Abstract
Adenovirus (Ad) is thought to be one of the most promising platforms for a malaria vaccine targeted against its liver stages, because of its ability to induce a strong T-cell response against a transgene. However, a further improvement of this platform is needed in order to elicit another arm of the immunity, i.e. humoral response, against malaria.
In order to augment immunogenicity and protective efficacy of Ad-based malaria vaccine, we inserted B cell, as well as CD4+ T cell, epitopes of Plasmodium falciparum circumsporozoite protein (PfCSP) into the capsid protein, Hexon, and the core protein, VII (pVII), of Ad, respectively, in addition to the PfCSP transgene. Insertion of PfCSP-derived B cell epitope to Hexon significantly enhanced the epitope-specific antibody response compared to AdPfCSP, an Ad vaccine expressing only PfCSP transgene. PfCSP-derived CD4+ T cell epitope insertion into pVII augmented not only PfCSP-specific CD4+ T cell response but also anti-PfCSP antibody response. Finally, mice immunized with AdPfCSP having both Hexon and pVII modifications were more protected than AdPfCSP or Hexon-modified AdPfCSP against challenge with transgenic rodent malaria parasites expressing the PfCSP.
Overall, this study has demonstrated that Hexon and pVII-modified AdPfCSP vaccine is a promising malaria vaccine which induces strong PfCSP-specific humoral, CD4+ T cell, and CD8+ T cell responses and protects against infection with transgenic malaria parasites expressing the PfCSP.
Keywords: adenovirus, malaria vaccine, capsid protein, core protein, circumsporozoite protein
Introduction
Malaria remains one of the most deadly tropical parasitic diseases in children in sub-Saharan Africa, with roughly 200 million clinical cases and almost 600,000 fatalities annually [1 – WHO: world malaria report]. Current strategies for malaria prevention and control include use of insecticide-treated nets (ITNs) and anti-malaria drugs. So far there is only one malaria vaccine that has been adopted a positive opinion for use in endemic countries. It is an RTS,S/AS01 vaccine, and the vaccine efficacy against all episodes of severe malaria to be approximately 50% in young children in Africa [2, 3]. Therefore, a more effective malaria vaccine is still needed to significantly reduce the global mortality and morbidity caused by malaria.
In order to develop effective malaria vaccine against the liver stages of malaria parasites, much efforts have been made not only to identify a new liver-stage-specific malaria antigen, but also to understand the nature of the protective immunity against malaria [4–6]. A circumsporozoite protein (CSP), a most abundant surface protein expressed by the sporozoites, is shown to mediate protective immunity against malaria [7]. In fact, the protection displayed by the RTS,S/AS01 vaccine described above, has been shown to be primarily associated with the titer of anti-CSP antibody [8].
CSP-specific CD8 + T-cell response has been shown to be another major contributor for the protective anti-malaria immunity in addition to anti-CSP antibody response in mice [9]. Various viral vectors expressing the whole CSP or its CD8+ T-cell epitope sequence have been shown to elicit CSP-specific CD8+ T-cell response and ultimately induce CD8+ T-cell mediated protection against malaria [10–14]. It is plausible that the inability of an RTS,S/AS01 vaccine to induce CSP-specific CD8+ T-cell response may be one of the reasons why the efficacy of the vaccine is not more than 50% [15].
CSP-specific CD4+ T-cell response has also been shown to contribute to protection against malaria in mice. Adoptive transfer of CSP-specific CD4+ T-cell clones to naïve mice conferred protection against malaria challenge [16, 17]. As for the role of CSP-specific CD4+ T cells in humans, volunteers vaccinated with the RTS,S/AS01 vaccine mounted a CSP-specific CD4+ T-cell response, which appears to be an independent factor associated with the protection against malaria [18].
It is noteworthy that a couple of recent studies has modified the RTS,S/AS01 vaccination regimen by combining it with viral vectored vaccines [19] or controlled human malaria parasite infection (CHMI)[20], as a prime-boost vaccination regimen, partly in an attempt to increase the cell-mediated immunity against malaria. In fact, these modified vaccination regimens have been shown to improve the protective efficacy of RTS,S/AS01 in malaria naïve human subjects who were vaccinated and challenged [19, 20]. Collectively, all these studies point toward the fact that protective anti-malaria immunity induced by CSP-based vaccines, is rather complex and consisting of humoral immune response and cell-mediated immune response, i.e. CD4+ and CD8+ T-cell responses.
We have previously reported that a recombinant adenovirus serotype 5 (Ad5) having an insertion of Plasmodium yoelii B-cell epitope repeats into the Hyper Variable Region 1 (HVR1) of its Hexon in addition to a P. yoelii CSP (PyCSP) transgene, induced a more potent cell-mediated and humoral immune responses and protected more mice from P. yoelii sporozoite challenge than a conventional Ad5 expressing a PyCSP alone [21]. More recently, other groups have constructed a recombinant Ad5 that has an insertion of either CSP-specific B-cell epitopes [22, 23] or a CSP-specific CD4+ T-cell epitope [24] into its Hexon and shown an increased protective efficacy not only in mice [22, 24] but also an increased immunogenicity in non-human primates [23].
In this study, we constructed Ad5 that has a modification not only in the Hexon, but also in the protein VII (pVII) by inserting P. falciparum CSP (PfCSP) B-cell epitope and CD4+ T-cell epitope, respectively, in addition to the PfCSP transgene. We then analyzed the effect of insertion on virus growth and PfCSP-specific immune responses in detail. Finally, we tested the protective efficacy of Hexon- and pVII-modified Ad5 expressing PfCSP by challenging mice with two different transgenic rodent parasites expressing the PfCSP or its repeat sequence.
Methods
Cell lines
A sub-cell line of HEK293, AD293 was obtained from Stratagene (La Jolla, CA, USA) and maintained with DMEM supplemented with 10% FBS and antibiotics.
Construction, production and purification of recombinant adenovirus
The adenovirus genome DNA used in this study is E1 and E3-deleted, replication deficient human adenovirus serotype 5 from Stratagene (pAdEasy-1) and an adenovirus shuttle vector, pShuttle-CMV (Stratagene) was used to insert a codon-optimized PfCSP transgene into adenovirus genome DNA plasmid [21].
The PfCSP amino acid sequence, from the position 1 to 374, of P. falciparum 3D7 strain was used as a template sequence for codon-optimization for protein expression in human with Integrated DNA Technologies’ (Coralville, IA USA) optimization software (Supplemental Figure 1). DNA fragments that encode whole PfCSP except for the GPI-anchored motif at the C-terminus were synthesized by Integrated DNA Technologies. The synthesized PfCSP cDNA was cloned into Kpn I and Hind III sites in adenovirus shuttle vectors, pShuttle-CMV, to construct AdPfCSP. The recombinant shuttle vector was linearized by Pme I digestion and used for homologous recombination with pAdEasy-1 vector in E. coli BJ5183 cells (Stratagene) to construct PfCSP recombinant adenovirus genome, AdPfCSP.
Insertion of (NANP)n in Hexon was done by replacing HVR1 sequence (from 138 to 164 a.a.) with the repeat sequence. DNA fragments containing (NANP)n in Hexon were prepared by two-step PCR amplifying the region containing Age I and Nde I sites of the Hexon gene. Firstly 5 prime HVR1 fragment, 3 prime HVR1 fragment and NANP-coding fragment were amplified by PCR separately. And then these resulting three fragments were connected by overlapping PCR. The PCR product was digested with Age I and Nde I, and then used to replace the native Age I-Nde I region of the Sfi I - Sfi I fragment (about 6.6 kbp), which was subcloned in pUC19 vector (Sfi I/pUC19). The sequence of the amplified region in the constructs was confirmed by sequencing. The Sfi I - Sfi I region of AdPfCSP adenovirus genome plasmid was replaced with the Sfi I - Sfi I fragment containing (NANP)n in HVR1 to construct AdPfCSP/(NANP)n.
The PfCSP-specific universal CD4+ T-cell epitope (EYLNKIQNSLSTEWSPCSVT) was inserted between 198 a.a. and the stop codon (CD4-PVII-1), 1 and 2 a.a. (CD4-PVII-2), 98 and 99 a.a. (CD4-PVII-3), or 140 and 141 a.a. (CD4-PVII-4). To insert the CD4 T cell epitope sequence into the C-terminus of pVII, the region containing Sfi I and Sal I sites was amplified by two-step PCR using primers which have the CSP epitope sequence. The PCR product was digested with Sfi I and Sal I, and then used to replace the native Sfi I-Sal I region of Sfi I/pUC19. After confirming the sequence, the Sfi I-Sfi I fragment of adenovirus genome was replaced with Sfi I-Sfi I fragment having the CSP epitope in pVII. To insert the PfCSP CD4+ T-cell epitope in the N-terminus or in the middle of pVII, about 7.7kb fragment of pAdEasy-1 was prepared by Rsr II digestion and cloned between the EcoR I and Hind III sites of pUC19 plasmid using Rsr II linker (Rsr II/pUC19). The region containing Asc I and Bgl II sites in Rsr II/pUC19 was amplified by two-step PCR using primers which have the epitope sequence. The PCR product was digested with Asc I and Bgl II, and then used to replace the native Asc I and Bgl II region in Rsr II/pUC19 plasmid. After confirming the sequence of the replaced region, the Rsr II fragment of HVR1-modified adenovirus DNA was replaced with the Rsr II fragment containing the epitope sequence.
Adenovirus genome DNA plasmids were linearized by Pac I digestion and used for transfection of AD293 cells in order to produce recombinant adenovirus. Adenovirus seed solution was prepared from the transfected AD293 cells showing cytopathic effects (CPE) by several rounds of freeze/thaw and used for further virus amplification. After the last virus amplification, adenovirus particles were purified by CsCl gradient centrifugation. The band was then collected and dialyzed against dialysis buffer (10 mM Tris-HCl, 150 mM NaCl, 10 mM MgCl2, 3% (w/v) Sucrose, pH7.8) to remove CsCl. Virus particle unit (v.p.) was calculated based on O.D.260 (1O.D.260=1.25×1012v.p./mL).
Measurement of Adenovirus production
AD293 cells were seeded into 12 well plates one day before transfection and linearized adenovirus genome DNA was transfected in triplicate using Lipofectamine 2000 (Invitrogen) at day 0. At day 3, cells were trypsinized and re-seeded into 24 well plates. At day 5, 7, 9 and 11, genomic DNA was extracted from the wells using QIAamp DNA Mini kit. The copy number of adenovirus genomic DNA in the extracted DNA was measured by real time PCR using 7500 Real Time PCR system (Applied Biosystems). The primer set for the real time PCR reaction was 100KF (AACTTCTACCCCGTATTTGCC) and 100KR (GATATCAGGTATGACAGCGCC), and the probe is 100KProbe (5′-[FAM]-AAGATACCCCTATCCTGCCGTGC-[BHQ-1]-3′).
PCR amplification of HVR1
(NANP)n insertion in HVR1 region was reconfirmed by PCR using purified adenoviruses as templates. The region was amplified with HexF12 (GTGCTGGACATGGCTTCCACGTAC) and Hex R13 (TTTAGGTGTTTGACCTTCGACACC) primers and the PCR products were analyzed on agarose gel.
Silver staining and Western blot analysis
Virions (2 × 109 v.p./lane) were lysed in 1x SDS sample buffer, denatured by heating at 70°C for 10 min, and applied onto a 4–12% gradient polyacrylamide gel under a reducing condition. After electrophoresis, the gel was stained with a silver staining kit (Invitrogen Carlsbad, CA, USA). For Western blotting, the separated proteins were blotted onto a nitrocellulose membrane. The membrane was blocked in 3% skim milk, and then incubated with Protein G-purified mouse monoclonal anti-NANP antibody (2A10)[7]. After washing, the blots were incubated with horseradish peroxidase (HRP) - labeled goat anti-mouse IgG antibody (Thermo Fisher Scientific Inc., Waltham, MA, USA), and the signal was developed using an ECL detection system (GE Healthcare).
Determination of Plaque Forming Unit
Plaque Forming Unit (PFU) of adenovirus was determined by end point dilution assay. 2×104 AD293 cells were seeded in 96 well plates and then ten-fold serial dilutions of adenovirus were added to the plates (12 wells for one dilution). The plates were incubated for 10 days at 37°C, 5% CO2 and then number of wells which showed cytopathic effect (CPE) was counted and PFU was calculated based on the following equation: PFU per mL = 10(X+0.8). X is the sum of the fractions of CPE positive wells at each dilution.
Mice and Immunizations
Six- to eight-week old female BALB/c mice were purchased from Taconic (Hudson, NY, USA) or Japan SLC Inc. (Hamamatsu, Shizuoka, Japan) and maintained under standard conditions in the Otsuka Pharmaceutical Co., Ltd. or in The Laboratory Animal Research Center of The Rockefeller University. All experimental procedures were performed in accordance with the Guidelines for Animal Care and Use of Otsuka Pharmaceutical Co., Ltd. or the protocol approved by the Institutional Animal Care and Use Committee at The Rockefeller University (Assurance no. A3081-01). CO2 was used for euthanasia, and all efforts were made to minimize suffering. For immunization, Adenovirus were diluted in PBS and injected intramuscularly at indicated doses.
Assessment of PfCSP-specific cellular and humoral immune responses
NANP-specific humoral response was determined by Enzyme-Linked Immunosorbent Assay (ELISA). Five micro-liter of blood was collected from tail vain of the immunized mice and diluted in 495 μl of PBS, and then the samples were centrifuged at 5,000 rpm for 5min to prepare diluted plasma samples (x100). Maxisorp ELISA plates were coated with 1μg/mL (T1B)4 CSP repeat peptide, which contains NANP repeat sequence [25] or 5μg/mL of a peptide, NANPNANPNANPNANPNANPNANP, in 0.1M Sodium Carbonate Buffer (pH 9.5) at 4°C for overnight. (T1B)4 CS repeat peptide was kindly provided by Dr. Elizabeth Nardin. Plates were washed and blocked with 1x Diluent (eBiosciences) for 2 hours at R.T. The plates were washed again and 100 μl of serially twofold-diluted plasma samples in 1x Diluent were added to the plates and the plates were incubated for one hour at room temperature. The plates were washed and incubated with 100 μl of HRP-labeled goat anti-mouse IgG antibody. The antibody titers were determined by the endpoint dilution of the sera.
The numbers of PfCSP-specific, IFN-γ-secreting CD8+ T cells in the spleens of immunized mice were determined by an ELISpot assay (eBiosciences), using a synthetic peptide corresponding to the CD8+ T cell epitope (NYDNAGTNL) within the PfCSP protein, as previously described [26]. For analysis of PfCSP-specific CD4+ T cells, a synthetic peptide corresponding to the universal CD4+ T cell epitope (EYLNKIQNSLSTEWSPCSVT) within the PfCSP protein was used [27] for ELISpot assays (eBiosciences). All peptides were synthesized by Sigma-Aldrich.
Challenge Experiment
Transgenic Plasmodium berghei sporozoites that express the repeat region of PfCSP (PfCS-repeat/Pb Spz) were obtained from Dr. Elizabeth Nardin [28]. Transgenic P. yoelii sporozoites that express a full-length PfCSP (PfCS/Py Spz) were generated, as we recently described [29]. Immunized mice were challenged by injecting 1× 103 PfCS-repeat//Pb Spz or 50 PfCSP/Py Spz intravenously.
Statistical analysis
All of the statistical analyses were done using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA). Bars in each figure represent geometric means (antibody titer) or means (ELISPOT). For statistical analysis of antibody titer, values were log-transformed and then one-way ANOVA followed by a Dunnett’s were applied to determine the differences between three or more groups. Unpaired t-test was used if the comparison was done between two groups. For statistical analysis of the challenge experiments, Log-rank (Mantel-Cox) test was applied without adjusting for multiple comparisons to compare infection-free survival between two groups.
Results
Insertion of PfCSP B-cell epitope repeats in Hexon
In our previous study, we replaced Hexon HVR1 sequence of Ad5 with three repeats of QGPGAP, which is an immunodominant B-cell epitope present in PyCSP, and showed enhanced protection by the modification [21]. In this study, PfCSP was used as antigen, in an attempt to develop experimental malaria vaccines that can be tested in clinical trials. The platform vector used in this study is E1 and E3-deleted, replication incompetent Ad5 vector, and codon-optimized PfCSP cDNA was used as a transgene (Supplemental Fig. 1). Hence, a recombinant adenovirus expressing the entire PfCSP except for the GPI-anchored motif at the C-terminus in its transgene, called AdPfCSP, was used as a platform vaccine in the current study. Then, we constructed adenoviruses with insertion of four to forty repeats of NANP, which is an immunodominant B-cell epitope present in PfCSP, to determine the maximal number of the repeats that can be inserted into HVR1 of AdPfCSP without affecting the production and infectivity of adenovirus (Table 1). As shown in Figure 1, adenovirus production from adenovirus packaging AD293 cells was significantly delayed or reduced in cells transfected with adenovirus genome with twenty-eight or more NANP repeats (Figure 1A). We could expand adenovirus carrying twenty-eight and thirty-four repeats of NANP, i.e. (NANP)28 and (NANP)34, but PCR analysis of Hexon revealed that a majority of these viruses had deletions in the repeat sequence (data not shown). We analyzed CsCl-ultracentrifuge-purified Hexon-modified adenoviruses with four to twenty-two repeats, i.e. (NANP)4 to (NANP)22, by PCR, Western Blot analysis and SDS-PAGE followed by Silver Staining. There were no deletion mutants in these purified viruses by PCR (Supplemental Fig. 2A), and the insertion of the B-cell epitope repeats was confirmed by Western Blot analysis with anti-NANP monoclonal antibody (2A10), in which intensity of corresponding bands increased as the number of repeats increases (Supplemental Fig. 2B). Finally, SDS-PAGE analysis revealed bands of Hexon protein shifted up in SDS-PAGE corresponding to the number of NANP repeats (Supplemental Fig. 2C). Bands corresponding to lower molecular weights below the band of modified Hexon were observed in both Western Blot assay and Silver Staining. These bands are considered, as degraded or incomplete products, but not as deletion mutants, because no deletion in adenoviral genome was detected by PCR analysis (Supplemental Fig. 2A). Based on these observations, we concluded that the upper limit of NANP repeats is approximately twenty-two for replacement of HVR1 in Hexon.
Table 1.
| Adenovirus | Transgene | Hexon | pVII |
|---|---|---|---|
| AdPfCSP | PfCSP | Wild-type | Wild-type |
| AdPfCSP/(NANP)4 | PfCSP | 4 repeats of NANP in HVR1 | Wild-type |
| AdPfCSP/(NANP)6 | PfCSP | 6 repeats of NANP in HVR1 | Wild-type |
| AdPfCSP/(NANP)8 | PfCSP | 8 repeats of NANP in HVR1 | Wild-type |
| AdPfCSP/(NANP)10 | PfCSP | 10 repeats of NANP in HVR1 | Wild-type |
| AdPfCSP/(NANP)12 | PfCSP | 12 repeats of NANP in HVR1 | Wild-type |
| AdPfCSP/(NANP)14 | PfCSP | 14 repeats of NANP in HVR1 | Wild-type |
| AdPfCSP/(NANP)16 | PfCSP | 16 repeats of NANP in HVR1 | Wild-type |
| AdPfCSP/(NANP)18 | PfCSP | 18 repeats of NANP in HVR1 | Wild-type |
| AdPfCSP/(NANP)20 | PfCSP | 20 repeats of NANP in HVR1 | Wild-type |
| AdPfCSP/(NANP)22 | PfCSP | 22 repeats of NANP in HVR1 | Wild-type |
| AdPfCSP/(NANP)28 | PfCSP | 28 repeats of NANP in HVR1 | Wild-type |
| AdPfCSP/(NANP)34 | PfCSP | 34 repeats of NANP in HVR1 | Wild-type |
| AdPfCSP/(NANP)40 | PfCSP | 40 repeats of NANP in HVR1 | Wild-type |
| AdPfCSP/(NANP)4/CD4-pVII-1 | PfCSP | 4 repeats of NANP in HVR1 | PfCSP CD4 epitope at C terminus |
| AdPfCSP/(NANP)4/CD4-pVII-2 | PfCSP | 4 repeats of NANP in HVR1 | PfCSP CD4 epitope at N terminus |
| AdPfCSP/(NANP)4/CD4-pVII-3 | PfCSP | 4 repeats of NANP in HVR1 | PfCSP CD4 epitope in the upstream of 1st NLS |
| AdPfCSP/(NANP)4/CD4-pVII-4 | PfCSP | 4 repeats of NANP in HVR1 | PfCSP CD4 epitope between the NLSs |
| AdPfCSP/(NANP)22/CD4-pVII-4 | PfCSP | 22 repeats of NANP in HVR1 | PfCSP CD4 epitope between the NLSs |
| Ad(NANP)22 | - | 22 repeats of NANP in HVR1 | Wild |
| Ad(NANP)22/CD4-pVII-4 | - | 22 repeats of NANP in HVR1 | PfCSP CD4 epitope between the NLSs |
Figure 1.
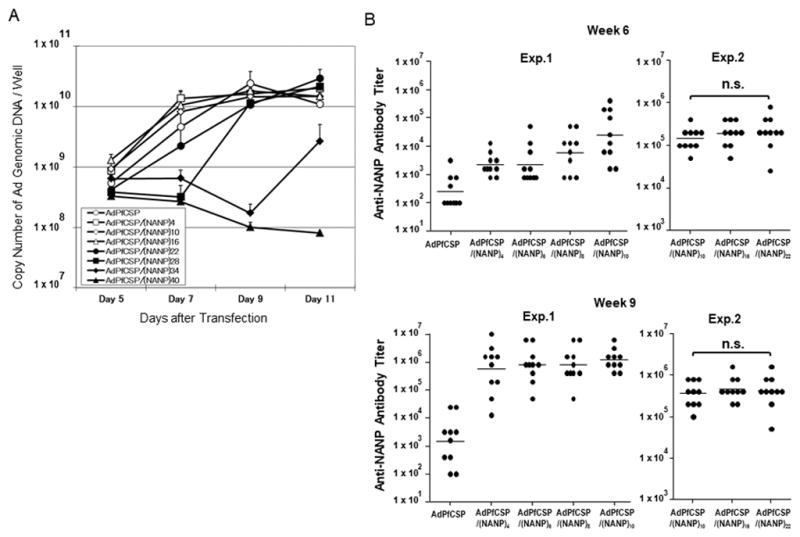
Adenovirus productivity after adenovirus genome transfection and Anti-NANP antibody titer after Hexon-modified adenovirus immunization. (A) Adenovirus packaging cell line AD293 was transfected with linearized adenovirus genomes, and then cells and supernatant were collected at indicated days after transfection. Adenovirus genomic DNA was extracted from the samples and used for quantification by Real Time PCR. Error bars represent standard deviation (n=3). (B) Groups of naïve BALB/c mice (ten per group) were immunized with 1×108, 1×109 or 1×1010 v.p. of various rAd intramuscularly at 3-week interval, and NANP-specific antibody responses were measured at week 6 and 9. Anti-NANP antibody titer was measured by ELISA using (T1B)4 antigen to coat ELISA plates and described as end-point dilution folds to detect the antigen. Antibody titers of 100 or below were plotted as 100. Bars represent geometric means.
Next we evaluated the effect of the number of the NANP repeats expressed in the Hexon of AdPfCS vaccine on the induction of anti-NANP antibody response in mice upon immunization. We first gave groups of mice a single immunizing dose of the Hexon-modified adenoviruses, at doses ranging from 1×108 to 1×1010 virus particles (v.p.). Both modified and unmodified adenoviruses induced a highest level of anti-NANP response when 1×1010 v.p. was given, and there were no significant differences with regards to the level of anti-NANP antibody induced among the groups (data not shown). This finding corroborates with our earlier results using P. yoelii B-cell repeats [21]. We then immunized mice with multiple doses of AdPfCSP, AdPfCSP/(NANP)4, AdPfCSP/(NANP)6, AdPfCSP/(NANP)8, or AdPfCSP/(NANP)10 with increasing doses, i.e. 1×108, 1×109, and 1×1010 v.p. at week 0, 3 and 6. Anti-NANP antibody titer was measured at week 6 and 9. The increasing doses were used here in an attempt to elude a potent pre-existing anti-A5 immunity that could be induced by a priming with a high dose of the adenovirus. All of the Hexon-modified Adenoviruses induced statistically higher antibody titer than AdPfCSP at week 6 and 9 (p<0.01, one-way ANOVA followed by Dunnett’s Multiple Comparison Test) and a trend was observed that more NANP repeats in Hexon induced higher anti-NANP antibody titer (Figure 1B, Exp. 1). We also tested AdPfCSP/(NANP)10, AdPfCSP/(NANP)16, AdPfCSP/(NANP)22 in a different set of experiment (Figure 1B, Exp. 2) and observed a trend that the more repeats induced the higher anti-NANP antibody titer, although the difference was not statistically significant.
Based on the observations above, we concluded that insertion of twenty-two repeats of NANP is the best modification in terms of adenovirus production, genomic stability and anti-NANP antibody induction.
Insertion of the PfCSP universal CD4 T cell epitope in pVII
A universal CD4 epitope, which can be presented onto a wide variety of MHC class II alleles, has been reported in PfCSP [27] and this epitope-specific CD4+ T-cell response was shown to be associated with clinical protection by RTS,S [8]. Therefore, we sought to enhance this epitope-specific CD4+ T-cell response by inserting the epitope into one of adenoviral proteins. As the adenoviral protein to be inserted, we chose adenovirus core protein VII (pVII), which is associated with adenovirus genomic DNA and mediates translocation to nucleus. A high copy number (about 700 to 800 copies per virion) of pVII presents in an adenovirus virion, making it advantageous for the induction of a potent CD4+ T-cell response [30]. Another advantage is that pVII is not a capsid protein, and, therefore, the modification of pVII would not affect adenovirus-cell interaction and/or adenovirus infectivity.
PSORT-II, a computer program for the prediction of protein localization sites [31], predicted pVII has multiple two Nuclear Localization Signals (NLS) in the middle of the protein, as shown in Figure 3A. We constructed adenovirus genomes carrying the PfCSP universal epitope in the C-terminus, N-terminus, upstream of the first NLS, or between the NLSs (Fig. 2A). All viruses except for the one carrying the epitope in the N-terminus, AdPfCSP/(NANP)4/CD4-pVII-2, produced infectious adenovirus after adenovirus genome transfection. Therefore AdPfCSP/(NANP)4/CD4-pVII-1, AdPfCSP/(NANP)4/CD4-pVII-3 and AdPfCSP/(NANP)4/CD4-pVII-4 were produced and purified by CsCl-ultracentrifuge for further analysis.
Figure 3.
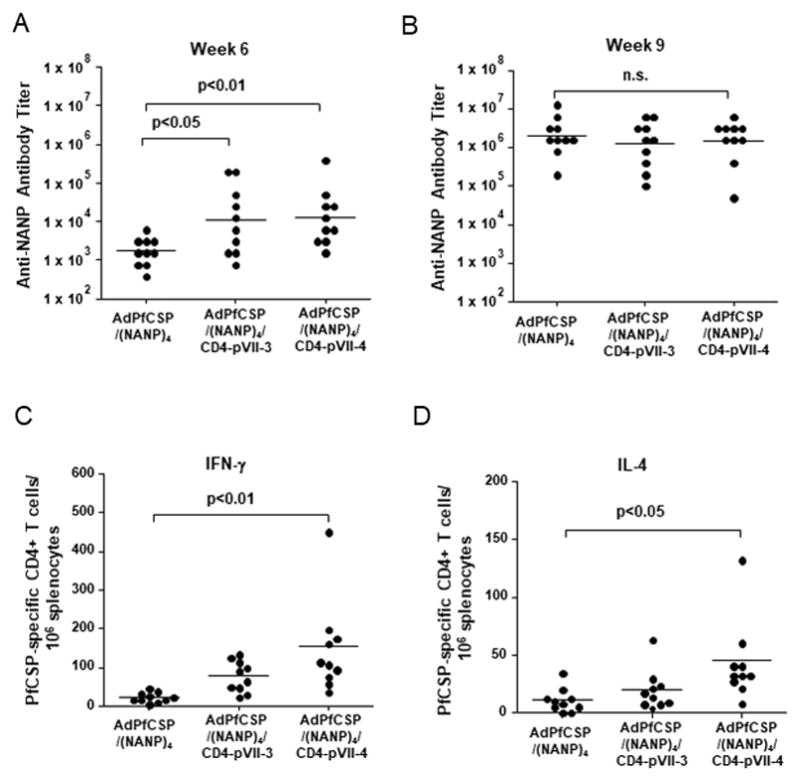
Humoral and cell-mediated immune responses induced by vaccination with pVII-modified adenoviruses
Groups of naïve BALB/c mice (ten per group) were immunized with 1×108, 1×109 or 1×1010 v.p. of pVII-modified adenoviruses intramuscularly at 3-week interval. (A) NANP-specific antibody responses were measured at week 6 by ELISA using (T1B)4 antigen and described as end-point dilution folds to detect the antigen. Values were log-transformed. (B) NANP-specific antibody titer at week 9. Bars represent geometric means. (C) PfCSP-specific IFN-γ-secreting CD4+ T-cell response was measured by IFN-γ ELISPOT assay using a synthetic peptide, corresponding to the PfCSP-derived, universal CD4+ T-cell epitope, EYLNKIQNSLSTEWSPCSVT, to stimulate splenocytes prepared from the immunized mice. (D) PfCSP-specific IL-4-secreting CD4+ T-cell response was measured by IL-4 ELISPOT assay using above mentioned peptide, corresponding to the PfCSP-specific CD4+ T-cell epitope. Bars represent means. In Figures 3 and 4, one-way ANOVA followed by a Dunnett’s test was employed to determine the differences among groups.
Figure 2.
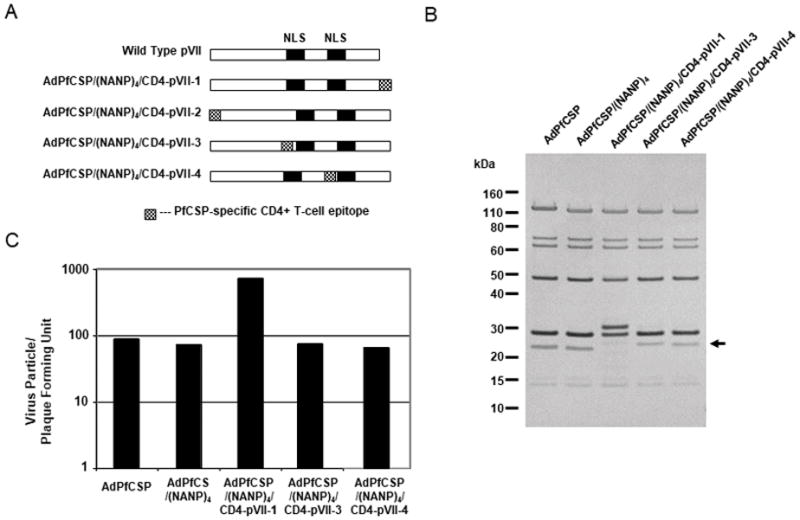
Characterization of adenovirus core proteinVII-modified adenoviruses
(A) Schematic structure of wild-type and modified pVII. Fill boxes show putative Nuclear Localization Signals (NLS). The first and second NLSs are located at 99 – 113a.a. and 141 – 158 a.a., respectively. Checkered boxes represent the PfCSP universal CD4 epitope inserted in pVII (between 198 a.a. and the stop, 1 and 2 a.a., 98 and 99 a.a., or 140 and 141 a.a. respectively). (B) Sliver-Staining of purified pVII-modified adenoviruses. 2×109 v.p. per lane were lysed in SDS-PAGE Sample Buffer and separated on 4 to 12% polyacrylamide gel. The gel was stained with Sliver Staining Kit (Invitrogen). (C) Plaque Forming Unit was determined by end point dilution assay. 2×104 AD293 cells were seeded in 96 well plates and then ten-fold serial dilutions of adenovirus were added to the plates (12 wells for one dilution). The plates were incubated for 10 days at 37°C, 5% CO2 and then number of wells with plaque was counted.
The purified pVII-modified adenoviruses were analyzed by SDS-PAGE to confirm that the epitope is correctly inserted into pVII. Shift of pVII bands on SDS-PAGE gel was observed in AdPfCSP/(NANP)4/CD4-pVII-3 and AdPfCSP/(NANP)4/CD4-pVII-4 (Fig. 2B). AdPfCSP/(NANP)4/CD4-pVII-1, which has the epitope in the C-terminus, displays a strange migration pattern, which is not explainable (Fig. 2B).
Next, we evaluated the effect of pVII modification on virus infectivity and production using packaging AD293 cells as target cells. Virus particle (v.p.) per plaque forming unit (p.f.u.) of pVII-modified adenoviruses except AdPfCSP/(NANP)4/CD4-pVII-1 was similar to wild-type adenovirus (Fig. 2C), which indicated that the modification did not affect adenovirus fitness at least in vitro. AdPfCSP/(NANP)4/CD4-pVII-1 grows slowly compared to the wild-type and other pVII-modified adenoviruses, indicating that the insertion of the epitope in the C-terminus affected virus infectivity and/or productivity (Fig. 2C).
The effect of the CD4+ T-cell epitope insertion into pVII of AdPfCSP(NANP)4 on the in vivo induction of PfCSP-specific humoral and cell-mediated immune responses was determined. AdPfCSP/(NANP)4, AdPfCSP/(NANP)4/CD4-pVII-3 and AdPfCSP/(NANP)4/CD4-pVII-4 were injected with increasing doses, i.e. 1×108, 1×109, and 1×1010 v.p. at week 0, 3 and 6, and anti-NANP antibody titer was measured at week 6 and 9 (Fig. 3A and B). Anti-NANP antibody titers induced in mice immunized with AdPfCSP/(NANP)4/CD4-pVII-3 or AdPfCSP/(NANP)4/CD4-pVII-4 were significantly higher than those induced in mice immunized with AdPfCSP/(NANP)4 at week 6 (Fig. 3A). However, no difference was observed in antibody titers among the groups at week 9 (Fig. 3B). When we collected splenocytes from the immunized mice at week 9 and performed ELISpot assays to determine the relative number of PfCSP-specific CD4+ T cells secreting IFN-γ or IL-4 (Fig. 3C and D), immunization with AdPfCSP/(NANP)4/CD4-pVII-4 was found to induce significantly higher levels of PfCSP-specific CD4+ T-cell responses that secrete IFN-γ and IL-4 than those induced by AdPfCSP/(NANP)4 immunization (Fig. 3C and D).
Evaluation of Hexon and pVII-modified PfCSP adenovirus
Based on the results described above, we hypothesized that adenovirus having twenty-two repeats of NANP in Hexon and the PfCSP-specific CD4+ T-cell epitope in pVII in addition to PfCSP expression should induce strong CD4+ and CD8+ T-cell responses against the PfCSP and high titers of anti-PfCSP antibody response. For this study, we have also constructed two control adenoviruses, Ad (NANP)22 having a modification in Hex on without the PfCSP transgene and Ad (NANP)22/CD4-pVII-4 having modifications in both Hexon and pVII without the PfCSP transgene n order to evaluate a contribution of the transgene to immune responses induced by Hexon and pVII-modified adenoviruses.
Mice were immunized with 1×1010 v.p. adenoviruses three times at 3-week interval. Humoral immune response against NANP was measured by ELISA at week 6 and 8 and PfCSP-specific CD4+ and CD8+ T cell responses were measured by ELISpot assay at week 8.
As for the humoral response, Ad(NANP)22 that has the PfCSP transgene (AdPfCSP/(NANP)22) and/or the PfCSP-specific CD4+ T-cell epitope in pVII (AdPfCSP/(NANP)22/CD4-pVII-4 or Ad(NANP)22/CD4-pVII-4), induced higher level of antibody than Ad(NANP)22 at week 6 and week 8 (Fig. 4A and 4B). The difference in antibody titer between Ad(NANP)22 and Ad(NANP)22/CD4-pVII-4 was statistically significant at week 6 and 8 (p<0.01, unpaired t-test, Fig. 4A and 4B). This indicates that the insertion of the PfCSP-specific CD4+ T-cell epitope in pVII contributed to the enhancement of humoral response against the PfCSP. Intriguingly, Ad(NANP)22/CD4-pVII-4 could induce a similar or slightly higher level of PfCSP-specific antibody response to AdPfCSP/(NANP)22 (n.s., unpaired t-test, Fig. 4A and 4B).
Figure 4.
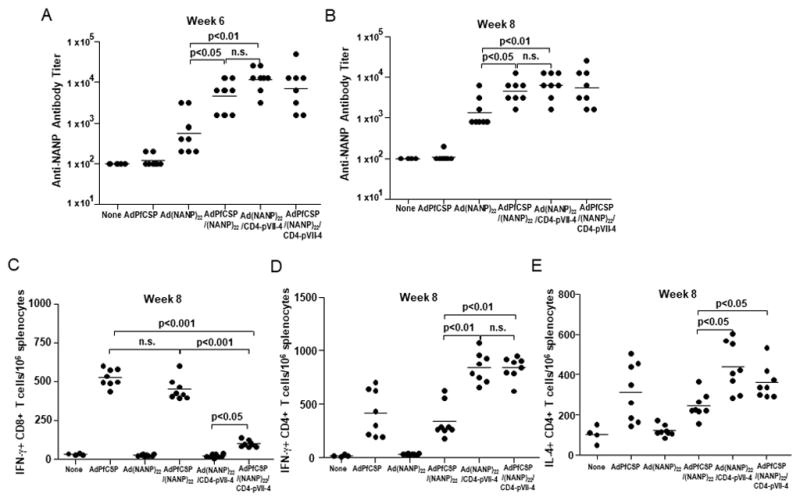
Humoral and cell-mediated immune responses induced by Hexon and pVII-modified Adenovirus
Groups of naïve BALB/c mice (ten per group) were immunized with 1×1010 v.p. of Hexon and/or pVII-modified adenoviruses intramuscularly three times at 3-week interval. (A) NANP-specific antibody responses were measured at week 6 by ELISA as described in Figure 3A. (B) NANP-specific antibody responses at week 8. Antibody titers of 100 or below were plotted as 100. Bars represent geometric means. Values were log-transformed. (C) PfCSP-specific IFN-γ-secreting CD8+ T-cell response was measured by IFN-γ ELISPOT assay using a synthetic peptide, representing the PfCSP-specific CD8+ T-cell epitope, NYDNAGTNL, to stimulate splenocytes prepared from the immunized mice. (D) PfCSP-specific IFN-γ-secreting CD4+ T-cell response was measured, as described in Figure 3C. (E) PfCSP-specific IL-4-secreting CD4+ T-cell response was measured, as described in Figure 3D. Bars represent means.
The level of PfCSP-specific CD8+ T-cell response induced by AdPfCSP/(NANP)22 was comparable with that induced by AdPfCSP, which indicates that the Hexon modification does not seem to undermine the adenovirus infectivity in vivo (Figure 4C). In contrast, immunization with AdPfCSP/(NANP)22/CD4-pVII-4, which has the CD4 epitope in pVII, induced a significantly lower level of PfCSP-specific CD8+ T-cell response compared to that induced by AdPfCSP/(NANP)22 (p<0.001, unpaired t-test, Fig. 4C). This indicates that the pVII modification has a negative effect on the PfCSP transgene expression upon adenovirus infection in vivo.
We confirmed that PfCSP-derived CD4+ T-cell epitope insertion into pVII augments PfCSP-specific CD4+ T-cell responses (Fig. 4D and 4E). AdPfCSP/(NANP)22/CD4-pVII-4 induced a significantly higher number of PfCSP-specific CD4+ T cells secreting IFN-γ (p<0.01, unpaired t-test, Fig. 4D) and IL-4 (p<0.05, unpaired t-test, Fig. 4E) than AdPfCSP/(NANP)22. It was also noted that Ad(NANP)22/CD4-pVII-4 induced a comparable level of CD4+ T-cell response, particularly those secreting IFN-γ to AdPfCSP/(NANP)22/CD4-pVII-4 (n.s., unpaired t-test, Fig. 4D).
Protection by Hexon and pVII-modified adenovirus against challenge with PfCSP-expressing transgenic mouse malaria parasites
Finally, we sought to investigate whether enhanced humoral and cellular immune responses induced by Hexon/pVII-modified adenoviral vaccine would contribute to an overall protection against malaria infection. For the challenge purpose, we first used transgenic P. berghei parasites that express only the repeat domain of PfCSP, but not N- or C-terminus of the PfCSP that contains the CD4+ T-cell epitope (Fig. 5A)[28]. Most recently, we were able to generate a novel transgenic P. yoelii parasite clone that expresses a full-length PfCSP, which contains both B-cell and CD4+ T-cell epitopes, and found that the infectivity of these full-length PfCSP-bearing Py sporozoites is as good as the wild-type counterpart [29]. Therefore, we have also used these highly infectious transgenic Py sporozoites for challenge in Fig. 5B. Groups of immunized mice, as well as naïve mice, were challenged by the tail vain injection of live transgenic sporozoites, followed by monitoring blood stage infection up to 10–12 days after the challenge by thin blood smears.
Figure 5.
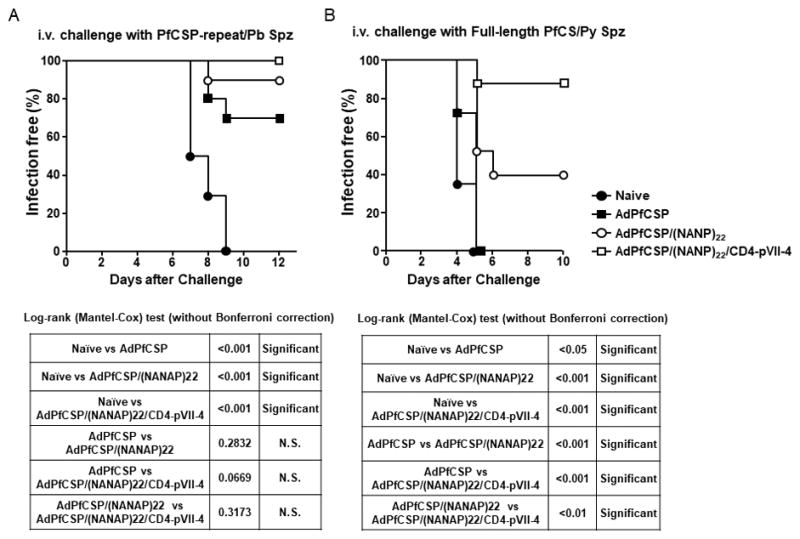
Protective anti-malaria immunity induced by Hexon and pVII-modified Adenovirus
Groups of naïve BALB/c mice (ten per group for A and fifteen per group for B) were immunized with 1×1010 v.p. of Hexon and/or pVII-modified adenoviruses intramuscularly three times at 3-week interval. Two weeks after last immunization, immunized, as well as naïve, mice were challenged intravenously with transgenic P. berghei sporozoites that express the PfCSP repeat, PfCS-repeat/Pb spz (A) or transgenic P. yoelii sporozoites that express the full-length PfCSP, PfCS/Py spz (B). From day 2 after challenge, the thin blood smears were taken daily and the presence or absence of parasites in the blood (parasitemia) was determined under the microscope after Giemsa-staining of the blood smears. Log-rank (Mantel-Cox) test was applied without adjusting for multiple comparisons to compare infection-free survival between two groups.
When we challenged groups of mice with 1 × 103 transgenic P. berghei sporozoites by i.v., we were not able to observe a significant increase in terms of the number of protected mice that were immunized by AdPfCSP/(NANP)22/CD4-pVII-4, compared to those immunized with AdPfCSP/(NANP)22 or AdPfCSP (Fig. 5A). Then, when we challenged groups of immunized and naïve mice with 50 live sporozoites of transgenic Py parasites that express an entire PfCSP by tail vein injection, we could observe that 87% (13 out of 15) of mice immunized with AdPfCSP/(NANP)22/CD4-pVII-4 protected from infection, whereas 40% (6 out of 15) of mice immunized with AdPfCSP/(NANP)22 and none of AdPfCSP-immunized mice were protected (Fig. 5B). Taken together these results indicate that the insertion of PfCSP-derived B-cell epitopes and CD4+ T-cell epitope in Hexon and pVII, respectively, not only increased the levels of malaria-specific humoral and cellular immune responses, but also augmented the level of protective anti-malaria immunity and provided a sterile protection to significantly higher number of vaccinated animal.
Discussion
Malaria remains to be one of the most prevalent and deadly infectious diseases especially in Africa and therefore effective vaccines against malaria infection are desperately needed.
Among a number of approaches, developing a CSP-based vaccine to target the pre-erythrocytic stages is thought to be one of the most promising approaches. For example, CSP is a major component of the most advanced malaria vaccine candidate, RTS,S/AS01, also called Mosquirix, which has been adopted a positive opinion for use in endemic countries by the European Medicines Agency [32]. A clinical phase 3 trial evaluated the vaccine efficacy against all episodes of severe malaria to be approximately 50% in young children in Africa [2, 3]. There was a correlation between antibody titers against PfCSP and the degree of protection [33].
In addition to the protective role of humoral response against the CSP, CD8+ T cells [10–14, 34–38] and CD4+ T cells, to some extent [16, 17, 39, 40], have been shown to contribute to the protective immunity against malaria in a mouse model. Apart from a conventional mouse model, we have most recently been able to show that a single immunizing dose of AdPfCSP induced anti-malaria immunity in humanized mice that mimic human immune system [41] and that the protective anti-malaria immunity was abolished by the depletion of human CD8+ T cells in vivo, indicating the protective role of human CD8+ T cells against malaria [42]. Retrospective analysis of immune responses in RTS,S clinical trials revealed that CSP-specific CD4+ T cell response is an independent factor associated with protection [18], thus suggesting the protective role of human CD4+ T cells against malaria.
Based on published observations above, we sought to develop a CSP-based malaria vaccine that is able to induce a respectable level of both CSP-specific humoral and cellular immune responses. For this purpose, we chose adenovirus as a vaccine platform because of its ability to induce a strong antigen-specific CD8+ T-cell response [11]. We have previously shown that vaccination of mice with capsid-modified adenovirus having an insertion of Plasmodium yoelii B-cell epitope repeats into the HVR1 of its Hexon in addition to a PyCSP transgene, induced a more potent cell-mediated and humoral immune responses and protected more mice from P. yoelii sporozoite challenge than the vaccination with a conventional Ad5 expressing a PyCSP alone [21]. In the current study, we used the CSP of a human malaria parasite, P. falciparum, as a model antigen. Hence, in addition to using a PfCSP gene as a transgene, we inserted a peptide, (NANP)n, representing the PfCSP B-cell epitope, into the Hexon and a peptide that corresponds to the PfCSP-specific universal CD4+ T-cell epitope into the pVII of Ad5. We made such modifications to Ad5 based on our hypothesis that adenovirus virion itself may act as a virus-like particle and, upon uptake by APCs, the modified Ad5 may present the PfCSP-specific CD4+ T-cell epitope via the exogenous pathway, thus being able to stimulate both PfCSP-specific CD4+ T-cell response and humoral response.
We could successfully insert up to twenty-two repeats of NANP in HVR1 of Hexon without affecting adenovirus fitness. To our knowledge, this is the first study where capacity of Hexon for foreign peptide insertion was analyzed systematically. Ability to hold up to about 90 amino acids in Hexon would widen a window of application of Hexon-modified adenovirus to vaccine development for other infectious diseases. As we have shown previously [21], the replacement of HVR1 in Hexon circumvented the negative effect of pre-existing anti-adenovirus antibody, thus benefiting the strategy of using adenovirus-based vaccines in clinical settings.
We confirmed our previous studies using P. yoelii CSP-derived repeat, (QGPGAP)3 [21], that adenoviruses having the NANP repeats in Hexon could induce significantly higher anti-NANP antibody titer. Intriguingly, the Hexon-modified adenovirus without transgene - Ad(NANP)22 - induced significantly lower antibody titer than the one with the transgene AdPfCSP/(NANP)22 or Ad(NANP)22/CD4-pVII-4 (Figure 4A and 4B). This indicates that the PfCSP-specific CD4+ T-cell response, induced by the PfCSP transgene or PfCSP-specific CD4+ epitope in VII, seemed to have strengthened the humoral response induced by the NANP epitope present in the Hexon. Here we must emphasize that the levels of anti-NANP response induced in mice received 3 immunizations of increasing doses (108–1010 v.p.) of Hexon-modified adenoviruses (Fig. 3B) were more than 2-log higher than those induced in mice received 3 immunizations of the same dose (1010 v.p.) of the same Hexon-modified adenoviruses (Fig. 4B). This phenomenon is seen most likely due to the fact that upon vaccination with a high dose (1010 v.p.) of adenovirus mice mounted a potent pre-existing anti-Ad5 immunity that substantially reduced the immunogenicity of the Hexon-modified adenovirus subsequently used even at the dose of 1010 v.p. injection, as we previously observed [21].
As for the CD4+ T-cell responses, an insertion of PfCSP-derived CD4+ T-cell epitope strongly augmented both IFN-γ and IL-4 secreting PfCSP-specific CD4+ T-cell responses as we anticipated (Figure 4D and 4E). The level of PfCSP-specific CD4+ T-cell response induced by Ad(NANP)22/CD4-pVII-4 was significantly higher than that induced by AdPfCSP/(NANP)22, suggesting that the exogenous pathway may be more efficient than the endogenous pathway in inducing the CD4+ T-cell response. In addition, the PfCSP-specific CD4+ T-cell epitope inserted into VII might have been expressed a high copy number (700 to 800 copies) per one virion than the PfCSP as a transgene. It should be noted that the CD4+ T-cell epitope we chose in this study is known as a universal CD4+ T-cell epitope [27], which means that the epitope can be promiscuously presented by a majority of MHC class II haplotypes across the board, and, therefore, a large human populations regardless of their HLA class II polymorphism can be effectively vaccinated with this Ad(NANP)22/CD4-pVII-4 vaccine.
The only pitfall of this AdPfCSP/(NANP)22/CD4-pVII-4 vaccine is that although there was no effect of the CD4+ T-cell epitope insertion into pVII on adenovirus infectivity and production in vitro, the insertion seemed to have reduced the infectivity or transgene expression in vivo. This was proven by the fact that AdPfCSP/(NANP)22/CD4-pVII-4 induced a significantly lower PfCSP-specific CD8+ T-cell response than AdPfCSP/(NANP)22 (Figure 4C).
Nevertheless, the most important is to learn which modified adenovirus vaccine could induce a most potent protective anti-malaria immunity. Since most adenovirus vaccines are made based on the sequence of PfCSP, we used transgenic rodent malaria parasites that express PfCSP as a challenge agent. When transgenic P. berghei parasites that express NANP repeat in addition to PbCSP [28] were used to challenge, a minimal difference was seen among the groups of mice received different adenovirus vaccines (Fig. 5A). However, when groups of mice were challenged with highly infectious transgenic P. yoelii parasites that express a full-length of PfCSP instead of PyCSP (Fig. 5B)[29], AdPfCSP/(NANP)22/CD4-pVII-4, an adenovirus having modifications at both Hexon and pVII, protected more mice than not only the mice received a conventional PfCSP adenovirus vaccine, but also those received Hexon-modified adenovirus (Fig. 5B). This discrepancy is most likely due to the fact that the transgenic P. berghei parasites that express only NANP repeat, whereas the transgenic P. yoelii parasites express an entire PfCSP that includes a CD4+ T-cell epitope that can contribute to the protection. We are currently investigating this issue more in detail.
In summary, we have constructed PfCSP-expressing adenoviruses having modification at Hexon and/or pVII to incorporate PfCSP-specific B-cell epitope and/or CD4+ T-cell epitope, respectively in this study, and shown that the doubly modified adenovirus vaccine can induce both humoral and cellular immune responses potently, and ultimately, protected a majority of mice from challenge with highly infectious transgenic rodent parasites that express PfCSP. Therefore, Hexon and pVII-modified adenoviral malaria vaccine that express not only PfCSP but also its B-cell and CD4+ T-cell epitopes, is a promising candidate as a next generation malaria vaccine to be tested in clinical trials in the future.
Supplementary Material
Acknowledgments
We thank Dr. Elizabeth Nardin and Dr. Ana Rodriguez of New York University for providing transgenic P. berghei and the parasite-infected mosquitoes, respectively. We also thank Tiffany Tsao for her technical assistance.
Funding
This research was supported by grants from NIH R01 AI081510 and Otsuka Pharmaceutical Co. Ltd (both to M.T.) and from KAKENHI 26293091, 26305009, and 25253027 (to M.Y.).
Abbreviations
- Ad
adenovirus
- rAd
recombinant adenovirus
- Ad5
adenovirus serotype 5
- CSP
circumsporozoite protein
- HVR
hyper-variable region
- PfCSP
Plasmodium. falciparum CSP
- pVII
adenovirus core protein VII
- v.p
virus particle
Footnotes
Author contributions
TS and MT designed research studies. TS, UR and MZ conducted experiments and acquired data. IK, SI and MY provided PfCS/Py Spz. TS and MT analyzed data and wrote the manuscript.
Conflict of interest
The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. World malaria report. 2014 http://www.who.int/malaria/publications/world_malaria_report_2014/
- 2.RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.RTS,S Clinical Trials Partnership. Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmuller B, Issifou S, et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012;367:2284–2295. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doolan DL, Hoffman SL. The complexity of protective immunity against liver-stage malaria. J Immunol. 2000;165:1453–1462. doi: 10.4049/jimmunol.165.3.1453. [DOI] [PubMed] [Google Scholar]
- 5.Overstreet MG, Cockburn IA, Chen YC, Zavala F. Protective CD8 T cells against Plasmodium liver stages: Immunobiology of an “unnatural” immune response. Immunol Rev. 2008;225:272–283. doi: 10.1111/j.1600-065X.2008.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuji M. A retrospective evaluation of the role of T cells in the development of malaria vaccine. Exp Parasitol. 2010;126:421–425. doi: 10.1016/j.exppara.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nardin EH, Nussenzweig V, Nussenzweig RS, Collins WE, Harinasuta KT, Tapchaisri P, Chomcharn Y. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J Exp Med. 1982;156:20–30. doi: 10.1084/jem.156.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olotu AI, Fegan G, Bejon P. Further analysis of correlates of protection from a phase 2a trial of the falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults. J Infect Dis. 2010;201:970–971. doi: 10.1086/651025. [DOI] [PubMed] [Google Scholar]
- 9.Kumar KA, Sano G, Boscardin S, Nussenzweig RS, Nussenzweig MC, Zavala F, Nussenzweig V. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature. 2006;444:937–940. doi: 10.1038/nature05361. [DOI] [PubMed] [Google Scholar]
- 10.Li S, Rodrigues M, Rodriguez D, Rodriguez JR, Esteban M, Palese P, Nussenzweig RS, Zavala F. Priming with recombinant influenza virus followed by administration of recombinant vaccinia virus induces CD8+ T-cell-mediated protective immunity against malaria. Proc Natl Acad Sci U S A. 1993;90:5214–5218. doi: 10.1073/pnas.90.11.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigues EG, Zavala F, Eichinger D, Wilson JM, Tsuji M. Single immunizing dose of recombinant adenovirus efficiently induces CD8+ T cell-mediated protective immunity against malaria. J Immunol. 1997;158:1268–1274. [PubMed] [Google Scholar]
- 12.Tsuji M, Bergmann CC, Takita-Sonoda Y, Murata K, Rodrigues EG, Nussenzweig RS, Zavala F. Recombinant Sindbis viruses expressing a cytotoxic T-lymphocyte epitope of a malaria parasite or of influenza virus elicit protection against the corresponding pathogen in mice. J Virol. 1998;72:6907–6910. doi: 10.1128/jvi.72.8.6907-6910.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao D, Barba-Spaeth G, Rai U, Nussenzweig V, Rice CM, Nussenzweig RS. Yellow fever 17D as a vaccine vector for microbial CTL epitopes: protection in a rodent malaria model. J Exp Med. 2005;201(2):201–209. doi: 10.1084/jem.20041526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearson FE, O’Mahony C, Moore AC, Hill AV. Induction of CD8(+) T cell responses and protective efficacy following microneedle-mediated delivery of a live adenovirus-vectored malaria vaccine. Vaccine. 2015;33:3248–3255. doi: 10.1016/j.vaccine.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 15.Moorthy VS, Ballou WR. Immunological mechanisms underlying protection mediated by RTS,S: a review of the available data. Malar J. 2009;8:312. doi: 10.1186/1475-2875-8-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Giudice G, Grillot D, Rénia L, Müller I, Corradin G, Louis JA, Mazier D, Lambert PH. Peptide-primed CD4+ cells and malaria sporozoites. Immunol Lett. 1990;25:59–63. doi: 10.1016/0165-2478(90)90092-5. [DOI] [PubMed] [Google Scholar]
- 17.Takita-Sonoda Y, Tsuji M, Kamboj K, Nussenzweig RS, Clavijo P, Zavala F. Plasmodium yoelii: peptide immunization induces protective CD4+ T cells against a previously unrecognized cryptic epitope of the circumsporozoite protein. Exp Parasitol. 1996;84:223–230. doi: 10.1006/expr.1996.0108. [DOI] [PubMed] [Google Scholar]
- 18.Garçon N, Heppner DG, Cohen J. Development of RTS,S/AS02: a purified subunit-based malaria vaccine candidate formulated with a novel adjuvant. Expert Rev Vaccines. 2003;2:231–238. doi: 10.1586/14760584.2.2.231. [DOI] [PubMed] [Google Scholar]
- 19.Rampling T, Ewer KJ, Bowyer G, Bliss CM, Edwards NJ, Wright D, Payne RO, Venkatraman N, de Barra E, Snudden CM, et al. Safety and High Level Efficacy of the Combination Malaria Vaccine Regimen of RTS,S/AS01B With Chimpanzee Adenovirus 63 and Modified Vaccinia Ankara Vectored Vaccines Expressing ME-TRAP. J Infect Dis. 2016;214:772–781. doi: 10.1093/infdis/jiw244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regules JA, Cicatelli SB, Bennett JW, Paolino KM, Twomey PS, Moon JE, Kathcart AK, Hauns KD, Komisar JL, Qabar AN, et al. Fractional Third and Fourth Dose of RTS,S/AS01 Malaria Candidate Vaccine: A Phase 2a Controlled Human Malaria Parasite Infection and Immunogenicity Study. J Infect Dis. 2016;214:762–771. doi: 10.1093/infdis/jiw237. [DOI] [PubMed] [Google Scholar]
- 21.Shiratsuchi T, Rai U, Krause A, Worgall S, Tsuji M. Replacing adenoviral vector HVR1 with a malaria B cell epitope improves immunogenicity and circumvents preexisting immunity to adenovirus in mice. J Clin Invest. 2010;120:3688–3701. doi: 10.1172/JCI39812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palma C, Overstreet MG, Guedon JM, Hoiczyk E, Ward C, Karen KA, Zavala F, Ketner G. Adenovirus particles that display the Plasmodium falciparum circumsporozoite protein NANP repeat induce sporozoite-neutralizing antibodies in mice. Vaccine. 2011;29:1683–1689. doi: 10.1016/j.vaccine.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karen KA, Deal C, Adams RJ, Nielsen C, Ward C, Espinosa DA, Xie J, Zavala F, Ketner G. A replicating adenovirus capsid display recombinant elicits antibodies against Plasmodium falciparum sporozoites in Aotus nancymaae monkeys. Infect Immun. 2015;83:268–275. doi: 10.1128/IAI.02626-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fonseca JA, Cabrera-Mora M, Kashentseva EA, Villegas JP, Fernandez A, Van Pelt A, Dmitriev IP, Curiel DT, Moreno A. A Plasmodium Promiscuous T Cell Epitope Delivered within the Ad5 Hexon Protein Enhances the Protective Efficacy of a Protein Based Malaria Vaccine. PLoS One. 2016;11:e0154819. doi: 10.1371/journal.pone.0154819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Oliveira GA, Clavijo P, Nussenzweig RS, Nardin EH. Immunogenicity of an alum-adsorbed synthetic multiple-antigen peptide based on B- and T-cell epitopes of the Plasmodium falciparum CS protein: possible vaccine application. Vaccine. 1994;12:1012–1017. doi: 10.1016/0264-410x(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 26.Hedstrom RC, Doolan DL, Wang R, Kumar A, Sacci JB, Jr, Gardner MJ, Aguiar JC, Charoenvit Y, Sedegah M, Tine JA, et al. In vitro expression and in vivo immunogenicity of Plasmodium falciparum pre-erythrocytic stage DNA vaccines. Int J Mol Med. 1998;2:29–38. doi: 10.3892/ijmm.2.1.29. [DOI] [PubMed] [Google Scholar]
- 27.Calvo-Calle JM, Hammer J, Sinigaglia F, Clavijo P, Moya-Castro ZR, Nardin EH. Binding of malaria T cell epitopes to DR and DQ molecules in vitro correlates with immunogenicity in vivo: identification of a universal T cell epitope in the Plasmodium falciparum circumsporozoite protein. J Immunol. 1997;159:1362–1373. [PubMed] [Google Scholar]
- 28.Persson C, Oliveira GA, Sultan AA, Bhanot P, Nussenzweig V, Nardin E. Cutting edge: a new tool to evaluate human pre-erythrocytic malaria vaccines: rodent parasites bearing a hybrid Plasmodium falciparum circumsporozoite protein. J Immunol. 2002;169:6681–6685. doi: 10.4049/jimmunol.169.12.6681. [DOI] [PubMed] [Google Scholar]
- 29.Zhang M, Kaneko I, Tsao T, Mitchell R, Nardin EH, Iwanaga S, Yuda M, Tsuji M. A highly infectious Plasmodium yoelii parasites, bearing Plasmodium falciparum circumsporozoite protein. Malaria J. 2016;15:201. doi: 10.1186/s12936-016-1248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Oostrum J, Burnett RM. Molecular composition of the adenovirus type 2 virion. J Virol. 1985;56:439–448. doi: 10.1128/jvi.56.2.439-448.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakai K, Horton P. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem Sci. 1999;24:34–36. doi: 10.1016/s0968-0004(98)01336-x. [DOI] [PubMed] [Google Scholar]
- 32.Birkett AJ. Status of vaccine research and development of vaccines for malaria. Vaccine. 2016;34:2915–2920. doi: 10.1016/j.vaccine.2015.12.074. [DOI] [PubMed] [Google Scholar]
- 33.White MT, Verity R, Griffin JT, Asante KP, Owusu-Agyei S, Greenwood B, Drakeley C, Gesase S, Lusingu J, Ansong D, et al. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect Dis. 2015;15:1450–1458. doi: 10.1016/S1473-3099(15)00239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero P, Maryanski JL, Corradin G, Nussenzweig RS, Nussenzweig V, Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989;341:323–326. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigues MM, Cordey AS, Arreaza G, Corradin G, Romero P, Maryanski JL, Nussenzweig RS, Zavala F. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int Immunol. 1991;3:579–85. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
- 36.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330:664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 37.Weiss WR, Sedegah M, Beaudoin RL, Miller LH, Good MF. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A. 1988;85:573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seguin MC, Klotz FW, Schneider I, Weir JP, Goodbary M, Slayter M, Raney JJ, Aniagolu JU, Green SJ. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei infected mosquitoes: involvement of interferon gamma and CD8+ T cells. J Exp Med. 1994;180:353–358. doi: 10.1084/jem.180.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuji M, Romero P, Nussenzweig RS, Zavala F. CD4+ cytolytic T cell clone confers protection against murine malaria. J Exp Med. 1990;172:1353–1357. doi: 10.1084/jem.172.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss WR, Sedegah M, Berzofsky JA, Hoffman SL. The role of CD4+ T cells in immunity to malaria sporozoites. J Immunol. 1993;151:2690–2698. [PubMed] [Google Scholar]
- 41.Huang J, Li X, Coelho-dos-Reis JG, Wilson JM, Tsuji M. An AAV vector-mediated gene delivery approach facilitates reconstitution of functional human CD8+ T cells in mice. PLoS One. 2014;9:e88205. doi: 10.1371/journal.pone.0088205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, Huang J, Zhang M, Funakoshi R, Sheetij D, Spaccapelo R, Crisanti A, Nussenzweig V, Nussenzweig RS, Tsuji M. Human CD8+ T cells mediate protective immunity induced by a human malaria vaccine in human immune system mice. Vaccine. 2016;34:4501–4506. doi: 10.1016/j.vaccine.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


