Abstract
Introduction
In vivo imaging biomarkers of various HIV neuropathologies, including dopaminergic dysfunction, are still lacking. Towards developing dopaminergic biomarkers of brain involvement in HIV, we assessed the pre and postsynaptic components of the dopaminergic system in the HIV-1 transgenic rat (Tg), a well-characterized model of treated HIV+ patients, using small-animal PET imaging.
Methods
15–18 month-old Tg and wild type (WT) rats were imaged with both [18F]-FP-CMT, a dopamine transporter (DAT) ligand (n=16), and [18F]-Fallypride, a D2/D3 dopamine receptor (D2/D3DR) ligand (n=16). 5–8 month-old Tg and WT rats (n=18) were also imaged with [18F]-FP-CMT. A subset of animals was imaged longitudinally at 7 and 17 months of age. Multiplex immunohistochemistry staining for DAT, tyrosine hydroxylase, D2DR, D3DR, GFAP, Iba1 and NeuN was performed on a subgroup of the scanned animals.
Results
[18F]-FP-CMT and [18F]-Fallypride binding potential (BPND) values were significantly lower in 15–18 month-old Tg compared to age-matched WT rats (p< 0.0001 and 0.001, respectively). [18F]-FP-CMT BPND values in 5–8 month-old rats, however, were not significantly different. Longitudinal age-related decrease in [18F]-FP-CMT BPND was exacerbated in the Tg rat. Immunohistochemistry showed decreased staining of dopaminergic markers in Tg rats. Rats with higher serum gp120 had lower mean BPND values for both ligands.
Conclusions
We found presynaptic and postsynaptic dopaminergic dysfunction/loss in older Tg compared to WT rats. We believe this to be related to neurotoxicity of viral proteins present in the Tg rats’ serum and brain.
Advances in Knowledge
Our findings confirm prior reports of neurobehavioral abnormalities suggestive of dopaminergic dysfunction in this model. They also suggest similarities between the Tg rat and HIV+ patients as far as dopaminergic dysfunction.
Implications for patient Care
The Tg rat, along with the above-described quantitative PET imaging biomarkers, can have a role in the evaluation of HIV neuroprotective therapies prior to human translation.
Keywords: Small animal PET, HIV, Dopaminergic system, imaging biomarkers
INTRODUCTION
Despite decreased prevalence of severe neurocognitive dysfunction in HIV positive (HIV+) patients after the widespread use of antiretroviral therapies [1], milder forms of HIV-associated neurocognitive disorder (HAND) remain problematic, with virologically-suppressed patients still showing persistent and often progressive functional deterioration over time [2, 3]. An early established feature of HIV infection is documented vulnerability of the dopaminergic system to the effect of the virus, which seems to selectively target the basal ganglia [4] resulting in parkinsonian-like symptomatology [5–8]. Dopaminergic dysfunction has also been described [9–13] in the HIV-1 transgenic rat (Tg), a non-infectious Tg rat model which expresses 7 of the 9 HIV-1 viral proteins including gp120, nef, and Tat and is known to develop clinically relevant neuropathologies [14] and cognitive deficits [9, 10, 15–17]. Using [18F]-Fallypride, a PET radiotracer targeting D2/D3 receptors (D2DR and D3DR), we previously described deficits in D2/D3 striatal receptors in the Tg rat, considered to be a model of treated HIV+ patients [17, 18].
While decreased [18F]-Fallypride binding in the striatum of the Tg rats compared to age-matched WT rats suggested postsynaptic dopaminergic dysfunction, we suspected that some of the decreased binding could be due to extensive astrocytic loss in this animal model [12] since astrocytes are known to express D2/D3 receptors [19–23]. Therefore, we wanted to confirm dopaminergic dysfunction by concurrently probing the postsynaptic component using [18F]-Fallypride and the presynaptic component using [18F]-FP-CMT, a dopamine transporter (DAT) ligand with good pharmacological selectivity, lack of brain penetrant metabolites and rapid kinetic profile [24]. We corroborated our in vivo imaging findings with immunohistochemical (IHC) analysis of neuronal and glial cell populations as well as DAT, D2DR, D3DR and tyrosine hydroxylase (TH) expression in a subset of the scanned animals. Our ultimate goal was to validate in vivo imaging biomarkers of dopaminergic dysfunction in this animal model that could potentially be used in the evaluation of neuroprotective therapies, prior to human translation.
MATERIALS AND METHODS
Radioligand synthesis
The synthesis of [18F]-Fallypride was performed as previously described [25]. Radiochemical purity was >99%. Specific activity at production (at the end of synthesis) varied between 44.4 and 92.5 GBq/μmol. Specific activity values at time of injection varied between 12.3 and 81.9 GBq/μmol.
[18F]-FP-CMT was synthesized in-house, as previously described [24]. Radiochemical purity was > 99%. Radiochemical yield was 8–12% (uncorrected, n > 10). Specific activity at production was 55–148 GBq/μmol. Specific activity values at time of injection varied between 22.5 and 110.2 GBq/μmol.
Animals
Male Tg and Fischer F344 wild type (WT) rats were purchased from Envigo (Indianapolis, IN) and housed in a temperature controlled environment with a 12-hour light/dark cycle, with free access to food and water. All PET scans were performed during the light cycle. All experimental procedures, including handling and care, were approved by the Animal Care and Use Committee of the Clinical Center of the National Institutes of Health.
The young animal group scanned with [18F]-FP-CMT PET consisted of 10 Tg rats (5–8 month-old, mean age = 6.4 months, weight = 330 ± 45 g) and 8 WT rats (5–8 month-old, mean age = 6.6 months, weight = 380 ± 33 g). The older animal group that underwent [18F]-FP-CMT scanning consisted of 9 Tg rats (15–18 month-old, mean age = 17.1 months, weight = 357 ± 55 g) and 7 WT rats (15–18 month-old, mean age = 17 months, weight = 447 ± 28 g). The same older animal group underwent [18F]-Fallypride imaging with 9 Tg rats (15–18 month-old, mean age = 16.9 months, weight = 357 ± 55 g) and 7 WT rats (15–18 month-old, mean age = 16.3 months, weight = 447 ± 28 g). Out of the young animals, 3 Tg and 2 WT rats underwent repeat [18F]-FP-CMT scanning imaging after approximately 10 months (mean ages: 7.4 and 17.6 months respectively).
Test-retest reproducibility between day 1 and day 2 of [18F]-FP-CMT scanning were analyzed for two 5 month-old WT rats.
Among the older group of animals, 6 Tg and 7 WT rats were euthanized and their brains used for IHC staining. Additionally, brain sections from a separate cohort of 4 Tg and 2 WT rats (1–3 month-old) along with 6 Tg and 4 WT rats (7–9 month-old) were also used for IHC staining.
Dynamic PET scanning
Prior to imaging, each rat was anesthetized with 2–2.5% isoflurane-oxygen mixture. The respiratory frequency of the animal under anesthesia was monitored periodically to account for intra-subject variability of the anesthesia level (target respiratory frequency range=40–60 breaths/min). PET experiments were conducted on a small animal Inveon PET/CT scanner (Siemens Medical Solutions, USA with 10 × 12.7 cm transaxial and axial field of view (FOV) and 1.4 mm FWHM spatial resolution at center FOV. Three rats were scanned per session with alternative order of Tg and WT rats. Each animal was secured to the pallet with the head placed symmetrically in the center FOV. Prior to positioning, the lateral tail vein was cannulated and the catheter was connected to a heparin lock. Both the cannula and catheter attachment were then securely fastened to the tail of the animal using medical tape. For [18F]-Fallypride studies, an average dose of 27.75 ± 7.4 MBq (1.39 nmol/Kg) was administered slowly via bolus injection (over 30 seconds), followed by 300 μL of saline flush. The average injected mass for the Tg rats was 0.43±0.18 μg compared to 0.28 ± 0.15 μg for WT rats (p=0.09). PET emission data was started with the beginning of the injection and continued for 90 min in list mode. For [18F]-FP-CMT scans, 31.45 ± 1.48 MBq (1.57 nmol/Kg) was administered, following the same procedure and PET emission was collected for 60 minutes in list mode. The average injected mass for the 5–8 month-old Tg rats was 0.16 ± 0.05 μg compared to 0.18 ± 0.11 μg for WT rats (p=0.68). The average injected mass for the 15–18 month-old Tg rats was 0.30 ± 0.12 μg compared to 0.23 ± 0.06 μg for WT rats (p=0.09).
Following data acquisition, the resulting emission sinogram from every frame was corrected for scatter, 18F decay, randoms and dead time. The resultant histogram sets were reconstructed for [18F]-Fallypride data from the scans into a dynamic sequence of 39 individual frames (10×10 seconds, 7×20 seconds, 6×10 seconds, 16×300 seconds) whereas for [18F]-FP-CMT, scans were reconstructed into a dynamic sequence of 33 individual frames (10×10 seconds, 7×20 seconds, 6×60 seconds, 10×300 seconds) using Fourier rebinning and two-dimensional ordered subject expectation maximization algorithm (OSEM-3D; 4 OSEM iterations, 18 MAP iterations, matrix: 128 × 128, and a target resolution of 0.8 mm). At the conclusion of the PET scan, the catheter was removed and the animal was allowed to recover from anesthesia under a heat lamp.
Image analysis
PET analysis was performed using PMOD 3.5. The co-registration was performed in the same way for both the [18F]-Fallypride and the [18F]--FP-CMT images. The first step was to spatially normalize the reconstructed images to a standard stereotaxic space using the Rat Schiffer MR Atlas provided in PMOD templates. Once co-registration was complete, VOIs were drawn on the relevant regions (e.g. dorsal and ventral striatum) using the co-registered MRI image for reference. The regions evaluated included the striatum (average of right and left striatal VOIs), septum, midbrain, colliculi and cerebellum. The cerebellar VOI was used as a reference region. Statistics on the drawn VOIs generated the radioactivity time activity curve (TAC) for each scan.
Images were analyzed using the kinetic modelling tool of PMOD 3.5 (PMOD technologies Ltd., Zurich, Switzerland). [26] To derive estimates for binding potential (BPND) of [18F]-fallypride, TACs were fit to Watabe’s reference tissue model with two compartments [27], an extension of the simplified reference model [28]. For [18F]-FP-CMT PET images, a simplified reference tissue reference model (SRTM) was used to fit the time activity curve for a region of interest based on only three parameters, k2, R1 and BPND as shown below [24]:
where,
k2 = rate constant for transfer from free to plasma compartment (min−1)
BPND =Binding potential with respect to non-displaceable compartment.
Gp120 protein analysis from HIV-1 transgenic rat serum by ELISA
Serum collection
9 Tg and 2 WT rats were placed under anesthesia before collecting blood by retro-orbital sampling. Roughly 2μl of the collected blood was placed into serum separator tubes and left to stand for 30 minutes to allow the blood to clot. The tubes were then centrifuged for 20 minutes at 1200g and the top layer (serum) was removed from the tube without disturbing the polymer barrier, and stored in a fresh tube.
ELISA
Serum gp120 levels were quantified by enzyme-linked immunosorbent assay (ELISA) using HIV-1 gp120 antigen capture assay (ABL, Rockville, MD) with slight modifications to the manufacturer’s instructions. The gp120 standards were diluted in rat sera (AbD Serotec, Raleigh, NC; SPF Fisher 344). Based upon the standard dilutions done for this assay, the gp120 detection limit was determined to be between 2000pg/ml and 15.125 pg/ml.
Immunofluorescence
Tg rats (n =6) and WT rats (n = 7), ranging in age from 15 to 18 months, were first anesthetized with isoflurane (3% with 700 cc/min O2). This was followed by transcardial perfusion using 100 ml of normal saline (pH = 7.4) and 350 ml of freshly prepared and filtered (0.45-μm filter) 4% paraformaldehyde (pH 7.4). The brains were removed and post-fixed overnight in 4% paraformaldehyde at 4 °C followed by three 1-h washes in normal saline at 4 °C. The brains were then cryoprotected in 10% sucrose and stored at 4 °C until they sank in the solution; they were subsequently placed in 20% and then 30% sucrose until they sank again in each solution. The brains were then embedded in optimal cutting temperature compound (OCT, Tissue-Tek®), and 10-μm-thick coronal serial sections were obtained. The sections containing the striatum (bregma 0.48 to 0.12 mm) were then selected for multiplex immunofluorescent staining. A separate cohort of 1–3 month-old [Tg (n=4) and WT (n=2)] and 7–9 month-old [Tg (n=6) and WT (n=4)] rats were also sacrificed to obtain brain sections as described above. The latter group of animals did not undergo PET imaging.
Immunolabeling protocols were applied to identify the dopamine transporter and D2/D3 receptors in fresh frozen brain slices using antibodies against DAT (Santa Cruz Biotech, Cat # sc-32259) (1:100 dilution), D2DR (EMD Millipore, Cat # MABN53) (1:200 dilution) and D3DR (EMD Millipore, Cat # MABN463) (1:200 dilution). We also used antibodies to detect GFAP (USBiological, Cat # G2032-27Q-ML550) (1:200 dilution), Iba1 (Wako Chemicals, Cat # 019-19741) (1:100 dilution), TH (Novus Biologicals, Cat # NB300-110) (1:100 dilution) and NeuN (EMD Millipore, Cat # ABN90P) (1:500 dilution). The above primary immunoreaction was visualized using appropriate fluorophore-conjugated secondary antibodies. The cell nuclei were counterstained using 1 ug/ml DAPI to facilitate cell counting. All fluorescence signals were imaged using an Axio Imager.Z2 upright scanning wide-field fluorescence microscope (Zeiss) equipped with an Orca Flash 4.0 high-resolution sCMOS camera (Hamamatsu), 200W X-cite 200DC broadband light source (Lumen Dynamics), and standard DAPI and Alexa Fluor filter sets (Semrock). After imaging, the image datasets were processed for image stitching and illumination correction and the images were imported into Adobe Photoshop CS6 to produce pseudo-colored composites.
Quantification of DAT and D2/D3 immunofluorescent staining was performed using FIJI image processing package, based on ImageJ (NIH, Bethesda, MD). The locations of the selected striatal ROIs were identical between all the animals. The RGB bitmap images were first converted to 8-bit grayscale, and the threshold was adjusted to include only cells of interest and eliminate the background. This was followed by calculating the fluorescence intensity and/or cell density (NeuN) within the ROIs. All images were processed using the same analysis parameters. Since staining of different animals was performed in separate sessions, Tg brain sections were only compared to WT sections from the same session. The results are reported as (Tg values)/(average concurrently stained WT values). The data is expressed as a ratio of fluorescence intensity or cell count (only for NeuN) ±SD.
Statistical analysis
Binding potential values are reported as mean ± SD for both [18F]-Fallypride and [18F]-FP-CMT scans. Statistical differences of binding potentials were assessed using student t-test with significance denoted at p<0.05. Test-retest reproducibility between day 1 and day 2 of [18F]-FP-CMT scanning were analyzed for 2 WT rats (age 5 month-old). Linear regression analysis was performed to assess correlation between [18F]-Fallypride and [18F]-FP-CMT BPND values.
Longitudinal changes in [18F]-FP-CMT binding were calculated for 5 animals (3 Tg and 2 WT) as follows: Percentage change= [BPND (at 17 months) - BPND (at 9 months)]/BPND (at 9 months)].
RESULTS
Time-Activity Curves
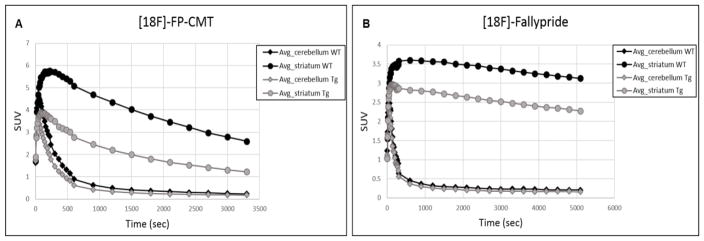
Average TACs of [18F]-FP-CMT (Fig 1A) and [18F]-Fallypride (Fig 1B) PET are shown for 15-18month-old Tg and age-matched WT rats. The WT rat TACs show higher uptake and faster washout of both ligands from the striatum. On the other hand, no appreciable difference in distribution or washout is seen in the cerebellum for either ligand.
Fig. 1.
Average Time activity curves (TACs) of (A) [18F]-FP-CMT and (B) [18F]-Fallypride in 15–18 month-old transgenic and age-matched WT rats. The TACs are shown for the striatum and cerebellum respectively
PET analysis results
[18F]-FP-CMT results
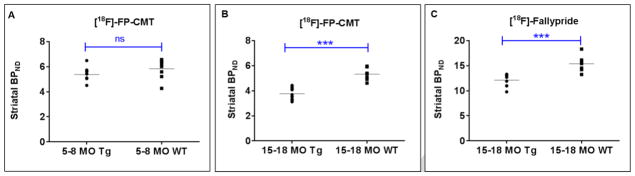
For the younger animals (5–8 month-old), the striatal BPND values for the Tg rats were on average lower than WT rats (Tg: Mean 5.37 +/− 0.53, WT: Mean 5.83 +/− 0.78), however the differences did not reach statistical significance (p=0.18) (Fig. 2A, 3A). In the older rats (15–18 month-old) however, striatal BPND values were significantly lower in the Tg compared to WT rats (p<0.0001) (Fig. 2B, 3B).
Fig. 2.
Representative PET images of (A) [18F]-FP-CMT in 5–8 month-old, (B) [18F]-FP-CMT in 15–18 month-old and (c) [18F]-Fallypride in 15–18 month-old Tg and age-matched WT rats are shown. While no appreciable differences are seen in the younger animal group scanned with [18F]-FP-CMT, the older group shows decreased [18F]-FP-CMT and [18F]-Fallypride striatal binding in the Tg compared to WT rats, suggesting pre and post synaptic dopaminergic dysfunction
Fig. 3.
Striatal binding potential values for [18F]-FP-CMT in 5–8 month-old Tg and WT rats show no statistical difference (A), whereas for 15–18 month-old rats, both [18F]-FP-CMT (B) and [18F]-Fallypride (C) binding are significantly lower in Tg compared to WT rats
The differences in the BPND values between Tg and WT rats in both age groups were not significantly different in the lower binding regions, including the septum, midbrain and colliculi (data not shown).
Test-retest of [18F]-FP-CMT assessed in 2 WT rats (5 month-old) showed good reproducibility with less than 8.4% variability within the striatal VOI.
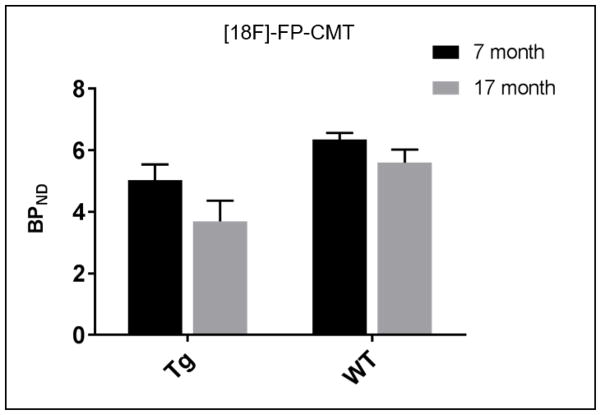
Longitudinal [18F]-FP-CMT showed that with age, BPND decreased for both WT and Tg animals. However, the decrease was more marked for Tg animals (average decrease of 27.2 % in BPND) compared to WT rats (average decrease of 11.6 % in BPND) (Fig. 4).
Fig. 4.
Longitudinal assessment of [18F]-FP-CMT binding shows more impressive loss of striatal DAT density in the Tg rats with aging than in the WT rats
[18F]-Fallypride results
The BPND values for [18F]-Fallypride binding in the group of older Tg rats that underwent [18F]-FP-CMT was significantly lower in the striatum when compared to age-matched WT rats (p=0.001) (Fig. 2C, 3C) while no differences were observed in other regions of the brain (data not shown).
Linear regression analysis
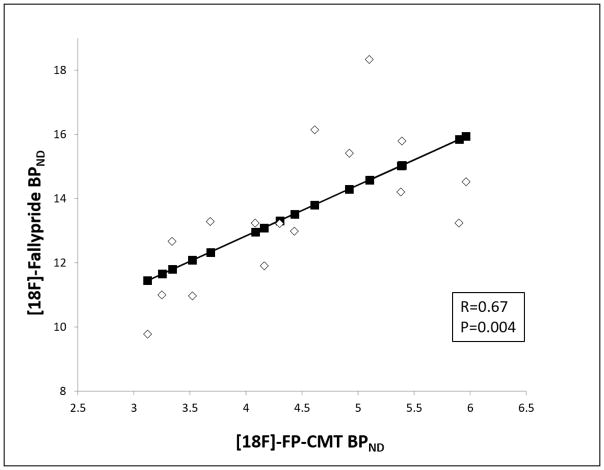
Among the animals that underwent both scans (Tg and WT), there was a positive correlation between [18F]-Fallypride and [18F]-FP-CMT BPND values (R=0.67, p=0.004) (Fig. 5). When the Tg rats were considered separately, the positive correlation was maintained (R=0.69, p=0.038).
Fig. 5.
Linear regression plot of [18F]-FP-CMT BPND and [18F]-Fallypride BPND values in the 15–18 month-old age-matched Tg and WT animals.
Gp120 ELISA
Out of the nine older Tg rats that underwent scanning using both ligands, five had serum gp120 levels below detection levels (15.125 pg/ml) while four had gp120 levels above detection level (mean = 47.21 +/− 24 pg/ml). The latter group had lower mean BPND values for [18F]-Fallypride (11.6 compared to 12.5) and lower mean BPND values for [18F]-FP-CMT (3.6 compared to 3.89), however the differences did not reach statistical significance.
Immunofluorescence staining
Multiplex immunohistochemical analysis of various targets in the brain sections of WT and Tg rats was performed to validate the PET results. IHC results from a different cohort of 1–3 month-old Tg and WT rats showed no differences in DAT staining although D2DR and D3DR staining were slightly decreased. GFAP expression was markedly decreased even at this young age while NeuN and Iba1 staining were not appreciably different between Tg and WT rats (Table. 1).
Table 1.
Ratio of fluorescence intensity or cell count (NeuN only) in Tg rats compared to WT rats at different ages (values reflect Tg/WT ratios ± SD)
| Age (months) | Ratio of fluorescence intensity or cell count [(Tg/WT) ± SD] | |||||
|---|---|---|---|---|---|---|
| D2DR | D3DR | DAT | NeuN | GFAP | Iba1 | |
| 1 to 3 | 0.60 ± 0.45 | 1.05 ± 1.02 | 0.56 ± 0.32 | 0.91 ± 0.12 | 0.33 ± 0.21 | 1.08 ± 0.64 |
| 7 to 9 | 0.45 ± 0.33 | 0.27 ± 0.15 | 0.45 ± 0.24 | 0.93 ± 0.26 | 0.45 ± 0.27 | 0.76 ± 0.38 |
| 15 to 17 | 0.56 ± 0.25 | 0.43 ± 0.16 | 0.37 ± 0.14 | 0.79 ± 0.32 | 0.54 ± 0.17 | 0.83 ± 0.46 |
7–9 month-old Tg rats showed more appreciable decrease in DAT, D2DR and D3DR staining when compared to WT rats, than younger (1–3 month-old) animals. Again GFAP staining was markedly decreased while NeuN and Iba1 staining ratios remained closer to 1 (Table. 1).
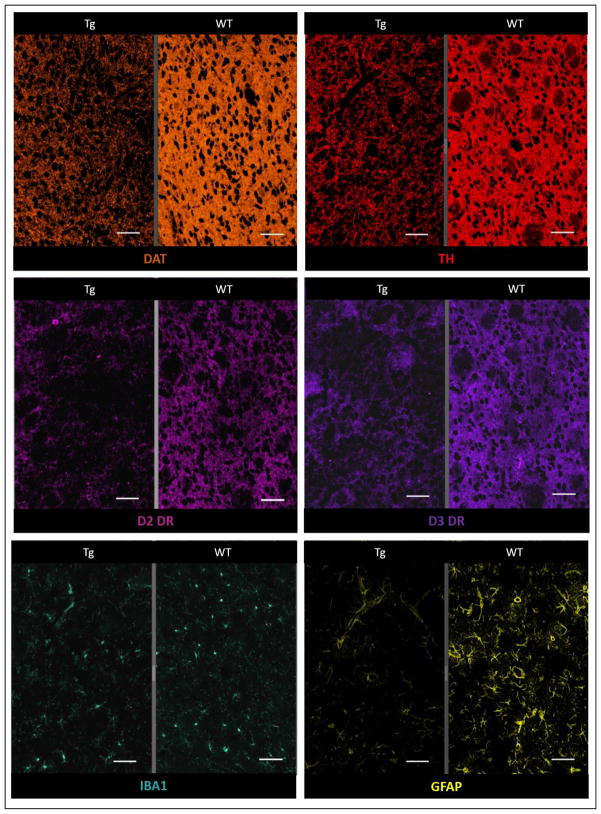
In the subset of the rats that underwent PET imaging (15–17 month-old), there was substantial lower DAT, D2DR and D3DR receptor expression in Tg compared to WT rats. Striatal GFAP staining was also substantially lower in Tg rats (Fig. 6, Table. 1).
Fig. 6.
Less intense IHC staining seen in the striatum of a 17 month-old Tg rat (left sided panel of each image subset) compared to age-matched WT rat (right sided panel of each image subset) for dopamine transporter (orange), Tyrosine hydroxylase (red), D2 Dopamine receptor (pink) and D3 Dopamine receptor (purple). Although staining for Iba1 (green) was comparable, marked loss of GFAP staining (yellow) is seen in the Tg rat (magnification = 50X, scale bar = 100 μm)
DISCUSSION
Using [18F]-FP-CMT and [18F]-Fallypride PET imaging in 15–18 month-old rats, we found significantly lower binding in the striatum of Tg rats when compared to age-matched WT rats, suggesting lower levels of both DAT and D2/D3 expression. This implies a complex pre and post synaptic dopaminergic dysfunction in the Tg rats, which we presume is related to chronic neurotoxicity associated with exposure to viral proteins [12, 17, 29, 30]. In the longitudinal component, although decreased [18F]-FP-CMT biding was seen in the WT rats, consistent with age-related degenerative changes, a more marked decrease in binding was noted in the Tg rats, suggesting an additional effect of the transgene, again likely related to chronic exposure to viral proteins. Immunohistochemistry staining confirmed our in vivo imaging findings with significantly lower DAT, D2DR and D3DR staining in the Tg rats when compared to the corresponding age-matched controls, a finding which worsened with age. This was also associated with decreased staining of TH, the rate limiting enzyme of cathecholamine synthesis, suggesting impaired dopamine synthesis.
The mechanism of dopaminergic damage in the setting of HIV infection is thought to be related to the neurotoxic effect of viral proteins, namely transactivator of transcription (Tat) and envelope glycoprotein 120 (gp120). This was shown in primary hippocampal rat cell cultures in which Tat triggered mitochondrial depolarization, increased intracellular production of reactive oxygen species (ROS) and protein oxidation, and caused neuronal degeneration [31]. Similarly, gp120 was neurotoxic to a mesencephalic neuronal/glial culture model, resulting in reduced function (decreased DA uptake), morphological changes and reduced viability of the dopaminergic neurons [32]. In another study, gp120 produced loss of nigrostriatal neurons, as shown both by histochemical analysis of brain sections for apoptosis and biochemical determination of dopamine concentration [33]. In the Tg rat, previous papers asserted prolonged exposure to viral proteins as the main cause of neuropathology [17, 18, 29, 34, 35], with dopaminergic system dysfunction being a characteristic finding in neurobehavioral studies [10, 11, 13]. We have previously shown expression of viral proteins in the brain, CSF and serum of the Tg rats, which could potentially explain the dopaminergic neuronal toxicity we identified by PET. In fact, the group of animals that had higher gp120 levels around the time of scanning (n=4) showed lower [18F]-Fallypride and [18F]-FP-CMT binding than those with gp120 levels below detection level (n=5). The differences in binding however did not reach statistical significance, possibly due to the small sample size.
In HIV+ patients, two previous papers have evaluated components of the dopaminergic system with PET imaging. The first study showed significantly lower DAT availability in putamen and ventral striatum in HIV patients with associated dementia (HAD) [36]. In the second study, HIV+ subjects with and without history of cocaine dependence had lower DAT in putamen compared to controls [37]. Up to our knowledge, there hasn’t been however a previous PET evaluation of dopaminergic loss in optimally treated, virologically-suppressed patients before. Our findings in the rat model, suggested as a model of treated HIV+ subjects, warrant translation into patients.
In younger Tg rats (5–8 month-old), lower Tg [18F]-FP-CMT binding was seen compared to WT rats but the difference was not statistically significant (Fig. 2A, 3A). This was discordant with our previous findings of significantly decreased [18F]-Fallypride binding in the Tg rats compared to WT rats at a similar age (5–9 months) [26]. Since we know D2/D3 receptors are expressed in many cell types other than dopaminergic neurons [19–23, 38] we believe this discrepancy could be related to loss/dysfunction of those cell types, such as astrocytes and GABAergic neurons. This could lead to D2/D3 deficits that are not fully reflective of dopaminergic loss. This is especially likely in this animal model in which we have consistently shown marked astrocytic loss [12]. In fact, staining in the same group of rats that underwent imaging showed markedly decreased GFAP staining (ratio of Tg/WT= 0.54 +/− 0.18) (Fig. 6, Table. 1). Although astrocytic loss is not a commonly encountered aspect of neuropathology, astrogliosis (astrocyte activation) on the other hand, is not unusual. Whether the contributing effect of those non-dopaminergic cells to D2/D3DR binding using ligands such as [18F]-Fallypride or 11C-Raclopride is significant or not might need to be considered.
One limitation to our paper include not using stereology techniques to account for possible shrinkage of the tissues thus affecting cell density calculations. However, since all the brains went through the same tissue preparation procedure before staining, the amount of shrinking of each tissue should be the same, and cell counts/staining densities in matching areas of interest should be comparable. Another inherent limitation is the slight difference in weight between Tg and WT rats (smaller body weights in Tg rats).
In conclusion, there are currently no established imaging biomarkers of HIV neuropathology that could be reliably used in the evaluation of experimental neuroprotective therapies [39–46]. Those neuroprotective approaches remain badly needed in HIV+ subjects still suffering neurocognitive dysfunction despite optimized treatment and peripheral control of the infection. Using small animal PET along with the HIV-1 transgenic rat, a well-studied animal model of treated HIV+ patients, we have shown quantifiable presynaptic and postsynaptic dopaminergic dysfunction/loss. The combination of this model with the above used PET ligands as in vivo imaging biomarkers of degeneration can thus be used to evaluate HIV neuroprotective therapies, prior to human translation.
Acknowledgments
Financial support: This research was supported by the Center for Infectious Disease Imaging (CIDI), an intramural research program at NIH.
We would like to thank the staff of the Imaging Probe Development Center (IPDC) at NIH especially Drs Falguni Bhattacharyya and Chandrasekhar Mushti for helping in the development of the synthesis of [18F]-FP-CMT. We also thank Dr Amy Hauck Newman (NIH) for providing the precursor for [18F]-FP-CMT.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All experimental procedures, including handling and care of the animals, were approved by the Animal Care and Use Committee of the Clinical Center of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010;67(6):699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 2.Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan IL, McArthur JC. HIV-associated neurological disorders: a guide to pharmacotherapy. CNS Drugs. 2012;26(2):123–34. doi: 10.2165/11597770-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, et al. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol. 2000;14(3):222–7. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- 5.Hersh BP, Rajendran PR, Battinelli D. Parkinsonism as the presenting manifestation of HIV infection: improvement on HAART. Neurology. 2001;56(2):278–9. doi: 10.1212/wnl.56.2.278. [DOI] [PubMed] [Google Scholar]
- 6.Koutsilieri E, ter Meulen V, Riederer P. Neurotransmission in HIV associated dementia: a short review. J Neural Transm. 2001;108(6):767–75. doi: 10.1007/s007020170051. [DOI] [PubMed] [Google Scholar]
- 7.Lopez OL, Smith G, Meltzer CC, Becker JT. Dopamine systems in human immunodeficiency virus-associated dementia. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12(3):184–92. [PubMed] [Google Scholar]
- 8.Mirsattari SM, Power C, Nath A. Parkinsonism with HIV infection. Mov Disord. 1998;13(4):684–9. doi: 10.1002/mds.870130413. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Chang L, Vigorito M, Kass M, Li H, Chang SL. Methamphetamine-induced behavioral sensitization is enhanced in the HIV-1 transgenic rat. J Neuroimmune Pharmacol. 2009;4(3):309–16. doi: 10.1007/s11481-009-9160-8. [DOI] [PubMed] [Google Scholar]
- 10.Moran LM, Aksenov MY, Booze RM, Webb KM, Mactutus CF. Adolescent HIV-1 transgenic rats: evidence for dopaminergic alterations in behavior and neurochemistry revealed by methamphetamine challenge. Curr HIV Res. 2012;10(5):415–24. doi: 10.2174/157016212802138788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moran LM, Booze RM, Webb KM, Mactutus CF. Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Exp Neurol. 2013;239:139–47. doi: 10.1016/j.expneurol.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid WC, Ibrahim WG, Kim SJ, Denaro F, Casas R, Lee DE, et al. Characterization of neuropathology in the HIV-1 transgenic rat at different ages. J Neuroimmunol. 2016;292:116–25. doi: 10.1016/j.jneuroim.2016.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb KM, Aksenov MY, Mactutus CF, Booze RM. Evidence for developmental dopaminergic alterations in the human immunodeficiency virus-1 transgenic rat. J Neurovirol. 2010;16(2):168–73. doi: 10.3109/13550281003690177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Hayes N, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proceedings of the National Academy of Sciences. 2001;98(16):9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moran LM, Booze RM, Mactutus CF. Time and time again: temporal processing demands implicate perceptual and gating deficits in the HIV-1 transgenic rat. J Neuroimmune Pharmacol. 2013;8(4):988–97. doi: 10.1007/s11481-013-9472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lashomb AL, Vigorito M, Chang SL. Further characterization of the spatial learning deficit in the human immunodeficiency virus-1 transgenic rat. J Neurovirol. 2009;15(1):14–24. doi: 10.1080/13550280802232996. [DOI] [PubMed] [Google Scholar]
- 17.Peng J, Vigorito M, Liu X, Zhou D, Wu X, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 2010;218(1–2):94–101. doi: 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Vigorito M, Connaghan KP, Chang SL. The HIV-1 transgenic rat model of neuroHIV. Brain Behav Immun. 2015;48:336–49. doi: 10.1016/j.bbi.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bal A, Bachelot T, Savasta M, Manier M, Verna JM, Benabid AL, et al. Evidence for dopamine D2 receptor mRNA expression by striatal astrocytes in culture: in situ hybridization and polymerase chain reaction studies. Brain Res Mol Brain Res. 1994;23(3):204–12. doi: 10.1016/0169-328x(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 20.Lin R, Karpa K, Kabbani N, Goldman-Rakic P, Levenson R. Dopamine D2 and D3 receptors are linked to the actin cytoskeleton via interaction with filamin A. Proc Natl Acad Sci U S A. 2001;98(9):5258–63. doi: 10.1073/pnas.011538198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mladinov M, Mayer D, Brčić L, Wolstencroft E, Man Nt, Holt I, et al. Astrocyte expression of D2-like dopamine receptors in the prefrontal cortex. Translational Neuroscience. 2010;1(3):238–243. [Google Scholar]
- 22.Lao CL, Lu CS, Chen JC. Dopamine D3 receptor activation promotes neural stem/progenitor cell proliferation through AKT and ERK1/2 pathways and expands type-B and -C cells in adult subventricular zone. Glia. 2013;61(4):475–89. doi: 10.1002/glia.22449. [DOI] [PubMed] [Google Scholar]
- 23.Elgueta D, Aymerich MS, Contreras F, Montoya A, Celorrio M, Rojo-Bustamante E, et al. Pharmacologic antagonism of dopamine receptor D3 attenuates neurodegeneration and motor impairment in a mouse model of Parkinson’s disease. Neuropharmacology. 2017;113(Pt A):110–123. doi: 10.1016/j.neuropharm.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 24.Cumming P, Maschauer S, Riss PJ, Tschammer N, Fehler SK, Heinrich MR, et al. Radiosynthesis and validation of (1)(8)F-FP-CMT, a phenyltropane with superior properties for imaging the dopamine transporter in living brain. J Cereb Blood Flow Metab. 2014;34(7):1148–56. doi: 10.1038/jcbfm.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukherjee J, Christian BT, Narayanan TK, Shi B, Collins D. Measurement of d-amphetamine-induced effects on the binding of dopamine D-2/D-3 receptor radioligand, 18F-fallypride in extrastriatal brain regions in non-human primates using PET. Brain research. 2005;1032(1–2):77–84. doi: 10.1016/j.brainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Lee DE, Reid WC, Ibrahim WG, Peterson KL, Lentz MR, Maric D, et al. Imaging Dopaminergic Dysfunction as a Surrogate Marker of Neuropathology in a Small-Animal Model of HIV. Mol Imaging. 2014;13:1–10. doi: 10.2310/7290.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watabe H, Channing MA, Der MG, Adams HR, Jagoda E, Herscovitch P, et al. Kinetic analysis of the 5-HT2A ligand [11C]MDL 100,907. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000;20(6):899–909. doi: 10.1097/00004647-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4(3 Pt 1):153–8. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 29.Royal W, 3rd, Zhang L, Guo M, Jones O, Davis H, Bryant JL. Immune activation, viral gene product expression and neurotoxicity in the HIV-1 transgenic rat. J Neuroimmunol. 2012;247(1–2):16–24. doi: 10.1016/j.jneuroim.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vigorito M, Connaghan KP, Chang SL. The HIV-1 transgenic rat model of neuroHIV. Brain Behav Immun. 2015;48:336–49. doi: 10.1016/j.bbi.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM. Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: the role of oxidative stress and D1 dopamine receptor. Neurotoxicology. 2006;27(2):217–28. doi: 10.1016/j.neuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Hu S, Sheng WS, Lokensgard JR, Peterson PK, Rock RB. Preferential sensitivity of human dopaminergic neurons to gp120-induced oxidative damage. J Neurovirol. 2009;15(5–6):401–10. doi: 10.3109/13550280903296346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mocchetti I, Bachis A, Nosheny RL, Tanda G. Brain-derived neurotrophic factor expression in the substantia nigra does not change after lesions of dopaminergic neurons. Neurotox Res. 2007;12(2):135–43. doi: 10.1007/BF03033922. [DOI] [PubMed] [Google Scholar]
- 34.Midde NM, Gomez AM, Harrod SB, Zhu J. Genetically expressed HIV-1 viral proteins attenuate nicotine-induced behavioral sensitization and alter mesocorticolimbic ERK and CREB signaling in rats. Pharmacol Biochem Behav. 2011;98(4):587–97. doi: 10.1016/j.pbb.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wayman WN, Chen L, Persons AL, Napier TC. Cortical consequences of HIV-1 Tat exposure in rats are enhanced by chronic cocaine. Curr HIV Res. 2015;13(1):80–7. doi: 10.2174/0929867322666150311164504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, et al. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127(Pt 11):2452–8. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- 37.Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, et al. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage. 2008;42(2):869–78. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong AC, Shetreat ME, Clarke JO, Rayport S. D1- and D2-like dopamine receptors are co-localized on the presynaptic varicosities of striatal and nucleus accumbens neurons in vitro. Neuroscience. 1999;89(1):221–33. doi: 10.1016/s0306-4522(98)00284-x. [DOI] [PubMed] [Google Scholar]
- 39.Meeker RB, Poulton W, Clary G, Schriver M, Longo FM. Novel p75 neurotrophin receptor ligand stabilizes neuronal calcium, preserves mitochondrial movement and protects against HIV associated neuropathogenesis. Exp Neurol. 2015;275:182–198. doi: 10.1016/j.expneurol.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wrasidlo W, Crews LA, Tsigelny IF, Stocking E, Kouznetsova VL, Price D, et al. Neuroprotective effects of the anti-cancer drug Sunitinib in models of HIV- neurotoxicity: potential for drug repositioning for the treatment of neurodegenerative disorders. Br J Pharmacol. 2014;171(24):5757–73. doi: 10.1111/bph.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu S, Sheng WS, Rock RB. CB2 receptor agonists protect human dopaminergic neurons against damage from HIV-1 gp120. PLoS One. 2013;8(10):e77577. doi: 10.1371/journal.pone.0077577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramadan E, Basselin M, Chang L, Chen M, Ma K, Rapoport SI. Chronic lithium feeding reduces upregulated brain arachidonic acid metabolism in HIV-1 transgenic rat. J Neuroimmune Pharmacol. 2012;7(3):701–13. doi: 10.1007/s11481-012-9381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agrawal L, Louboutin JP, Reyes BA, Van Bockstaele EJ, Strayer DS. HIV-1 Tat neurotoxicity: a model of acute and chronic exposure, and neuroprotection by gene delivery of antioxidant enzymes. Neurobiol Dis. 2012;45(2):657–70. doi: 10.1016/j.nbd.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Heron PM, Turchan-Cholewo J, Bruce-Keller AJ, Wilson ME. Estrogen receptor alpha inhibits the estrogen-mediated suppression of HIV transcription in astrocytes: implications for estrogen neuroprotection in HIV dementia. AIDS Res Hum Retroviruses. 2009;25(11):1071–81. doi: 10.1089/aid.2009.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schifitto G, Navia BA, Yiannoutsos CT, Marra CM, Chang L, Ernst T, et al. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. Aids. 2007;21(14):1877–86. doi: 10.1097/QAD.0b013e32813384e8. [DOI] [PubMed] [Google Scholar]
- 46.Nosheny RL, Ahmed F, Yakovlev A, Meyer EM, Ren K, Tessarollo L, et al. Brain-derived neurotrophic factor prevents the nigrostriatal degeneration induced by human immunodeficiency virus-1 glycoprotein 120 in vivo. Eur J Neurosci. 2007;25(8):2275–84. doi: 10.1111/j.1460-9568.2007.05506.x. [DOI] [PubMed] [Google Scholar]