Abstract
Rationale
Cocaine addiction is a chronic psychiatric disorder characterized by pathological motivation to obtain cocaine and behavioral and neurochemical hypersensitivity to cocaine-associated cues. These features of cocaine addiction are thought to be driven by aberrant phasic dopamine signaling. We previously demonstrated that blockade of the hypocretin receptor 1 (HCRTr1) attenuates cocaine self-administration and reduces cocaine-induced enhancement of dopamine signaling. Despite this evidence, the effects of HCRTr1 blockade on endogenous phasic dopamine release are unknown.
Objective
In the current studies we assessed whether blockade of HCRTr1 alters spontaneous and cue-evoked dopamine release in the nucleus accumbens core of freely moving rats.
Methods
We first validated the behavioral and neurochemical effects of the novel, highly selective, HCRTr1 antagonist RTIOX-276 using cocaine self-administration and fast-scan cyclic voltammetry (FSCV) in anesthetized rats. We then used FSCV in freely moving rats to examine whether RTIOX-276 impacts spontaneous and cue-evoked dopamine release. Finally, we used ex vivo slice FSCV to determine whether the effects of RTIOX-276 on dopamine signaling involve dopamine terminal adaptations.
Results
Doses of RTIOX-276 that attenuate the motivation for cocaine reduce spontaneous dopamine transient amplitude and cue-evoked dopamine release. Further, these doses attenuated cocaine-induced dopamine uptake inhibition at the level of DA terminals.
Conclusion
Our results provide support for the standing hypothesis that HCRTr1 blockade suppresses endogenous phasic dopamine signals, likely via actions at dopamine cell bodies. These results also elucidate a second process through which HCRTr1 blockade attenuates the effects of cocaine by reducing cocaine sensitivity at dopamine terminals.
Keywords: Fast scan cyclic voltammetry, orexin, self-administration, dopamine transporter, addiction, drug abuse
Introduction
Cocaine addiction is a chronic psychiatric disorder with few effective treatment options (Dawson et al. 2007). The disorder is characterized by a pathological motivation to obtain cocaine, as well as a behavioral and neurobiological hypersensitivity to cocaine-associated cues (Jasinska et al. 2014; Robinson et al. 2014). Symptoms of cocaine addiction are likely a result of neurobiological adaptations that occur following the dramatic increases in striatal dopamine (DA) that are produced by cocaine-induced DA transporter (DAT) blockade. Thus, mesolimbic DA signaling is believed to be an important participant in the pathology of cocaine addiction (Chen et al. 2006; Ritz et al. 1987; Volkow et al. 2000).
DA neurons that project from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) are heavily implicated in the reinforcing properties of cocaine (Kalivas and O’Brien 2008; Richardson and Roberts 1996; Ritz et al. 1987). These DA neurons fire in a single-spike mode at 2–10 Hz and in burst patterns with two to six action potentials at 15–30 Hz (Freeman and Bunney 1987; Grace and Bunney 1984). The single-spike firing mode is thought to sustain a level of DA ‘tone’ in terminal fields, whereas burst patterns are thought to produce brief, phasic increases in terminal field DA concentrations (Grace 1991; Schultz 2007). While cocaine-induced alterations in DA tone surely influence the addictive properties of cocaine (Hurd and Ungerstedt 1989; Wise et al. 1995), mounting evidence suggests that phasic DA release events associated with cocaine availability play an essential role in the chronic nature of cocaine addiction (Covey et al. 2014; Willuhn et al. 2010). Specifically, phasic DA release that occurs in response to cocaine-associated cues has been shown to drive cocaine-taking (Phillips et al. 2003), and cocaine-induced changes in phasic DA release frequency and amplitude may function to cement drug-context associations following cocaine (Covey et al. 2014; Keiflin and Janak 2015; Willuhn et al. 2010). This suggests that potential pharmacotherapies that reduce cocaine self-administration may exert their behavioral effects via suppression of phasic DA signals. Several studies suggest that inhibition of the hypocretin receptor 1 (HCRTr1) may be one such potential pharmacotherapy.
The hypocretin/orexin (HCRT) system is comprised of the HCRT-1 and HCRT-2 peptides which bind to two G-protein coupled receptors, HCRTr1 and HCRT receptor 2 (de Lecea et al. 1998; Sakurai et al. 1998). HCRT signaling has been implicated in arousal and sleep/wake transitions, stress, homeostatic regulation, cognition, and motivation (Berridge et al. 2010; Mahler et al. 2014), and accumulating evidence indicates that HCRT signaling influences cocaine reward-related behavior (España et al. 2011; España et al. 2010; Mahler et al. 2012; Shaw et al. 2016). This modulation of cocaine taking appears to be predominately associated with actions at HCRTr1 versus HCRT receptor 2 (Prince et al. 2015; Smith et al. 2009), and extensive investigation into these effects has shown that HCRTr1 blockade reduces distinct features of cocaine self-administration (Bentzley and Aston-Jones 2015; Borgland et al. 2009; Brodnik et al. 2015; Calipari and España 2012; Mahler et al. 2012). Specifically, blockade of HCRTr1 does not reduce cocaine self-administration under low-effort conditions (Brodnik et al. 2015; España et al. 2010; Smith et al. 2009), but significantly reduces cocaine self-administration under conditions that require high-effort (Borgland et al. 2009; Brodnik et al. 2015; España et al. 2010). Furthermore, HCRTr1 blockade reduces both context- and cue-induced reinstatement of extinguished cocaine-taking (Smith et al. 2009; Smith et al. 2010). Together, this evidence suggests that HCRTr1 antagonists might serve to treat both pathological motivation to obtain cocaine and hypersensitivity to cocaine-associated contexts and cues.
In line with the observed HCRTr1 modulation of cocaine self-administration, HCRTr1 signaling also influences mesolimbic DA neurotransmission. HCRT-containing neurons send relatively dense projections to the VTA (Fadel and Deutch 2002; Peyron et al. 1998) where HCRT exerts excitatory influence on VTA DA neurons directly (Korotkova et al. 2003), through a suppression of GABAergic input onto DA neurons (Tung et al. 2016), and/or by enhancing glutamatergic drive onto DA neurons (Borgland et al. 2009; Borgland et al. 2006). The excitatory action of HCRT is manifested in vivo as both increases in DA neuron single-spike firing rate and bursting (Moorman and Aston-Jones 2010; Muschamp et al. 2007; Muschamp et al. 2014). These excitatory effects are likely driven by actions at HCRTr1 (Borgland et al. 2009; Tung et al. 2016), as blockade of HCRTr1 reduces VTA DA neuron activity (Moorman and Aston-Jones 2010). In consideration of this evidence, it has been posited that the effects of HCRTr1 inhibition on cocaine self-administration are mediated through modulation of mesolimbic DA activity (Baimel et al. 2012; Borgland et al. 2009; Borgland et al. 2006; Calipari and España 2012; España 2012; España et al. 2011; España et al. 2010; Moorman and Aston-Jones 2010; Prince et al. 2015; Shaw et al. 2016). Indeed, blockade of HCRTr1 attenuates cocaine-induced increases in DA tone and reduces cocaine-induced DA uptake inhibition following stimulated DA release (España et al. 2010; Prince et al. 2015). Nevertheless, it remains unclear how HCRTr1 blockade modulates endogenous phasic DA release events.
Given the importance of endogenous phasic DA signaling in cocaine addiction (Covey et al. 2014; Keiflin and Janak 2015; Willuhn et al. 2010), we sought to directly measure the effects of HCRTr1 blockade on phasic DA release in freely moving rats. For these studies, we used RTIOX-276, a novel HCRTr1 antagonist that offers the advantage of high HCRTr1 selectivity and that has previously been shown to reduce conditioned place preference for cocaine (Perrey et al. 2015a; Perrey et al. 2015b; Perrey et al. 2013). We first confirmed that systemic administration of RTIOX-276 reduces motivation for cocaine and alters DA signaling in a similar fashion as the more commonly used HCRTr1 antagonist, SB-334867. Following this validation, we used fast scan cyclic voltammetry (FSCV) in freely moving rats to examine the effects of HCRTr1 blockade on spontaneous and cue-evoked phasic DA release. We then investigated the nature of this HCRTr1 modulation of DA signaling though examination of acute HCRTr1 blockade effects on DA terminals and DA terminal sensitivity to cocaine using ex vivo FSCV. These studies demonstrate that HCRTr1 blockade exerts bimodal modulation of cocaine-associated DA signaling via regulation of phasic DA release and reduction of DA terminal sensitivity to cocaine.
Materials and Methods
Animals
Male Sprague–Dawley rats (350–450g, Harlan, Frederick, MD) were given ad libitum access to food and water and maintained on a reverse 12/12 h light/dark cycle (lights on at 15:00 h). All protocols and animal care procedures were maintained in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals: Eighth Edition (The National Academies Press, Washington, DC, 2011) and approved by the Institutional Animal Care and Use Committee at Drexel University College of Medicine.
Chemicals and Drug Preparation
RTIOX-276 was synthesized by Drs. Zhang and Perrey (Research Triangle Institute). This HCRTr1 antagonist offers greater than 1000x affinity for HCRTr1 over HCRT receptor 2 (Perrey et al. 2013), and has negligible activity at a panel of ~50 other common receptors and transporters (screened via the National Institute of Mental Health Psychoactive Drug Screening). RTIOX-276 was first dissolved in a 1.3mL solution of 5% Tween20 in H2O + 0.3ml 1M HCl before the addition of 0.3ml of 1M NaOH (final pH = 7.6). Cocaine was provided by the National Institute on Drug Abuse and was dissolved in saline.
Cocaine Self-Administration
Rats were anesthetized with ketamine (80 mg/kg) and xylazine (10 mg/kg) and implanted with an intravenous (i.v.) silastic catheter (ID, 0.012 in OD, 0.025 in, Access Technologies, Skokie, IL) in the right jugular vein that exited through the skin of the dorsal scapulae region. Rats received postsurgical antibiotic (Neo-Predef, Pharmacia & Upjohn Company, New York, NY and 5mg/kg enrofloxacin, Bayer HealthCare LLC, Shawnee Mission, KS) and analgesic (5 mg/kg; Ketoprofen, Patterson Veterinary, Devens, MA) and recovered for 3 days prior to training.
After catheterization, rats were individually housed in chambers equipped with a counterbalanced swivel that held a stainless steel spring connected to the catheter. Rats were trained to self-administer cocaine on a fixed ratio 1 (FR1) schedule of reinforcement, in which a single lever press resulted in a single injection of 0.75 mg/kg cocaine (in saline) over approximately 5 s followed by a 20 s inter-trial interval. We chose the 0.75 mg/kg dose of cocaine to test the effects of RTIOX-276 to compare with previous reports indicating the effectiveness of HCRT manipulations on self-administration at this dose (Brodnik et al. 2015; España et al. 2011; España et al. 2010; Prince et al. 2015). FR1 training sessions began at 10:00 h and were concluded once rats obtained 20 injections within the 6 hr session. Following stable responding on the FR1 schedule (20 injections per session over 3 consecutive days), rats were switched to a progressive ratio (PR) schedule of reinforcement. In the PR sessions, rats were given 6-h access to a response lever beginning at 10:00 h, and single 0.75 mg/kg cocaine injections were contingent upon increasing number of responses: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, and 603 (Richardson and Roberts 1996). After 3 days of stable responding (within ± 2 breakpoints with no ascending or descending trends), rats were given an i.p. injection of vehicle or one of three doses (5, 10, or 20 mg/kg) of RTIOX-276, 30 min prior to the start of the session (9:30 h). Rats received all drug treatments in a counterbalanced design with a minimum of 3 days between treatments.
During the early portion of a PR session rats receive injections with relatively few lever presses, and thus can readily titrate to preferred blood levels of cocaine. As the PR session continues, response requirements are increased, and thus obtaining a cocaine injection requires a greater degree of effort. To distinguish cocaine self-administration under low- and high-effort phases of the PR session, we analyzed responding using a two-phase analysis procedure as previously described (Brodnik et al. 2015). Briefly, the cumulative number of cocaine infusions obtained across each session was averaged in 5-min bins. To quantitatively define the temporal profile of the two phases, we fitted the cumulative injection data from the vehicle treatment group with multiple linear functions. Lines were fitted starting from the first 5 min bin, and we selected the line that had an R2 greater than 0.99 and that encompassed the greatest amount of time. The data encompassed by the line was defined as the ‘consumption phase’. We measured the infusion rate (infusions/hr) and total number of infusions obtained during the consumption phase as a measure of low-effort cocaine consumption. The later, high-effort phase of responding was defined as the ‘appetitive phase’. For this we used standard PR outcome measures in the number of injections obtained across the entire 6 hr session (breakpoint) and total number of lever presses across the session as a measure of high-effort appetitive phase cocaine self-administration. The effects of RTIOX-276 were assessed using one-way repeated measures ANOVA (vehicle and each dose of RTIOX-276). When statistical significance was obtained, Dunnett’s post hoc tests were conducted using vehicle as the control.
Anesthetized FSCV surgery and procedures
To examine pharmacologically-induced changes in DA release and uptake, FSCV studies were conducted in anesthetized rats. Rats were anesthetized with i.p. urethane (~1.5 g/kg), implanted with a catheter in the right jugular vein, and then placed in a stereotaxic apparatus (Brodnik and España 2015; Garris et al. 2003). Once in the apparatus, rats were implanted with a bipolar stimulating electrode (Plastics One, Roanoke, VA) in the VTA (+5.2 A/P, +1.1 M/L, −7.5 to −8.0 D/V), a carbon fiber electrode in the NAc core (+1.3 A/P, +1.3 M/L, −6.5 to −7.0 D/V), and a reference electrode in the contralateral cortex (+2.5 A/P, −2.5 M/L, −2.0 D/V). The carbon fiber electrode potential was linearly scanned from −0.4 to 1.2 V and back to −0.4 V vs Ag/AgCl. Cyclic voltammograms were recorded at the carbon fiber electrode every 100 ms with a scan rate of 400 V/s using a voltammeter/amperometer (Chem-Clamp; Dagan Corporation, Minneapolis, MN). Carbon fiber and stimulating electrode positions were maximized until a 1 s, 60 Hz monophasic (4 ms; 500–750 μA) stimulation train elicited a robust DA signal as previously described (España et al. 2011; España et al. 2010; Prince et al. 2015; Shaw et al. 2016). After collecting stable baselines (< 10% variation) in the NAc core at 5 min intervals, rats received an i.p. injection of vehicle or one of three doses (5, 10, or 20 mg/kg) of RTIOX-276 and DA signaling continued to be monitored. Cocaine (1.5 mg/kg) was delivered i.v. 30 min after the RTIOX-276 injection. This dose of cocaine was selected to compare to previous studies examining the effects of HCRT manipulations on DA signaling (España et al. 2011; España et al. 2010; Prince et al. 2015). Electrically-evoked DA responses were recorded at 30 s, 1 min, and 5 min after cocaine injection, and every 5 min thereafter.
Extracellular concentrations of DA were estimated by comparing the current at the peak oxidation potential for DA in consecutive voltammograms with electrode calibrations obtained from a known concentration of DA (3 μM), as has been performed previously (Brodnik and España 2015; España et al. 2011; España et al. 2010; Prince et al. 2015). Stimulated DA release following vehicle or RTIOX-276 was calculated as the percent change from baseline, with baseline (100%) defined as the average of 3 samples that occurred prior to the injection of the antagonist. Stimulated DA release following cocaine was calculated as the percent change from the post-vehicle or post-RTIOX-276 DA release time points that preceded the cocaine injection. Changes in maximal uptake rate following RTIOX-276 injection were expressed as Vmax and changes in uptake inhibition following cocaine were expressed as apparent Km. To examine the effects of antagonists on DA signaling prior to cocaine, stimulated DA release and Vmax were assessed using a two-way mixed design ANOVA comparing DA release or Vmax from the 3 baseline recordings prior to drug treatment and DA release or Vmax for the 30 min following drug treatment (average baseline vs. pre-cocaine). Drug (vehicle or RTIOX-276) treatment was the between subjects variable and Time was the repeated measures variable. To examine the effects of RTIOX-276 on cocaine-induced changes in DA signaling, stimulated DA release and DA uptake inhibition were assessed using a two-way mixed design ANOVA over the course of the experiment such that Drug (vehicle or RTIOX-276) was the between subjects variable and Time was the repeated measures variable. Where appropriate, Dunnett’s post-hoc analyses using vehicle as the control were conducted to examine differences between drug treatments across time.
Freely-moving FSCV Surgery and Procedures
Rats were anesthetized with 5% isoflurane and anesthesia was maintained at 2.0% isoflurane for the duration of the surgery. Rats were first implanted with an i.v. catheter as in Methods: Cocaine Self-Administration and then placed in a stereotaxic apparatus for chronic electrode implantation. Bilateral chronic carbon fiber electrodes were placed in the NAc core (+1.3 A/P, ±1.3 M/L, −6.5 to −7.0 D/V), and a reference electrode was implanted in the posterior cortex (−2.5 A/P, −2.5 M/L, −2.0 D/V). Electrodes were secured into place using dental acrylic cement. Rats received postsurgical antibiotic (Neo-Predef, Pharmacia & Upjohn Company, and 5mg/kg enrofloxacin, Bayer HealthCare LLC) and analgesic (5 mg/kg; Ketoprofen, Patterson Veterinary) and were allowed to recover for 21–24 days before testing (Clark et al. 2010).
During experimentation the electrode potential was linearly scanned (−0.4 to 1.3 V and back to −0.4 V vs Ag/AgCl) and cyclic voltammograms were recorded at the carbon fiber electrode every 100 ms with a scan rate of 400 V/s using a voltammeter/amperometer (Electronics and Materials Engineering, Seattle, WA). Concentrations of DA were estimated by comparing the current at the peak oxidation potential for DA in consecutive voltammograms with electrode calibrations obtained from a 3 μm concentration of DA. Chemometric analysis was employed to isolate DA from the voltammetric signals using a standard training set obtained from electrically-evoked DA release in the NAc core of an awake behaving rat as previously described (Clark et al. 2010; Keithley et al. 2009; Willuhn et al. 2010).
Our initial freely-moving FSCV experiments were designed to determine the effects of HCRTr1 blockade on baseline and cocaine-induced changes in endogenous phasic DA release. Rats were connected to cabling for FSCV recordings, placed into operant boxes, and habituated to the environment for 1 hr. During habituation, electrodes were cycled at 60 Hz for 30 min, and then at 10 Hz for 15 min before proceeding. After habituation, baseline FSCV recordings were taken for 30 min followed by an i.p. injection of either vehicle, 10, or 20 mg/kg RTIOX-276. Thirty min after pretreatment with vehicle or RTIOX-276 rats received a single, non-contingent i.v. injection of 0.75 mg/kg cocaine and recordings continued for an additional 30 min. The 0.75 mg/kg dose of cocaine was selected to compare with the current self-administration findings. In all cases, FSCV recordings consisted of 60 sec data files. Rats were treated with vehicle and each dose of RTIOX-276 using a counterbalanced design with 3 days between treatments.
Spontaneous DA phasic release events were identified using Demon Voltammetry and Analysis software (Yorgason et al. 2011) written in Lab VIEW language (National Instruments, Austin, TX) and based on previously described approaches (Robinson et al. 2003; Shnitko and Robinson 2015). Briefly, voltammograms from spontaneous DA release events in which the amplitude exceeded 5 times the background noise were compared to a template cyclic voltammogram obtained from electrically-evoked DA release in the NAc core of an awake, behaving rat. Spontaneous DA release events with voltammograms that displayed correlations greater than or equal to an r2 of 0.7 were used for subsequent analysis. Spontaneous DA release events meeting these criteria where then analyzed for amplitude and frequency during the 5 min of collection immediately before vehicle or RTIOX-276 injections (baseline), the last 5 min of post-vehicle or post-RTIOX-276 collections immediately before cocaine injection (pretreatment), and the first 5 min of collection immediately following cocaine delivery (cocaine). The effects of RTIOX-276 were assessed using two-way repeated measures ANOVA with Time as one repeated measures variable (5 baseline, 5 pretreatment, and 5 cocaine collections) and Drug (Vehicle, 10 and 20 mg/kg RTIOX-276) as the other repeated measures variable. When statistical significance was obtained, Holm-Bonferroni post hoc tests were conducted to examine differences between the last baseline, the last pretreatment, and the first cocaine collections.
A second set of experiments was designed to test the effects of HCRTr1 blockade on cue-evoked DA release. Rats were placed into operant boxes and allowed to habituate to the environment for 1 hr per day for 3 consecutive days. Rats began conditioning sessions at approximately 09:00 h on the first day following habituation. Electrodes were cycled at 60 Hz for 30 min, and then at 10 Hz for 15 min before proceeding. After electrode cycling, FSCV recordings began and a new conditioning session was started. Each session was initiated with the presentation of an 8 sec compound cue comprised of illumination of stimulus light and the extension of a lever located immediately below the stimulus light. After 8 sec, the cue light was extinguished and the lever retracted. This was immediately followed by an approximately 5 sec injection of 0.75 mg/kg cocaine. This dose of cocaine was selected to compare to the current self-administration findings. The pairing of the cue and cocaine reward occurred 10 times during each session, separated by a variable inter-trial interval of 416–860 seconds. After the 10 trials were completed, rats were returned to their home cage until the next day of conditioning. After 4 consecutive days of conditioning, animals were pretreated with i.p. vehicle or 20 mg/kg RTIOX-276, 30 min before the onset of the conditioning session (09:30 h). All rats were treated twice, once with vehicle and once with 20 mg/kg RTIOX-276, using a counterbalanced design with 3 days of conditioning between treatments.
Data were analyzed for cue-evoked DA release by creating 8 sec, peri-event FSCV data files aligned to cue presentation such that 4 sec preceded and 4 sec followed the cue. These peri-event files were then averaged across the 10 trials per session independently for each of the experimental treatments days. The amplitude of cue-evoked DA release for the vehicle and RTIOX-276 experiments was expressed as a percent of baseline relative to the prior day’s FSCV recording to control for potential shifts in the magnitude of cue-evoked DA release across days. The effect of RTIOX-276 on cue-evoked DA transient amplitude was assessed using a paired student’s t-test (vehicle vs. RTIOX-276).
Ex vivo slice FSCV procedures
Rats were pretreated with i.p. injections of either vehicle or RTIOX-276 (20 mg/kg) and sacrificed 30 min after treatment. Brains were rapidly removed following decapitation and were transferred to oxygenated, ice-cold artificial cerebral spinal fluid (aCSF) containing (in mM) NaCl (126), KCl (2.5), NaH2PO4 (1.2), CaCl2 (2.4), MgCl2 (1.2), NaHCO3 (25), glucose (11), l-ascorbic acid (0.4), pH adjusted to 7.4. A vibrating microtome was used to produce 400 μm-thick sections containing the NAc. Slices were allowed to rest at room temperature for 1 hr before being transferred into a testing chamber flushed with aCSF (32° C). A bipolar stimulating electrode (Plastics One) was placed on the surface of the tissue and a carbon fiber microelectrode was implanted in the NAc between stimulating electrode leads. DA release was evoked every 5 min using a single electrical pulse (400 μA, 4 ms, monophasic). After recording 3 stable baseline responses (3 stimulations with <10% variation), cocaine was cumulatively applied to the tissue (0.3–30 μM) as previously described (Brodnik and España 2015; Brodnik et al. 2016). Stimulated DA release and DA uptake measures (Vmax and Km) were determined as described for the anesthetized FSCV experiments. Differences in baseline DA release and uptake were assessed using independent samples t-tests, and differences in the effects of cocaine were assessed using a two-way ANOVA with drug as the between subjects variable and cocaine concentration as the repeated measures variable.
Results
RTIOX-276 reduces motivation to self-administer cocaine
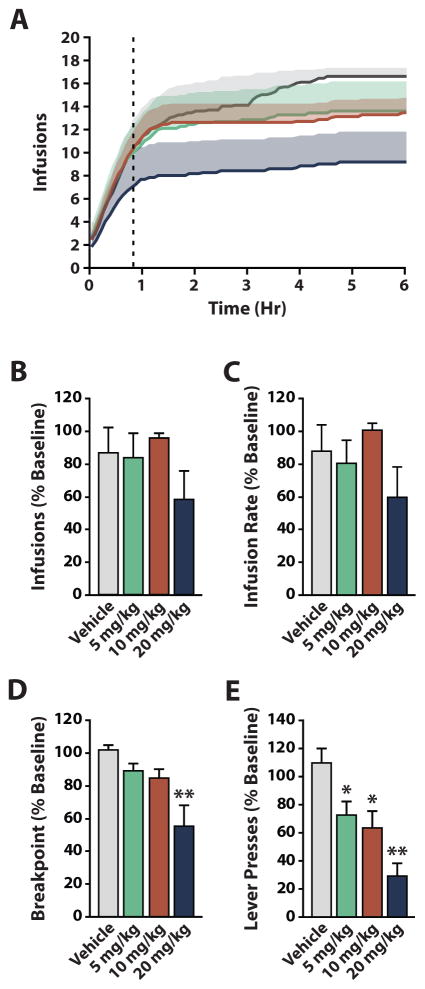
The most commonly used HCRTr1 antagonist, SB-334867, has been repeatedly shown to influence reward and reinforcement processes (Bentzley and Aston-Jones 2015; Borgland et al. 2009; Brodnik et al. 2015; España et al. 2010; Smith et al. 2009; Smith et al. 2010). Despite its utility, SB-334867 has been criticized for having poor solubility, potential off-target effects (Gotter et al. 2012; Lebold et al. 2013), and hydrolytic instability (McElhinny et al. 2012). To limit potential confounds due to these issues we used the novel, highly selective HCRTr1 antagonist RTIOX-276 (Perrey et al. 2015a; Perrey et al. 2013). The effects of RTIOX-276 on cocaine self-administration have not been assessed previously, thus we first sought to: 1) confirm that RTIOX-276 reduced cocaine self-administration in a manner similar to SB-334867; and 2) determine the behaviorally relevant doses of RTIOX-276 to use in subsequent experiments investigating phasic DA release. To achieve this, we examined whether systemic administration of RTIOX-276 (vehicle, 5, 10, or 20 mg/kg; n=7) alters low and high-effort responding for 0.75 mg/kg cocaine on a PR schedule of reinforcement. To determine effects on low-effort cocaine self-administration, we measured total cocaine infusions and the rate of infusion during the consumption phase of PR responding (Brodnik et al. 2015). Vehicle injections did not significantly alter consumption phase responding for cocaine. Similar to SB-334867 (Brodnik et al. 2015), we found no significant effect of RTIOX-276 on consumption phase responding for 0.75 mg/kg cocaine (Figure 1A–C). To determine effects on high-effort cocaine self-administration, we measured breakpoint and total lever presses across the entire session as measures of appetitive phase responding. Vehicle injections did not significantly alter appetitive phase responding for cocaine. We found no significant effects of vehicle, 5, or 10 mg/kg RTIOX-276 on breakpoint, but found a significant reduction in breakpoint with 20 mg/kg RTIOX-276 (Figure 1A, D). We also found no effect of vehicle on lever presses, but did find a significant decrease in lever presses following all three doses of RTIOX-276 (Figure 1E). These data confirm that RTIOX-276 modifies effortful cocaine self-administration in a similar manner to, and with lower doses than, SB-334867. Further, these data show that the 20 mg/kg dose of RTIOX-276 provides robust decreases in motivation for cocaine under high-effort conditions.
Figure 1. Systemic administration of RTIOX-276 decreases high-effort responding for cocaine.
(A) Average number of infusions across the 6-hour self-administration session for vehicle, 5, 10, and 20 mg/kg RTIOX-276 treatment (n=7). The dotted line represents the end of the low-effort consumption phase and the beginning of the high-effort appetitive phase. Shaded region represents standard error of the mean. (B) Effect of HCRTr1 blockade on the number of infusions received by the end of consumption phase. One-way repeated measures ANOVA revealed no significant difference between vehicle and RTIOX-276 treatments. (C) Effect of HCRTr1 blockade on infusion rate across the consumption phase. One-way repeated measures ANOVA revealed no effect of vehicle or RTIOX-276 on infusion rate. (D) Effect of HCRTr1 blockade on breakpoint. One-way repeated measures ANOVA revealed a significant effect of RTIOX-276 treatment on breakpoints (F(3,24) = 6.803, p = 0.0018). (E) Effect of HCRTr1 blockade on total lever presses. One-way repeated measures ANOVA revealed a significant effect of RTIOX-276 treatment on total lever presses (F(3,24) = 10.04, p < 0.001). Grouped data are presented as mean ± SEM. Dunnet’s post hoc tests: *p < 0.05, **p < 0.01.
HCRTr1 blockade attenuates cocaine-induced DA uptake inhibition
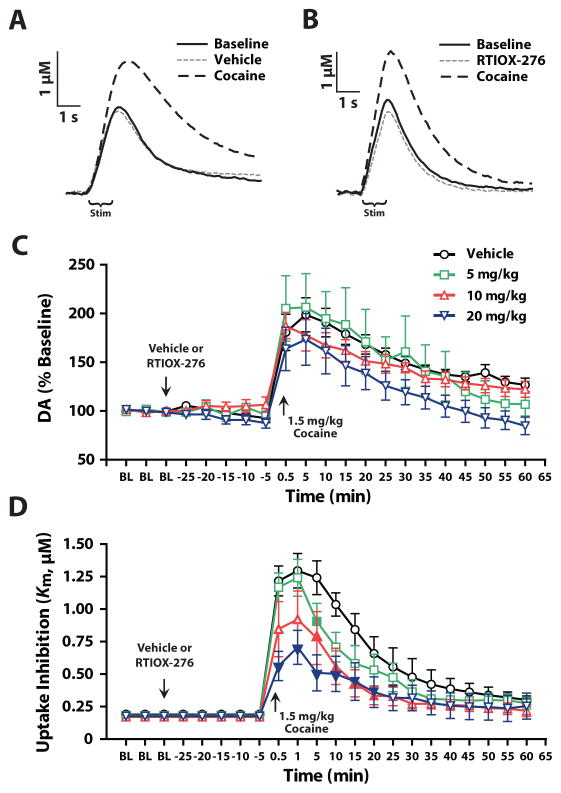
To further verify that HCRTr1 blockade modulates DA signaling, we used in vivo FSCV in anesthetized rats to examine the effects of RTIOX-276 on stimulated DA release and uptake as well as cocaine-induced DA uptake inhibition. Rats received an i.p. injection of vehicle (n=8), 5 (n=7), 10 (n=8), or 20 mg/kg (n=8) RTIOX-276, 30 min prior to receiving an i.v. injection of 1.5 mg/kg cocaine. As shown in Figure 2A–C, we found that RTIOX-276 did not alter stimulated DA release prior to cocaine, nor did it affect cocaine-induced increases in stimulated DA release. RTIOX-276 also had no effect on maximal uptake rate (Vmax) prior to cocaine-injection (data not shown). In contrast, we found that RTIOX-276 produced dose-dependent reductions in cocaine-induced DA uptake inhibition (Figure 2A, B, D). Together with our behavioral experiments (Figure 1), these data demonstrate that RTIOX-276 drives changes in cocaine self-administration and DA signaling in a similar manner to SB-334867 (España et al. 2010; Prince et al. 2015).
Figure 2. HCRTr1 blockade does not alter cocaine-induced increases in DA release, but does reduce cocaine-induced DA uptake inhibition.
(A) Example traces of evoked DA release and uptake for baseline, vehicle pretreatment, and cocaine. (B) Example traces of evoked DA release and uptake for baseline, RTIOX-276 pretreatment, and cocaine. (C) Stimulated DA release expressed as a percent of baseline for vehicle, 5, 10, and 20 mg/kg RTIOX-276. Two-way mixed design ANOVA with Drug as the between subjects variable and Time as the repeated measures variable indicated a significant effect of Time (F(2.1,57.6) = 46.5, p < 0.001), but no effect of Drug (F(3,27) = 1.4, p = 0.27) or Time x Drug interaction (F(6.4,57.6) = 1.2, p = 0.32). (D) DA uptake inhibition (Km) for vehicle, 5, 10, and 20 mg/kg RTIOX-276 pretreated animals. Two-way mixed design ANOVA with Drug as the between subjects variable and Time as the repeated measures variable indicated a significant difference in DA uptake inhibition for Time (F(21,567) = 51.9, p < 0.001), Drug (F(3,27) = 6.0, p = 0.003) and Time x Drug interaction (F(63,567) = 2.2, p < 0.001). Data was Greenhouse-Geisser corrected due to violation of sphericity. Arrows indicate timepoints where vehicle or RTIOX-276, and cocaine were administered. Grouped data are presented as mean ± SEM. Filled symbols indicate a significant effect versus control as determined by Dunnet’s post hoc tests: *p < 0.05.
HCRTr1 blockade reduces spontaneous phasic DA release and blocks cocaine-induced increases in spontaneous phasic DA release
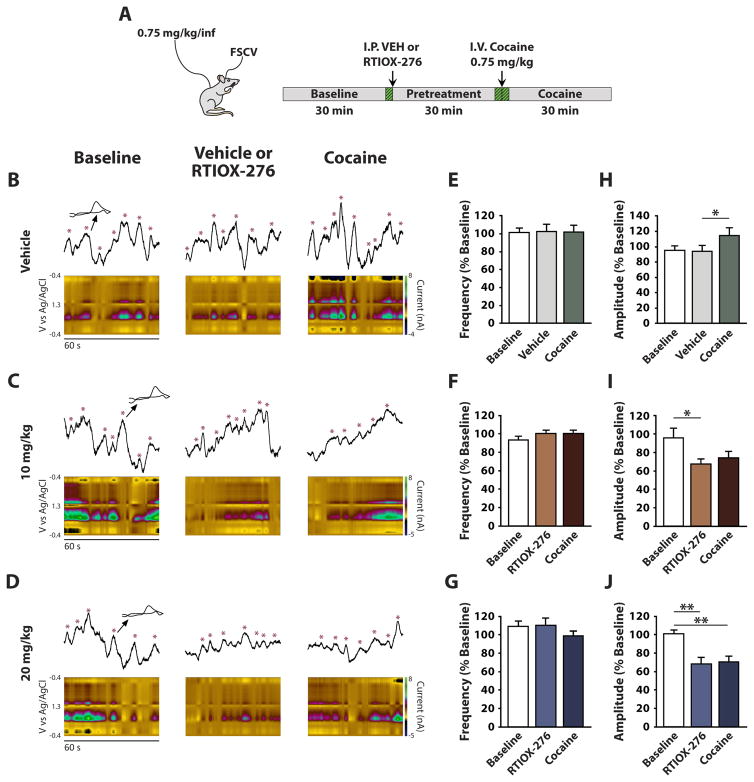
One emerging hypothesis suggests that aberrant motivation to obtain cocaine may be a consequence of increases in DA transient activity that occurs following drug administration (Covey et al. 2014; Keiflin and Janak 2015; Willuhn et al. 2010). We examined the effects of HCRTr1 blockade on spontaneous phasic DA transients before and after cocaine to determine if HCRTr1 blockade might reduce motivation for cocaine by altering its pharmacologic effect on phasic DA signaling. We measured the frequency and amplitude of phasic DA transients following an i.p. injection of vehicle, 10, or 20 mg/kg RTIOX-276 (n=8). After 30 min of post-vehicle or post-RTIOX-276 recording, rats received a single i.v. injection of 0.75 mg/kg cocaine and spontaneous DA transient activity was monitored (Figure 3A). We found that neither vehicle, RTIOX-276, nor cocaine altered DA transient frequency (Figure 3B–G). Likewise, we found that vehicle injections did not alter the amplitude of phasic DA transients (Figure 3B and H). In contrast, we observed a significant reduction in DA transient amplitude following both 10 mg/kg (Figure 3C and I) and 20 mg/kg RTIOX-276 (Figure 3D and J). Further, administration of cocaine to vehicle-pretreated rats significantly increased DA transient amplitude (Figure 3B and H), but did not affect DA transient amplitude in rats pretreated with RTIOX-276 (Figure 3C, D, I and J). These data indicate that blockade of HCRTr1 both reduces DA transient amplitude in cocaine-free conditions and blocks cocaine-induced increases in DA transient amplitude.
Figure 3. HCRTr1 blockade decreases the amplitude of, and blocks cocaine-induced increases in, spontaneous phasic DA amplitude.
(A) Diagram of the experimental set-up and timeline for freely-moving FSCV experiments measuring spontaneous DA transients. Green hashed segments illustrate the 1-min time points for which post hoc analysis was performed. Example voltammetric color plots and raw data traces of spontaneous phasic DA events following treatment with (B) Vehicle, (C) 10 mg/kg, or (D) 20 mg/kg RTIOX-276. For each panel, data is shown during baseline (left), pretreatment (middle), and cocaine (right) collections. Effects of (E) Vehicle, (F) 10 mg/kg, or (G) 20 mg/kg RTIOX-276 on the frequency of spontaneous phasic DA transients. Two-way repeated measures ANOVA with Time and Drug as the repeated measures variables indicated no significant effects of Time (F (14,98) = 0.9, p = 0.59), Drug (F (2,14) = 1.9, p = 0.17) and Time x Drug interaction (F(28,196) = 1.0, p = 0.43) following RTIOX-276 treatment. Effects of (H) Vehicle, (I) 10 mg/kg, or (J) 20 mg/kg RTIOX-276 on the amplitude of spontaneous phasic DA transients. Two-way repeated measures ANOVA with Time and Drug as the repeated measures variables indicated that there was a significant effect of Time (F (14,98) = 8.7, p < 0.001), Drug (F (2,14) = 13.5, p < 0.001) and Time x Drug interaction (F(28,196) = 2.1, p < 0.002) following RTIOX-276 treatment. Grouped data are presented as mean ± SEM. Holm-Bonferroni post hoc tests: *p < 0.05, **p < 0.01.
HCRTr1 blockade decreases cue-evoked phasic DA release amplitude
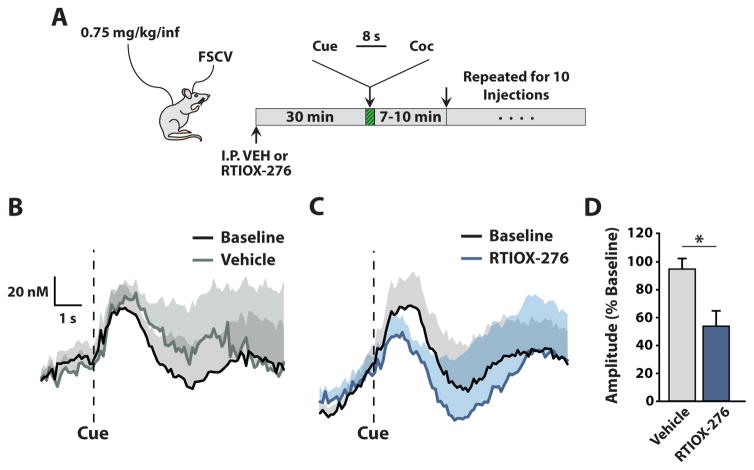
Cue-evoked phasic DA release events have been shown to drive cocaine-taking behavior (Phillips et al. 2003). To determine whether HCRTr1 blockade affects cue-evoked phasic DA release, we measured the amplitude of cue-evoked DA transients using FSCV in freely-moving animals that had been conditioned to associate a cue with a non-contingent i.v. injection of cocaine. Animals were given i.p. vehicle or 20 mg/kg RTIOX-276 (n=8) in a counterbalanced design, 30 min prior to starting a new session (Figure 4A). Cue-evoked DA events were averaged across all 10 trials in one session. The amplitudes of cue-evoked DA transients were analyzed as a percent of baseline, which was defined as the amplitude of the average cue-evoked DA transient on the day prior to the experimental session. Using this approach we found that HCRTr1 blockade significantly reduced cue-evoked phasic DA transient amplitude (Figure 4B–D).
Figure 4. HCRTr1 blockade reduces the amplitude of cue-evoked phasic DA events.
(A) Experimental set-up and timeline for freely-moving FSCV experiments measuring cue-evoked, phasic DA release. (B) Average amplitude of cue-evoked DA transients for baseline (black) and vehicle pretreatment (gray). (C) Average amplitude of cue-evoked DA transients for baseline (black) and RTIOX-276 pretreatment (blue). Shaded regions represent standard error of the mean. (D) Amplitude of cue-evoked DA transients for vehicle and RTIOX-276 pretreatment expressed as a percent of baseline. Grouped data are presented as mean ± SEM. Paired Student’s t-test revealed a significant effect of RTIOX-276 treatment on amplitude of cue-evoked DA events (t(8) = 2.5 p=0.039). *p < 0.05.
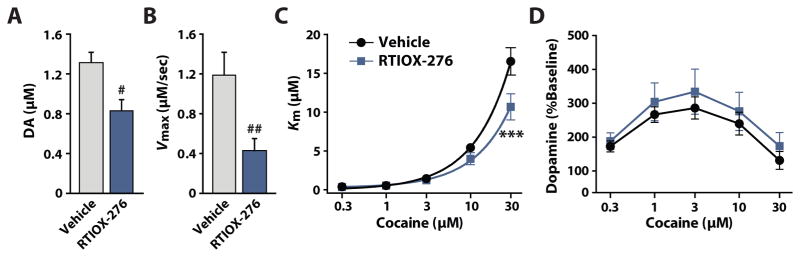
Acute HCRTr1 blockade reduces DA terminal sensitivity to cocaine
Alterations in the amplitude of phasic DA transients may be a product of drug-induced changes in either; 1) DA neuron activity; 2) DA vesicle release probability; 3) DA uptake rate; or 4) a combination of these parameters. In our previous work (España et al. 2010; Prince et al. 2015), and in the current studies (Figure 2), we found that systemic HCRTr1 blockade reduces cocaine-induced DA uptake inhibition, and a wealth of literature indicates that cocaine’s effect on DA clearance is primarily driven by cocaine’s action at the DAT (Bergman et al. 1989; Calligaro and Eldefrawi 1987; Cline et al. 1992; Kuhar et al. 1991; Ritz et al. 1987; Wilcox et al. 1999). We used ex vivo FSCV to directly test for HCRTr1 antagonist-induced modulation of DA terminal cocaine sensitivity. Rats were pretreated with either vehicle (n=6) or 20 mg/kg RTIOX-276 (n=6), 30 min prior to sacrifice, and DA release and uptake were examined in brain slices prepared from these subjects. We found that RTIOX-276 reduced baseline stimulated DA release (Figure 5A) and DA uptake (Figure 5B). We also observed that RTIOX-276 significantly reduced DA uptake inhibition produced by cocaine (Figure 5C), but did not alter the effects of cocaine on stimulated DA release (Figure 5D). These results indicate that acute blockade of HCRTr1 drives DA terminal adaptations including reduced DA release and uptake at baseline and a reduction in cocaine-induced DA uptake inhibition.
Figure 5. HCRTr1 blockade reduces baseline stimulated DA release and uptake, as well as cocaine-induced DA uptake inhibition.
(A) Baseline DA release for vehicle and RTIOX-276 pretreatment conditions. Student’s t-test revealed a significant effect on DA release (t(10) = 2.98, p = 0.014). (B) Maximal uptake rate (Vmax) for vehicle and RTIOX-276 pretreatment conditions. Student’s t-test revealed a significant effect on Vmax (t(10) = 3.27, p = 0.009). (C) Cocaine-induced DA uptake inhibition (Km) for vehicle and RTIOX-276 pretreatment conditions. Two-way mixed design ANOVA with Drug as the between subjects variable and cocaine Concentration as a repeated measures variable revealed a significant effect of Drug (F(4,10) = 4.24, p = 0.049), Concentration (F(4,40) = 109.9, p < 0.001), and a significant Drug x Concentration interaction (F(4,40) = 5.52, p = 0.0011). (D) Cocaine-induced changes in stimulated DA release for vehicle and RTIOX-276 pretreatment conditions. Two-way mixed design ANOVA with Drug as the between subjects variable and cocaine Concentration as the repeated measures variable showed no significant effect on DA release. Grouped data are presented as mean ± SEM. Student’s t-test: #p < 0.05, ##p < 0.01. Dunnet’s post hoc test: ***p < 0.001.
Discussion
In the current studies we examined the effects of HCRTr1 blockade on endogenous phasic DA release using the highly selective HCRTr1 antagonist RTIOX-276. We determined that RTIOX-276 decreases motivation to self-administer cocaine and modifies cocaine’s effect on DA clearance similar to the more commonly used HCRTr1 antagonist SB-334867. We then tested whether RTIOX-276 modulates endogenous phasic DA signals. We observed that blockade of HCRTr1 decreases the amplitude of spontaneous DA transients prior to and following cocaine and reduces the amplitude of cue-evoked DA transients. Finally, we found that RTIOX-276 modulation of cocaine sensitivity is derived from adaptations that occur at the level of DA terminals, as our ex vivo FSCV studies showed that DA terminal cocaine sensitivity is reduced following acute blockade of HCRTr1. Combined, these observations demonstrate that HCRTr1 blockade alters DA signaling in two distinct ways. First by dampening spontaneous and cue-evoked phasic DA signals independent of the pharmacologic effects of cocaine, and second by attenuating cocaine’s effect on phasic DA signals by reducing cocaine sensitivity at DA terminals.
HCRTr1 blockade reduces motivation for cocaine and reduces cocaine-induced DA uptake inhibition
Previous studies investigating the effects of HCRTr1 blockade on cocaine self-administration report that the HCRTr1 antagonist SB-334867 significantly decreases motivation for cocaine and reduces cocaine-induced DA uptake inhibition. In the current studies we evaluated the effects of RTIOX-276 on low- and high-effort cocaine self-administration as well as on cocaine-induced DA uptake inhibition in anesthetized rats. For self-administration experiments, rats were treated with RTIOX-276 and tested using a PR schedule (Richardson and Roberts 1996). Similar to previous studies using SB-334867 (Aston-Jones et al. 2009; Brodnik et al. 2015; España et al. 2010), we found that RTIOX-276 does not reduce low-effort responding for cocaine. This finding is consistent with reports indicating that HCRTr1 blockade does not affect locomotor activity (Calipari and España 2012) or sleep/wake activity (Brodnik et al. 2015), suggesting that reduced effects on motivation for cocaine are not due to general deficits in arousal. Importantly, however, RTIOX-276 produced a significant and dose-dependent decrease in high-effort, appetitive phase responding for 0.75 mg/kg cocaine indicating a reduction in the motivation for cocaine. The underlying processes that differentiate the effects of HCRTr1 blockade in low- vs high-effort responding for cocaine remain unclear, particularly given that there is no direct evidence for preferential involvement of the HCRT system during high-effort drug self-administration conditions. Nevertheless, some studies suggest that HCRT neurons may be most active during high arousal conditions including exploratory behavior, exposure to a novel environment, or during stress (España et al. 2003; Estabrooke et al. 2001; Ida et al. 2000; Zhu et al. 2002). Consequently, it is possible that HCRT systems are engaged to a greater degree during high-effort responding and thus HCRT manipulations may be more effective under these conditions.
In our anesthetized FSCV experiments we tested the effect of RTIOX-276 on stimulated DA release and cocaine-induced DA uptake inhibition. We found that the effects of RTIOX-376 on cocaine-induced uptake inhibition closely resemble our previous findings using SB-334867, with RTIOX-376 pretreatment producing a reduction in cocaine-induced DA uptake inhibition (España et al. 2010; Prince et al. 2015). This similarity between SB-334867 and RTIOX-276 suggests that the effects of SB-334867 on motivation for cocaine observed in previous studies are likely to be attributable to blockade of HCRTr1, rather than to off-target effects.
HCRTr1 blockade disrupts endogenous phasic DA release
Endogenous phasic DA signals are believed to be a product of DA neuron bursting (Grace 2000; Schultz 2007). Multiple computational modeling studies predict that DA neuron bursting is driven by glutamatergic NMDA receptor currents (Canavier and Landry 2006; Kuznetsov et al. 2006), and this model is supported by in vitro (Johnson et al. 1992; Mereu et al. 1997; Prisco et al. 2002; Wang et al. 1994) and in vivo (Chergui et al. 1993) studies that heavily implicate NMDA receptors in the production of DA neuron bursting. In vitro studies have shown that application of HCRT-1 peptide potentiates NMDA receptor-mediated neurotransmission by activation of HCRTr1 (Borgland et al. 2006). In line with this, VTA infusions of HCRT-1 increase DA neuron bursting in vivo (Moorman and Aston-Jones 2010; Muschamp et al. 2007; Muschamp et al. 2014). Together these data suggest that HCRTr1 activation may potentiate endogenous phasic DA signals, and thus we predicted that blockade of HCRTr1 would depress endogenous phasic DA release.
We found that HCRTr1 blockade reduced the amplitude of both spontaneous DA transients and cue-evoked DA release. The reduction in spontaneous DA transient amplitude occurred in the absence of cocaine, demonstrating a direct effect of HCRTr1 blockade on DA signaling rather than an interaction with the effects of cocaine. Likewise, in our studies of cue-evoked DA release, DA transients were temporally synced to the start of the cue such that DA release began at cue onset and terminated prior to the start of the i.v. cocaine infusions. It is thus likely that RTIOX-276-induced reductions in cue-evoked DA release also occur independently of cocaine’s pharmacologic effects (Willuhn et al. 2010). Based on these observations, we hypothesize that reductions in both spontaneous and cue-evoked DA release are due to a depression of DA neuron bursting that results from HCRTr1 blockade as previously suggested (Borgland et al. 2009; Borgland et al. 2006; España et al. 2010; Moorman and Aston-Jones 2010).
Evidence suggests that phasic DA signals in the NAc are critical for cue-induced incentive motivation for both cocaine (Phillips et al. 2003) and natural (Syed et al. 2016) rewards. In the current studies we demonstrate that HCRTr1 blockade reduced phasic DA signals and in accordance with potential effects on natural rewards we previously reported that HCRTr1 blockade reduces motivation for sucrose (España et al. 2010). Interestingly, however, this reduction in motivation for sucrose was observed in sated rats but did not occur when rats were food restricted (España et al. 2010). It has recently been shown that cue-evoked phasic DA signals track the need-based motivational value of natural rewards such that when the physiological need for a nutrient is high the phasic DA release produced by a reward predictive cue is larger (Aitken et al. 2016; Cone et al. 2016). Combined, these separate lines of evidence suggest that HCRTr1 blockade-induced reductions in phasic DA signals may be sufficient for disrupting motivation for natural rewards under satiated conditions, but that any reduction in phasic DA signal strength that occurs in the deprived state does not sufficiently reduce motivation for rewards. Ongoing studies in our laboratory seek to directly address this hypothesis.
HCRTr1 blockade attenuates cocaine-induced DA uptake inhibition
Previous investigation into the effects of cocaine on endogenous phasic DA signaling have reported that cocaine increases both the frequency and amplitude of spontaneous DA release events (Aragona et al. 2008; Stuber et al. 2004). In subjects pretreated with vehicle, we found that a single i.v. infusion of 0.75 mg/kg cocaine produced a significant increase in average DA transient amplitude but did not affect DA transient frequency. We were initially surprised by this result; however previous reports showing increases in DA transient frequency used i.v. doses of cocaine between ~1.1 and 2.0 mg/kg (Aragona et al. 2008; Stuber et al. 2004; Stuber et al. 2005). Given that increases in DA transient frequency are dependent on cocaine concentrations (Stuber et al. 2005), it is possible that the lack of measurable changes in DA transients frequency in the present studies could be attributed to the relatively lower dose of cocaine used (0.75 mg/kg). Further investigation of the effects of HCRTr1 blockade on DA transient frequency using higher doses of cocaine could help to elucidate this issue.
We found that HCRTr1 blockade also suppressed cocaine-induced increases in DA transient amplitude. While HCRTr1 blockade-induced reductions in endogenous phasic DA release are likely associated with modulation of DA neuron firing, it is unlikely that attenuation of cocaine’s effect on spontaneous phasic DA transient amplitude can be explained solely by DA neuron firing changes. Cocaine primarily modulates DA signaling at the level of DA terminals by blocking the DAT and thereby inhibiting DA uptake, therefore modulation of cocaine’s acute effects on DA signaling would most likely occur via processes that regulate both DA release and uptake at the level of the terminal.
To determine if HCRTr1 blockade influences cocaine-induced changes in DA release and/or uptake we used ex vivo FSCV to directly test whether acute HCRTr1 blockade produced DA terminal adaptations that result in reduced terminal sensitivity to cocaine. This approach allowed us to separate effects of systemic HCRTr1 blockade on DA terminals from effects that may be a direct product of ongoing reductions in DA neuron activity. Here we found that HCRTr1 blockade reduced baseline DA release and uptake in the absence of cocaine. Further, we found a reduction in DA uptake inhibition produced by cocaine but no change in the effects of cocaine on DA release. Together, these results indicate that, in addition to altering DA neuron synaptic properties, HCRTr1 blockade produces DA terminal alterations that functionally reduce DA uptake inhibition produced by cocaine.
It is tempting to suggest that our results show that HCRTr1 antagonism modifies DA terminal cocaine sensitivity by acting on HCRTr1 in DA terminal fields. Indeed, HCRT-induced changes in NAc shell DA signaling has been shown using ex vivo FSCV (Patyal et al. 2012). Nevertheless, this effect is observed when DA release is elicited using repeated, high frequency electrical pulses but does not occur when DA release is elicited with a single electrical pulse as used in the current experiments (Patyal et al. 2012). Further, while HCRTr1-induced modulation of DA signaling has been observed at NAc shell terminals, there is no evidence for terminal modulation of DA signaling by HCRT in the NAc core. This is in line with multiple studies showing a paucity of HCRTr1 expression in the NAc core (Ch’ng and Lawrence 2015; Marcus et al. 2001; Trivedi et al. 1998) but see (D’Almeida et al. 2005). Moreover, using FSCV in anesthetized rats, we have previously shown that reductions in cocaine-induced DA uptake inhibition are produced following direct VTA microinfusions of the HCRTr1 antagonist SB-334867 (España et al. 2010) with no possibility of antagonist spread into the NAc core. Together, this evidence suggests that acute inhibition of HCRTr1 signaling at DA cell bodies in the VTA leads to a reduction in DA terminal cocaine sensitivity.
The mechanisms through which HCRTr1 blockade at DA cell bodies exerts alterations in terminal cocaine sensitivity are not clear. One possibility is that NAc terminal adaptations might be a product of changes in gene expression that occur in VTA DA neurons that project to the NAc core. This is an unlikely explanation, however, given that in this and other studies we observe changes in cocaine sensitivity in the NAc within 30 min of i.p. administration of HCRTr1 antagonists (Figure 2, 3, and 5). For changes in gene expression to influence DA terminal function, the resulting protein products must physically reach DA terminals. The most efficient means by which this might occur is via fast axonal transport, which proceeds at a rate of 0.035–0.139 mm/min (Roy 2014). As the approximate length of axon projections from the VTA to the NAc core of the rat is ~7.2 mm (Swanson 1998), we expect that the minimum time by which fast axonal transport can lead to the transfer of proteins from DA neuron cell bodies in the VTA to DA terminals in the NAc is ~52 min. Given that we observed changes in DA terminal cocaine sensitivity within 30 min of RTIOX-276 delivery, we conclude that it is unlikely that changes in gene expression could be the predominant mechanism through which acute HCRTr1 inhibition reduces DA terminal cocaine sensitivity.
An alternate possibility is that changes in DA neuron firing rate induced by HCRTr1 blockade in the absence of cocaine engenders adaptations in cocaine sensitivity at the terminal, as others have shown that changes in DA neuron excitability can affect DAT function (Richardson et al. 2016). HCRTr1 blockade reduces DA neuron excitability, and thus it is possible that this effect could lead to changes in terminal calcium signaling that result in terminal DAT modifications that have been proposed to modulate DAT sensitivity to cocaine. Potential mechanisms for altered DAT sensitivity to cocaine include differential DAT phosphorylation state (Moritz et al. 2013), shifts in inward/outward facing DAT (Liang et al. 2009), and changes in oligomer/monomer ratios (Chen and Reith 2007). DAT modification that is driven by changes in DA neuron excitability occurs within seconds of changes in DA neuron excitability (Richardson et al. 2016), and thus we expect that this process could occur within the 30 min window that HCRTr1 blockade-induced reductions in terminal cocaine sensitivity have been observed. Ongoing experiments seek to test this possible explanation for altered terminal cocaine sensitivity following HCRTr1 blockade.
Bimodal modulation of DA signaling influences cocaine self-administration
Based on the current and previous work, we propose that HCRTr1 blockade reduces the motivation to self-administer cocaine via bimodal modulation of DA signaling that occurs through: 1) suppression of cue-evoked DA release; and 2) suppression of cocaine’s acute pharmacologic effect on phasic DA transients. With regard to the influence of suppressed cue-evoked DA release on cocaine self-administration, a long-standing body of work has shown that reward-related cues can activate motivational states that promote drug-seeking (Saunders and Robinson 2013). The presentation of these reward-related stimuli can activate bursting in the VTA (Schultz 2007; Schultz et al. 1997), as well as phasic DA release in the NAc (Aragona et al. 2009; Phillips et al. 2003). Furthermore, DA signaling in the NAc has been shown to be necessary and sufficient to promote behavioral responses to cues (Nicola et al. 2005). Over time, these cues can also serve as incentive stimuli that induce conditioned motivational states that promote and prolong reward-seeking behaviors (Saunders and Robinson 2013). Moreover, reward-related cues themselves can become reinforcing over time by maintaining behavioral responding even in the absence of rewards (Di Ciano and Everitt 2004). Importantly, there is evidence to suggest that HCRTr1 signaling is important for these motivating properties of reward-related cues, as recent studies indicate that reductions in cocaine self-administration produced by HCRTr1 blockade depend on the presence of cocaine-paired cues (Bentzley and Aston-Jones 2015). In line with these observations, we found that HCRTr1 blockade reduced cue-evoked DA release in response to cocaine-paired cues. While modulation of DA transient release is likely to impact multiple aspects of motivated behavior (Cameron et al. 2014; Ko and Wanat 2016; Robinson et al. 2011; Shnitko and Robinson 2015) the data presented herein supports the notion that suppression of cue-evoked DA signals by HCRTr1 antagonists influences motivation for cocaine. Thus, we propose that one mechanism by which HCRTr1 antagonism may reduce motivation for cocaine is through the suppression of cue-evoked DA release.
Decreased motivation to self-administer cocaine may also be attributable to alterations in the pharmacologic effects of cocaine. Ascending mesolimbic projections have been highly implicated in motivated behaviors (Di Chiara 1998; Wise 2004), and cocaine-induced increases in DA signaling are a critical component of cocaine reinforcement (Chen et al. 2006; Ritz et al. 1987; Volkow et al. 2000). In particular, one emerging hypothesis suggests that drug-evoked augmentation of DA transients serve to drive an overvaluation of drug-associated context and cues (Covey et al. 2014; Keiflin and Janak 2015; Willuhn et al. 2010). This overvaluation of drug-associated environments is proposed to facilitate and maintain aberrant reward learning that drives pathological motivation for cocaine (Covey et al. 2014; Keiflin and Janak 2015). In the current studies, we observed an attenuation of cocaine-induced increases in the amplitude of these spontaneous phasic DA release events and reductions in DA terminal sensitivity to cocaine. Thus, we propose that a second mechanism by which HCRTr1 antagonism may reduce motivation for cocaine is through a suppression of cocaine-enhanced phasic DA signaling following cocaine.
Conclusion
The current studies demonstrate that HCRTr1 blockade decreases motivation to self-administer cocaine and dampens endogenous phasic DA release within the NAc core. Treatment with RTIOX-276 decreased the amplitude of cue-evoked DA events, as well as attenuated cocaine-induced enhancements of DA signaling. These effects of disrupted HCRTr1 transmission on DA signaling are likely to be attributable to both changes in DA neuron firing patterns and to neuroadaptations that occur at the level of the DA terminal. Combined, these observations provide support for the hypothesis that inhibition of HCRTr1 may function to modulate pathological cocaine-taking behaviors via modulation of mesolimbic DA signaling.
Acknowledgments
We would like to thank Douglass Fox for his expert technical assistance. This work was supported by the National Institutes on Drug Abuse (DA031900) to R.A.E. and (DA032837) to Y.Z. and by the Drexel University Dean’s Fellowship for Excellence in Collaborative or Themed Research to Z.D.B.
Footnotes
Compliance with Ethical Standards
All protocols and animal care procedures were maintained in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals: Eighth Edition and approved by the Institutional Animal Care and Use Committee at Drexel University College of Medicine.
References
- Aitken TJ, Greenfield VY, Wassum KM. Nucleus accumbens core dopamine signaling tracks the need-based motivational value of food-paired cues. Journal of neurochemistry. 2016;136:1026–36. doi: 10.1111/jnc.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Day JJ, Roitman MF, Cleaveland NA, Wightman RM, Carelli RM. Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur J Neurosci. 2009;30:1889–99. doi: 10.1111/j.1460-9568.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56(Suppl 1):112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimel C, Borgland SL, Corrigall W. Cocaine and nicotine research illustrates a range of hypocretin mechanisms in addiction. Vitamins and hormones. 2012;89:291–313. doi: 10.1016/B978-0-12-394623-2.00016-0. [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci. 2015;41:1149–56. doi: 10.1111/ejn.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251:150–155. [PubMed] [Google Scholar]
- Berridge CW, España RA, Vittoz NM. Hypocretin/orexin in arousal and stress. Brain Res. 2010;1314:91–102. doi: 10.1016/j.brainres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Brodnik ZD, Bernstein DL, Prince CD, España RA. Hypocretin receptor 1 blockade preferentially reduces high effort responding for cocaine without promoting sleep. Behavioural brain research. 2015;291:377–84. doi: 10.1016/j.bbr.2015.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodnik ZD, España RA. Dopamine uptake dynamics are preserved under isoflurane anesthesia. Neurosci Lett. 2015;606:129–34. doi: 10.1016/j.neulet.2015.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodnik ZD, Ferris MJ, Jones SR, España RA. Reinforcing Doses of Intravenous Cocaine Produce Only Modest Dopamine Uptake Inhibition. ACS chemical neuroscience. 2016 doi: 10.1021/acschemneuro.6b00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, España RA. Hypocretin/orexin regulation of dopamine signaling: implications for reward and reinforcement mechanisms. Front Behav Neurosci. 2012;6:54. doi: 10.3389/fnbeh.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calligaro DO, Eldefrawi ME. High affinity stereospecific binding of [3H] cocaine in striatum and its relationship to the dopamine transporter. Membr Biochem. 1987;7:87–106. doi: 10.3109/09687688709039986. [DOI] [PubMed] [Google Scholar]
- Cameron CM, Wightman RM, Carelli RM. Dynamics of rapid dopamine release in the nucleus accumbens during goal-directed behaviors for cocaine versus natural rewards. Neuropharmacology. 2014;86:319–28. doi: 10.1016/j.neuropharm.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavier CC, Landry RS. An Increase in AMPA and a Decrease in SK Conductance Increase Burst Firing by Different Mechanisms in a Model of a Dopamine Neuron In Vivo. Journal of Neurophysiology. 2006;96:2549–2563. doi: 10.1152/jn.00704.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch’ng SS, Lawrence AJ. Distribution of the orexin-1 receptor (OX1R) in the mouse forebrain and rostral brainstem: A characterisation of OX1R-eGFP mice. Journal of chemical neuroanatomy. 2015;66–67:1–9. doi: 10.1016/j.jchemneu.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Chen N, Reith ME. Substrates and inhibitors display different sensitivity to expression level of the dopamine transporter in heterologously expressing cells. J Neurochem. 2007;101:377–88. doi: 10.1111/j.1471-4159.2006.04384.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci U S A. 2006;103:9333–8. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chergui K, Charlety PJ, Akaoka H, Saunier CF, Brunet JL, Buda M, Svensson TH, Chouvet G. Tonic activation of NMDA receptors causes spontaneous burst discharge of rat midbrain dopamine neurons in vivo. Eur J Neurosci. 1993;5:137–44. doi: 10.1111/j.1460-9568.1993.tb00479.x. [DOI] [PubMed] [Google Scholar]
- Clark JJ, Sandberg SG, Wanat MJ, Gan JO, Horne EA, Hart AS, Akers CA, Parker JG, Willuhn I, Martinez V, Evans SB, Stella N, Phillips PE. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat Methods. 2010;7:126–129. doi: 10.1038/nmeth.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline EJ, Terry P, Carroll FI, Kuhar MJ, Katz JL. Stimulus generalization from cocaine to analogs with high in vitro affinity for dopamine uptake sites. Behavioural pharmacology. 1992;3:113–116. [PubMed] [Google Scholar]
- Cone JJ, Fortin SM, McHenry JA, Stuber GD, McCutcheon JE, Roitman MF. Physiological state gates acquisition and expression of mesolimbic reward prediction signals. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:1943–8. doi: 10.1073/pnas.1519643113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DP, Roitman MF, Garris PA. Illicit dopamine transients: reconciling actions of abused drugs. Trends Neurosci. 2014;37:200–10. doi: 10.1016/j.tins.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Almeida V, Hipolide DC, Raymond R, Barlow KB, Parkes JH, Pedrazzoli M, Tufik S, Nobrega JN. Opposite effects of sleep rebound on orexin OX1 and OX2 receptor expression in rat brain. Brain research Molecular brain research. 2005;136:148–57. doi: 10.1016/j.molbrainres.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Grant BF. Rates and correlates of relapse among individuals in remission from DSM-IV alcohol dependence: a 3-year follow-up. Alcoholism, clinical and experimental research. 2007;31:2036–45. doi: 10.1111/j.1530-0277.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van Den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. Journal of psychopharmacology (Oxford, England) 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47(Suppl 1):202–13. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- España RA. Hypocretin/orexin involvement in reward and reinforcement. Vitamins and hormones. 2012;89:185–208. doi: 10.1016/B978-0-12-394623-2.00010-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Melchior JR, Roberts DCS, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology. 2011:1–12. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DCS, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Valentino RJ, Berridge CW. Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience. 2003;121:201–217. doi: 10.1016/s0306-4522(03)00334-8. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Freeman AS, Bunney BS. Activity of A9 and A10 dopaminergic neurons in unrestrained rats: further characterization and effects of apomorphine and cholecystokinin. Brain Res. 1987;405:46–55. doi: 10.1016/0006-8993(87)90988-7. [DOI] [PubMed] [Google Scholar]
- Garris PA, Budygin EA, Phillips PEM, Venton BJ, Robinson DL, Bergstrom BP, Rebec GV, Wightman RM. A role for presynaptic mechanisms in the actions of nomifensine and haloperidol. Neuroscience. 2003;118:819–829. doi: 10.1016/s0306-4522(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Gotter AL, Webber AL, Coleman PJ, Renger JJ, Winrow CJ. International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin receptor function, nomenclature and pharmacology. Pharmacol Rev. 2012;64:389–420. doi: 10.1124/pr.111.005546. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95(Suppl 2):S119–28. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–90. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, Ungerstedt U. Cocaine: an in vivo microdialysis evaluation of its acute action on dopamine transmission in rat striatum. Synapse. 1989;3:48–54. doi: 10.1002/syn.890030107. [DOI] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N. Possible involvement of orexin in the stress reaction in rats. Biochem Biophys Res Commun. 2000;270:318–323. doi: 10.1006/bbrc.2000.2412. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neuroscience and biobehavioral reviews. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, Seutin V, North RA. Burst firing in dopamine neurons induced by N-methyl-D-aspartate: role of electrogenic sodium pump. Science. 1992;258:665–7. doi: 10.1126/science.1329209. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–80. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Keiflin R, Janak PH. Dopamine Prediction Errors in Reward Learning and Addiction: From Theory to Neural Circuitry. Neuron. 2015;88:247–63. doi: 10.1016/j.neuron.2015.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithley RB, Heien ML, Wightman RM. Multivariate concentration determination using principal component regression with residual analysis. Trends Analyt Chem. 2009;28:1127–1136. doi: 10.1016/j.trac.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko D, Wanat MJ. Phasic Dopamine Transmission Reflects Initiation Vigor and Exerted Effort in an Action- and Region-Specific Manner. J Neurosci. 2016;36:2202–11. doi: 10.1523/JNEUROSCI.1279-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AS, Kopell NJ, Wilson CJ. Transient High-Frequency Firing in a Coupled-Oscillator Model of the Mesencephalic Dopaminergic Neuron. Journal of Neurophysiology. 2006;95:932–947. doi: 10.1152/jn.00691.2004. [DOI] [PubMed] [Google Scholar]
- Lebold TP, Bonaventure P, Shireman BT. Selective orexin receptor antagonists. Bioorganic & medicinal chemistry letters. 2013;23:4761–9. doi: 10.1016/j.bmcl.2013.06.057. [DOI] [PubMed] [Google Scholar]
- Liang YJ, Zhen J, Chen N, Reith ME. Interaction of catechol and non-catechol substrates with externally or internally facing dopamine transporters. J Neurochem. 2009;109:981–94. doi: 10.1111/j.1471-4159.2009.06034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nature neuroscience. 2014;17:1298–303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- McElhinny CJ, Jr, Lewin AH, Mascarella SW, Runyon S, Brieaddy L, Carroll FI. Hydrolytic instability of the important orexin 1 receptor antagonist SB-334867: possible confounding effects on in vivo and in vitro studies. Bioorganic & medicinal chemistry letters. 2012;22:6661–4. doi: 10.1016/j.bmcl.2012.08.109. [DOI] [PubMed] [Google Scholar]
- Mereu G, Lilliu V, Casula A, Vargiu PF, Diana M, Musa A, Gessa GL. Spontaneous bursting activity of dopaminergic neurons in midbrain slices from immature rats: role of N-methyl-D-aspartate receptors. Neuroscience. 1997;77:1029–36. doi: 10.1016/s0306-4522(96)00474-5. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: diurnal influences. J Neurosci. 2010;30:15585–99. doi: 10.1523/JNEUROSCI.2871-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz AE, Foster JD, Gorentla BK, Mazei-Robison MS, Yang JW, Sitte HH, Blakely RD, Vaughan RA. Phosphorylation of dopamine transporter serine 7 modulates cocaine analog binding. The Journal of biological chemistry. 2013;288:20–32. doi: 10.1074/jbc.M112.407874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Dominguez JM, Sato SM, Shen RY, Hull EM. A role for hypocretin (orexin) in male sexual behavior. J Neurosci. 2007;27:2837–2845. doi: 10.1523/JNEUROSCI.4121-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, Kamenecka TM, Borgland SL, Kenny PJ, Carlezon WA., Jr Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci U S A. 2014;111:E1648–55. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Taha SA, Kim SW, Fields HL. Nucleus accumbens dopamine release is necessary and sufficient to promote the behavioral response to reward-predictive cues. Neuroscience. 2005;135:1025–33. doi: 10.1016/j.neuroscience.2005.06.088. [DOI] [PubMed] [Google Scholar]
- Patyal R, Woo EY, Borgland SL. Local hypocretin-1 modulates terminal dopamine concentration in the nucleus accumbens shell. Front Behav Neurosci. 2012;6:82. doi: 10.3389/fnbeh.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrey DA, Decker AM, Li JX, Gilmour BP, Thomas BF, Harris DL, Runyon SP, Zhang Y. The importance of the 6- and 7-positions of tetrahydroisoquinolines as selective antagonists for the orexin 1 receptor. Bioorg Med Chem. 2015a;23:5709–24. doi: 10.1016/j.bmc.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrey DA, German NA, Decker AM, Thorn D, Li JX, Gilmour BP, Thomas BF, Harris DL, Runyon SP, Zhang Y. Effect of 1-substitution on tetrahydroisoquinolines as selective antagonists for the orexin-1 receptor. ACS chemical neuroscience. 2015b;6:599–614. doi: 10.1021/cn500330v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrey DA, German NA, Gilmour BP, Li JX, Harris DL, Thomas BF, Zhang Y. Substituted tetrahydroisoquinolines as selective antagonists for the orexin 1 receptor. J Med Chem. 2013;56:6901–16. doi: 10.1021/jm400720h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van Den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Prince CD, Rau AR, Yorgason JT, España RA. Hypocretin/Orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS chemical neuroscience. 2015;6:138–46. doi: 10.1021/cn500246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisco S, Natoli S, Bernardi G, Mercuri NB. Group I metabotropic glutamate receptors activate burst firing in rat midbrain dopaminergic neurons. Neuropharmacology. 2002;42:289–96. doi: 10.1016/s0028-3908(01)00192-7. [DOI] [PubMed] [Google Scholar]
- Richardson BD, Saha K, Krout D, Cabrera E, Felts B, Henry LK, Swant J, Zou MF, Newman AH, Khoshbouei H. Membrane potential shapes regulation of dopamine transporter trafficking at the plasma membrane. Nature communications. 2016;7:10423. doi: 10.1038/ncomms10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Venton BJ, Heien ML, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clinical chemistry. 2003;49:1763–73. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Zitzman DL, Williams SK. Mesolimbic dopamine transients in motivated behaviors: focus on maternal behavior. Frontiers in psychiatry. 2011;2:23. doi: 10.3389/fpsyt.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: Individual differences. Neuropharmacology. 2014;76(Pt B):450–9. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S. Seeing the unseen: the hidden world of slow axonal transport. Neuroscientist. 2014;20:71–81. doi: 10.1177/1073858413498306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1. doi: 10.1016/s0092-8674(02)09256-5. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in resisting temptation: implications for addiction. Neuroscience and biobehavioral reviews. 2013;37:1955–75. doi: 10.1016/j.neubiorev.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A Neural Substrate of Prediction and Reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Shaw JK, Ferris MJ, Locke JL, Brodnik ZD, Jones SR, España RA. Hypocretin/orexin knock-out mice display disrupted behavioral and dopamine responses to cocaine. Addict Biol. 2016 doi: 10.1111/adb.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitko TA, Robinson DL. Regional variation in phasic dopamine release during alcohol and sucrose self-administration in rats. ACS chemical neuroscience. 2015;6:147–54. doi: 10.1021/cn500251j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2010;58:179–184. doi: 10.1016/j.neuropharm.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PEM, Carelli RM, Wightman RM. Rapid Dopamine Signaling in the Nucleus Accumbens during Contingent and Noncontingent Cocaine Administration. Neuropsychopharmacology. 2004;30:853–863. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Wightman RM, Carelli RM. Extinction of cocaine self-administration reveals functionally and temporally distinct dopaminergic signals in the nucleus accumbens. Neuron. 2005;46:661–669. doi: 10.1016/j.neuron.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps:Structure of the rat brain. Elsevier Science Publishers B.V; Amsterdam: 1998. [Google Scholar]
- Syed EC, Grima LL, Magill PJ, Bogacz R, Brown P, Walton ME. Action initiation shapes mesolimbic dopamine encoding of future rewards. Nature neuroscience. 2016;19:34–6. doi: 10.1038/nn.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Tung LW, Lu GL, Lee YH, Yu L, Lee HJ, Leishman E, Bradshaw H, Hwang LL, Hung MS, Mackie K, Zimmer A, Chiou LC. Orexins contribute to restraint stress-induced cocaine relapse by endocannabinoid-mediated disinhibition of dopaminergic neurons. Nature communications. 2016;7:12199. doi: 10.1038/ncomms12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin R, Fowler JS, Franceschi D, Franceschi M, Logan J, Gatley SJ, Wong C, Ding YS, Hitzemann R, Pappas N. Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci. 2000;67:1507–15. doi: 10.1016/s0024-3205(00)00731-1. [DOI] [PubMed] [Google Scholar]
- Wang T, O’Connor WT, Ungerstedt U, French ED. N-methyl-D-aspartic acid biphasically regulates the biochemical and electrophysiological response of A10 dopamine neurons in the ventral tegmental area: in vivo microdialysis and in vitro electrophysiological studies. Brain Res. 1994;666:255–62. doi: 10.1016/0006-8993(94)90780-3. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Paul IA, Woolverton WL. Comparison between dopamine transporter affinity and self-administration potency of local anesthetics in rhesus monkeys. European journal of pharmacology. 1999;367:175–81. doi: 10.1016/s0014-2999(98)00967-4. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Current topics in behavioral neurosciences. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nature reviews Neuroscience. 2004;5:483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. Demon Voltammetry and Analysis software: Analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011 doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Onaka T, Sakurai T, Yada T. Activation of orexin neurones after noxious but not conditioned fear stimuli in rats. Neuroreport. 2002;13:1351–1353. doi: 10.1097/00001756-200207190-00027. [DOI] [PubMed] [Google Scholar]