Abstract
Rationale
Clinical trials and human laboratory studies have established that varenicline can reduce rates of alcohol use among heavy drinkers. Less is known about the mechanisms by which varenicline has this effect on drinking behavior. Reactivity to alcohol cues is often cited as the primary cause of relapse among those being treated for alcohol use disorder, and several front-line treatments for alcohol use disorder work, at least in part, by minimizing cue-induced alcohol craving.
Objective
The current double-blind, placebo-controlled human laboratory study tested the effects of varenicline on alcohol cue reactivity in a group of heavy-drinking adult smokers and nonsmokers.
Methods
As part of a larger series of sequential human laboratory experiments testing the effects of varenicline on drinking outcomes, participants were assigned (between-participant) to receive either active varenicline (2 mg/day) or placebo. Following a titration period, participants (n = 77) attended a laboratory session during which they were exposed to alcohol and neutral cues using a standard cue-reactivity paradigm.
Results
Alcohol-cue exposure increased craving for alcohol in both medication groups. However, participants receiving varenicline showed a smaller increase in alcohol craving, compared to participants receiving placebo. The medication effect did not differ between smokers and nonsmokers. Among smokers, alcohol cue exposure also increased tobacco craving. Varenicline did not attenuate this effect.
Conclusions
Results support the use of varenicline for reducing alcohol use in heavy drinkers and identify a potential mechanism by which varenicline reduces drinking. Varenicline continues to show promise as a pharmacological treatment for alcohol use disorder.
Keywords: cue reactivity, alcohol, human laboratory, varenicline, alcohol craving, smokers
Introduction
Varenicline (Chantix; Pfizer, New York) is a partial agonist at α4β2 nicotinic acetylcholine receptors (nAChRs) that was developed as a smoking cessation aid (Gonzales et al. 2006). Growing evidence suggests that it may also be an effective medication for reducing alcohol consumption. Preclinical studies demonstrated that varenicline can reduce alcohol consumption in rats (Steensland et al. 2007), and these findings were later confirmed in the human laboratory (McKee et al. 2009; Verplaetse et al. 2016a). Subsequently, several clinical trials have found that varenicline can help treatment-seeking individuals with alcohol use disorder (AUD) reduce their drinking (Fucito et al. 2011; Litten et al. 2013), although another clinical trial found that participants receiving varenicline did not differ from those receiving placebo on primary drinking outcomes (de Bejczy et al. 2015). Nonetheless, the bulk of the evidence suggests that varenicline likely reduces rates of alcohol use among heavy drinkers, particularly among individuals who also smoke (Fucito et al. 2011; McKee et al. 2009; Mitchell et al. 2012), a finding that has been supported by a systematic review of the literature (see Erwin & Slaton, 2014).
Although the results of these clinical trials have generally supported the notion that varenicline can reduce rates of drinking, less is known about the mechanisms by which the drug exerts this effect. Unlike current front-line medications for AUD, varenicline targets nAChRs, so the behavioral mediator of its effect may differ from other drugs used to treat AUD (e.g., naltrexone). Acknowledging the role of nAChRs in alcohol reinforcement (Soderpalm et al. 2000), several studies have tested the ability of varenicline to reduce the acute pleasurable effects of alcohol, similar to the well-documented effects of naltrexone (Volpicelli et al. 1995). Varenicline reduced positive alcohol effects (McKee et al. 2009) and increased aversive alcohol effects (Childs et al. 2012). A related possibility is that varenicline reduces alcohol craving. Indeed, two clinical trials found that varenicline reduced tonic alcohol craving (Fucito et al. 2011; Litten et al. 2013). Human laboratory studies have found that varenicline can reduce the phasic increase in craving typically elicited in “high risk” situations (e.g., following alcohol prime; McKee et al. 2009). Likewise, preclinical research has found that microinfusions of varenicline delivered to the nucleus accumbens attenuated alcohol-seeking in a rat model of cue-induced relapse (Lacroix et al., 2017).
Exposure to alcohol cues also can result in phasic increases in alcohol craving, particularly among individuals with AUD (Reid et al. 2006; Sinha and Li 2007; Witteman et al. 2015). This relationship is important for those attempting to reduce drinking, as cue-induced craving is known to be a major contributor to relapse (Cooney et al. 1997; Drummond 2000; Garland et al. 2012). As such, blocking cue-induced increases in craving have been identified as a potential mechanism by which pharmacological and behavioral interventions could reduce rates of drinking (Rohsenow et al. 2000). Cue-induced alcohol craving can be quantified in the laboratory using alcohol-cue exposure paradigms (Monti et al. 1987). Participants are exposed to alcohol-related cues (e.g., viewing an image of an alcohol beverage or observing an alcohol beverage being prepared in front of them), and changes in their level of craving following cue exposure are observed.
The current double-blind, placebo-controlled human laboratory study tested the effects of varenicline on cue-induced alcohol craving in a sample of non-treatment seeking adult heavy drinkers. These data were collected as part of a larger series of sequential laboratory studies examining the effects of varenicline on alcohol use (McKee et al. 2009; Verplaetse et al. 2016a,b). The primary goal of this study was to determine whether varenicline reduces alcohol-cue-induced craving. Participants received active varenicline (2 mg/day) or placebo and completed a cue reactivity paradigm while their craving for alcohol was assessed. The sample included both smokers and nonsmokers. Because prior research in this area has typically been conducted with participants who both use alcohol and smoke, we included smoking status as a grouping variable to determine whether any medication effects differ between smokers and nonsmokers. Among smokers, we also measured alcohol-cue-induced changes in tobacco craving. People who use both substances endorse high rates of alcohol and tobacco co-administration (Shiffman and Balabanis 1995). Alcohol may acutely potentiate the incentive salience properties of tobacco and vice versa (Henningfield et al. 1983; Kouri et al. 2004). Indeed, prior research has found evidence for cross-substance cue reactivity, specifically for alcohol and tobacco (reviewed in Verplaetse and McKee 2016). Reducing any alcohol-induced increases in tobacco craving would provide additional support for using varenicline to reduce drinking and smoking among people using both drugs.
We hypothesized that all participants would report more alcohol craving following exposure to alcohol cues, compared to neutral cues. However, we predicted that the participants receiving varenicline would show a smaller increase in craving following alcohol cue exposure relative to participants receiving placebo. In past studies, the largest effects of varenicline on drinking outcomes have been found in samples of heavy-drinking smokers (McKee et al. 2009). Effects sizes in mixed samples have been more modest or nonsignificant (de Bejczy et al. 2015; Verplaetse et al. 2016a). As such, we predicted that varenicline would be more effective at reducing craving in daily smokers. We hypothesized that exposure to alcohol cues would increase tobacco craving among smokers (Rohsenow et al. 1997) and that this cue-induced increase in craving would be attenuated among smokers receiving active varenicline. Finally, prior research has identified mediators and moderators of varenicline efficacy in randomized clinical trials. Brandon and colleagues (2011) found that participants could guess their medication condition despite double-blind administration, which mediated the effect of medication on cue-induced tobacco craving. Based on the findings of Falk and colleagues (2015), we also conducted exploratory analyses using several different participant variables (i.e., age, gender, past 6-month alcohol use disorder, % heavy drinking days, cigarettes per day) to test whether any of these participant characteristics moderated medication effects of cue-induced craving.
Method
Participants
Volunteers were eligible to participate if they were ≥ 21 years of age and were able to read and speak English. All participants were heavy drinkers who reported consuming > 7 (women) or 14 (men) drinks per week and > 3 (women) or > 4 (men) drinks per episode in the last 30 days. Participants were recruited as part of larger series of sequential human laboratory studies, the results of which are reported elsewhere (McKee et al. 2009; Verplaetse et al. 2016a; Verplaetse et al. 2016b). Data reported in McKee et al. (2009) and Verplaetse et al. (2016a) were collected 8 post-randomization and reported on ad-libitum drinking outcomes. Data reported in Verplaetse et al. (2016b) were collected between 2 and 3 weeks post-randomization and reported on reactivity to a high dose of alcohol. Data reported in the current manuscript has not been previously published and were collected on a separate laboratory session (day 10 post-randomization). Exclusion criteria included illicit drug use (except for occasional cannabis use), past 30-day use of psychoactive drugs, treatment-seeking for alcohol or smoking, current psychiatric disorder, current suicidal or homicidal ideation, being pregnant or breastfeeding, or medical conditions contraindicating alcohol use (e.g., liver enzymes ≥ 3× normal) or varenicline administration (e.g., known allergy to varenicline). Volunteers who were likely to exhibit clinically significant alcohol withdrawal during the study as determined by the Clinical Institute Withdrawal Assessment for Alcohol Scale (Sullivan et al. 1989) did not participate.
Procedures
Eligibility screening
The Human Investigation Committee of Yale University approved this study. Written informed consent was obtained at the start of the intake session. Demographic information and other baseline measures were obtained at intake. These measures included a timeline follow-back (TLFB) (Sobell and Sobell 1992) of alcohol use and smoking during the 30 days before intake, the Fagerström Test of Nicotine Dependence (Heatherton et al. 1991), the Alcohol Use Disorder Identification Test (Saunders et al. 1993), and the Structured Clinical Interview for the DSM-IV (SCID-I; First et al. 2002). Drinking behavior from the TLFB was used to calculate the percentage of drinking days characterized by heavy use (i.e., 4/5 or more drinks for women and men, respectively; % heavy drinking days). Participants were classified as smokers or non-smokers based on Centers for Disease Control and Prevention criteria (CDC, 2009). Those who had smoked at least 100 cigarettes in their lifetime who reported any cigarette use in the past 30 days were classified as smokers. Those when did not meet these criteria were classified as non-smokers. The SCID-I was used to screen for any psychiatric disorders and specifically for past six-month AUD. Physical examination included an electrocardiogram, urine toxicology, pregnancy test, and basic blood chemistries.
Medication
The medication condition was double-blind and placebo-controlled. Participants were randomized to receive varenicline (2 mg/day) or placebo (0 mg/day). Varenicline was titrated to steady-state levels over 7 days (1 mg/day varenicline: 0.5 mg daily for days 1 – 5 and 0.5 mg twice daily for days 6 and 7; 2 mg/day varenicline: 0.5 mg daily for days 1 and 2, 0.5 mg twice daily for days 3-5, and 1 mg twice daily on days 6 through day 10). Medication compliance was monitored with pill counts and riboflavin marker on days 5 and 10 (Del Boca et al. 1996). All participants were at least 80% medication adherent. Participants were asked at the end of the laboratory session to report whether they believed they received varenicline or placebo.
Cue reactivity session
On day 10, each participant completed a 3-hour laboratory cue reactivity session. Participants arrived at the laboratory at 2:00 pm (0 min) and completed screening measures (i.e., urine drug and breath alcohol screen). They verbally confirmed abstinence from alcohol use for at least 24 hours proceeding the session. Baseline assessments of alcohol and tobacco craving were collected. Smokers were instructed to smoke one cigarette + 18 min to standardize the time between the last cigarette and the start of the cue-reactivity session to 15 minutes, which prevented nicotine withdrawal effects that are known to influence alcohol craving in heavy-drinking smokers (McKee et al., 2008; Palfai et al., 2000). All participants completed a five-minute relaxation period before beginning the cue reactivity paradigm.
The cue reactivity procedure was adapted from a similar procedure used successfully by other research groups (Monti et al. 1987; Rohsenow et al. 2000). Participants were seated in a comfortable chair in a relaxed laboratory setting. They received instructions and guided practice at the beginning of the session. To reduce the likelihood of carryover effects documented in cue exposure research (Monti et al. 1987; Sayette et al. 2010), all participants completed a neutral cue exposure trial followed by an alcohol cue exposure trial. The neutral trial began +30 min. Participants were presented with a bottle of water and a glass. The experimenter poured the bottle of water into the glass, and the participant was instructed to handle the glass for 3 minutes. An audio recording was used that consisted of 13 tone pairings occurring during the exposure period. The tone occurred in pseudo-random intervals. Participants were instructed to start smelling the beverage following the first tone of each pair and to stop smelling the beverage following the second tone. The experimenter then removed the tray, and participants completed the neutral cue craving assessment. After a five-minute break, participants began the alcohol cue exposure trial + 44 min. This trial was identical to the neutral cue exposure trial except that participants were presented with their preferred alcoholic beverage and a beverage-appropriate glass. After the alcohol stimuli were removed, participants completed the alcohol cue craving assessment. A second block of cue exposure trials was completed from + 58 min to + 72 min. This pair of trials was identical to the first. The experimenter observed participants during the entire procedure to ensure that they did not drink any beverage in either condition.
Adverse Events
Adverse events were assessed in person on days 1, 5, and 10 and by phone on day 2 (Levine and Schooler 1986). Common varenicline side effects include nausea, abnormal dreams, insomnia, constipation, flatulence, and vomiting, in addition to cardiac and neuropsychiatric adverse events (Pfizer Inc, 2014). Severity was assessed on a 4-point scale (1 = mild, 2 = minimal, 3 = moderate, 4 = severe).
Craving Measures
Alcohol craving
Alcohol craving was measured using an item from the Alcohol Urge Questionnaire (Bohn et al. 1995). Consistent with prior laboratory studies (Cooney et al., 1997; Rohsenow et al., 2000), a single item (the first item presented during each assessment) was used rather than the entire measure because participants' responses suggested inattention to later items (systematic inconsistencies between items based on reverse coding). Participants marked on a visual analogue scale (range = 1-100) how much they agreed with the statement “All I want to do now is have a drink” with responses ranging from 1 (strongly disagree) to 100 (strongly agree).
Tobacco craving
Tobacco craving was assessed using the Brief Questionnaire of Smoking Urges (QSU-B; Cox et al. 2001). This measure consists of twelve items that evaluate two facets of smoking urge on a visual analogue scale (range = 1-100), including desire and intention to smoke due to expected reward (Factor 1) or anticipation that smoking would relieve negative affect (Factor 2). We elected to use all items from the QSU-B to examine differences between craving factors and because there were no reverse-scored items included on this measure.
Data Analyses
Frequency and severity ratings of adverse events were analyzed using t-tests and chi-square tests. Demographic variables and baseline substance use were compared between medication groups using independent samples t tests to test for any pre-treatment differences in participant characteristics or substance use severity. Alcohol craving at the beginning of the cue reactivity session was analyzed using 2 (medication condition: placebo versus varenicline) × 2 (smoking group: nonsmoker versus smoker) between-participant analysis of variance (ANOVA). Tobacco craving at this same time point was analyzed using independent samples t tests (placebo vs. varenicline). Primary outcome variables were measures of tobacco and alcohol craving following alcohol and neutral cue exposure. These primary outcome variables were analyzed using 2 (medication condition) × 2 (smoking group) × 2 (cue condition: neutral versus alcohol cue exposure) × 2 (block) ANOVAs. Prior research has suggested that multi-block cue exposure studies can produce carryover effects that complicate analyses (Sayette et al. 2010) and often only analyze a single block of trials in this paradigm (e.g., Monti et al. 1993). We tested for any main effect and interaction effect of block. In the absence of any interaction effect involving block, we planned to limit subsequent analyses to the first block in the interest of clarity and to avoid any interpretive problems related to carryover. Any significant interaction effect was probed using a priori t tests, and effect sizes are indicated using Cohen's dz. Ratings of tobacco craving were analyzed using 2 (medication condition) × 2 (cue condition) ANOVA. Only data from smokers were included in these analyses.
Supplemental analyses were conducted to explore potential mediators and moderators of medication effects. Prior research (Brandon et al., 2011) found that perceived medication condition influenced varenicline responses, so we examined whether participants' perceptions of whether they were on active medication or placebo influenced their responses to varenicline or placebo. We also examined whether participant characteristics (i.e., age, gender, past 6-month alcohol dependence diagnosis, proportion of drinking days binging) moderated the effects of varenicline on cue-induced craving. These supplemental analyses were a series of 2 (medication condition) × 2 (cue condition) × 2 (potential moderator) ANOVAs. For continuous moderators, groups were formed using median splits, and group status was included as a variable in the ANOVA.
Results
Demographic and Baseline Substance Use Variables
Demographic and baseline substance use variables are presented in Table 1. As seen in this table, there were no significant difference between medication conditions in any demographic characteristic. There was no significant group difference in any baseline substance use variable.
Table 1. Baseline demographic, alcohol use, and smoking variables.
| Placebo (n = 38) | Varenicline (n = 39) | |
|---|---|---|
|
|
||
| Age (years) | 32.61 (10.21) | 34.85 (11.17) |
| Sex (% male) | 64% | 67% |
| Race | ||
| White | 23 (64%) | 27 (73%) |
| Other | 13 (36 %) | 8 (22%) |
| Marital status | ||
| Not married | 28 (74%) | 34 (89%) |
| Married | 10 (26%) | 5 (13%) |
| Alcohol Use | ||
| Disorder | ||
| Yes | 24 (63%) | 21 (54%) |
| No | 14 (37%) | 18 (46%) |
| Alcohol use | ||
| AUDIT | 16.50 (5.71) | 14.87 (4.92) |
| Drinks/week | 26.87 (21.70) | 28.63 (17.11) |
| Drinking episodes/week | 4.81 (1.75) | 4.60 (1.75) |
| % heavy drinking days | 0.54 (0.36) | 0.58 (0.33) |
| Smoking status | ||
| Smokers | 18 (47%) | 21 (54%) |
| Nonsmokers | 20 (53%) | 18 (46%) |
| Smoking | ||
| FTND | 3.75 (2.41) | 4.53 (2.67) |
| Cig/day | 10.46 (8.36) | 11.91 (8.19) |
Note. Quantity/frequency measures of alcohol use and smoking are based on timeline follow-backs assessing patterns of use four weeks before starting medication. Alcohol Use Disorder indicates number of participants who met criteria for any alcohol use disorder on the SCID-I during the 6 months before intake. AUDIT is the Alcohol Use Disorder Identification Test. FTND is the Fagerström Test of Nicotine Dependence. For smoking variables, reported means and t-test comparisons are for smokers in each group. Chi square and t-test analyses found no significant differences between medication groups.
Treatment Emergent Adverse Events
All participants completed their assigned doses, and none required a dose adjustment. No participants discontinued the study because they were unable to tolerate the medication. Severity rations were all in the minimal to mild range and did not differ across medication groups.
Craving before Cue Exposure
Alcohol craving at the beginning of the cue exposure session is reported in Figure 1. The ANOVA of alcohol craving found no significant main effect of medication condition F (1, 73) = 2.15, p = 0.147, smoking group, F (1, 73) = 1.19, p = 0.279, or medication group × smoking group interaction, F (1, 73) = 0.25, p = .621. Tobacco craving at the beginning of the cue exposure session is reported in Figure 2. The t tests of tobacco craving found no significant difference between medication conditions in QSU-B Factor 1, t (37) = 0.37, p = 0.711, or Factor 2, t (37) = 0.18, p = 0.856.
Figure 1.
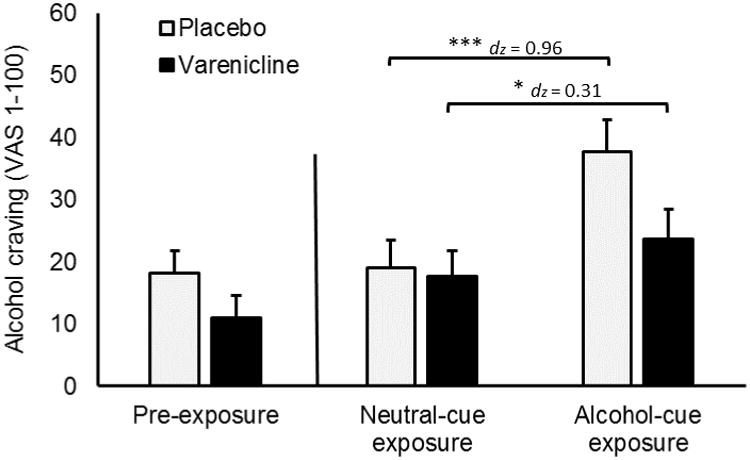
Varenicline and cue exposure effects on alcohol craving. Capped bars represent SEM. Brackets indicate significant differences. No comparisons were made between pre-exposure craving and craving following either cue exposure condition. Responses were provided on a visual analogue scale (1-100). Brackets describe effects of cue exposure condition within each medication group.
*** p < 0.001, * p < 0.050.
Figure 2.
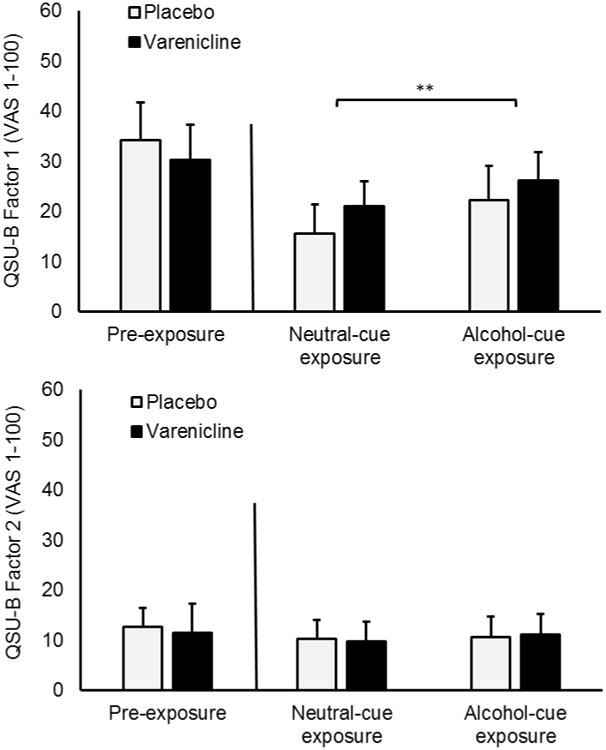
Varenicline and cue exposure effects on tobacco craving. Reported means are for smoking subset of the sample. Capped bars represent SEM. Bracket represents significant effect Responses were provided on a visual analogue scale (1-100). No comparisons were conducted between pre-exposure craving and craving following either cue exposure condition because participants smoked a cigarette between these two measurement periods.
** p < 0.010
Changes in Craving across Blocks
For alcohol craving, there was a significant main effect of block, F (1. 73) = 9.64, p = 0.003, because participants reported lower alcohol craving overall during the first block (M = 24.47, SD = 27.60) compared to the second block (M = 29.74, SD = 27.60). There was no significant interaction effect involving block (ps > 0.050). This pattern of findings suggests a carryover effect such that exposure to the first alcohol cue increased alcohol craving during subsequent trials. For QSU-B Factor 1, there was a similar main effect of block, F (1, 37) = 12.42, p = 0.001, because scores during the first block (M = 21.01, SD = 28.3) were lower than those during the second block (M = 29.29, SD = 33.30). No effect involving block was significant for QSU-B Factor 2 scores (ps > 0.050). These findings suggest that the observed effects were similar between blocks despite a general increase in craving following the initial alcohol cue exposure (i.e., carryover effect). As such, subsequent analyses only include results of the first block of trials.
Alcohol Craving following Cue Exposure
Alcohol craving scores are presented in Figure 1. There was a main effect of cue condition, F (1, 73) = 34.73, p < 0.001, because participants reported more alcohol craving following alcohol cue compared to neutral cue exposure. There was no significant main effect of medication, F (1, 73) = 1.58, p = 0.213. There was, however, a cue condition × medication interaction, F (1, 73) = 8.57, p = 0.005. As seen in Figure 1, this interaction confirms that participants receiving varenicline experienced a smaller increase in craving following alcohol cue exposure, compared to those receiving placebo. There were no significant main effects or interaction effects involving smoking status, Fs (1, 73) ≤ 1.16, ps > 0.050, suggesting that smokers did not differ from nonsmokers in their cue reactivity or in their response to varenicline. Alcohol craving scores reported separately by smoking status group are reported in a supplemental table. A priori t tests examined found a significant increase in craving from the neutral to alcohol cue in both the placebo group, t (36) = 5.25, p < 0.001, dz = 0.959, and the varenicline group, t (38) = 2.60, p = 0.013, dz = 0.310.
Tobacco Craving following Alcohol Cue Exposure
Self-reported tobacco craving scores on the QSU-B are presented in Figure 2. The ANOVA of QSU-B Factor 1 scores revealed a significant main effect of cue condition, F (1, 37) = 10.21, p = 0.003. Participants reported higher levels of tobacco craving following alcohol cue exposure, compared to neutral cue exposure. There was no significant main effect of medication, F (1, 37) = 0.18, p = 0.674, or medication × cue exposure condition interaction, F (1, 37) = 0.05, p = 0.834. The ANOVA of QSU-B Factor 2 scores found no significant main effect or interaction, Fs (1, 37) ≤ 0.75, ps > 0.050.
The sample included several lighter smokers, as indicated by the low average cigarettes per day (M = 11.26 cig/day, SD = 8.19). A possible reason for the lack of varenicline effect on tobacco craving is that such an effect is only evident among heavy smokers. To test this possibility, we conducted a median split on cigarettes per day among smokers to create a group of light (M = 4.18 cig/day, SD = 4.55) and heavy smokers (M = 17.63 cig/day, SD = 4.68). We included smoking group factor in a follow-up 2 (medication) × 2 (cue) × 2 (light versus heavy smoker) ANOVA of QSU-B Factor 1 scores. There was a significant main effect of cig/day group, F (1, 34) = 10.90, p = 0.002, because overall craving was higher in heavy smokers (M = 31.44, SD = 30.98) relative to light smokers (M = 10.39, SD = 21.33). No interaction effect involving cig/day group was significant, Fs (1, 34) ≤ 2.99, ps > 0.050.
Perceived Medication Condition and Potential Effect Moderators
One participant was not willing to guess his or her medication condition. 56.4% of participants receiving varenicline and 40.5% of those receiving placebo reported that they received varenicline. A chi-square test of independence found no significant association between perceived and actual medication condition, χ2 (1, N = 76) = 1.91, p = 0.167. Including perceived medication condition as a factor in the ANOVAs did not change the pattern of results. No significant main effect or interaction involving perceived medication condition on alcohol craving was observed, ps > 0.050.
Additional participant characteristics were examined as moderators of varenicline's effect on cue-induced craving for alcohol. These ANOVAs found no significant main effect or interaction involving age, Fs (1, 73) ≤ 0.02, ps > 0.050, gender, Fs (1, 73) ≤ 0.51, ps > 0.050, % heavy drinking days Fs (1, 73) ≤ 1.72, ps > 0.050, or past 6 month AUD, Fs (1, 73) ≤ 2.22, ps > 0.050. The cue condition × medication interaction remained significant in all models (ps ≤ .006).
Discussion
This study examined the effects of 2 mg/day varenicline on alcohol cue-induced craving for both alcohol and tobacco. Results of the study were generally consistent with our hypotheses. Participants in both medication conditions reported similar levels of alcohol craving prior to any cue exposure and following neutral cue exposure. The lack of medication effect on baseline (tonic) craving differs from the findings of prior research (Litten et al. 2013). Following alcohol cue exposure, however, participants receiving varenicline reported significantly less craving, compared to participants receiving placebo. This finding suggests that varenicline attenuates cue-induced alcohol craving. The ability of varenicline to block increases in craving following cue exposure is important because cue-induced craving is a major contributor to relapse among individuals with alcohol use disorder (Childress et al. 1993; Niaura et al. 1988). This finding points to a mechanism by which varenicline reduces drinking as shown in previous human laboratory studies and clinical trials (e.g., Litten et al. 2013; McKee et al. 2009). Despite our prediction that varenicline would be more effective for reducing alcohol craving in smokers, results suggest that it was equally effective at reducing cue-induced alcohol craving in smokers and nonsmokers. Other participant characteristics (i.e., gender, age, % heavy drinking days, past 6 month AUD diagnosis) did not moderate the medication effects, suggesting that the effect of varenicline on cue-induced alcohol craving is robust across different groups of heavy drinkers.
Cue induced craving is thought to be caused by associative learning over repeated pairings of drug cues with positive drug effects during self-administration episodes. It is well-established that naltrexone can minimize alcohol craving via opioid receptor antagonism (Ray et al. 2010). We have demonstrated here that varenicline, an nAChR partial agonist, exerts a similar effect on cue-induced craving as naltrexone, presumably through a different neurobiological mechanism. Cholinergic signaling in the ventral tegmental (VTA) area can affect incentive processing by modulating dopaminergic release in the nucleus accumbens (NAcc) (Gotti and Clementi 2004), which is implicated in salience-attribution and processing of drug-related cues (Ito et al. 2000). As such, varenicline may have reduced cue-induced craving by indirectly reducing dopamine transmission in the NAcc. Additional support for this interpretation of our findings comes from a preclinical study conducted by Löf and colleagues (2007) examining the role of nAChR receptors in alcohol cue reactivity in rats. This study found that systemically administered mecamylamine, a nonselective nAChR antagonist, reduced preference for an alcohol-associated cue (i.e., an alcohol-paired lever) and diminished NAcc dopamine release in response to the alcohol cue in treated rats. Results of the current study are consistent with a similar process in humans. Specifically, varenicline may have indirectly downregulated NAcc dopamine release in response to alcohol cue reactivity by altering cholingeric tone in regions with input to the VTA. Additional preclinical research will be useful for determining whether varenicline exerts a similar effect on cue-induced NAcc dopamine release as nAChR antagonists such as mecamylamine.
We also examined the effects of alcohol cue exposure on tobacco craving in smokers. Numerous studies have documented pharmacological and behavioral interactions between nicotine and alcohol (reviewed in Verplaetse and McKee 2016) that may result in associative learning, resulting in increased tobacco craving following exposure to alcohol cues. The current study replicated the results of prior human laboratory studies showing this cross-substance cue reactivity (Drobes 2002; Rohsenow et al. 1997). Considering the frequency with which alcohol and tobacco are co-administered in heavy drinking smokers, it is not surprising that alcohol cues provoked smoking urge in the current study. Interestingly, varenicline did not attenuate alcohol-cue induced increases in craving for tobacco. This finding is surprising given that varenicline's primary indication is as a smoking cessation aid and that it has reduced cue-induced tobacco craving in prior research (Franklin et al., 2011). One reason for this lack of medication effect may be that participants were nicotine satiated due to their recent smoking. This study design element may have masked medication effects on cue-induced tobacco craving that are evident following overnight deprivation (Brandon et al., 2011). A longer deprivation period also may have allowed us to observe cue-induced increases in anticipated-relief craving (i.e., QSU-Factor 2). On the other hand, a preclinical study found that varenicline reduced cue-induced relapse to alcohol-seeking but not nicotine-seeking in rats (Wouda et al. 2011). Additional research will be needed to elucidate the effects of varenicline on cue-invoked craving for different drugs of abuse.
This research provides important information regarding the effects of varenicline on cue-induced craving in heavy drinkers; however, our findings should be interpreted considering some limitations. First, we only assessed self-reported craving following cue exposure. Cue reactivity studies often assess physiological states (e.g., heart rate, skin temperature, salivary flow) to corroborate self-reported changes in craving, although these measures may be less sensitive to cue-induced craving changes compared to self-report (Carter and Tiffany 1999). Future research should use multiple assessment techniques to better characterize cue-induced changes in craving. Second, we did not counterbalance the order of the neutral and alcohol cue reactivity condition sessions (following the procedures described by Rohsenow et al. 2000), so we cannot rule out an order effect. We chose not to counterbalance cue conditions because doing so may have introduced carryover effects. The potential confounds introduced by using a fixed order are preferable to those created by counterbalancing the presentation order (Sayette et al. 2010). In future studies, it may be preferable to use a completely between-subjects design, although doing so would significantly increase the number of participants needed to achieve adequate power. An alternative strategy may be to conduct multiple cue exposure sessions on separate days to minimize carryover effects. Finally, recent research has suggested that participants are frequently able to guess their drug condition assignment in clinical trials of varenicline, which may impact treatment outcomes (Brandon et al., 2011; Correa et al., 2014). Although we found no evidence in the current study that drug expectancies influenced our outcomes, it will continue to be important in future research to assess whether participants accurately guess their medication condition.
In conclusion, findings from this study support the use of varenicline for reducing alcohol use among heavy drinkers and identify a potential mechanism by which varenicline exerts this effect. Results of this study are consistent with preclinical literature (Lof et al. 2007) demonstrating that medication targeting nAChRs can reduce alcohol-cue reactivity as well as clinical trials showing that varenicline reduces drinking in those with alcohol use disorder (Litten et al. 2013; Mitchell et al. 2012). However, varenicline may be less effective at blocking alcohol-induced priming of tobacco craving. Varenicline continues to show promise as a pharmacological treatment for AUD, and researchers should continue to evaluate its efficacy.
Supplementary Material
Supplemental Table 1. Varenicline effects on alcohol craving at baseline and following cue
Acknowledgments
Funding: Supported by NIH grants R01AA017976 (SAM); R01AA022285 (SAM); R01AA015596 (SAM); T32DA007238 (WR); T32AA015496 (EH); UL1TR001863 (Sherwin)
Dr. McKee has consulted to Embera and Cerecor, has had investigator-initiated grants from Pfizer and Cerecor, and has ownership interest in Lumme.
Footnotes
Dr. Roberts and Dr. Harrison have no conflict of interest.
References
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent Alcoholics. Alcohol Clin Exp Res. 1995;19:600–66. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC, Karver SB, Small BJ. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology (Berl) 2011;218:391–403. doi: 10.1007/s00213-011-2327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–40. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Adult tobacco use information. [Accessed April 27, 2017];2009 Available at: http://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm.
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogra. 1993;137:73–73. [PubMed] [Google Scholar]
- Childs E, Roche DJ, King AC, de Wit H. Varenicline potentiates alcohol-induced negative subjective responses and offsets impaired eye movements. Alcohol Clin Exp Res. 2012;36:906–14. doi: 10.1111/j.1530-0277.2011.01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106:243–50. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Correa JB, Heckman BW, Marquinez NS, Drobes DJ, Unrod M, Roetzheim RG, Brandon TH. Perceived medication assignment during a placebo-controlled laboratory study of varenicline: Temporal associations of treatment expectancies with smoking-related outcomes. Psychpharmacol. 2014;231:2559–66. doi: 10.1007/s00213-013-3420-2. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- de Bejczy A, Lof E, Walther L, Guterstam J, Hammarberg A, Asanovska G, Franck J, Isaksson A, Soderpalm B. Varenicline for treatment of alcohol dependence: a randomized, placebo-controlled trial. Alcohol Clin Exp Res. 2015;39:2189–99. doi: 10.1111/acer.12854. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20:1412–7. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Drobes DJ. Cue reactivity in alcohol and tobacco dependence. Alcohol Clin Exp Res. 2002;26:1928–9. doi: 10.1097/01.ALC.0000040983.23182.3A. [DOI] [PubMed] [Google Scholar]
- Drummond DC. What does cue-reactivity have to offer clinical research? Addiction. 2000;95(2):S129–44. doi: 10.1080/09652140050111708. [DOI] [PubMed] [Google Scholar]
- Erwin BL, Slaton RM. Varenicline in the treatment of alcohol use disorders. Ann Pharmacother. 2014;48:1445–55. doi: 10.1177/1060028014545806. [DOI] [PubMed] [Google Scholar]
- Falk DE, Castle IJ, Ryan M, Fertig J, Litten RZ. Moderators of varenicline treatment effects in a double-blind, placebo-controlled trial for alcohol dependence: an exploratory analysis. J Addict Med. 2015;9:296–303. doi: 10.1097/ADM.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Williams J, Gibbon M. Structured clinical interview for DSM-IV-TR (SCID-I)-research version. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O'Brien CP, Childress AR. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiat. 2011;68:516–26. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O'Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology (Berl) 2011;215:655–63. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Franken IH, Howard MO. Cue-elicited heart rate variability and attentional bias predict alcohol relapse following treatment. Psychopharmacology (Berl) 2012;222:17–26. doi: 10.1007/s00213-011-2618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR Grp VPS. Varenicline, an alpha 4 beta 2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. JAMA-J Am Med Assoc. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–96. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Chait LD, Griffiths RR. Cigarette smoking and subjective response in alcoholics: effects of pentobarbital. Clin Pharmacol Ther. 1983;33:806–12. doi: 10.1038/clpt.1983.110. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–95. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouri EM, McCarthy EM, Faust AH, Lukas SE. Pretreatment with transdermal nicotine enhances some of ethanol's acute effects in men. Drug Alcohol Depend. 2004;75:55–65. doi: 10.1016/j.drugalcdep.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Lacroix F, Pettorelli A, Maddux JN, Heidari-Jam A, Chaudhri N. Varenicline reduces context-induced relapse to alcohol-seeking through actions in the nucleus accumbens. Neuropsychopharmacol. 2017;42:1037–48. doi: 10.1038/npp.2016.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22:343–81. [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid-Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R Abuse NNIA. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med. 2013;7:277–86. doi: 10.1097/ADM.0b013e31829623f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lof E, Olausson P, deBejczy A, Stomberg R, McIntosh JM, Taylor JR, Soderpalm B. Nicotinic acetylcholine receptors in the ventral tegmental area mediate the dopamine activating and reinforcing properties of ethanol cues. Psychopharmacology (Berl) 2007;195:333–43. doi: 10.1007/s00213-007-0899-4. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison ELR, O'Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiat. 2009;66:185–90. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, O'Malley SS, Shi J, Mase T, Krishnan-Sarin S. Effects of transdermal nicotine replacement on alcohol responses and alcohol self-administration. Psychopharmacology (Berl) 2008;196:189–200. doi: 10.1007/s00213-007-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl) 2012;223:299–306. doi: 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol. 1987;96:122–6. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Rubonis AV, Niaura RS, Sirota AD, Colby SM, Abrams DB. Alcohol cue reactivity: effects of detoxification and extended exposure. J Stud Alcohol. 1993;54:235–45. doi: 10.15288/jsa.1993.54.235. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97:133–52. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Palfai TP, Monti PM, Ostafin B, Hutchinson K. Effects of nicotine deprivation on alcohol-related information processing and drinking behavior. J Abnorm Psychol. 2000;109:96–105. doi: 10.1037//0021-843x.109.1.96. [DOI] [PubMed] [Google Scholar]
- Ray LA, Chin PF, Miotto K. Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS Neurol Disord Drug Targets. 2010;9:13–22. doi: 10.2174/187152710790966704. [DOI] [PubMed] [Google Scholar]
- Reid MS, Flammino F, Starosta A, Palamar J, Franck J. Physiological and subjective responding to alcohol cue exposure in alcoholics and control subjects: evidence for appetitive responding. J Neural Transm. 2006;113:1519–35. doi: 10.1007/s00702-005-0439-5. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Colby SM, Monti PM, Swift RM, Martin RA, Mueller TI, Gordon A, Eaton CA. Predictors of compliance with naltrexone among alcoholics. Alcohol Clin Exp Res. 2000;24:1542–9. [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Colby SM, Gulliver SB, Sirota AD, Niaura RS, Abrams DB. Effects of alcohol cues on smoking urges and topography among alcoholic men. Alcohol Clin Exp Res. 1997;21:101–7. [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Griffin KM, Sayers WM. Counterbalancing in smoking cue research: a critical analysis. Nicotine Tob Res. 2010;12:1068–79. doi: 10.1093/ntr/ntq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Balabanis M. Associations between alcohol and tobacco. In: Fertig J, Allen J, editors. Alcohol and tobacco: from basic science to clinical practice. Government Printing Office; Washington, DC: U.S: 1995. pp. 17–36. [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Vol. 1992. Humana Press; Totowa: 1992. pp. 41–72. [Google Scholar]
- Soderpalm B, Ericson M, Olausson P, Blomqvist O, Engel JA. Nicotinic mechanisms involved in the dopamine activating and reinforcing properties of ethanol. Behav Brain Res. 2000;113:85–96. doi: 10.1016/s0166-4328(00)00203-5. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha 402 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. P Natl Acad Sci USA. 2007;104:12518–23. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–7. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Verplaetse TL, McKee SA. An overview of alcohol and tobacco/nicotine interactions in the human laboratory. Am J Drug Alcohol Abuse. 2016:1–11. doi: 10.1080/00952990.2016.1189927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Pittman BP, Shi JM, Tetrault JM, Coppola S, McKee SA. Effect of lowering the dose of varenicline on alcohol self-administration in drinkers with alcohol use disorders. J Addict Med. 2016a;10:166–73. doi: 10.1097/ADM.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Pittman BP, Shi JM, Tetrault JM, Coppola S, McKee AS. Effect of varenicline combined with high-dose alcohol on craving, subjective intoxication, perceptual motor response, and executive cognitive funcion in adults with alcohol use disorders: preliminary findings. Alcohol Clin Exp Res. 2016b;40:1567–6. doi: 10.1111/acer.13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Watson NT, King AC, Sherman CE, O'Brien CP. Effect of naltrexone on alcohol “high” in alcoholics. Am J Psychiatry. 1995;152:613–5. doi: 10.1176/ajp.152.4.613. [DOI] [PubMed] [Google Scholar]
- Witteman J, Post H, Tarvainen M, de Bruijn A, Perna Ede S, Ramaekers JG, Wiers RW. Cue reactivity and its relation to craving and relapse in alcohol dependence: a combined laboratory and field study. Psychopharmacology (Berl) 2015;232:3685–96. doi: 10.1007/s00213-015-4027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D, Schoffelmeer ANM, Pattij T, De Vries TJ. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology (Berl) 2011;216:267–77. doi: 10.1007/s00213-011-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Varenicline effects on alcohol craving at baseline and following cue


