Abstract
Objectives
We evaluated the relationship of systolic blood pressure, diastolic blood pressure, pulse pressure, and treatment with antihypertensives with knee osteoarthritis incidence in a United States cohort.
Methods
We performed a longitudinal study (2004 – 2010) nested within the Osteoarthritis Initiative Study including only individuals without knee osteoarthritis at baseline. Systolic blood pressure, diastolic blood pressure and pulse pressure were assessed at baseline, 12-, 24-, and 36-month visits. Knee radiographs at baseline, 12-, 24-, 36- and 48 month visits defined radiographic osteoarthritis, Kellgren and Lawrence grade ≥ 2. We performed logistic regression, adjusting for age, sex, body mass index, NSAID use, number of antihypertensive medications, diabetic medications, and cholesterol medications.
Results
1930 people (6040 observations) were included. Annual incidence rates of radiographic osteoarthritis by systolic blood pressure quartiles (lowest to highest) were 2.1%, 3.4%, 3.7%, and 3.7%. Fully adjusted odds ratios of incident radiographic OA for the 2nd–4th quartiles were 1.6, 1.7, and 1.6 relative to the lowest quartile (p for trend = 0.03). Pulse pressure results were similar. There was no association with diastolic blood pressure. Compared to those not taking any antihypertensive medications, those taking ≥ 3 had decreased odds (0.4, 0.1–1.0) of developing incident OA.
Conclusions
In a United States cohort, higher systolic blood pressure and pulse pressure are associated with increased incidence of radiographic knee osteoarthritis while treatment with ≥ 3 antihypertensive medications was associated with reduced incidence. These findings suggest a new and promising avenue for research on disease modification in knee osteoarthritis.
Keywords: Hypertension, Pulse Pressure, Osteoarthritis
INTRODUCTION
Osteoarthritis (OA) is the most common form of arthritis, and a major public health problem, with at least 30 million adults in the US with clinical OA.[1] Health care expenditures are estimated at $189 billion annually in the US.[2] Further, OA is the most frequent cause of dependency in lower limb tasks, especially in the elderly,[3] and accounts for 68 million work loss days per year in the US and more than 5% of the annual retirement rate.[4] It is also the most frequent reason for arthroplasty, of which more than 700,000 are performed annually in the US,[5] equivalent to 2.2 per 1000 persons,[6] approximately triple the rates seen in Scandinavian countries where the rates are 0.7 per 1000 persons,[7–9] still a very common procedure.
The burden of knee OA is particularly heavy because only a limited number of effective treatments have been identified, including physical therapy,[10] weight loss,[11] nonsteroidal anti-inflammatory drugs (NSAIDs),[12] and arthroplasty.[13] Because the natural history of knee OA is long, much of the emphasis of randomized controlled trials has been on those with established disease and persistent symptoms. However, the opportunity to intervene may occur early in disease, supporting attempts to identify risk factors for incident OA.
A study from Japan, the Research on Osteoarthritis and Osteoporosis Against Disability (ROAD) Study, reported an association between the accumulation of metabolic syndrome factors and incident knee OA.[14] Its main focus was evaluating the relationship of metabolic syndrome as a whole, not the individual factors, as it related to incident knee OA. Hypertension was the most common factor observed occurring in 67% of the cohort. In univariate analyses, systolic but not diastolic blood pressure was higher in those who developed knee OA compared to those who did not. To validate these findings, and because results from a Japanese cohort may not be generalizable to other populations it is important to replicate the study in a different cohort, adjusting for potential confounders. Further, these findings raise the hypothesis that an increase in pulse pressure, defined as systolic blood pressure minus diastolic blood pressure, and generally regarded as an indicator of arterial stiffness[15] may be a risk factor for incident knee OA. To our knowledge, the relationship between pulse pressure and knee OA has never been evaluated. We also expected that treatment of elevated systolic pressure and pulse pressure would be associated with a reduced incidence of knee OA.
Therefore, the objectives of our study were to evaluate whether higher systolic blood pressure, diastolic blood pressure and pulse pressure and treatment with anti-hypertensives were associated with incident radiographic knee OA in a United States cohort.
MATERIALS AND METHODS
Study Design
This is a longitudinal study (2004 – 2010) nested within the Osteoarthritis Initiative (OAI), a publicly available multi-center observational study. Full details of the study design of the OAI are available online at https://oai.epi-ucsf.org/datarelease/docs/StudyDesignProtocol.pdf
In this study, we included all participants of the OAI who had at least a baseline clinical visit and a 12 month follow up visit where blood pressure assessments and osteoarthritis assessments were available. Because this was a study of incident knee OA, only those who did not have OA in either knee at baseline were included in this study. This study was approved by the Institutional Review Board at Baylor College of Medicine (K23 H-30423). Each participant provided written informed consent.
Systemic Blood Pressure Measurements
Participants were asked to refrain from drinking any caffeine, eating, doing heavy physical activity, smoking or ingesting alcohol for 30 minutes prior to the blood pressure measurement. Additionally, in the clinic, they were asked to sit upright comfortably in a chair with back supported and rest for five minutes with legs and ankles uncrossed and their feet flat on the floor prior to the blood pressure assessment. A maximal inflation level was determined by adding 30mm of Hg to the palpated systolic blood pressure. Subsequently a manual systolic and diastolic blood pressure was measured using conventional mercury sphygmomanometers, stethoscopes, and the appropriate size blood pressure cuffs. Pulse pressure was defined as systolic blood pressure minus diastolic blood pressure. Blood pressure measurements were assessed at OAI baseline, 12, 24, and 36 month visits. These data are publicly available on the OAI website (http://oai.epi-ucsf.org/datarelease/) under filenames AllClinical00_SAS (Version 0.2.2), AllClinical01_SAS (Version 1.2.1), AllClinical03_SAS (Version 3.2.1), and AllClinical05_SAS (Version 5.2.1).
Physical Activity Assessment
The Physical Activity Scale for the Elderly (PASE)[16] reflecting amount and level of physical activity over the prior 7 days was assessed at OAI baseline, 12, 24, and 36 month visits. These data were available from the same files used to obtain blood pressure data.
Medication Data
Detailed medication inventory data for prescription medicines taken during the 30 days prior to a clinic visit was obtained at OAI baseline, 12, 24, and 36 month visits. Each medication was mapped to ingredients through use of a medication-coding dictionary adapted from the Women’s Health and Aging Study,[17] and these ingredients were then hierarchically-organized and numerically-coded using the Iowa Drug Information Service database of ingredients.[18] These data are publicly available on the OAI website (http://oai.epi-ucsf.org/datarelease/) under filenames MIF00_SAS (Version 0.2.2), MIF01_SAS (Version 1.2.1), MIF03_SAS (Version 3.2.1), and MIF05_SAS (Version 5.2.1).
Antihypertensive medications
GL individually reviewed medication ingredients to identify the following groups of anti-hypertensive medications: calcium channel blocker, beta blocker, angiotensin converter enzyme inhibitor, angiotensin receptor II antagonist, alpha antagonist, diuretic, or miscellaneous antihypertensive medication (i.e. hydralazine, minoxidil, and nitrates).
Non-steroidal anti-inflammatory drugs (NSAIDs)
Participants were asked whether they took either over-the-counter or prescription NSAIDs on most days of the month in the last month. GL also individually reviewed medication ingredients to identify traditional NSAIDs and Cox-2 inhibitors. If participants reported an NSAID (including diclofenac, etodolac, ibuprofen, indomethacin, ketorolac, ketoprofen, meloxicam, nabumetome, naproxen, piroxicam, rofecoxib, or celecoxib) in their medication inventory or answered affirmative to the dichotomous questions about NSAIDs, they were categorized as being exposed to an NSAID.
Diabetic medications
GL individually reviewed medication ingredients to identify all of the following diabetic medications: glipizide, glyburide, glimepiride, glibonuride, repaglinide, nateglinide, metformin, rosiglitazone, pioglitazone, and insulin. If participants were on any of these medications, they were categorized as taking a diabetic medication. Because serum glucose levels were not available for these participants, this was the best alternative method available for assessing diabetes status at the baseline, 12, 24 and 36 month follow up visits.
Cholesterol medications
Using the medication inventory, GL identified the following medications as lipid lowering medications: atorvastatin, fluvastatin, lovastatin, rosuvastatin, simvastatin, niacin, colesevelam, colestipol, cholestyramine, fenofibrate, and gemfibrozil. If participants were on any of these medications, they were categorized as taking cholesterol medications. Classification of hyperlipidemia status based on medication administration was our best effort to appropriately classify people’s hyperlipidemia status since we did not have information on fasting lipid panels at any study visit.
Knee Radiographs
Weight-bearing, fixed-flexion, posterior-anterior radiographs of bilateral knees were obtained at the OAI baseline, 12, 24, 36, and 48 month visits. Central readers [19] scored these for overall radiographic severity using Kellgren-Lawrence grades (0 – 4) using the Osteoarthritis Research Society International Atlas.[20] These data are publicly available on the OAI website (http://oai.epi-ucsf.org/datarelease/), filename kXR_SQ_BU06_SAS (Version 6.3)). The reliability for these readings (read-reread) was substantial[21] (weighted kappa [intra-rater reliability] = 0.71 [95%CI 0.55 – 0.87]).[22] Those knees with a Kellgren and Lawrence grade ≥ 2 were defined as having radiographic OA (ROA). If a knee had a total joint replacement, it was assigned as having ROA.
Outcome Definition
Incident ROA was a person-based definition reflecting the development of ROA in either knee 12 months after the exposure assessment.
Covariates
Sex was based on self-report from the OAI baseline visit. Date of birth from OAI baseline visit was used to calculate participant age at each OAI visit. Body mass index (BMI) at each visit was calculated as weight (measured at each visit) divided by most recently measured height squared (kg/m2) (measured at every other OAI visit).
Statistical Analysis
We evaluated the influence of systolic blood pressure, diastolic blood pressure, and pulse pressure quartiles including time points at baseline, months 12, 24, and 36, on the development of incident ROA in at least one knee 12 months after blood pressure assessments, meaning months 12, 24, 36 and 48. Each time point was treated as a separate observation. (E.g. we evaluated whether a participant’s quartile of systolic blood pressure at baseline was predictive of incident OA at the 12 month visit. Then if the participant did not develop OA, we evaluated whether his/her quartile of systolic blood pressure at 12 months was predictive of incident OA at the 24 month visit, etc.) We performed repeated measures longitudinal analyses using generalized estimating equations to adjust for correlations across visits within the same person over time.[23] Once participants developed incident ROA, they were censored from the analyses. Each participant could contribute up to 4 observation periods. The Cochran-Armitage trend test was used to assess for statistically significant trends.[24, 25] We performed the analyses unadjusted and then adjusted for potential confounders including age, sex, body mass index, PASE, NSAIDs use, number of antihypertensive medications (0, 1, 2 or ≥3), diabetic medication use, and cholesterol medication use at the baseline, 12, 24, and 36 month visits. Participants missing either radiographic readings or blood pressure readings were excluded from the analyses at that particular visit.
Because systolic blood pressure and pulse pressure quartiles 2–4 did not exhibit a typical dose response relationship, we performed post hoc logistic regression analyses collapsing those quartiles into one group.
All analyses were performed using SAS version 9.4. P-values <0.05 were considered statistically significant.
RESULTS
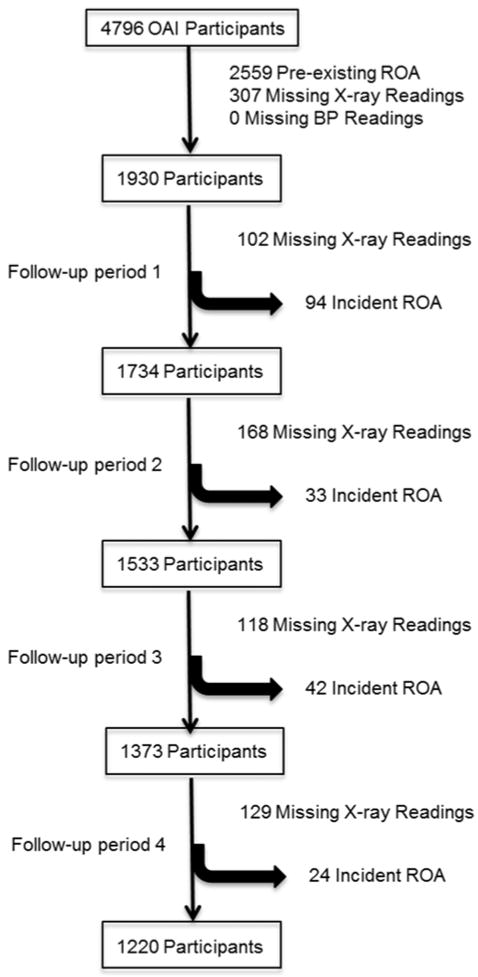
In total 1930 participants contributed 6040 observations. A flow diagram for the cohort is included in figure 1. Mean age was 59.2 (9.1) years, mean body mass index was 27.2 (4.5) kg/m2 and 42% of the group were male.
Figure 1.
Flow diagram of participants included.
Number of antihypertensive medications, diabetic medication use, and cholesterol medication use were all associated with increased systolic blood pressure and pulse pressure (Table 1).
Table 1.
Relationship of medications with systolic blood pressure (SBP) and pulse pressure (PP).
| % taking number of anti HTN meds | % taking DM meds | % taking chol meds | ||||
|---|---|---|---|---|---|---|
| SBP | 0 | 1 | 2 | ≥3 | ||
| Quartile 1 (76 – 111) | 79% (1374/1737) | 13% (225/1737) | 6% (105/1737) | 2% (33/1737) | 3% (44/1737) | 22% (378/1737) |
| Quartile 2 (112 – 121) | 70% (1099/1571) | 16% (244/1571) | 11% (165/1571) | 4% (63/1571) | 5% (83/1571) | 31% (481/1571) |
| Quartile 3 (122 – 130) | 61% (854/1396) | 21% (287/1396) | 12% (173/1396) | 6% (82/1396) | 5% (68/1396) | 32% (453/1396) |
| Quartile 4 (131 – 208) | 49% (656/1336) | 26% (342/1336) | 16% (218/1336) | 9% (120/1336) | 8% (110/1336) | 34% (458/1336) |
| PP | ||||||
| Quartile 1 (10 – 38) | 78% (1441/1849) | 13% (242/1849) | 7% (130/1849) | 2% (36/1849) | 2% (40/1849) | 22% (402/1849) |
| Quartile 2 (39 – 44) | 72% (855/1190) | 18% (212/1190) | 7% (83/1190) | 3% (40/1190) | 4% (53/1190) | 28% (337/1190) |
| Quartile 3 (45 – 54) | 64% (1011/1588) | 19% (307/1588) | 12% (194/1588) | 5% (76/1588) | 5% (78/1588) | 30% (481/1588) |
| Quartile 4 (55 – 136) | 48% (676/1413) | 24% (337/1413) | 18% (254/1413) | 10% (146/1413) | 10% (134/1413) | 39% (550/1413) |
There was a statistically significant increased incidence of ROA with increased systolic blood pressure and pulse pressure quartiles (Table 2 and 3), but not with diastolic blood pressure (Table 4). The associations between both systolic blood pressure and pulse pressure and incident knee ROA persisted after adjusting for age, sex, body mass index, PASE, NSAID use, number of antihypertensive medications, diabetic medications, and cholesterol medications (Tables 2 and 3). Post-hoc analyses comparing systolic blood pressure quartiles 2–4 (ROA incidence of 3.6%) against quartile 1 (2.1%) resulted in unadjusted and adjusted odds ratios (ORs) of 1.7 (95% confidence interval [CI] 1.2 – 2.5) and 1.6 (95%CI 1.1 – 2.4) respectively. Comparing pulse pressure quartiles 2–4 (ROA incidence 3.6%) against quartile 1 (2.2%), unadjusted and adjusted ORs were 1.7 (95%CI 1.2 – 2.4) and 1.6 (95%CI 1.1 – 2.4).
Table 2.
Odds Ratios (ORs) of Incident ROA across SBP Quartiles. There were 1930 participants, contributing 6040 observation periods, for a total of 192 events.
| Incidence of ROA by Systolic BP Quartile | Unadjusted OR (95% CI) | OR (95% CI) Adjusted for Age, Sex, BMI, PASE, NSAID use, number of anti-hypertensive meds, diabetes meds, cholesterol meds | |
|---|---|---|---|
| SBP Quartile 1 (76 – 111) | 37/1737 (2.1%) | Referent | Referent |
| SBP Quartile 2 (112 – 121) | 54/1571 (3.4%) | 1.6 (1.1 – 2.5) | 1.6 (1.0 – 2.5) |
| SBP Quartile 3 (122 – 130) | 51/1396 (3.7%) | 1.7 (1.1 – 2.7) | 1.7 (1.1 – 2.6) |
| SBP Quartile 4 (131 – 208) | 50/1336 (3.7%) | 1.8 (1.2 – 2.7) | 1.7 (1.0 – 2.6) |
| p-for trend = 0.007 | p-for trend = 0.03 |
Table 3.
Odds Ratios of Incident ROA across Pulse Pressure (PP) Quartiles. There were 1930 participants, contributing 6040 observation periods, for a total of 192 events.
| Incidence of ROA by PP Quartile | Unadjusted OR (95% CI) | OR (95% CI) Adjusted for Age, Sex, BMI, PASE, NSAID use, number of anti-hypertensive meds, diabetes meds, cholesterol meds | |
|---|---|---|---|
| PP Quartile 1 (10 – 38) | 40/1849 (2.2%) | Referent | Referent |
| PP Quartile 2 (39 – 44) | 47/1190 (4.0%) | 1.9 (1.2 – 2.8) | 1.8 (1.2 – 2.8) |
| PP Quartile 3 (45 – 54) | 50/1588 (3.2%) | 1.5 (1.0 – 2.2) | 1.4 (0.9 – 2.2) |
| PP Quartile 4 (55 – 136) | 55/1413 (3.9%) | 1.8 (1.2 – 2.8) | 1.8 (1.1 – 2.8) |
| p-for trend = 0.01 | p-for trend = 0.03 |
Table 4.
Odds Ratios of Incident ROA across Diastolic Pressure (DBP) Quartiles. There were 1930 participants, contributing 6040 observation periods, for a total of 192 events.
| Incidence of ROA by DBP Quartile | Unadjusted OR (95% CI) | OR (95% CI) Adjusted for Age, Sex, BMI, PASE, NSAID use, number of anti-hypertensive meds, diabetes meds, cholesterol meds | |
|---|---|---|---|
| DBP Quartile 1 (36 – 68) | 54/1601 (3.4%) | Referent | Referent |
| DBP Quartile 2 (69 – 74) | 48/1544 (3.1%) | 0.9 (0.6 – 1.4) | 0.9 (0.6 – 1.4) |
| DBP Quartile 3 (75 – 80) | 54/1583 (3.4%) | 1.0 (0.7 – 1.5) | 1.0 (0.6 – 1.4) |
| DBP Quartile 4 (82 – 116) | 36/1276 (2.7%) | 0.8 (0.5 – 1.2) | 0.7 (0.5 – 1.2) |
| p-for trend = 0.5 | p-for trend = 0.3 |
In the fully adjusted models, greater number of anti-hypertensive medications was associated with a lower risk of incident ROA. Participants taking ≥3 anti-hypertensive medications had decreased odds (0.4, 0.1–1.0) of developing incident ROA compared to those not taking any antihypertensive medications. However, adding anti-hypertensive medications into the model did not substantively change the point estimates for the risk of ROA related to systolic blood pressure and pulse pressure. Diabetic medication use and cholesterol medication use were not statistically significant variables in the adjusted models and also did not substantively change the point estimates for the risk of ROA related to systolic blood pressure and pulse pressure, although each individually is related to systolic blood pressure and pulse pressure (Table 1).
DISCUSSION
We have demonstrated that higher systolic blood pressure and pulse pressure, but not diastolic blood pressure, were associated with increased incidence of ROA in a US based cohort, the OAI. Further, use of three or more antihypertensive agents was associated with a lower incidence of ROA. Prior uncontrolled studies have described that hypertension is common among people with OA.[26, 27] The Japanese ROAD study[14] presented univariate unadjusted analysis identifying an association between incident ROA and systolic but not diastolic blood pressure. After adjusting for potential confounders including physical activity and NSAID use, with a known side effect of hypertension [28, 29], as well as diabetes and cholesterol medications, our study has replicated this relationship, and we are the first to show an association of pulse pressure with incident ROA.
Systolic and diastolic blood pressures are the maximal and minimal pressures in the aorta during the cardiac cycle. Pulse pressure is the difference between the two. We found elevated systolic blood pressure and pulse pressure but not diastolic blood pressure to associate with incident knee ROA. A pulse pressure of greater than 38 conferred a 70% increased odds for incident ROA. Similarly, a systolic blood pressure of greater than 111 conferred a 70% increased odds for incident ROA. Many people on the largest number of medications had substantially elevated systolic blood pressure and pulse pressures, consistent with real world management of hypertension.[30] Interestingly, those taking ≥3 anti-hypertensive medications had decreased odds (0.4, 0.1–1.0) of developing incident OA compared to those not taking any antihypertensive medications, suggesting that treatment of hypertension may reduce the incidence of knee OA.
The pattern of having an elevated systolic blood pressure and pulse pressure without an elevated diastolic blood pressure is consistent with isolated systolic hypertension, though the specific definition is a systolic blood pressure > 160 when the diastolic blood pressure < 95.[31] This is a phenomenon attributed to arterial stiffness,[32] aortic stiffness and endothelial dysfunction.[15] Isolated systolic hypertension confers an increased risk for cardiovascular death.[33] Although there is controversy surrounding whether OA is associated with increased cardiovascular death[34, 35], some studies do suggest this,[36, 37] perhaps because OA is a marker of arterial stiffness which would be in keeping with a growing concept that there might be a vascular pathology to OA.[38] Hussain et al found that those who received incident knee arthroplasties for knee OA had smaller caliber retinal arterioles compared to those who did not receive arthroplasties.[39] Though caliber of retinal arterioles is not a direct measure of vascular pathology at the site of the joint, it is a measure of small arteries and bearing in mind that using arthroplasty as a marker of OA is suboptimal,[40] this study supports the idea of a vascular pathology to OA. It will be of great interest to better understand differences that exist within specific joint structures, including bone marrow lesions, menisci, synovitis, ligament damage, and attrition in those with and without elevated systolic blood pressure and pulse pressure prior to the development of knee OA. While the OAI has a wealth of data, MRIs which can ascertain these lesions, at present have only been read on a fraction of the available images. Basic science research on the effect of increased systolic blood pressure and pulse pressure on key joint structures may also be illuminating.
There some limitations to this study. Serum glucose and cholesterol levels were not measured. Therefore we used diabetic and cholesterol medication use which were available at each visit, as surrogate assessments for these conditions. Both medication groups were associated with systolic blood pressure and pulse pressure; however, neither was associated with incident ROA. The lack of associations of diabetic and cholesterol medication use with incident ROA may have resulted from confounding by indication. Therefore, it is still possible that hyperlipidemia[41–43] and diabetes[44] are risk factors for incident ROA. There is the potential that targeting hyperlipidemia and diabetes, other components of metabolic syndrome, might be helpful in preventing incident OA, important questions to address in future research. An additional limitation is that because this an observational study where participants were initiated on medications in a real world setting for hypertension to treat elevated blood pressure, we cannot be certain that initiation of antihypertensive agents for the purpose of reducing the risk of knee OA will actually be effective. A double blinded randomized controlled trial would be needed to address this question. However, it would be prudent to derive a greater understanding of the pathophysiology behind this relationship before embarking on such an intervention study, particularly because the threshold at which SBP seems to confer a greater risk of knee OA is lower than the treatment target for elevated SBP. It is not clear that the risk-benefit ratio is sufficient to recommend this intervention at present.
Given the high prevalence of systemic hypertension and knee OA, the current paucity of practical knee OA preventative strategies and disease modifying therapies, the potential implications of our findings are substantial. Though future epidemiologic studies are needed to confirm our findings, this study provides evidence to suggest a new and promising avenue for research on disease modification in knee OA.
Acknowledgments
Funding Statement
This work was supported by the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases [K23 AR062127 to G.L., K24 AR053593 to M.S.]; the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and the Center for Innovations in Quality, Effectiveness and Safety [CIN 13-413], Michael E. DeBakey VA Medical Center, Houston Texas, the location of Dr. Lo’s academic office; and The Osteoarthritis Initiative, a public-private partnership comprised of five contracts [N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262] funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. These contracts supported collection and management of data collected in this study. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation. The views expressed in this article are those of the author(s) and do not necessarily represent the views of the Department of Veterans Affairs, the National Institutes of Health, or the United States government.
Funding: NIH/NIAMS; Department of Veterans Affairs
Footnotes
Authors’ Contributions
Grace H. Lo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Lo conducted and is responsible for the data analyses.
Study concept and design: Lo, McAlindon, Katz, Petersen, Suarez-Almazor
Acquisition of data: Lo
Analysis and interpretation of data: Lo, McAlindon, Katz, Driban, Price, Eaton, Petersen, Ballantyne, and Suarez-Almazor
Drafting of the manuscript: Lo.
Critical revision of the manuscript for important intellectual content: Lo, McAlindon, Katz, Driban, Price, Eaton, Petersen, Ballantyne, and Suarez-Almazor
Statistical analysis: Lo, Petersen, Suarez-Almazor
Disclosure of Potential Conflicts of Interests
None of the authors have a conflict of interest that could influence this work.
References
- 1.Cisternas MG, Murphy L, Sacks JJ, Solomon DH, Pasta DJ, Helmick CG. Alternative Methods for Defining Osteoarthritis and the Impact on Estimating Prevalence in a US Population-Based Survey. Arthritis Care Res (Hoboken) 2016;68(5):574–580. doi: 10.1002/acr.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: Evidence from national survey data. Arthritis Rheum. 2009;60(12):3546–3553. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- 3.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, Kelly-Hayes M, Wolf PA, Kreger BE, Kannel WB. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84(3):351–358. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mankin HJ. Clinical features of osteoarthritis. In: Kelly WN, Harris EDJ, Ruddy S, Sledge CB, editors. Textbook of Rheumatology. 4. Philadelphia, PA: W.B. Saunders Co; 1993. pp. 1374–1384. [Google Scholar]
- 5.Most Frequent Procedures Performed in U.S. Hospitals, 2011. Healthcare Cost and Utilization Project Statistical Brief #165. 2013 Oct; [ http://www.hcup-us.ahrq.gov/reports/statbriefs/sb165.pdf]
- 6.U.S. Census Bureau. [Accessed on December 23, 2014];U.S. and World Population Clock. [ http://www.census.gov/popclock/]
- 7.Robertsson O, Bizjajeva S, Fenstad AM, Furnes O, Lidgren L, Mehnert F, Odgaard A, Pedersen AB, Havelin LI. Knee arthroplasty in Denmark, Norway and Sweden. A pilot study from the Nordic Arthroplasty Register Association. Acta Orthop. 2010;81(1):82–89. doi: 10.3109/17453671003685442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. [Accessed on December 23, 2014];Statistics Norway. [ http://www.ssb.no/en/befolkning/statistikker/folkendrkv]
- 9. [Accessed on December 23, 2014];Statistics Sweden. [ http://www.scb.se/en_/]
- 10.Larmer PJ, Reay ND, Aubert ER, Kersten P. A systematic review of guidelines for the physical management of osteoarthritis. Arch Phys Med Rehabil. 2013 doi: 10.1016/j.apmr.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, Beavers DP, Hunter DJ, Lyles MF, Eckstein F, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. Jama. 2013;310(12):1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Towheed TE, Hochberg MC. A systematic review of randomized controlled trials of pharmacological therapy in osteoarthritis of the knee, with an emphasis on trial methodology. Semin Arthritis Rheum. 1997;26(5):755–770. doi: 10.1016/s0049-0172(97)80043-1. [DOI] [PubMed] [Google Scholar]
- 13.Nilsdotter AK, Toksvig-Larsen S, Roos EM. Knee arthroplasty: are patients’ expectations fulfilled? A prospective study of pain and function in 102 patients with 5-year follow-up. Acta Orthop. 2009;80(1):55–61. doi: 10.1080/17453670902805007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshimura N, Muraki S, Oka H, Tanaka S, Kawaguchi H, Nakamura K, Akune T. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthritis Cartilage. 2012;20(11):1217–1226. doi: 10.1016/j.joca.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Wallace SM, Yasmin, McEniery CM, Maki-Petaja KM, Booth AD, Cockcroft JR, Wilkinson IB. Isolated systolic hypertension is characterized by increased aortic stiffness and endothelial dysfunction. Hypertension. 2007;50(1):228–233. doi: 10.1161/HYPERTENSIONAHA.107.089391. [DOI] [PubMed] [Google Scholar]
- 16.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 17.Crentsil V, Ricks MO, Xue QL, Fried LP. A pharmacoepidemiologic study of community-dwelling, disabled older women: Factors associated with medication use. Am J Geriatr Pharmacother. 2010;8(3):215–224. doi: 10.1016/j.amjopharm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Wanke LA. Self-instructional guide to the Iowa Drug Information Service. Drug Intell Clin Pharm. 1977;11(11):688–689. doi: 10.1177/106002807701101114. [DOI] [PubMed] [Google Scholar]
- 19.Central reading of knee X-rays for Kellgren and Lawrence grade and individual radiographic features of tibiofemoral knee OA. [ http://oai.epi-ucsf.org/datarelease/SASDocs/kXR_SQ_BU_descrip.pdf]
- 20.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A):A1–56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 22.Project 15 Test-Retest Reliability of Semi-quantitative Readings from Knee Radiographs. [ https://oai.epi-ucsf.org/datarelease/ImageAssessments.asp]
- 23.Zhang Y, Glynn RJ, Felson DT. Musculoskeletal disease research: should we analyze the joint or the person? J Rheumatol. 1996;23(7):1130–1134. [PubMed] [Google Scholar]
- 24.Cochran WG. Some methods of strengthening the common χ2 tests. Biometrics. 1954;10:417–451. [Google Scholar]
- 25.Armitage P. Tests for linear trends in proportions and frequencies. Biometrics. 1955;11:375–386. [Google Scholar]
- 26.Marks R, Allegrante JP. Comorbid disease profiles of adults with end-stage hip osteoarthritis. Med Sci Monit. 2002;8(4):CR305–309. [PubMed] [Google Scholar]
- 27.Weinberger M, Tierney WM, Booher P. Common problems experienced by adults with osteoarthritis. Arthritis Care Res. 1989;2(3):94–100. doi: 10.1002/anr.1790020304. [DOI] [PubMed] [Google Scholar]
- 28.Pope JE, Anderson JJ, Felson DT. A meta-analysis of the effects of nonsteroidal anti-inflammatory drugs on blood pressure. Arch Intern Med. 1993;153(4):477–484. [PubMed] [Google Scholar]
- 29.Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Intern Med. 1994;121(4):289–300. doi: 10.7326/0003-4819-121-4-199408150-00011. [DOI] [PubMed] [Google Scholar]
- 30.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. Jama. 2003;290(2):199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 31.Wilking SV, Belanger A, Kannel WB, D’Agostino RB, Steel K. Determinants of isolated systolic hypertension. Jama. 1988;260(23):3451–3455. [PubMed] [Google Scholar]
- 32.Mitchell GF, Conlin PR, Dunlap ME, Lacourciere Y, Arnold JM, Ogilvie RI, Neutel J, Izzo JL, Jr, Pfeffer MA. Aortic diameter, wall stiffness, and wave reflection in systolic hypertension. Hypertension. 2008;51(1):105–111. doi: 10.1161/HYPERTENSIONAHA.107.099721. [DOI] [PubMed] [Google Scholar]
- 33.Antikainen R, Jousilahti P, Tuomilehto J. Systolic blood pressure, isolated systolic hypertension and risk of coronary heart disease, strokes, cardiovascular disease and all-cause mortality in the middle-aged population. J Hypertens. 1998;16(5):577–583. doi: 10.1097/00004872-199816050-00004. [DOI] [PubMed] [Google Scholar]
- 34.Xing D, Xu Y, Liu Q, Ke Y, Wang B, Li Z, Lin J. Osteoarthritis and all-cause mortality in worldwide populations: grading the evidence from a meta-analysis. Sci Rep. 2016;6:24393. doi: 10.1038/srep24393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu R, Kwok WY, Vliet Vlieland TP, Kroon HM, Meulenbelt I, Houwing-Duistermaat JJ, Rosendaal FR, Huizinga TW, Kloppenburg M. Mortality in osteoarthritis patients. Scand J Rheumatol. 2015;44(1):70–73. doi: 10.3109/03009742.2014.922213. [DOI] [PubMed] [Google Scholar]
- 36.Cerhan JR, Wallace RB, el-Khoury GY, Moore TE, Long CR. Decreased survival with increasing prevalence of full-body, radiographically defined osteoarthritis in women. Am J Epidemiol. 1995;141(3):225–234. doi: 10.1093/oxfordjournals.aje.a117424. [DOI] [PubMed] [Google Scholar]
- 37.Ravi B, Croxford R, Austin PC, Lipscombe L, Bierman AS, Harvey PJ, Hawker GA. The relation between total joint arthroplasty and risk for serious cardiovascular events in patients with moderate-severe osteoarthritis: propensity score matched landmark analysis. Br J Sports Med. 2014;48(21):1580. doi: 10.1136/bjsports-2014-f6187rep. [DOI] [PubMed] [Google Scholar]
- 38.Findlay DM. Vascular pathology and osteoarthritis. Rheumatology (Oxford) 2007;46(12):1763–1768. doi: 10.1093/rheumatology/kem191. [DOI] [PubMed] [Google Scholar]
- 39.Hussain SM, Wang Y, Shaw JE, Magliano DJ, Wong TY, Wluka AE, Graves S, Tapp RJ, Cicuttini FM. Retinal arteriolar narrowing and incidence of knee replacement for osteoarthritis: a prospective cohort study. Osteoarthritis Cartilage. 2015;23(4):589–593. doi: 10.1016/j.joca.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Gossec L, Paternotte S, Bingham CO, 3rd, Clegg DO, Coste P, Conaghan PG, Davis AM, Giacovelli G, Gunther KP, Hawker G, et al. OARSI/OMERACT initiative to define states of severity and indication for joint replacement in hip and knee osteoarthritis. An OMERACT 10 Special Interest Group. J Rheumatol. 2011;38(8):1765–1769. doi: 10.3899/jrheum.110403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clockaerts S, Van Osch GJ, Bastiaansen-Jenniskens YM, Verhaar JA, Van Glabbeek F, Van Meurs JB, Kerkhof HJ, Hofman A, Stricker BH, Bierma-Zeinstra SM. Statin use is associated with reduced incidence and progression of knee osteoarthritis in the Rotterdam study. Ann Rheum Dis. 2012;71(5):642–647. doi: 10.1136/annrheumdis-2011-200092. [DOI] [PubMed] [Google Scholar]
- 42.Kadam UT, Blagojevic M, Belcher J. Statin use and clinical osteoarthritis in the general population: a longitudinal study. J Gen Intern Med. 2013;28(7):943–949. doi: 10.1007/s11606-013-2382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gierman LM, Kuhnast S, Koudijs A, Pieterman EJ, Kloppenburg M, van Osch GJ, Stojanovic-Susulic V, Huizinga TW, Princen HM, Zuurmond AM. Osteoarthritis development is induced by increased dietary cholesterol and can be inhibited by atorvastatin in APOE*3Leiden.CETP mice--a translational model for atherosclerosis. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203248. [DOI] [PubMed] [Google Scholar]
- 44.Williams MF, London DA, Husni EM, Navaneethan S, Kashyap SR. Type 2 diabetes and osteoarthritis: a systematic review and meta-analysis. J Diabetes Complications. 2016;30(5):944–950. doi: 10.1016/j.jdiacomp.2016.02.016. [DOI] [PubMed] [Google Scholar]