.
In summary, the present results demonstrate that changing the social environment in subordinate monkeys results in significant increases in D2/D3R availability, consistent with previous results (Morgan et al. 2002; Czoty et al. 2010; Nader et al. 2012b). In contrast, changing the social environment in previously dominant monkeys did not substantially affect D2/D3R measures, suggesting that social history plays a role in determining the absolute measures of D2/D3R availability after new social groups are formed. On the whole, however, these findings have encouraging clinical implications. The results indicate that, even in monkeys who have been exposed to chronic social stress for several years, the transition to a relatively enriched environment (i.e. attaining dominance or simply moving to a new social group) can result in adaptations in brain dopamine systems that have been associated with a decreased sensitivity to cocaine reinforcement in animal models and humans. Moreover, these data support the view that attention to environmental variables may represent an important part of a successful treatment strategy. Finally, it should be noted that these studies were conducted in male monkeys, and that the relationships noted may not translate to female subjects (e.g. Nader et al. 2012b). Future studies should examine the plasticity of DA D2/D3R function, as well as consequences of changes in social status in female subjects.
Acknowledgments
The authors wish to acknowledge the technical assistance of Michelle Bell and Kim Black and Jay Kaplan and Susan Nader for assistance with these studies over the years. We also thank Drs. Hui Chen and Jim Anthony for statistical assistance. This research was supported by R37 DA010584.
Footnotes
The authors declare no conflicts of interest.
References
- Banks ML, Negus SS. Insights from preclinical choice models on treating drug addiction. Trends Pharmacol Sci. 2017 doi: 10.1016/j.tips.2016.11.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. 2013;65:255–290. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Nader MA. Relationship between social rank and cortisol and testosterone concentrations in male cynomolgus monkeys (Macaca fascicularis) J Neuroendocrinol. 2009;21:68–76. doi: 10.1111/j.1365-2826.2008.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, McCabe C, Nader MA. Assessment of the relative reinforcing strength of cocaine in socially housed monkeys using a choice procedure. J Pharmacol Exp Ther. 2005;312:96–102. doi: 10.1124/jpet.104.073411. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Morgan D, Shannon EE, Gage HD, Nader MA. Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology. 2004;174:381–388. doi: 10.1007/s00213-003-1752-z. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Gage HD, Nader MA. Differences in D2 dopamine receptor availability and reaction to novelty in socially housed male monkeys during abstinence from cocaine. Psychopharmacology. 2010;208:585–592. doi: 10.1007/s00213-009-1756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Nader MA. Individual differences in the effects of environmental stimuli on cocaine choice in socially housed male cynomolgus monkeys. Psychopharmacology. 2012;224:69–79. doi: 10.1007/s00213-011-2562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Nader MA. Effects of dopamine D2/D3 receptor ligands on food-cocaine choice in socially housed male cynomolgus monkeys. J Pharmacol Exp Ther. 2013;344:329–338. doi: 10.1124/jpet.112.201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Nader MA. Effects of oral and intravenous administration of buspirone on food-cocaine choice in socially housed male cynomolgus monkeys. Neuropsychopharmacology. 2015;40:1072–1083. doi: 10.1038/npp.2014.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinsin ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Baxter AJ, Lee Y, Hall W, Sara GE, Johns N, Flaxman A, Whiteford HA, Vos T. The global epidemiology and burden of psychostimulant dependence: Findings from the Global Burden of Disease Study 2010. Drug Alcohol Dep. 2014;137:36–47. doi: 10.1016/j.drugalcdep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- Gould RW, Czoty PW, Porrino LJ, Nader MA. Social status in monkeys: effects of social confrontation on brain function and cocaine reward. Neuropsychopharmacology. 2017 doi: 10.1038/npp.2016.285. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, Mahoney JJ, 3rd, Newton TF, De la Garza., 2nd Pharmacotherapeutics directed at deficiencies associated with cocaine dependence: focus on dopamine, norepinephrine and glutamate. Pharmacol Ther. 2012;134:260–277. doi: 10.1016/j.pharmthera.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder C. Rationale in support of the use of selective dopamine D3 receptor antagonists for the pharmacotherapeutic management of substance use disorders. Naunyn-Schmiedeberg's Arch Pharmacol. 2013;386:167–176. doi: 10.1007/s00210-012-0803-6. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM. Social status, environment, and atherosclerosis in cynomolgus monkeys. Atherosclerosis. 1982;2:359–368. doi: 10.1161/01.atv.2.5.359. [DOI] [PubMed] [Google Scholar]
- Kromrey SA, Czoty PW, Nader SH, Register TC, Nader MA. Preclinical laboratory assessments of predictors of social rank in female cynomolgus monkeys. Am J Primatol. 2016;78:402–417. doi: 10.1002/ajp.22514. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hitzemann R, Bendriem B, Gatley SJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev. 2013;37:1622–1644. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach RH, Nader MA, Ehrenkaufer RL, Line SW, Smith CR, Luedtke RR, Kung MP, Kung HF, Lyons D, Morton TE. Comparison of two fluorine-18 labeled benzamide derivatives that bind reversibly to dopamine D2 receptors: in vitro binding studies and positron emission tomography. Synapse. 1996;24:322–333. doi: 10.1002/(SICI)1098-2396(199612)24:4<322::AID-SYN2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Martinez D, Orlowska D, Narendran R, Slifstein M, Liu F, et al. Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biol Psychiatry. 2010;67:275–278. doi: 10.1016/j.biopsych.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH, van Erp AM, Blank AD, McInerney SC. d-amphetamine “cue” generalizes to social defeat stress: behavioral sensitization and attenuated accumbens dopamine. Psychopharmacology. 1999;147:190–199. doi: 10.1007/s002130051160. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Nader MA, Czoty PW, Gould RW, Riddick NV. PET imaging studies of dopamine receptors in primate models of addiction. Phil Trans Royal Soc B. 2008;363:3223–3232. doi: 10.1098/rstb.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Czoty PW, Nader SH, Morgan D. Nonhuman primate models of social behavior and cocaine abuse. Psychopharmacology. 2012a;224:57–67. doi: 10.1007/s00213-012-2843-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Grant KA, Gage HD, Ehrenkaufer RL, Kaplan JR, Mach RH. PET imaging of dopamine D2 receptors with [18F]fluoroclebopride in monkeys: effects of isoflurane- and ketamine-induced anesthesia. Neuropsychopharmacology. 1999;21:589–596. doi: 10.1016/S0893-133X(98)00101-8. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun T, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nature Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Nader MA, Nader SH, Czoty PW, Riddick NV, Gage HD, Gould RW, Blaylock BL, Kaplan JR, Garg PK, Davies HML, Morton D, Garg S, Reboussin BA. Social dominance in female monkeys: dopamine receptor function and cocaine reinforcement. Biol Psychiatry. 2012b;72:414–421. doi: 10.1016/j.biopsych.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddick NV, Czoty PW, Gage HD, Kaplan JR, Nader SH, Icenhower M, Pierre PJ, Bennett A, Garg PK, Nader MA. Behavioral and neurobiological characteristics influencing social hierarchy formation in female cynomolgus monkeys. Neuroscience. 2009;158:1257–1265. doi: 10.1016/j.neuroscience.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Substance Abuse and Mental Health Services Administration. Substance Abuse and Mental Health Services Administration. U.S. Dept. of Health and Human Services; Rockville, MD: 2013. Reliability of Key Measures in the National Survey on Drug Use and Health. [PubMed] [Google Scholar]
- Sapolsky RM. Endocrine aspects of social instability in the olive baboon (Papio anubis) Am J Primatol. 1983;5:365–379. doi: 10.1002/ajp.1350050406. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Cortisol concentrations and the social significance of rank instability among wild baboons. Psychoneuroendocrinology. 1992;17:701–709. doi: 10.1016/0306-4530(92)90029-7. [DOI] [PubMed] [Google Scholar]
- Smith MA, Pennock MM, Walker KL, Lang KC. Access to a running wheel decreases cocaine-primed and cue-induced reinstatement in male and female rats. Drug Alcohol Depend. 2012;121:54–61. doi: 10.1016/j.drugalcdep.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs DJ, Bardo MT. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol Biochem Behav. 2009;92:377–382. doi: 10.1016/j.pbb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology. 1997;130:203–212. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain D2 dopamine receptors. Amer J Psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Zlebnik NE, Carroll ME. Prevention of the incubation of cocaine seeking by aerobic exercise in female rats. Psychopharmacology. 2015;232:3507–3513. doi: 10.1007/s00213-015-3999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]