Summary
Purpose
The aromatase inhibitors (AI) exemestane (EXE), letrozole (LET), and anastrozole suppress estrogen biosynthesis and are effective treatments for estrogen receptor (ER)-positive breast cancer. Prior work suggests that anastrozole blood concentrations are associated with the magnitude of estrogen suppression. The objective of this study was to determine whether the magnitude of estrogen suppression, as determined by plasma estradiol (E2) concentrations, in EXE or LET treated patients is associated with plasma AI concentrations.
Methods
Five hundred post-menopausal women with ER-positive breast cancer were enrolled in the prospective Exemestane and Letrozole Pharmacogenetic (ELPh) Study conducted by the COnsortium on BReast cancer phArmacogomics (COBRA) and randomly assigned to either drug. Estrogen concentrations were measured at baseline and after 3 months of AI treatment and drug concentrations were measured after 1 or 3 months. EXE or LET concentrations were compared with 3-month E2 concentration or the change from baseline to 3 months using several complementary statistical procedures.
Results
Four-hundred patients with on-treatment E2 and AI concentrations were evaluable (EXE n=200, LET n=200). Thirty (7.6%) patients (EXE n=13, LET n=17) had 3-month E2 concentrations above the lower limit of quantification (LLOQ) (median: 4.75; range: 1.42–63.8 pg/mL). EXE and LET concentrations were not associated with on-treatment E2 concentrations or changes in E2 concentrations from baseline (all p>0.05).
Conclusions
Steady-state plasma AI concentrations do not explain variability in E2 suppression in post-menopausal women receiving EXE or LET therapy, in contrast with prior evidence in anastrozole treated patients.
Keywords: aromatase inhibitor, exemestane, letrozole, pharmacokinetics, estradiol, breast cancer
Introduction
The third generation aromatase inhibitors (AIs) exemestane (EXE), letrozole (LET), and anastrozole are very effective for the treatment of estrogen receptor-positive (ER+) breast cancer. In the adjuvant treatment of post-menopausal patients, 5 years of AIs improve absolute 10-year survival by approximately 2.1% compared to 5 years of tamoxifen[1]. When combined with estrogen suppression in pre-menopausal patients AIs have an estimated 10%–15% benefit in breast cancer free interval compared to tamoxifen[2]. However, AIs are associated with common side effects such as arthralgias, myalgias, and hot flashes, and more serious side effects including increased risk of fragility fractures[3].
AIs are used exclusively in the treatment of hormone receptor-positive breast cancers, many of which rely on endogenous estrogens including estradiol (E2) and estrone (E1) to stimulate estrogen receptor (ER) signaling and proliferation. AIs inhibit aromatase-mediated conversion of androgens to estrogens, suppressing systemic estrogen concentrations to below detectable levels in most patients[4,5]. Robust estrogen suppression is presumed to be necessary for treatment efficacy and hypothesized to be the causal mechanism for AI-related toxicities[6,7].
In the prospective Exemestane and Letrozole Pharmacogenetics (ELPh) study of post-menopausal patients randomized to either EXE or LET conducted by the COnsortium on BReast cancer phArmacogomics (COBRA), there was substantial variability in the magnitude of estrogen suppression[8]. A subset of patients had measurable E2, E1, and estrone-sulfate (E1-S) plasma concentrations after 3 months of AI treatment, with some patients exhibiting increased estrogen concentrations from baseline.
Given the central role of estrogens and ER signaling in breast cancer pathogenesis, the observed lack of estrogen suppression in a subset of patients receiving AI therapy could potentially contribute to treatment failure. Therefore, it is critical to identify the mechanism by which some patients have persistently elevated estrogen concentrations during AI treatment. Prior work has suggested that patients whose estrogen concentrations remain stable or increase while receiving anastrozole treatment was associated with significantly lower systemic anastrozole concentrations[9]. Therefore, the objective of the current study was to analyze data from the ELPh trial in order to extend this hypothesis to include EXE and LET. Specifically, we hypothesized that incomplete E2 suppression in post-menopausal patients with ER+ breast cancer receiving EXE or LET treatment is due to insufficient systemic concentrations of these AIs.
Methods
Patient cohort
This secondary correlative analysis of on-treatment concentrations of AIs and E2 was carried out in the previously published ELPh cohort of post-menopausal women diagnosed with stage 0-III hormone receptor positive breast cancer[10,11]. Post-menopausal status was defined as one of the following: age > 60 years, prior bilateral oophorectomy, amenorrhea for 1 year with intact uterus and ovaries, or serum estradiol and FSH concentrations consistent with post-menopausal status and either amenorrhea for 6 months or prior hysterectomy at the time of enrollment. Patients considering AI treatment upfront or following tamoxifen at three cancer centers (Indiana University Cancer Center, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, and the University of Michigan Comprehensive Cancer Center) from August 2005-July 2009 were enrolled in the ELPh study (ClinicalTrials.gov identifier: NCT00228956), an open-label randomized clinical trial conducted by the Consortium on Breast Cancer Pharmacogenomics (COBRA). The Institutional Review Boards of each participating site approved the protocol and all patients provided written informed consent prior to enrollment.
Patients were randomized to receive oral EXE 25 mg/day or LET 2.5 mg/day for 2 years, with stratification based on previous treatment with chemotherapy, tamoxifen, and bisphosphonates. Surgery and/or radiation and systemic chemotherapy were completed prior to enrollment. Data and samples collected from ELPh have been used for several correlative analyses of AI treatment outcomes[11–14].
Estrogen Concentration Sample Collection and Measurement
Whole blood samples were collected prior to AI treatment initiation (baseline) and after 3 months of AI treatment. Collection and measurement of estradiol (E2), was previously described[8]. E2 was measured in plasma isolated immediately after sample collection by inVentiv Health (Princeton, NJ) using an established ultrasensitive gas chromatography, tandem mass spectroscopy (MS/MS) assay[15]. Calibration curves were obtained by performing weighted linear regression and lower (LLOQ) and upper (ULOQ) limits of quantification were estimated for each analytical run. Although the previous analysis reported multiple LLOQs, they were not meaningfully different. For this analysis the higher LLOQ was used for all results for simplicity: E2 (LLOQ: 1.25 pg/mL, ULOQ 80 pg/mL).
Drug Concentration Sample Collection and Measurement
Concentrations of EXE and LET were measured in plasma isolated from blood samples collected after 1 or 3 months of treatment. Patients were instructed to take their daily AI dose two hours before the blood draw for estimation of an approximate steady-state maximum concentration (Cmax). EXE was measured using a validated LC/MS/MS method as previously described [16] with LLOQ = 2.5 ng/mL. LET was measured by high performance liquid chromatography (LC) with fluorescent detection as previously described[17], with LLOQ = 7.0 ng/ml.
Statistical Methods
For all quantitative analyses, concentrations below the LLOQ or above the ULOQ were set at the LLOQ or ULOQ, respectively. The association between steady-state drug concentrations and 3-month E2 concentrations, or E2 change from baseline to month 3, was analyzed in several complementary ways, with model assumption checks used to select appropriate analyses. The first series of analyses were conducted comparing steady-state drug concentrations with 3-month E2 concentrations. All patients with steady-state drug concentrations and 3-month E2 concentrations were included in these analyses. First, the association of drug concentration with a binary outcome corresponding to whether the 3-month E2 was below the LLOQ (Yes vs. No) was conducted via logistic regression. Second, the association of 3-month E2 concentration with a binary predictor corresponding to whether the patient had measurable drug concentration was conducted via Wilcoxon Tests. Finally, the association of binary indicators of whether the steady-state drug and 3-month E2 concentrations were above the respective LLOQs were compared via Fisher’s exact tests.
Drug concentrations were then compared with changes in E2 concentrations from baseline to three months. Only patients with concentrations at both time points, and measurable concentrations at baseline, were included in these analyses. The association of drug concentrations with an ordinal outcome of the change in E2 concentration from baseline to month 3 defined as; E2 increased, E2 decreased but not to below LLOQ, and E2 decreased to below LLOQ, was conducted via cumulative logistic regression. A similar analysis was then performed after collapsing the two groups of patients whose E2 decreased, directly comparing them with the patients whose E2 increased via Mann-Whitney U Test. Finally, steady-state drug concentration was compared with the percent change in E2 concentration from baseline to 3 months via Spearman correlation. Analyses were conducted using two-sided tests with an uncorrected significance threshold of p<0.05 in SAS version 9.4, and R version 3.3.1.
Results
Steady State AI Concentrations
Of the 500 patients enrolled in the ELPh clinical trial, 476 had plasma AI concentrations measured at 1 or 3 months. AI concentrations were below LLOQ in 25 (11%) patients receiving EXE (LLOQ=2.5 ng/mL) and 2 patients (1%) receiving LET (LLOQ=7.0 ng/mL). In the patients whose AI concentrations were above the LLOQ, the median concentration of EXE was 8.05 ng/mL (range: 2.7–72.0 ng/mL) and LET was 92.0 ng/mL (range: 28.4–349.2 ng/mL).
3-Month E2 Concentrations
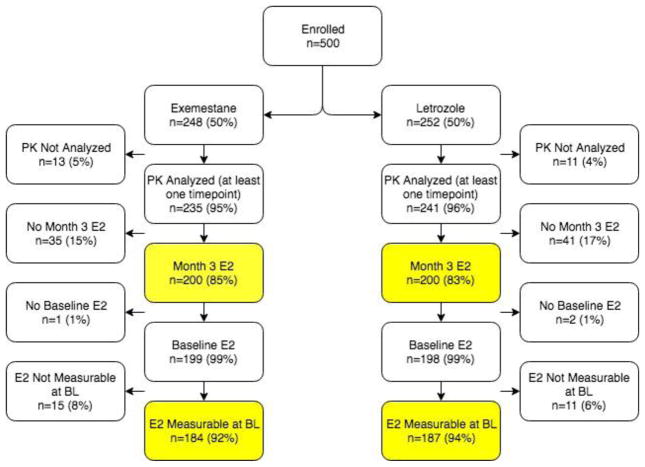
Of the 476 patients with steady-state AI concentrations, 400 also had 3-month E2 concentration data, as described in the CONSORT diagram in Figure 1. The demographic data for these patients are reported in Table 1. Participants were 89% Caucasian and had a mean age of 59.8 years (range 35–89), which did not differ by arm. At the 3-month E2 measurement, 370 patients (EXE=187, let=183) had E2 concentrations below the LLOQ (1.25 pg/mL). In the remaining 30 (EXE=13, let=17) patients, the median E2 was 4.75 pg/mL (range: 1.42–63.8 pg/mL). The 3-month E2 concentrations and changes from baseline to month 3 have been previously reported in detail, including a comparison between the two AIs[8].
Fig. 1. CONSORT Diagram describing flow of patients from the clinical trial into the analysis.
Of the 500 enrolled patients, 400 had steady-state drug concentrations (PK) and 3-month estradiol (E2). Of these, 371 patients had measurable baseline E2. Abbreviations: PK: Pharmacokinetics (drug concentration measurement), E2: Estradiol, BL: Baseline
Table 1.
Demographics of Patients Included in the Analyses
| Characteristic | 3-month E2 Analysis (n=400) | E2 Change from Baseline to Month 3 Analysis (n=371) | |
|---|---|---|---|
| Treatment Arm | Exemestane | 200 (50%) | 184 (50%) |
| Letrozole | 200 (50%) | 187 (50%) | |
| Self-Reported Race | White | 354 (88%) | 327 (88%) |
| Black | 36 (9%) | 34 (9%) | |
| Other/Unknown | 10 (3%) | 10 (3%) | |
| Age (years) | Mean (sd); [range] | 59.8 (8.5) [35–89] | 59.7 (8.3) [35–89] |
| Body Mass Index (kg/m2) | Mean (sd); [range] | 30.1 (6.5) [18.4–55.9] | 30.4 (6.5) [18.4–55.9] |
| Prior Chemotherapy | Yes | 179 (45%) | 161 (43%) |
| No | 221 (55%) | 210 (57%) | |
| Prior Tamoxifen | Yes | 140 (35%) | 132 (36%) |
| No | 258 (65%) | 238 (64%) | |
| AI Collection Time Point (months) | 1 | 24 (6%) | 23 (6%) |
| 3 | 376 (94%) | 348 (94%) | |
Association of Drug and 3-Month E2 Concentrations
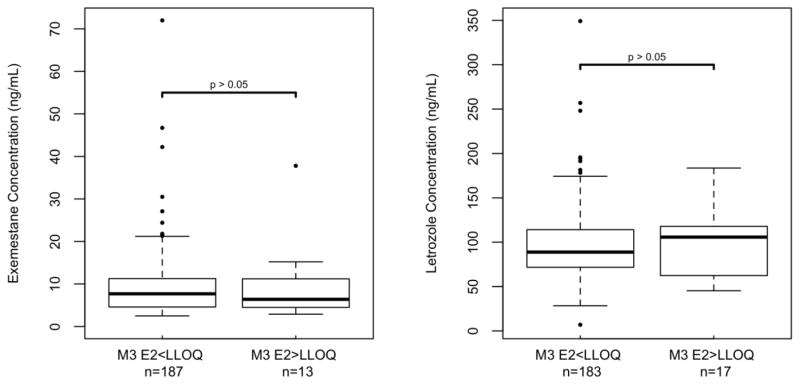
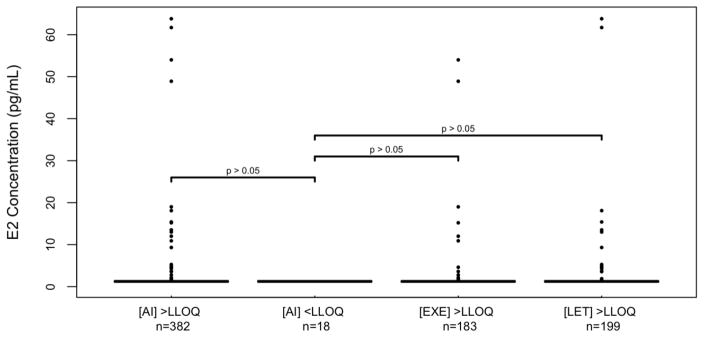
In the 400 patients with steady state drug concentrations and 3-month E2 concentrations, median steady-state concentrations of EXE and LET were not significantly different in patients who did and did not achieve E2 suppression <LLOQ, respectively (EXE: 7.7 vs. 6.4 ng/ml, p=0.79, LET: 88.8 vs. 105.7 ng/mL, p=0.63, Table 2, Figure 2). Similarly, median 3-month E2 concentrations were not different when comparing the 18 patients with concentrations of either AI below LLOQ (median 3-month E2=1.25 pg/mL, range: 1.25–1.25 pg/mL) with the patients with measurable concentrations for EXE (n=183, median E2=1.25 pg/mL, range: 1.25–54.0 pg/mL, p=0.26), LET (n=199, median E2=1.25 pg/mL, range: 1.25–63.8 pg/mL, p=0.77) or either drug (n=382, median E2=1.25 pg/mL, range: 1.25–63.8 pg/mL, p=0.22, Table 3, Figure 3). Finally, patients with 3-month E2 concentrations above LLOQ were not more likely to have steady-state concentrations of EXE (p=0.61) or LET (p=1.0) below LLOQ (data not shown).
Table 2.
AI Concentrations in Patients Stratified by 3-Month Estradiol Suppression Below Quantification or E2 Change from Baseline to 3 Months
| 3-Month E2a | Change in E2 from Baseline to 3 months b | |||||||
|---|---|---|---|---|---|---|---|---|
| E2<LLOQ | E2>LLOQ | p-valuec | Increase | Decrease to >LLOQ | Decrease to <LLOQ (Reference category) | p-value d | ||
| Exemestane (ng/mL) | n | 187 | 13 | 0.79 | 6 | 7 | 171 | 0.86 |
| Median | 7.7 | 6.4 | 11.8 | 4.6 | 7.7 | |||
| Range | 2.5, 72.0 | 2.9, 37.8 | 3.8, 37.8 | 2.9, 10.6 | 2.5, 72.0 | |||
| Letrozole (ng/mL) | n | 183 | 17 | 0.63 | 8 | 9 | 170 | 0.71 |
| Median | 88.8 | 105.7 | 85.6 | 105.7 | 87.7 | |||
| Range | 7.0, 349.2 | 45.4, 183.6 | 45.4, 183.6 | 46.1, 149.4 | 7.0,256.9 | |||
Abbreviations: E2: Estradiol, LLOQ: Lower limit of quantification (E2=1.25 pg/mL)
Using the patients with AI concentrations and 3-month E2 (n=400)
Using the patients with AI concentrations, 3-month E2, and measurable baseline E2 (n=371)
Refers to the hypothesis: Means of PK concentration are similar between E2<LLOQ and E2>LLOQ at 3-months.
P-value evaluating the association between PK and all three ordinal groups via cumulative logistic regression (ordinal outcome, PK as predictor).
Fig. 2. Comparison of AI concentrations in patients who did and did not achieve estradiol below the lower limit of quantification.
Box and whisker plots present the median and interquartile range. Steady-state concentrations of exemestane (left) or letrozole (right) were not significantly different in patients who did (M3 E2<LLOQ) and did not (M3 E2>LLOQ) achieve 3-month estradiol concentrations below the lower limit of quantification (both p>0.05). Summary data and p-values are reported in Table 2. Abbreviations: M3 E2<LLOQ: 3-month estradiol below lower limit of quantification (i.e. <1.25 pg/mL)
Table 3.
Estradiol concentrations in Patients Stratified by AI Concentrations Below Quantification
| n | Median E2 (pg/mL) | E2 Range (pg/mL) | p-value (vs. AI<LLOQ) | |
|---|---|---|---|---|
| AI<LLOQ | 18 | 1.25 | 1.25, 1.25 | Reference |
| AI>LLOQ | 382 | 1.25 | 1.25, 63.8 | 0.22 |
| EXE>LLOQ | 183 | 1.25 | 1.25, 54.0 | 0.26 |
| LET>LLOQ | 199 | 1.25 | 1.25, 63.8 | 0.77 |
Abbreviations: AI: Aromatase inhibitor, E2: Estradiol, EXE: Exemestane, LET: Letrozole, LLOQ: Lower limit of quantification (EXE=2.5 ng/mL, LET=7.0 ng/mL)
Fig. 3. Comparison of 3-month estradiol concentrations in patients who did and did not have measurable AI concentrations.
All estradiol concentrations that were not at the LLOQ (1.25 pg/mL, horizontal line) are represented by individual dots. 3-Month estradiol concentrations were not significantly different in patients who had steady state drug concentrations below the LLOQ ([AI]<LLOQ) when compared with patients who had steady-state AI ([AI]>LLOQ, p=0.22), exemestane ([EXE]>LLOQ, p=0.26) or letrozole ([LET]>LLOQ, p=0.77) concentrations above the LLOQ. Abbreviations: [AI]: Aromatase inhibitor concentration, E2: Estradiol, EXE: Exemestane, LET: Letrozole, LLOQ: lower limit of quantification
Change in E2 Concentrations From Baseline to Month 3
Of the 400 patients with steady-state drug and 3-month E2 concentrations, 371 also had a measurable baseline E2 concentration. This subset was similar to the larger cohort of patients who had 3-month E2 concentrations (Table 1). In these 371 patients, 4% (14/371) had an increase in E2 from baseline to three months, 4% (16/371) had a decrease but not to below LLOQ, and 92% (341/371) had a decrease to below LLOQ. Representing these changes as a percentage change in E2, wherein E2 suppression below LLOQ =−100% and increases in E2 from baseline are positive values, the median change in E2 was −74% (range:−100%, 2320%).
Association of Drug and Change in E2 Concentrations
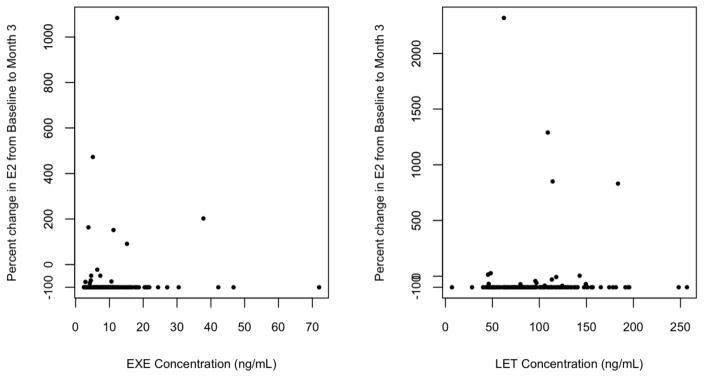
Considering only those patients with measurable E2 at baseline (n=371), median steady-state EXE or LET concentrations were not different in those patients whose E2 increased, decreased to >LLOQ, or decreased to <LLOQ (EXE: 11.8 vs. 4.6 vs. 7.7 ng/mL, p=0.86, let: 85.6 vs. 105.7, vs. 87.7 ng/mL, p=0.71) (Table 2). These findings do not change meaningfully if the two groups of patients whose E2 decreased are combined and compared directly with the patients whose E2 increased (data not shown). Finally, there was no statistical correlation between steady-state plasma AI concentrations and percent change in E2 from baseline to 3-months (EXE: p=0.63, LET: p=0.97, Figure 4).
Fig. 4. Comparison of percent change in estradiol concentration from baseline with AI concentration.
Percent change in estradiol concentration from baseline to month 3 of AI treatment were not associated with the steady-state concentration of exemestane (left, p=0.63) or letrozole (right, p=0.97). Abbreviations: E2: Estradiol, EXE: Exemestane, LET: Letrozole
Discussion
AIs inhibit aromatase-mediated conversion of androgens to estrogens, resulting in suppression of systemic estrogen concentrations that impedes ER-mediated proliferation of ER+ breast tumors. Most patients on AI treatment have estrogen concentrations below measurable levels[18,5], even when measured using high-sensitivity estrogen measurement assays[15]. In the prospectively enrolled ELPh cohort of post-menopausal patients treated with EXE or LET, there was substantial variability in the on-treatment concentrations of E2, E1, and E1S, with approximately 10–20% of patients continuing to have measurable estrogen concentrations during active treatment[8]. We hypothesized that persistence of measurable estrogen concentrations during AI treatment was due to insufficient AI systemic concentrations, perhaps due to rapid metabolism or lack of adherence to AI therapy.
Plasma estradiol concentrations after 3 months of AI treatment, and concentration changes from baseline to three months, were compared with steady-state concentrations of EXE and LET using a variety of complementary approaches. AI concentrations were similar in the patient subsets whose E2 remained above the LLOQ or increased during treatment, when compared with the patients whose estrogens decreased as they are expected to. Additionally, counter to our hypothesis, the patients whose AI concentrations were below our assay’s limits of quantification, did not exhibit higher E2 concentrations. In fact, all 18 of these patients (17 on EXE, 1 on letrozole) had E2 concentrations below LLOQ. Based on these results there is no evidence that persistence of measurable systemic estrogen concentrations during AI treatment is due to insufficient EXE or LET concentrations.
While our study is the first to examine EXE and LET, our results are somewhat inconsistent with those reported by Ingle et al. in secondary analyses of a cohort of post-menopausal patients treated with the other 3rd generation AI, anastrozole. In the original analysis of 191 patients, there was no association between anastrozole trough concentrations and on-treatment or treatment-induced changes in E1 or E2[19]. However, two patients with undetectable anastrozole concentrations had minimal estrogen suppression from treatment. Both patients had high concentrations of an inactive anastrozole metabolite, suggesting they were rapid anastrozole metabolizers, supporting our overall hypothesis. A statistical association between anastrozole and estrogen concentrations was detected in an expansion cohort of 649 patients. Systemic anastrozole concentrations were significantly lower in the subsets of patients whose E2 (8.9%, p=1.6×10−5), E1 (5.8%, p=6.7×10−5), or E1-conjugates (4.3%, p=1.3×10−5) did not decrease during treatment[9]. Intriguingly, these patients had significantly lower baseline concentrations of each estrogen, suggesting these findings may not reflect AI treatment response. A comparable analysis in our cohort did not detect a significant difference in EXE or LET concentrations in patients whose E2 (n=14) did not decrease from baseline. Additionally, parallel analyses of E1 and E1-S at 3-months or change from baseline in our cohort did not detect any meaningful associations with plasma EXE or LET concentrations (data not shown).
These discrepant findings could be due to several differences between the studies. The prior analysis was conducted in anastrozole treated patients whereas our patients were treated with the other two 3rd generation AIs, EXE and LET. Estrogen concentrations may be more sensitive to changes in anastrozole concentration, which would be consistent with the more robust estrogen suppression caused by LET compared with anastrozole[20] and the comparable suppression from EXE and LET[8]. Additionally, the prior analysis used trough drug concentrations (i.e. minimum concentrations collected prior to dosing) whereas our analysis utilized peak concentrations (i.e. maximum concentrations collected 2–4 hours after dosing). The relationship between systemic drug concentrations and treatment outcomes is often stronger with one of these measurement time points[21]; however, given their extended half-lives and daily dosing, the AIs accumulate from repeated dosing and steady-state peak and trough concentrations should not be substantially different.
There are a number of other potential alternate reasons for the discrepant findings. It is possible that they were attributable to differences in adherence. Measurement of systemic drug concentration at a single time point reflects recent treatment compliance but is minimally informative for adherence over a prolonged treatment period. In our study estrogen concentrations were measured after 3 months of AI treatment, whereas in the prior analysis estrogens were measured up to 6 months after treatment initiation. As adherence is known to decrease with continued treatment[22], it is possible that more patients from the prior study had discontinued treatment by the time of sample collection, and thus had measurable estrogens and low drug concentrations. It is also possible that measurable estrogen concentrations in patients taking AIs were due to exogenous estrogen administration, not lack of treatment response. Fifteen patients (3%) reported use of vaginal estrogens including Estring, Vagifem, or Estrace, which were allowed for vaginal symptoms but strongly discouraged based on the study protocol. Finally, there were two women younger than age 50 enrolled in the trial who were postmenopausal at the time of study entry according to the eligibility criteria and who had not undergone bilateral oophorectomy, but who developed elevated E2 concentrations. While it is possible that they could have recovered ovarian function without resumption of menses[23], it is unlikely that this would have substantially altered the overall findings and results of a secondary analysis restricted to patients >60 years old were not meaningfully different (data not shown).
We have previously identified genetic and clinical predictors of systemic concentrations of LET[17] and EXE[16] in the ELPh cohort. These variables could be useful for personalizing AI dosing, if drug concentrations are predictive of a meaningful clinical outcome. However, currently, there is little evidence to support doing so[24]. In this analysis, we attempted to demonstrate that systemic drug concentrations were predictive of the magnitude of estrogen suppression, which in turn may be a surrogate of treatment outcomes. Robust estrogen suppression is assumed to be critical for efficacious AI treatment based on the recognition that ER+ breast cancer cells are more likely to rely on estrogenic signaling and the established efficacy of AIs and agents that antagonize the estrogen receptor (i.e. tamoxifen)[1,25]. However, despite their more robust estrogen suppression, EXE and LET have not demonstrated superior efficacy when compared with anastrozole in clinical trials[26–28]. Similarly, there is limited direct evidence verifying the hypothesis[6,7] that AI toxicities are associated with estrogen suppression[24,29–31]. Additional research is needed to verify the putative associations between the AI drug concentrations or estrogen suppression with meaningful treatment outcomes, to warrant further discovery of the causal factors associated with these endophenotypes.
In this prospectively enrolled cohort of post-menopausal patients with ER+ breast cancer treated with EXE and LET, steady-state drug concentrations were not associated with the magnitude of estrogen suppression during treatment. Persistence of measurable estrogen concentrations in patients taking AIs, particularly in patients with drug concentrations indicative of treatment adherence, may be due to some other mechanism. It is possible that some patients had residual ovarian function at baseline or regained ovarian function during treatment. Weight is another confounding factor, as patient’s body mass index was associated with estrogen concentrations at baseline but not during AI treatment[8] Another potential mechanism is germline genetic variability in CYP19A1, which codes for aromatase[32–34]. Ongoing sequencing analyses of germline DNA collected from patients in the ELPh cohort will further investigate this possibility. Discovery of the causal mechanism for persistent measurable systemic estrogen concentrations in patients on AI treatment, and validation that estrogen suppression is predictive of meaningful clinical outcomes, would warrant development of individualized treatment approaches for patients with ER+ breast cancer to optimize therapeutic outcomes.
Acknowledgments
This research was supported by Pharmacogenetics Research Network Grant No. U-01 GM61373 (D.A.F.) and Clinical Pharmacology Training Grant No. 5T32-GM08425 (D.A.F.) from the National Institute of General Medical Sciences, National Institutes of Health (NIH), from Grants No. M01-RR000042 (University of Michigan), M01-RR00750 (Indiana University), and M01-RR00052 (Johns Hopkins University) from the National Center for Research Resources (NCRR), a component of the NIH, the Breast Cancer Research Foundation (BCRF) (N003173 to JMR and DFH), the National Cancer Institute (5T32CA083654-12, PI Jeremy Taylor), the National Institute of General Medical Sciences (GM099143 to J.M.R.) and the National Institutes of Health through the University of Michigan’s Cancer Center Support Grant (P30 CA046592) by the use of the following Cancer Center Core: University of Michigan DNA Sequencing Core. In addition, these studies were supported by grants from Pfizer (D.F.H.), Novartis Pharma AG (D.F.H.), the Fashion Footwear Association of New York/QVC Presents Shoes on Sale (D.F.H.). Drugs were supplied by Novartis and Pfizer.
We also wish to posthumously recognize David Flockhart, MD, PhD, who co-chaired the COnsortium on BReast cancer phArmacogomics (COBRA) and passed during preparation of this manuscript.
Footnotes
This work was submitted in preliminary form to 2017 American Society for Clinical Oncology Annual Meeting
Competing Interest:
DFH and VS have each received research funding from Pfizer and Novartis Pharma AG, including for this study. The authors declare no other competing interest.
References
- 1.Early Breast Cancer Trialists’ Collaborative G. Dowsett M, Forbes JF, Bradley R, Ingle J, Aihara T, Bliss J, Boccardo F, Coates A, Coombes RC, Cuzick J, Dubsky P, Gnant M, Kaufmann M, Kilburn L, Perrone F, Rea D, Thurlimann B, van de Velde C, Pan H, Peto R, Davies C, Gray R. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet (London, England) 2015;386(10001):1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 2.Regan MM, Francis PA, Pagani O, Fleming GF, Walley BA, Viale G, Colleoni M, Lang I, Gomez HL, Tondini C, Pinotti G, Price KN, Coates AS, Goldhirsch A, Gelber RD. Absolute Benefit of Adjuvant Endocrine Therapies for Premenopausal Women With Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Early Breast Cancer: TEXT and SOFT Trials. J Clin Oncol. 2016;34(19):2221–2231. doi: 10.1200/jco.2015.64.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lintermans A, Neven P. Safety of aromatase inhibitor therapy in breast cancer. Expert opinion on drug safety. 2015;14(8):1201–1211. doi: 10.1517/14740338.2015.1053458. [DOI] [PubMed] [Google Scholar]
- 4.Geisler J, King N, Dowsett M, Ottestad L, Lundgren S, Walton P, Kormeset PO, Lonning PE. Influence of anastrozole (Arimidex), a selective, non-steroidal aromatase inhibitor, on in vivo aromatisation and plasma oestrogen levels in postmenopausal women with breast cancer. Br J Cancer. 1996;74(8):1286–1291. doi: 10.1038/bjc.1996.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geisler J, Haynes B, Anker G, Dowsett M, Lonning PE. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20(3):751–757. doi: 10.1200/JCO.2002.20.3.751. [DOI] [PubMed] [Google Scholar]
- 6.Henry NL, Giles JT, Stearns V. Aromatase inhibitor-associated musculoskeletal symptoms: etiology and strategies for management. Oncology (Williston Park, NY) 2008;22(12):1401–1408. [PubMed] [Google Scholar]
- 7.Lintermans A, Neven P. Pharmacology of arthralgia with estrogen deprivation. Steroids. 2011;76(8):781–785. doi: 10.1016/j.steroids.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 8.Robarge JD, Desta Z, Nguyen AT, Li L, Hertz D, Rae JM, Hayes DF, Storniolo AM, Stearns V, Flockhart DA, Skaar TC, Henry NL. Effects of exemestane and letrozole therapy on plasma concentrations of estrogens in a randomized trial of postmenopausal women with breast cancer. Breast Cancer Res Treat. 2017;161(3):453–461. doi: 10.1007/s10549-016-4077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingle JN, Kalari KR, Buzdar AU, Robson ME, Goetz MP, Desta Z, Barman P, Dudenkov TT, Northfelt DW, Perez EA, Flockhart DA, Williard CV, Wang L, Weinshilboum RM. Estrogens and their precursors in postmenopausal women with early breast cancer receiving anastrozole. Steroids. 2015;99(Pt A):32–38. doi: 10.1016/j.steroids.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry NL, Giles J, Ang D, Mohan M, Dadabhoy D, Robarge J, Hayden J, Lemler S, Shahverdi K, Powers P, Li L, Flockhart D, Stearns V, Hayes D, Storniolo AM, Clauw D. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast cancer research and treatment. 2008;111(2):365–372. doi: 10.1007/s10549-007-9774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, Hayden J, Tarpinian K, Yakim E, Flockhart DA, Stearns V, Hayes DF, Storniolo AM. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(9):936–942. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry NL, Chan HP, Dantzer J, Goswami CP, Li L, Skaar TC, Rae JM, Desta Z, Khouri N, Pinsky R, Oesterreich S, Zhou C, Hadjiiski L, Philips S, Robarge J, Nguyen AT, Storniolo AM, Flockhart DA, Hayes DF, Helvie MA, Stearns V. Aromatase inhibitor-induced modulation of breast density: clinical and genetic effects. British journal of cancer. 2013;109(9):2331–2339. doi: 10.1038/bjc.2013.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry NL, Skaar TC, Dantzer J, Li L, Kidwell K, Gersch C, Nguyen AT, Rae JM, Desta Z, Oesterreich S, Philips S, Carpenter JS, Storniolo AM, Stearns V, Hayes DF, Flockhart DA. Genetic associations with toxicity-related discontinuation of aromatase inhibitor therapy for breast cancer. Breast cancer research and treatment. 2013;138(3):807–816. doi: 10.1007/s10549-013-2504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oesterreich S, Henry NL, Kidwell KM, Van Poznak CH, Skaar TC, Dantzer J, Li L, Hangartner TN, Peacock M, Nguyen AT, Rae JM, Desta Z, Philips S, Storniolo AM, Stearns V, Hayes DF, Flockhart DA. Associations between genetic variants and the effect of letrozole and exemestane on bone mass and bone turnover. Breast cancer research and treatment. 2015;154(2):263–273. doi: 10.1007/s10549-015-3608-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchett D, Goss PE, Wang S. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 2007;72(8):666–671. doi: 10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Hertz DL, Kidwell KM, Seewald NJ, Gersch CL, Desta Z, Flockhart DA, Storniolo AM, Stearns V, Skaar TC, Hayes DF, Henry NL, Rae JM. Polymorphisms in drug-metabolizing enzymes and steady-state exemestane concentration in postmenopausal patients with breast cancer. Pharmacogenomics J. 2016 doi: 10.1038/tpj.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desta Z, Kreutz Y, Nguyen AT, Li L, Skaar T, Kamdem LK, Henry NL, Hayes DF, Storniolo AM, Stearns V, Hoffmann E, Tyndale RF, Flockhart DA. Plasma letrozole concentrations in postmenopausal women with breast cancer are associated with CYP2A6 genetic variants, body mass index, and age. Clinical pharmacology and therapeutics. 2011;90(5):693–700. doi: 10.1038/clpt.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geisler J, King N, Anker G, Ornati G, Di Salle E, Lonning PE, Dowsett M. In vivo inhibition of aromatization by exemestane, a novel irreversible aromatase inhibitor, in postmenopausal breast cancer patients. Clin Cancer Res. 1998;4(9):2089–2093. [PubMed] [Google Scholar]
- 19.Ingle JN, Buzdar AU, Schaid DJ, Goetz MP, Batzler A, Robson ME, Northfelt DW, Olson JE, Perez EA, Desta Z, Weintraub RA, Williard CV, Flockhart DA, Weinshilboum RM. Variation in anastrozole metabolism and pharmacodynamics in women with early breast cancer. Cancer research. 2010;70(8):3278–3286. doi: 10.1158/0008-5472.CAN-09-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geisler J, Helle H, Ekse D, Duong NK, Evans DB, Nordbo Y, Aas T, Lonning PE. Letrozole is superior to anastrozole in suppressing breast cancer tissue and plasma estrogen levels. Clin Cancer Res. 2008;14(19):6330–6335. doi: 10.1158/1078-0432.ccr-07-5221. [DOI] [PubMed] [Google Scholar]
- 21.McCormack JP, Jewesson PJ. A critical reevaluation of the “therapeutic range” of aminoglycosides. Clin Infect Dis. 1992;14(1):320–339. doi: 10.1093/clinids/14.1.320. [DOI] [PubMed] [Google Scholar]
- 22.Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134(2):459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith IE, Dowsett M, Yap YS, Walsh G, Lonning PE, Santen RJ, Hayes D. Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhoea: caution and suggested guidelines. J Clin Oncol. 2006;24(16):2444–2447. doi: 10.1200/jco.2005.05.3694. [DOI] [PubMed] [Google Scholar]
- 24.Kadakia KC, Snyder CF, Kidwell KM, Seewald NJ, Flockhart DA, Skaar TC, Desta Z, Rae JM, Otte JL, Carpenter JS, Storniolo AM, Hayes DF, Stearns V, Henry NL. Patient-Reported Outcomes and Early Discontinuation in Aromatase Inhibitor-Treated Postmenopausal Women With Early Stage Breast Cancer. Oncologist. 2016;21(5):539–546. doi: 10.1634/theoncologist.2015-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–784. doi: 10.1016/s0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goss PE, Ingle JN, Pritchard KI, Ellis MJ, Sledge GW, Budd GT, Rabaglio M, Ansari RH, Johnson DB, Tozer R, D’Souza DP, Chalchal H, Spadafora S, Stearns V, Perez EA, Liedke PE, Lang I, Elliott C, Gelmon KA, Chapman JA, Shepherd LE. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27--a randomized controlled phase III trial. J Clin Oncol. 2013;31(11):1398–1404. doi: 10.1200/jco.2012.44.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Shaughnessy J, Yardley D, Burris H, De Boer R, Amadori D, McIntyre K, Ejlertsen B, Gnant M, Jonat W, Pritchard K, Dowsett M, Hart L, Poggio S, Valagussa P, Salomon H, Wamil B, Smith I. Abstract PD2-01: Randomized phase 3 trial of adjuvant letrozole versus anastrozole in postmenopausal patients with hormone receptor positive, node positive early breast cancer: Final efficacy and safety results of the femara versus anastrozole clinical evaluation (Face) trial. Cancer Research. 2016;76(4 Supplement):PD2-01-PD02-01. doi: 10.1158/1538-7445.sabcs15-pd2-01. [DOI] [PubMed] [Google Scholar]
- 28.Smith I, Yardley D, Burris H, De Boer R, Amadori D, McIntyre K, Ejlertsen B, Gnant M, Jonat W, Pritchard KI, Dowsett M, Hart L, Poggio S, Comarella L, Salomon H, Wamil B, O’Shaughnessy J. Comparative Efficacy and Safety of Adjuvant Letrozole Versus Anastrozole in Postmenopausal Patients With Hormone Receptor-Positive, Node-Positive Early Breast Cancer: Final Results of the Randomized Phase III Femara Versus Anastrozole Clinical Evaluation (FACE) Trial. J Clin Oncol. 2017 doi: 10.1200/jco.2016.69.2871. JCO2016692871. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Lu K, Song Y, Zhao S, Ma W, Xuan Q, Tang D, Zhao H, Liu L, Zhang Q. RANKL and OPG Polymorphisms Are Associated with Aromatase Inhibitor-Related Musculoskeletal Adverse Events in Chinese Han Breast Cancer Patients. PLoS One. 2015;10(7):e0133964. doi: 10.1371/journal.pone.0133964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lintermans A, Vanderschueren D, Verhaeghe J, Van Asten K, Jans I, Van Herck E, Laenen A, Paridaens R, Billen J, Pauwels S, Vermeersch P, Wildiers H, Christiaens MR, Neven P. Arthralgia induced by endocrine treatment for breast cancer: A prospective study of serum levels of insulin like growth factor-I, its binding protein and oestrogens. Eur J Cancer. 2014;50(17):2925–2931. doi: 10.1016/j.ejca.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Henry NL, Conlon A, Kidwell KM, Griffith K, Smerage JB, Schott AF, Hayes DF, Williams DA, Clauw DJ, Harte SE. Effect of estrogen depletion on pain sensitivity in aromatase inhibitor-treated women with early-stage breast cancer. J Pain. 2014;15(5):468–475. doi: 10.1016/j.jpain.2014.01.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang JZ, Deogan MS, Lewis JR, Chew S, Mullin BH, McNab TJ, Wilson SG, Ingley E, Prince RL. A non-synonymous coding change in the CYP19A1 gene Arg264Cys (rs700519) does not affect circulating estradiol, bone structure or fracture. BMC medical genetics. 2011;12 doi: 10.1186/1471-2350-12-165. 165-2350-2312-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma CX, Adjei AA, Salavaggione OE, Coronel J, Pelleymounter L, Wang L, Eckloff BW, Schaid D, Wieben ED, Adjei AA, Weinshilboum RM. Human aromatase: gene resequencing and functional genomics. Cancer research. 2005;65(23):11071–11082. doi: 10.1158/0008-5472.CAN-05-1218. 65/23/11071 [pii] [DOI] [PubMed] [Google Scholar]
- 34.Hertz DL, Henry NL, Rae JM. Germline genetic predictors of aromatase inhibitor concentrations, estrogen suppression and drug efficacy and toxicity in breast cancer patients. Pharmacogenomics. 2017;18(5):481–499. doi: 10.2217/pgs-2016-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]