Abstract
Metamemory entails cognitively assessing the strength of one’s memories. We tested the ability of nine Long-Evans rats to distinguish between remembering and forgetting by presenting a decline option that allowed a four-choice odor-based delayed match to sample (DMTS) tests to be by-passed. Rats performed significantly better on tests they chose to take than on tests they were forced to take, indicating metacognitive responding. However, rather than control by internal mnemonic cues, one alternative explanation is that decline use is based on external test-specific cues that become associated with increased rewards overtime. To examine this possibility, we tested rats on three generalization tests in which external contingencies were inconsistent and therefore could not serve as discriminative cues. Rats transferred adaptive use of the decline response in tests that eliminated memory by presenting no sample, increased memory by presenting multiple samples, and both weakened and strengthened memory by varying the retention interval. Further, subjects chose to take or decline the test before encountering the memory test, providing evidence that rats based their metacognitive responding on internal cues rather than external ones. To our knowledge, this is the first robust evidence for metamemory in rats using the DMTS decline-test paradigm in which several possible sources of external stimulus control can be ruled out.
Keywords: metamemory, executive control, metacognition, monitoring, explicit memory, declarative memory
Metamemory is the ability to monitor the strength of one’s memories, an ability routinely demonstrated by humans (e.g. Sutton & Shettleworth, 2008). For example, members of a trivia team with competing answers to the same question may gauge their memory strengths before submitting a final answer. In doing this, they have made real-time assessments of their level of knowledge concerning a specific subject. Such metacognitive abilities rely on a central executive that controls and optimizes one’s cognitive processes (e.g. Benjamin et al., 1998; Dunlosky & Bjork, 2008; Flavell, 1979; Nelson, 1992; Schwartz, 1994) and is thought to be one of humans’ most cognitively complex abilities (Melcalfe & Kober, 2005).
While humans are able to demonstrate metacognitive ability by verbal communication, the extent to which non-human animals possess metamemory remains a debated topic (Caruthers, 2008; Jozefowiez et al., 2009; Smith et al., 2014). The initial nonverbal hurdle has been largely overcome with clever designs suitable for nonverbal species that rely on operational definitions of metacognition based on observable behavior. Many species have demonstrated metacognitive responding (for reviews see: Crystal & Foote, 2009; Hampton, 2009; Kornell 2009; Smith 2009; Roberts et al., 2012, Smith et al. 2014), but the current challenge lies in teasing apart potential sources of stimulus control that govern putative metacognitive responding.
The earliest group of researchers to assess metacognitive abilities in nonhumans gave subjects the option to decline tests. Presumably, if subjective knowledge states can be monitored, tests should be declined when confidence is low. Rhesus monkeys, bottlenose dolphins, and humans show metacognitive responding by successfully declining difficult visual or auditory discrimination tests (e.g. recognizing auditory stimulus as high- and low-pitched; Shields et al. 1997) when the stimuli were near the discriminatory threshold. Results of similar tasks demonstrate that, in addition to monkeys, birds, and rats selectively avoid difficult psychological discriminations (e.g. monkeys: Brown et al., 2017; rats: Foote & Crystal, 2007; pigeons and bantams: Nakamura et al., 2011).
These response patterns may be a result of monitoring one’s own knowledge state, which is an internal and private source of stimulus control. However, they also may be explainable by reliance on external cues that are publicly available (Hampton, 2009). Because introspective cues are internal and private, they can only be inferred by first excluding public ones. One possible external cue is the fact that difficult trials in discrimination tasks may be declined simply because intermediate stimuli, like pixel densities that are not unmistakably dense or sparse, become associated with the decline response over time. Stimulus magnitude, image similarity, and other features inherent to the test stimuli itself (Sutton & Shettleworth, 2003; Kornell et al, 2007) are examples of environmental cue associations (Hampton, 2009) that may serve as a discriminative stimulus to decline certain trials which include no internal component. Associative simulation models (Jozefowiez et al., 2009; Le Pelley, 2012; Smith et al, 2008) have also been found to predict use of the decline response on psychophysical discriminations such as those in Smith et al. (1997) and Foote and Crystal (2007). The best way to rule out control of metacognitive responding (i.e. declining difficult tests) by external cues, like environmental cue associations, is to provide generalization tests across which external test-specific features are not consistent (Brown et al., 2017; Hampton, 2009). In addition, use of metamemory paradigms in which relative memory strength serves as the natural continuum of discriminanda is advantageous (Brown et al., 2017; Hampton, 2001; Smith et al., 1998; Templer & Hampton, 2012). This is because metamemory tests offer fewer ostensible stimulus features and potential cues in the test itself compared to psychophysical tests. Instead, memory strength is responsible for creating variation in performance.
Generalization of metacognitive responding across varied experimental conditions is one of the main reasons evidence for metamemory in rhesus monkeys has been so strong (Smith et al., 2014). The other reason is because of positive results when prospective metamemory tests have been used (Basile et al., 2015; Hampton, 2001). In a prospective metamemory test the secondary metamemory options are present before the primary memory test (Goto & Watanabe, 2012; Hampton, 2009; Shettleworth, 2010, p.249). In contrast, in a concurrent metamemory test the primary memory test and secondary metamemory options are present at the same time. In Hampton’s (2001) study monkeys chose to take or decline the test before engaging with the primary Delayed Match to Sample (DMTS) memory test. After training on the DMTS test, delays between study and test were titrated to an intermediate level that was above chance but below ceiling. Monkeys were then presented with a metacognitive response at the end of the delay that allowed them to accept the memory test, and receive a preferred reward if correct, or decline the memory test which resulted in a guaranteed, but less preferred reward. Monkeys were more accurate on trials they chose to take than they were on other trials that were presented in which there was no option to decline the memory test. A performance advantage on chosen trials as compared to forced trials results from adaptively declining difficult tests and indicates metacognitive responding. This is because forced trials are a combination of accuracy on tests animals would have declined had they had the option and trials they would have chosen to take. Monkeys also transferred adaptive decline use to conditions in which memory was manipulated by eliminating memory of the sample (by presenting no sample) or increasing memory difficulty (by increasing the delay).
The prospective, rather than concurrent, metamemory method is advantageous because it rules out potential sources of external stimulus control like behavioral cue associations and response competition (Hampton, 2009). Behavioral cue associations include behavioral tendencies a subject might engage in when the animal doesn’t know how to respond, like taking longer to respond, vacillating, or hesitating in any way (Goto & Watanabe, 2012; Hampton, 2009; Terrace & Son, 2009). Longer response latencies on such trials, when the correct answer is not immediately evident, could therefore serve as a publicly available cue to decline when the metacognitive response is concurrent with the memory test, especially since response latencies in such MTS-based tasks are longer for incorrect than correct tests (e.g. Hampton & Hampstead, 2006). Response competition refers to the fact that, when the primary memory test is in direct competition with the secondary metacognitive response, subjects might learn to associate absence of an inclination to select a choice response with selection of the decline response. In contrast, when the test is less difficult animals might simply have a strong tendency to select the correct choice response without making the internally-guided decision to take the test because they know they remember. Hampton’s (2001) and Basile et al. (2015) results are especially strong because of the use of prospective tests where control by behavioral cue associations and response competition are impossible.
Metamemory and metacognitive tests with multiple generalization procedures (Basile et al., 2015; Brown et al., 2017; Hampton 2001; Templer & Hampton, 2012) combined with results from other information-seeking paradigms, and retrospective confidence judgment paradigms, have led several students of comparative metacognition to contend that evidence for introspective metacognition in Old World monkeys (Kornell, Son, & Terrace, 2007; Rosati & Santos, 2016; Smith, Shields, & Washburn, 1998) and apes (Beran et al., 2013; Call, 2010; Call & Carpenter, 2001; Marsh & MacDonald, 2012; Suda-King, 2008; Suda-King et al., 2013) is strong and functionally similar to that of humans (Fujita, 2009; Roberts et al, 2009; Smith et al., 2014). Such compelling results, including those of Hampton (2001), Smith et al. (2013), and Smith et al., (2006) have since been shown to be impossible to predict from modeling using only associative processes (Smith et al., 2014).
Evidence for metacognition in other species like pigeons (Castro & Wasserman, 2013; Inman & Shettleworth, 1999; Iwasaki et al., 2013; Roberts et al., 2009; Roberts et al., 2012; Sutton & Shettleworth, 2008), New World monkeys (Basile et al., 2008; Beran et al., 2016; Paukner et al., 2006; Vining & Marsh, 2015) and rats (Foote & Crystal, 2007; Foote & Crystal, 2012) has been less robust, making it unclear whether metamemory is phylogenetically limited to Old World monkeys and apes, or whether is it a more ancient cognitive ability. Demonstrations of some aspects of metacognitive behavior in pigeons (Adams & Santi, 2011; Zentall and Stagner, 2010) and capuchin monkeys (Beran, Perdue, Church, & Smith, 2016) have been more convincing, but certainly not as substantial as those in macaque monkeys (Beran et al., 2016). In the few studies that exist, evidence for metacognition in rats is also mixed (Foote & Crystal, 2017; 2012; Kirk et al., 2014; Yuki & Okanoya, 2017). The lack of robust results in rats are somewhat surprising given that researchers have found many similarities between human and rat explicit memory in recent years (e.g., Crystal, 2013; Crystal et al., 2013; Eacott & Norman, 2004; Eichenbaum, 2005; Fortin et al., 2004; Panoz-Brown et al., 2016). One reason that the metacognitive results in rats are equivocal is due to the methods employed and fact that few attempts have been made to systematically rule out external sources of stimulus control.
Sprague-Dawley rats showed improved performance on classification tests of short vs. long sound durations when they chose to take the test than on trials they were forced to complete. Rats also successfully declined difficult intermediate durations (Foote & Crystal, 2007). In a later study, Foote and Crystal expanded upon this paradigm by also including trials in which subjects were forced to repeat the sample stimulus or could choose to repeat the stimulus. Three of seven rats were more accurate at making the duration discriminations when they were forced to repeat it than when they chose to repeat it (Foote & Crystal, 2012). While such responding could be explained by control by internal cues, rats were not more likely to repeat the stimulus on difficult trials, which would be a clear prediction of internal stimulus control. Further, with a temporal discrimination task, cueing by environmental associations is difficult to rule out because animals might simply have learned that middle stimuli (i.e. intermediate durations) signal decline use and this hypothesis was not tested with generalization tests (Hampton, 2009).
In a separate study, Long-Evans rats obtained information that signaled correct response locations by pressing a lever in a T-maze or a Radial Arm Maze, when it worked to their advantage in gaining food (Kirk et al., 2014). However, when the food reward that resulted from obtaining more information via a lever press was eliminated, the behavior diminished in some cases. Because rats did not complete generalization tests like those used in other information-seeking paradigms (Basile et al., 2015) it is possible that external sources of stimulus control could have controlled information-seeking behavior. In a very recent study, rats did generalize adaptive use of the decline response on a delayed-match-to-position test when the sample was omitted, but this metacognitive behavior was restricted to a six-choice test and was not found in a two-choice test (Yuki & Okanoya, 2017). This study made a significant step in the right direction, but positive results were obtained by only two rats in one generalization test.
Given the lack of consensus on the extent to which rats show performance consistent with metacognitive responding by internal sources of stimulus control, in Experiment 1 we employed a Delayed Matching to Sample (DMTS) test with Long-Evans rats modeled after the influential paradigm used in monkeys by Hampton (2001) and later adapted and expanded with a novel generalization test by Templer & Hampton (2012). Rats were tested in a four-choice odor based paradigm in which they dug in sand-filled cups, either to receive a preferred reward or to decline the test and obtain a less preferred, but guaranteed reward. To make the memory test reasonably difficult so that rats would experience both remembering and forgetting, the same four odors appeared on every trial, increasing memory interference. Subjects that have have access to internal memory cues should choose to take tests in which they remember the sampled odor and decline tests in which they forget, which would result in a performance advantage on tests in which the decline option is available as compared to tests on which it is not available. Experiments 2–4 presented three generalization tests in which memory strength was directly manipulated to control for possible cueing by environmental cue associations.
General methods
Subjects
Subjects were nine male Long-Evans rats (Rattus norvegicus) that arrived from Charles River Laboratories on post-natal day (PND) 21. The original testing group began with ten rats, but one was eliminated from testing after repeated failure to pass food preference testing. Rats were housed individually in enriched environments throughout development and into adulthood when they were tested on experiments described here, which occurred between PND 329–458. Subjects were housed in one colony room, maintained at 21.6 degrees C and kept on a 12:12 reversed light:dark cycle, with light onset at 8 PM. Cages were large (17×12.75×24in; Ferret Nation) with multiple platforms and solid flooring, enclosed by wire meshing. Rats had constant access to enrichment in their cages, which included additional platforms, running wheels, plastic toys/enclosures, an open-topped plastic shoebox cage (17×8×8in) and wooden chew toys. Corncob bedding (Enrich-o’cobs) was provided at the bottom of all enclosures, including the shoebox cage that was used to transport the rats into the testing room. The large size of the cages and surrounding metal bars provided ample opportunities for climbing and exercise.
Rat chow (LabDiet) was provided ad libitum until rats reached adulthood (approximately PND 60), after which point weighed rations of chow were provided daily after testing was complete. Rats were weighed weekly and chow rations were adjusted accordingly to maintain their body weights at approximately 90% of their estimated free-feeding weight. Water was available ad libitum, except for when the rats were being tested. All procedures described in the current study were approved by the Institutional Animal Care and Use Committee (IACUC) of Providence College.
Subjects had previous experience on unrelated learning and memory tasks. With the goal of piloting the methods for the experiments presented here, four of the subjects included in this study had participated in a previous DMTS metacognitive task. Seven of the subjects had also received limited training on the odor-based MTS procedure.
Apparatus
All training and testing sessions occurred inside a social interaction chamber (Noldus; 30×45in, Fig. 1) divided into three separate rooms. The center room was divided into the sample (10×15in) and delay (10×15in) chambers by a cardboard insert that could be easily lifted by pulling the insert up along metal tracks secured to the walls divided the rooms. A small rectangular hole connected the delay chamber and the large room to the right: the testing chamber (10×45in), where the choice and decline cups were located. A removable opaque Plexiglas door could easily be lifted to allow the rat to travel from the delay chamber to the testing chamber.
Fig. 1.
Photograph of the testing apparatus, which was divided into the sample chamber, delay chamber, and testing chamber (on the right). The testing chamber was divided into two sides: 1) the decline option area, which was bumpy in texture and covered in red contact paper, and contained the decline cup and 2) the MTS task area, which was corrugated in texture, covered with black contact paper, and contained the four choice cups.
The sample, choice, and decline cups were ceramic (2.75in in diameter and 1.5in tall) and filled half-way with play sand. The sample and choice cups were filled with play sand and the decline cup was filled with orange play sand that was slightly finer. To further differentiate the test and the decline option, two different floor textures (Fig. 1) were created. Where the choice cups were located, the floor was covered in black contact paper with elevated stripes created by layering duct tape underneath, while the floor where the decline option was located was covered in red contact paper with sand granules underneath. The four odors that were used throughout all experiments were: cinnamon, thyme, paprika, and coffee. Scented sand was created by mixing approximately three cups of sand with a small pinch of each scented material. All choice cups contained tiny crumbled pieces of cereal (that could not be eaten) to control for odor of the correct choice cup that contained a food reward. The scented sand was replaced each week.
General Procedure
Rats were tested between 8:30 AM and 5 PM six days a week. Subjects were transported from their home cages to the testing room and given at least 30 minutes to acclimate before being tested under red-light conditions. Rats were fed food rations after testing each day.
Rats were placed in the sample room and a right-angled flat-spatula holding the sample cup was lowered to the floor so that the subject could dig to the bottom of the sand-scented cup to obtain a half piece of cereal. A food reward was provided to ensure that subjects sufficiently sampled the odor. After the food reward was retrieved, the door that connected the sample chamber to the delay chamber was opened and the rat remained in the delay chamber for the duration of the retention interval. After the retention interval, a small opaque door connecting the delay chamber and the testing chamber was opened so that the rat could enter the testing chamber. The testing chamber contained the choice cups on the right side of the room and decline cup on the left side of the room. On trials in which the decline response was not available, there was no decline cup present. On trials in which the decline response was available, a quarter piece of cereal was buried in the unscented decline cup. Each of the four choice cups was filled with a scented sand (i.e. cinnamon, coffee, paprika, thyme). In one of the cups, the scent matched that of the sample cup (e.g. coffee), and this cup also contained the buried preferred food reward: a whole piece of cereal. Remaining choice cups were odor controlled with crumbled cereal mixed into the sand, so that all four cups smelled equally of the food reward. Rats indicated choices by digging and were prevented from attempting to dig in more than one cup. After digging in any one of the cups, and consuming the food reward if correct, the rat was immediately removed from the chamber to prevent multiple choices. Rats waited in their home cage adjacent to the testing chamber during the four-minute inter-trial interval (ITI) before being moved back into the sample chamber to begin the next trial.
The sample odor was semi-randomized and each of the odors appeared as the sample three times within a block of 12 trials. The locations of the incorrect and correct choice cups were also counterbalanced and randomized such that each odor was presented at each location an equal number of times.
Data Analysis and Behavior Scoring
During each trial, the first cup in which the rat started digging was considered his choice. Rats were immediately removed from the chamber after digging in the first cup to prevent them from selecting another choice cup or the decline cup. Proportions were arcsine transformed before statistical analyses to better approximate the normality assumption underlying parametric statistics (Keppel and Wickens 2004, p. 155).
Training Procedures
Preference Testing and Preference Checks
A hierarchy of food rewards was established so that the larger reward (whole piece of cereal) could be used as the “preferred” option in the correct choice cup and the smaller reward (1/4 piece of cereal) could be used as the “less preferred” in the decline cup. Depending on the individual rat’s preference, the cereal reward was either a FrootLoop (Kellogg’s), Capn’ Crunch (Quaker Oats), or dehydrated marshmallow (Medley Hills Farm). The two rewards were placed in two corners of the chamber and the rat was placed on the opposite side and then allowed to eat one of the rewards. Once the rat chose the larger reward four out of five trials for two consecutive sessions, MTS training began. As mentioned above, one rat did not establish a preference and was therefore not included in the following experiments. This testing began on PND 288 and lasted until PND 298.
Preference checks were performed each week throughout testing to ensure that preferred food rewards did not change. Preference checks operated in the same way as preference testing, but were comprised of two trials that occurred after normal test sessions were conducted. All animals maintained their original preferences throughout testing.
Training to Dig in Sand-filled Cups
Rats were trained to dig in sand-filled cups to obtain a food reward at the bottom. First, these sand filled cups were placed in home cages overnight. After two nights of successful reward retrieval, rats were required to dig for food rewards in the sample chamber of the apparatus twice before MTS training began.
Match to Sample (MTS) Training
Although seven rats had already undergone MTS training several months earlier, all were retrained to ensure that their behavior was still under the control of the task contingencies. Trials occurred as explained above in general procedure. The delay (retention interval) between study and test was as short as possible: as long as it took to open the door to the testing chamber, which was less than a few seconds. Rats received MTS trials until the sample odor was accurately matched to the choice odor for at least three out of six trials for two consecutive six-trial sessions.
Delay Titration in Delayed Match to Sample (DMTS)
This phase was identical to MTS training except that the delays between study and test were increased for each rat in order to ensure that performance was above chance (25%) but below ceiling. Delays were therefore titrated until performance stabilized between 40% and 70% correct. Beginning with the effective delay of zero seconds from MTS training, the delay was increased to 30 seconds if performance exceeded 70% for two consecutive sessions of six trials. Subsequent delay increases were calculated by multiplying 1.5 times the current delay. If performance fell below 40% on two consecutive sessions, the delay was decreased back to its previous retention interval. When the delay had not been changed for two sessions, this delay was set as the criterion delay, specific to each rat (Table 1).
Table 1.
Criterion delays and decline rates in Experiments 1–4 and accuracies in Experiments 1 and 4.
| Stage of testing | Specifics | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | Mean |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experiment 1 | Criterion delay (s) | 0 | *60 | 0 | 0 | 60 | *90 | 30 | 90 | 0 | |
| Forced accuracy | .55 | .58 | .43 | .6 | .45 | .65 | .5 | .5 | .5 | .52 | |
| Chosen accuracy | .51 | .58 | .63 | .7 | .51 | .69 | .78 | .53 | .6 | .61 | |
| Decline rate | .31 | .2 | .11 | .13 | .21 | .16 | .25 | .08 | .3 | .2 | |
| Experiment 2 | Criterion delay (s) | 0 | 60 | 0 | 0 | 60 | 90 | 30 | 90 | 0 | |
| Decline rate no sample trials | .3 | .2 | .33 | .5 | .25 | .33 | .15 | .38 | .5 | .32 | |
| Decline rate normal trials | .13 | .18 | .06 | .2 | .2 | .13 | 0 | .29 | .2 | .15 | |
| Experiment 3 | Criterion delay (s)-Phase 1 | 20 | 80 | 20 | 20 | 80 | 110 | 50 | 110 | 20 | |
| Criterion delay (s)-Phase 2 | 120 | 160 | 90 | 100 | 120 | 150 | 90 | 150 | 60 | ||
| Decline rate double sample trials | .05 | .13 | 0 | .3 | .07 | .05 | .03 | .3 | 0 | .1 | |
| Decline rate normal trials | .21 | .33 | .05 | .5 | .13 | .24 | .11 | .62 | .1 | .26 | |
| Experiment 4 | Criterion delay (s) | 120 | 160 | 90 | 100 | 120 | 150 | 90 | 150 | 60 | |
| Forced accuracy (0 sec) | .69 | .56 | .83 | .88 | .5 | .88 | .65 | .73 | .6 | .70 | |
| Forced accuracy criterion delay | .43 | .5 | .64 | .5 | .43 | .21 | .57 | .43 | .29 | .44 | |
| Forced accuracy long delay (4 min) | .45 | .63 | .33 | .41 | .33 | .45 | .38 | .33 | .36 | .41 | |
| Chosen accuracy (0 sec) | .72 | .97 | .71 | .97 | .77 | .82 | .84 | .78 | .88 | .83 | |
| Chosen accuracy (criterion delay) | .71 | .97 | .61 | .75 | .83 | .75 | .76 | .74 | .67 | .75 | |
| Chosen accuracy long delay (4 min) | .8 | .67 | .83 | 1 | .69 | .88 | .81 | .17 | .5 | .71 | |
| Decline rate short delay (0 sec) | 0 | .06 | .07 | 0 | .19 | .07 | .03 | .09 | 0 | .06 | |
| Decline rate criterion delay | .11 | .23 | .2 | .6 | .13 | .19 | .12 | .49 | .2 | .24 | |
| Decline rate long delay (4 min) | .78 | .72 | .67 | .9 | .42 | .69 | .51 | .64 | .5 | .64 |
Criterion delay was readjusted during Experiment 1
Decline Use Training
While delay titration was in progress, subjects were also trained to select the decline response option so that they could learn that this cup always held a less preferred, but guaranteed food reward. After each six-trial DMTS session, rats were given two forced decline trials. After sampling the odor and waiting for the currently titrated delay, the rat entered the testing chamber and encountered only the unscented decline cup (no choice cups were present). The decline cup contained the less preferred, yet guaranteed reward, a quarter of a cereal. After the rat consumed the cereal, he was removed from the chamber.
Experiment 1
Experiment 1 was the first situation in which rats had the opportunity to demonstrate metacognitive responding. Subjects were given the option to decline the DMTS test on two-thirds of the trials, but in the remaining third of trials, the decline option was not available. If rats have metamemory, then when given the option, they should decline tests when memory for the sample is weak, and take tests when memory is comparatively strong and they are more likely to answer the memory test correctly. Such adaptive use of the decline response would cause significantly higher performance on trials in which the decline option is available compared to trials on which it is not. This is because performance on forced tests includes trials that rats might have declined if they had the option. If rats do not have metamemory, decline use should not track internal memory strength and should instead be chosen at random, causing performance on chosen and forced trials to be equivalent.
Methods
Subjects and apparatus
All subjects and apparatus were the same as those used in training.
Procedure
The procedure was identical to that used in training except that four forced trials in which the decline cup was not available were randomly intermixed with eight choice trials in which the decline cup was available. Rats were tested on one 12-trial session a day and received a total of ten sessions.
To ensure that each rat’s performance remained within in the range of 40% to 70%, accuracy over three consecutive sessions (12 forced trials) was calculated using a sliding window after each session was completed. If a subject’s performance fell outside of the range for three consecutive sessions, the rat reentered the delay titration phase (consisting of 6 forced trials) to gauge performance. Delay titration occurred as described above and continued until performance stabilized within the desired range for one 12-trial session. At this point the rat re-entered testing in Experiment 1, beginning at session one with the adjusted delay. The performance of two rats exceeded 70% on forced trials, which lead to an increase in their respective delays (Table 1).
Results and Discussion
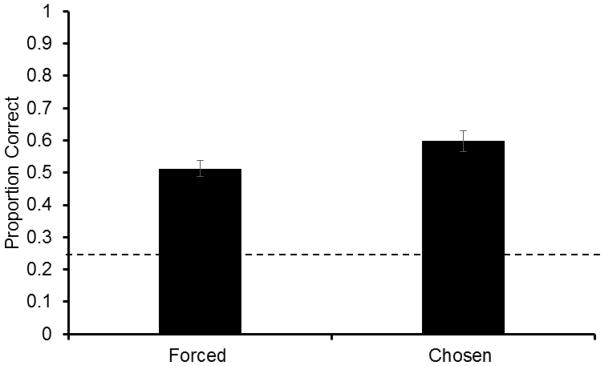
Performance was significantly higher on chosen trials than on forced trials (Fig. 2; two-tailed, paired-sample t-test: t8=3.20, P=0.013), indicating that rats adaptively used the decline option on chosen trials when memory was weak (average decline use is displayed in Table 1). There was no main effect of odor on forced trials (RMANOVA, F3,35=1.09, P=0.368), indicating that odors were equally memorable to subjects. Correspondingly, no odors were declined more than others (Cinnamon: M=.31, Coffee: M=.22; Paprika: .24; Thyme: .20; RMANOVA, F3,35=0.87, P=0.469), indicating that the effect was not driven simply by a tendency to decline one odor over another.
Fig. 2.
Proportion correct (±SEM) on forced and chosen trials in Experiment 1. The dotted line represents chance performance.
The results of Experiment 1 are consistent with the hypothesis that rats possess the ability to monitor their memories. Results mirror findings by Foote & Crystal (2007) that rats perform better on chosen tests as compared to forced tests, but this is the first demonstration of a chosen performance advantage on a memory-based test in rats. Because there are fewer ostensible environmental stimuli that could have cued metacognitive responding here than in the psychophysical arena (i.e. intermediate duration signals decline use), it is more likely that subjects here were responding to the detection of internal memory strengths. However, while variation in performance is driven by memory strength rather than externally presented stimuli, there may be environmental cues that subjects are attending to of which we are not aware. The following experiments test for generalization of appropriate decline use across multiple contexts in which no association with external stimuli was consistent. By manipulating memory in several ways, we test for the possibility that behavior is controlled by external stimuli or if is in fact driven by an internal assessment of memory strength.
Experiment 2
In Experiment 1 rats used the decline response adaptively, which lead to higher accuracy on chosen trials than forced trials. In order to control for external cues that may be correlated with the probability of reinforcement, Experiment 2 utilized “no-sample” trials, in which no sample was presented prior to the memory test. Here, memory strength is brought under direct experimental control because rats have no memory of a sample odor on these trials. If adaptive use of the decline response in Experiment 1 was controlled by an environmental cue association, rats should decline no-sample trials less frequently than normal trials because no relevant cue of the sample is available as a discriminative cue. Alternatively, if adaptive use of the decline response was controlled by an internal assessment of memory strength, rats should decline no-sample trials significantly more than normal trials because there are no external stimulus features of the sample that could be associated with reinforcement, as no sample is presented.
Methods
Subjects and Apparatus
The subjects and apparatus were the same as those used in Experiment 1.
Procedure
The procedure was similar to Experiment 1 in all but two ways; forced choice trials were not included, and no-sample trials were semi-randomly intermixed among normal choice trials. Each twelve-trial session therefore consisted of eight normal sample trials and four no-sample trials. During no-sample trials, a rat was placed in the sample chamber and after approximately one minute (the time it took to normally present a sample), he entered the delay chamber to wait for his criterion delay. A random choice cup was baited so that rats had a 25% chance of obtaining a reward if they did not decline. Subjects completed one session a day and received a total of ten sessions.
Results and Discussion
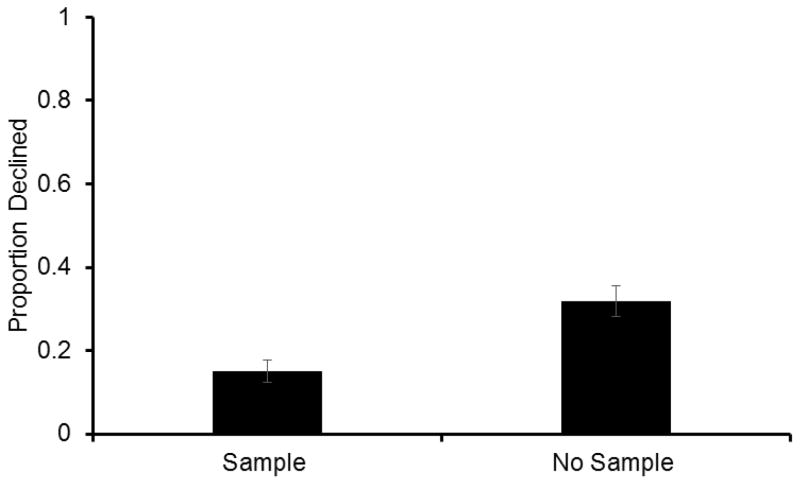
Rats declined no-sample trials significantly more often than the normal sample trials (Fig. 3, two-tailed, paired-sample t-test; t8=5.15, P=0.001), suggesting that use of the decline response is modulated by identifying an absence of memory, rather than by external cues that signal trial difficulty.
Fig. 3.
Proportion (±SEM) of choice trials on which rats declined the memory test when a sample was presented (sample) and when no sample was presented (no-sample) in Experiment 2.
While subjects declined no-sample trials more frequently than they did normal trials, average proportion declined on no-sample trials was .32, which is lower than would be expected. Presumably, if memory is truly being monitored and no sample was presented, subjects should almost always decline no-sample trials, which would result in decline rates close to 1.0, as seen in monkeys in an analogous experimental manipulation (Templer & Hampton, 2012). However, we rewarded animals randomly on no-sample tests so that the test phase of no-sample trials was identical to normal sample trials, while monkeys never received a reward on no-sample trials (Templer & Hampton, 2012). It is possible that rats declined these trials a third of the time, rather than 100% of the time, because they were attempting to remember the last presented sample and use that information to make their choice at test. In the current design, memory interference between trials is very high as the same four odors are present during each choice phase of every trial, increasing the possibility that rats were trying to remember the previously presented odor from the last normal trial in which a sample odor was experienced. To determine if rats were erring based on interference from the previous trial, we examined which odor was selected on trials that were not declined. During no-sample trials, if rats accepted the test, they were significantly more likely to choose the scent from the previous trial than chance (one sample t-test; t8=2.85, P=0.020), suggesting that behavior was likely under the control of the last presented odor on no-sample trials.
A related hypothesis worth considering is that subjects declined no-sample tests on the basis of weak signal strength of the last presented sample. In other words, the rat could be declining no-sample tests with no regard to the current no-sample test itself. However, if this was the case, such responding would necessitate metacognitive responding based on assessment of memory strength for previously presented trials. While this plausible adopted tactic by rats was unforeseen by the experimenters, there is no way to determine if decline use was under the control of the last presented sample or the omitted sample on the current trial. However, the strategy of remembering previously presented samples is consistent with the fact that subjects did not decline all or close to all no-sample tests.
In addition to the 25% chance of receiving a reward on a no-sample trial and the confirmed proactive interference, a general tendency towards risk taking behavior may have also contributed to rats’ tendency to take no-sample tests. Previous studies have indicated that if rats are satiated in terms of caloric intake, they may be more likely to take risks for possible but not guaranteed food rewards; when presented with choices similar to those presented here, rats are risk-prone (Mazur, 1988). Given that rats were fed full rations of food each day to maintain healthy weight, it is plausible that choosing to take the test, and having a 25% chance of receiving the quarter piece of cereal, was well worth the risk in most cases.
While we considered plausible reasons why rats did not decline the majority of no-sample trials, our primary metamemory hypothesis that no-sample trials should be declined significantly more than normal sample trials was strongly supported. Decline use tracked experimental manipulation of memory strength, reinforcing the hypothesis that rats chose to decline tests in which they knew they did not remember.
Experiment 3
In Experiment 2 we aimed to eliminate memory by not providing a sample, simulating trials on which subjects happened to forget the sample. Due to proactive inference from the previous trial’s sample on no-sample trials, it is likely that rats were attempting to remember a sample, and therefore were not fully aware of a true absence of memory. In Experiment 3, we therefore brought memory under direct experimental control in the opposite direction: increasing memory by providing two sample-study periods rather than one. If use of the decline response is controlled by an internal awareness of memory strength, rats should transfer adaptive use of the decline response to this new condition. Double-sample trials should be declined significantly less than normal sample trials as the second sample should increase memory (Roberts, 1972). However, if adaptive use of the decline response is controlled by some external cue, such as experiencing an unusual situation of receiving two samples, rats should decline double-sample trials significantly more than normal trials. Importantly, the hypothesis-driven predictions are in direct opposition to each other. Metacognitive responding by an assessment of memory strength predicts less decline use on double-sample trials as compared to normal sample tests. Responding based on environmental cue associations of probe tests predicts more decline use on double-sample trials as compared to normal sample tests. These bidirectional predictions will provide more convincing evidence of what decline use is tracking: either an internal memory-based cue or an external cue.
Methods
Subjects and Apparatus
The subjects and apparatus were the same as those used in Experiments 1 and 2.
Procedure
By the start of Experiment 3, rats had been tested on the MTS odor task for four months. Because we suspected performance may have increased due to experience, all rats underwent a performance check of ten forced trials with a slightly longer delay than their criterion delay (criterion delay plus 20 seconds). The results of the performance check revealed that all accuracies were within the range of 40% to 70% with the new delay, so testing for Experiment 3 began using this new delay.
Details of these trials were similar to Experiment 2 with two exceptions. Instead of no-sample trials, subjects received double-sample trials. Eight trials per session were normal sample trials, while the remaining four trials were double-sample trials. Additionally, to avoid satiation and hold reward value constant across sample periods, each presentation of the sample on double-sample trials contained a quarter piece of cereal rather than the half piece on normal trials. During the double-sample trials, the rat received the first sample scent and consumed the sample cup reward (quarter piece of cereal). Immediately after the rat had finished eating, a second sample of the same scent and a quarter piece of cereal was presented to the rat. The rest of the trial progressed as usual. Rats received one twelve-trial session a day and were tested for ten sessions.
Halfway through testing in Experiment 3, when five sessions had been completed, we noticed that decline use on normal sample trials had increased compared to Experiment 2. Therefore, we completed a second performance check halfway through Experiment 3 and confirmed that performance on forced trials had increased. Delays were subsequently re-titrated for each rat such that performance again fell between 40 and 70% correct. Rats were then tested on the remaining five sessions (Phase 2) with increased delays from the first half of Experiment 3 (considered Phase 1; see Table 1).
Results and Discussion
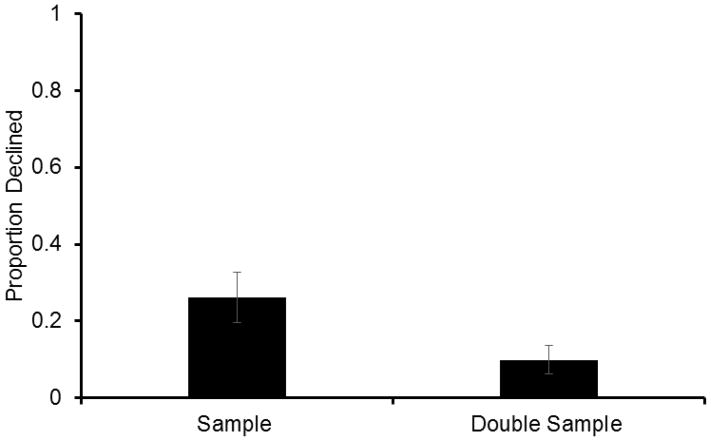
Subjects declined normal sample trials significantly more than they declined double-sample trials in both Phase 1 with the criterion delay + 20 seconds (two-tailed, paired-sample t-test; t8=−6.71, P=0.000) and Phase 2 with the further increased criterion delay (see Table 1; two-tailed, paired-sample t-test; t8=−6.65, P=0.000). Due to the analogous results across phases, we collapsed the data into a single set, which again indicated normal sample trials were declined significantly more than double-sample trials (Fig 4; two-tailed, paired-sample t-test; t8=6.71, P=0.000). That rats did not decline double-sample trials significantly more than normal sample trials, even though they could have appeared unique and unusual, indicates that metacognitive responding was not dependent upon external features unique to the probe trials. Instead, subjects were more likely to choose to take tests after a sample was presented twice and memory was increased. This provides additional evidence that decline use is likely based on an internal assessment of memory strength.
Fig. 4.
Proportion (±SEM) of choice trials on which rats declined the memory test when a sample was presented (sample) and when two samples were presented (double-sample) in Experiment 3.
In addition to initial metacognitive evidence presented in Experiment 1, rats transferred adaptive use of the decline response in both Experiments 2 and 3, providing growing refutations of potential sources of behavioral cue associations. Use of the decline response seems to be flexible enough to respond to bidirectional experimental manipulations of memory, as seen in the previous two experiments. To test bidirectional manipulations of memory in a single experiment, we attempted to both increase and decrease memory in Experiment 4.
Experiment 4
Memory in Experiment 4 was manipulated subtly by changing the delay between sample and test. Rats were tested on trials with their normal criterion delay, with no delay, and with a long delay of four minutes. To be sure that decreasing and increasing the delay altered memory strength, we included both forced trials and chosen trials at each of the three delays. If rats have metamemory they should decline fewer trials when memory is strong at the short delay and decline many trials when memory is weak at the long delay. Rats adaptively using the decline response should also display a performance advantage on chosen tests as compared to forced tests, mirroring the original finding in Experiment 1.
Methods
Subjects and Apparatus
The subjects and apparatus were the same as those used in the first three experiments.
Procedure
Procedures followed those described in general procedure. Rats received one 18-trial session per day for a total of ten sessions. Within each session, there were six trials at each delay: the short delay (0 seconds), the adjusted criterion delay (used in Experiment 3, Phase 2), and the long delay (4 minutes). Forced and choice trials were also semi-randomly intermixed (constrained so that no more than two identical trial types occurred in a row) during each session so that there was a total of four choice trials and two forced trials per delay over the course of the experiment.
Results and Discussion
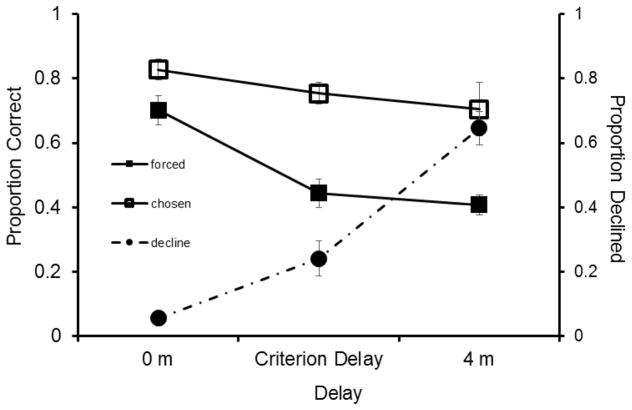
Subjects were more accurate on short delay trials than they were on long delay trials overall, and were more accurate on chosen trials than on forced trials (Fig. 5; 2 × 3 factorial ANOVA: main effect of delay: F2,48=7.81, P=0.001; main effect of trial type [forced vs. chosen]: F1,48=32.51, P=0.000; delay x trial type interaction: F2,48=1.54, P=0.225). Rats were most likely to decline trials when memory was weak at long delays and least likely when memory was strong at short delays (one-way ANOVA: F2,24=43.82, P=0.000). When considering decline rates and accuracy on forced tests as dependent measures, decline use tracked accuracy (2 × 3 factorial ANOVA: main effect of delay: F3,48=40.22, P=0.000; main effect of trial type [forced vs. decline]: F1,48=51.08, P=0.000; delay x trial type interaction: F1,48=11.33, P=0.002), indicating rats used the decline response adaptively and according to memory strength. Examining Figure 5, it is unknown why performance on forced and chosen criterion trials did not differ dramatically from performance at the long delay (proportion correct respectively, forced: .44 vs. .40, P=.53; chosen: .75 vs. .70; P=.79), but decline use did increase dramatically (decline criterion delay: .24 vs. decline 4m delay:.64; P=.000).
Fig. 5.
Proportion correct (±SEM) on forced and chosen trials after a short delay (0 minutes), intermediate delay (individually titrated criterion delays, which included 60, 90, 100, 120, 150, and 160 seconds), and long delay (4 minutes), and proportion of trials declined in Experiment 4. The proportion of correct trials on forced tests (filled squares), chosen tests (open squares) correspond to the primary y-axis and the proportion of trials declined (filled circles) correspond to the secondary y-axis.
In Experiment 4 memory was manipulated in a graded manner by varying delay times that corresponded to varying levels of difficulty, as confirmed by performance on forced trials. Memory on long delay trials here theoretically mirrors the results found in Experiment 2 in which memory was decreased by presenting no sample; while memory on short delay trials here theoretically mirrors the results found in Experiment 3 in which memory was increased by presenting two samples. As in Experiment 2 and 3, use of the decline response tracked trial difficulty as subjects were less likely to decline trials with shorter delays (.057) and more likely to decline trials with longer delays (.64). Importantly, decline use doubled on long delay trials as compared to no-sample trials in Experiment 2 (.32), suggesting that subjects could detect a weak memory state and that high interference is likely to account for relatively low decline rates on no-sample trials. The results of this experiment further support the hypothesis that internal memory monitoring is the cause of the metacognitive responding as opposed to external cue associations.
General discussion
In each of the four experiments presented here, results are consistent with the hypothesis that rats declined tests in which memory was weak and accepted tests in which memory was strong. In Experiment 1, performance on chosen trials exceeded that of performance on forced trials, suggesting that rats adaptively used the decline option when memory was weak. Three additional experiments examined transfer of decline use in generalization tests across which external stimuli were varied and not associated with memory status. In Experiment 2, rats declined no-sample trials more frequently than normal sample trials, indicating that memory state was weak much like in occasional normal sample trials when animals happened to forget the sample odor. However, a potential issue with this manipulation is that if subjects noticed something odd about these probe trials, such as the absence of the sample cup, they might have declined based on that novel feature rather than the absence of memory. We therefore increased memory in Experiment 3 by presenting two samples, thus predicting bidirectional external vs. internal cueing hypotheses. If rats selected the decline response whenever something odd or unexpected occurred, subjects should have declined these double-sample trials significantly more than normal sample trials. Instead, rats declined double-sample trials significantly less, thereby directly refuting this external cue hypothesis and lending support to the hypothesis that decline use was based on memory strength. Finally, in Experiment 4, both forced and choice trials were reintroduced as in Experiment 1 with the added manipulation of both increasing and decreasing the retention interval. Rats rarely declined trials after short delays and frequently declined trials after long delays. Subjects also performed significantly more accurately on chosen than forced tests, further reinforcing the conclusion that rats used the decline response adaptively and that its use is flexible enough to vary according to different memory strengths trial-to-trial. Successful transfer of the decline response across these test situations reveals that use of the decline response is most likely controlled by internal memory state rather than external cues like environmental cue associations.
A prospective metacognitive choice
Normally, when the decline response and memory test are present at the same time, the test is considered a concurrent metacognitive task (e.g. Basile et al., 2008; Hampton et al., 2004; Smith et al., 1997; Templer & Hampton; 2012). However, due to the fact that the metacognitive choice (turn to the left or right) occurred before, and was not the same response as the primary test responses, the present task can more aptly be described as a pseudo-prospective. If the rat chose to take the test, he would then travel to the right side of the testing room and systematically start sampling odors at one end of the line of choice cups and work his way through the options until arriving at his selection, which was indicated by digging. Locations of odors were always counterbalanced and randomized, so for rats to encounter the test, they needed to physically sample the choice odors. Although rats were physically able to change their take-the-test or decline-the-test decision, as they could travel to the decline side of the room after they had inhaled scents from the choice cups, this almost never occurred. Instead, 99.95% of the time rats made a decision to take or decline the test immediately upon entering the testing chamber: if they turned right to take the test they stayed right and chose one of the four choice cups; if they turned left they stayed right and dug in the decline cup.
Evaluation of internal and external sources of stimulus control
Using a broad definition of metacognition in which control for metacognitive responding can occur by both internal or external sources of stimulus control (Hampton, 2009), we are able to consider several explanations for adaptive use of the decline response within a unified framework. In animals, metacognitive responding by internal cues can only be inferred by excluding possible sources of external stimulus control (Hampton, 2009). Here we thoroughly consider the extent to which publicly available cues may have been available to subjects in this paradigm.
The environmental cue association hypothesis posits that animals respond to external test-specific cues to guide decline use (Hampton, 2009). This hypothesis is extremely unlikely to account for the results of the present study because rats made a pseudo-prospective choice and generalized adaptive decline use across several tests. In Experiment 1, rats appropriately used the decline response, demonstrated by increased performance on chosen compared to forced tests, mirroring what was found in an analogous visual task in monkeys (Hampton, 2001; Templer & Hampton, 2012). Rats were no more accurate after sampling any of the four odors and decline use did not vary as a function of odor. Though we can safely eliminate the environmental cue of individual odors cueing decline use, there may have been other environmental cue associations inherent to the testing stimuli of which we were unaware. In Experiment 2, we eliminated the sample, and found that rats declined these no-sample trials more than when the sample was presented, suggesting that features of the sample itself could not have served as a discriminative cue for decline use. Subjects did just the opposite in Experiment 3 when the sample odor was presented twice to increase memory of it, further reinforcing evidence that decline use was not controlled by stimulus-specific cues. When retention intervals were both increased and decreased, subjects used the decline response adaptively and according to memory strength. This strong generalization of the decline response across tests in which environmental cues were not constant suggests that rats based decline use on an internal memory cue.
According to the behavioral cue association hypothesis, subjects may use self-generated behaviors elicited by the primary memory test as cues to select the decline response. The most common possibilities of such publicly observable cues an animal could emit are hesitation or vacillation (Goto & Watanabe, 2012; Hampton, 2009; Terrace & Son, 2009). If the test is truly prospective, behavioral cue associations are not possible (Hampton, 2009). Here, the design is reminiscent of a concurrent task, but also resembles a prospective one. Because the choice and decline cups were in the same room, and subjects may have been able to smell the same four odors in the center of the room on every trial, it is possible that some external behavior was generated before the subject turned left or right to either the decline or choice cups. This could have resulted in the behavioral cue of relatively long vs. short response latencies to turn left or right. Unfortunately, with the manual design we employed, we were unable to collect response latency data, which limits the certainty with which we can rule out the potential behavioral cue of response latencies cueing decline use.
The response competition hypothesis posits that animals seeing (in the case of a visual task) or smelling (for our purposes) a correct answer overpowers other responses and the absence of this propensity leads to decline use (Hampton, 2009). This hypothesis was originally developed for tasks in which the metacognitive choice and selection of a primary test response are the same (e.g. Smith et al., 1998; Templer & Hampton, 2012), and are thus are in direct competition with each other. In the present task, the metacognitive choice (turn left or right) is not the same response as selection of the choice or decline cups. While this pseudo-prospective design may limit such external cueing to some extent, it is possible that response competition could have cued decline use. Though the same odors were present on every trial, and in order to determine which cup contained which odors rats had to physically sample each odor before choosing, it is possible rats could have detected the sample odor at the metacognitive decision point before they turned left or right. This external odor cue could have therefore activated a relatively salient sample representation and corresponding strong inclination to move towards the choice cups. Thus, response competition could result in a metacognitive behavior without complete control by an internal memory cue. Future studies should develop designs to improve upon the prospective nature of this paradigm by having the decline and choice cups in different rooms so that the response competition hypothesis can be tested more directly.
The final hypothesis to consider is rote response (Basile et al., 2015), which posits that, over many training trials, animals learn which response leads to maximized reward. In designing our study, we aimed to decrease the likelihood of this influence by presenting animals with the minimum number of trials required to sufficiently analyze the data. It is unlikely that associative learning guided adaptive decline use as rats shifted behavior to adjust for new circumstances immediately and in several conditions. In Experiment 2 the rote response hypothesis would posit that overtime animals learn that no sample tests should be declined to maximize reward. However, unlike other tests with omitted samples (e.g. Templer & Hampton 2012 and Yuki & Okanoya, 2017), here we chose to randomly reward one of the choice cups so that animals could not learn that choosing to take the test on no-sample trials never results in a reward, thereby eliminating the possibility of rote response in Experiment 2. In Experiment 3, we directly tested this hypothesis by pitting rote response against cueing by internal memory strength. If rats had learned to simply decline all non-typical sample (probe) tests, then they should have declined double-sample trials significantly more than normal sample trials. Subjects declined these probe tests less frequently, which is instead consistent with responding based on increased memory strength. In Experiment 4, short and long delay trials were randomly intermixed among criterion delay trials, making it difficult to learn that long delays resulted in incorrect responses and no rewards and short delay resulted in correct responses and rewards in just 16 forced trials at each delay.
That subjects quickly and adaptively transferred decline use across several generalization tests use allows us to safely eliminate the environmental cue association hypothesis as a possible explanation for the results of Experiments 1–4. We cannot rule out behavioral cue associations and response competition with certainty. However, based on the fact that rats made a prospective decision to take or decline the test, these hypotheses are less likely to apply to this data. Though rote response learning is a potential threat to many comparative cognition studies, it is unlikely that this can account for the aforementioned behaviors because animals experienced so few trials on several different generalization tests. We conclude that subjects most likely based their decision to take or decline the memory test on an internal memory cue.
Internal mnemonic cues: executive or associative control
A recurring debate in the field of animal metacognition is whether metacognitive behavior can be explained entirely by associative principles of learning and conditioning without the need to appeal to “more cognitive” mechanisms (Jozefowiez et al., 2009; Le Pelley, 2012; Smith et al., 2008). In response to this issue, some have taken the approach of obfuscating the reinforcement contingencies (Beran et al., 2014; Beran et al., 2006; Smith et al., 2006), thus attempting to make “low-level” accounts based on reinforcement less likely. These debates, and the experimental designs developed to circumvent them, often assume that “high-level” accounts are more interesting than “low-level” accounts. We see no need for such stratification, as there is little utility in differentiating between the worthiness of hypotheses. Any given behavior is likely supported by multiple mechanisms, and identifying the cues that support metacognitive decision making is important regardless of the answer (Basile et al., 2015; Basile & Hampton, 2014; Hampton, 2009). We therefore do not deny that pairing weak memory states with a particular response and strong memory states with another response follows well-recognized associative principles, but this does not negate the inference that that memory state is monitorable (Basile et al., 2015). Furthermore, we endorse the argument by Basile et al. (2015) that for many memory-monitoring paradigms “current associationist accounts are objectively indistinguishable from accounts of introspective memory monitoring, and may actually be in agreement” (p.16; see also Basile & Hampton, 2014).
The goal of the experiments presented here was to minimize external sources of stimulus control by using multiple generalization tests so that an internal source of stimulus control, if present, could be inferred. We have shown when memory state was experimentally manipulated, rats used that variation as a discriminative cue to respond adaptively, indicating that memory state was monitored. While we have argued that rats know when they remember, and have identified the monitored cue as an internal memory state, it is still unclear how much their decision represents a controlled “executive” process or a more automatic rule. We look forward to future studies that are designed to determine the degree to which associative or executive control support metacognitive behavior.
Broader implications and conclusions
Here we present a novel and valid test for investigating the presence of metamemory in rodents, which was modeled after a visual-based task in monkeys by Hampton (2001). Many methodological details were refined from previous iterations piloted to optimize this design which should help others aiming to replicate and expand upon this paradigm. For example, an interesting challenge we faced was the fact that several rats could remember odors for over 20 minutes, even with a minimal inter-trial interval, when odors were drawn from a pool of 24. We therefore found that for experimenters to be able to test nine rats in a day’s time, a set-size of four was optimal. High interference allowed us to more quickly titrate memory delays to an optimal level so that rats experienced both remembering and forgetting the sample. We suspect that using olfaction made to-be-remembered samples particularly salient and facilitated learning, especially in such an ecologically relevant task where subjects had to forage for food rewards. In future studies it would be interesting to examine metacognitive ability as a function of working memory (Smith, Redford, Beran, & Washburn, 2010) by increasing and decreasing the set-size as researchers have done in separate memory paradigms (Basile & Hampton, 2010).
One of the major controversies in comparative cognition is the degree to which nonhuman animals have declarative or explicit memory. We have examined this important question by operationalizing the monitorability feature of explicit (i.e. declarative) memory (Hampton, 2003). Researchers have made significant progress in other aspects of explicit memory in rats, including its source memory component (Crystal et al., 2013), episodic memory (Babb & Crystal, 2005, 2006; Eacott & Norman, 2004; Eichenbaum, 2005; Panoz-Brown et al., 2016), memory for the future (Crystal, 2013), and recollective processes (Eacott et al., 2005; Fortin et al., 2004). While putative metacognitive behavior has been reported in other studies (Foote & Crystal, 2007; 2012; Kirk et al., 2014; Yuki & Okanoya, 2017) the former two studies are especially subject to non-introspective accounts, and no previous studies have been able to rule out several possible sources of external stimulus control. Lack of evidence that clearly supports metacognitive or non-metacognitive accounts had led students of comparative metacognition to question whether metacognitive interpretations in rats are even appropriate (Foote & Crystal, 2012). Until now, robust evidence for metamemory in the rat had yet to be presented.
Evidence presented here suggests that rats respond adaptively to variation in memory strength caused by several experimental manipulations of memory. We argue that this behavior is very unlikely to be explainable by external sources of stimulus control, and is best explained by an internal source of stimulus control: memory state. These results, suggesting rats know when they remember, add to existing neurobiological evidence that the explicit memory system is largely conserved across humans and rats (Manns & Eichenbaum, 2006) and sets the stage for future cognitive and neurobiological studies of explicit memory. Together these studies will be shed light on the evolution of metamemory and will help provide an essential translational animal model for investigations of the treatment and prevention of disorders of memory.
Acknowledgments
Regina Paxton Gazes, Taylor B. Wise, and Benjamin M. Basile provided helpful comments on earlier drafts of this manuscript. Emily Kathryn Brown provided useful consultation during planning of Experiment 1 with regard to selecting an appropriate set-size. Kristin Palframan helped collect data in Experiment 1 and 2 and provided useful consult during planning of experiments. Research reported in this study was supported by an Institutional Development Award (IDeA) Network for Biomedical Research Excellence from the National Institute of General Medical Sciences of the National Institutes of Health under grant numbers P20GM103430 and P20GM203430 and a Medical Research Grant from the Rhode Island Foundation (2014-4397). These experiments comply with US law.
References
- Adams A, Santi A. Pigeons exhibit higher accuracy for chosen memory tests than for forced memory tests in duration matching-to sample. Learn Behav. 2011;39:1–11. doi: 10.1007/s13420-010-0001-7. [DOI] [PubMed] [Google Scholar]
- Babb SJ, Crystal JD. Discrimination of what, when, and where: Implications for episodic like memory in rats. Learn Motiv. 2005;26:177–189. [Google Scholar]
- Babb SJ, Crystal JD. Episodic-like memory in the rat. Curr Biol. 2006;16(13):1317–1321. doi: 10.1016/j.cub.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Basile BM, Hampton RR. Metacognition as discrimination: Commentary on Smith et al. Comp Psych. 2014;128:135–137. doi: 10.1037/a0034412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile BM, Hampton RR. Rhesus monkeys (Macaca mulatta) show robust primacy and recency in memory for lists from small, but not large, image sets. Behav Process. 2010;83(2):183–190. doi: 10.1016/j.beproc.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile BM, Hampton RR, Suomi S, Murray EA. An assessment of memory awareness in tufted capuchin monkeys (Cebus apella) Anim Cogn. 2008 doi: 10.1007/s10071-008-01801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile BM, Schroeder GR, Brown EK, Templer VL, Hampton RR. Evaluation of Seven Hypotheses for Metamemory Performance in Rhesus Monkeys. J Exp Psychol Gen. 2015;144(1):85–102. doi: 10.1037/xge0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin AS, Bjork RA, Schwartz BL. The mismeasure of memory: When retrieval fluency is misleading as a metacognitive index. J Exp Psychol Gen. 1998;127:55–68. doi: 10.1037/00963445.127.1.55. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Menzel CR, Parrish AE, Perdue BM, Sayers K, Smith JD, Washburn DA. Primate cognition: attention, episodic memory, prospective memory, self-control, and metacognition as examples of cognitive control in nonhuman primates. Wiley Interdisciplinary Reviews Cognitive Science. 2016;7(5):294–316. doi: 10.1002/wcs.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Perdue BM, Smith JD. What are my chances? Closing the gap in uncertainty monitoring between rhesus monkeys (Macaca mulatta) and capuchin monkeys (Cebus apella) J Exp: Anim Learn and Cogn. 2014;40:303–316. doi: 10.1037/xan0000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Perdue BM, Church BA, Smith JD. Capuchin Monkeys (Cebus apella) Modulate Their Use of an Uncertainty Response Depending on Risk. J Exp: Anim Learn and Cogn. 2016;42(1):32–43. doi: 10.1037/xan0000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Smith JD, Perdue BM. Language-trained chimpanzees (Pan troglodytes) name what they have seen but look first at what they have not seen. Psychol Sci. 2013;24:660–666. doi: 10.1177/0956797612458936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Smith JD, Redford JS, Washburn DA. Rhesus macaques (Macaca mulatta) monitor uncertainty during numerosity judgments. J Exp Anim Behav Process. 2006;32:111–119. doi: 10.1037/0097-7403.32.2.111. [DOI] [PubMed] [Google Scholar]
- Brown EK, Templer VL, Hampton RR. An assessment of domain-general metacognitive responding in rhesus monkeys. Behav Process. 2017;35:132–144. doi: 10.1016/j.beproc.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call J. Do apes know that they can be wrong? Anim Cogn. 2010;13:689–700. doi: 10.1007/s10071010-0317-x. [DOI] [PubMed] [Google Scholar]
- Call J, Carpenter M. Do apes and children know what they have seen? Anim Cogn. 2001;3:207–220. doi: 10.1007/s100710100078. [DOI] [Google Scholar]
- Carruthers P. Meta-cognition in animals: A skeptical look. Mind Lang. 2008;23(1):58–89. [Google Scholar]
- Castro L, Wasserman EA. Information-seeking behavior: Exploring metacognitive control in pigeons. Anim Cogn. 2013;16:241–254. doi: 10.1007/s10071-012-0569-8. [DOI] [PubMed] [Google Scholar]
- Crystal JD. Remembering the past and planning for the future in rats. Behav Process. 2013;93:39–49. doi: 10.1016/j.beproc.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal JD, Alford WT, Zhou WY, Hohmann AG. Source Memory in the Rat. Curr Biol. 2013;23(5):387–391. doi: 10.1016/j.cub.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal JD, Foote AL. Metacognition in animals: Trends and challenges. Comp Cogn Behav Rev. 2009;4:54–55. doi: 10.3819/ccbr.2009.40005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlosky J, Bjork RA, editors. Handbook of memory and metamemory. New York, NY: Psychology Press; 2008. [Google Scholar]
- Eacott MJ, Easton A, Zinkivskay A. Recollection in an episodic-like memory task in the rat. Learn Mem. 2005;12(3):221–223. doi: 10.1101/lm.92505. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Norman G. Integrated memory for object, place, and context in rats: A possible model of episodic-like memory? J Neurosci. 2004;24(8):1948–1953. doi: 10.1523/jneurosci.297503.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and episodic memory: Cognitive and neural mechanisms. Neuropsychopharmacol. 2005;30:S37–S37. [Google Scholar]
- Flavell JH. Metacognition and cognitive monitoring: A new area of cognitive-developmental inquiry. Am Psychol. 1979;34:906–911. doi: 10.1037/0003-066X.34.10.906. [DOI] [Google Scholar]
- Foote AL, Crystal JD. Metacognition in the rat. Curr Biol. 2007;17(6):551–555. doi: 10.1016/j.cub.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote AL, Crystal JD. “Play it Again”: a new method for testing metacognition in animals. Anim Cogn. 2012;15(2):187–199. doi: 10.1007/s10071-011-0445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431(7005):188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K. Metamemory in tufted capuchin monkeys (Cebus apella) Anim Cogn. 2009;12:575–585. doi: 10.1007/s10071-009-0217-0. [DOI] [PubMed] [Google Scholar]
- Goto K, Watanabe S. Large-billed crows (Corvus macrorhynchos) have retrospective but not prospective metamemory. Anim Cogn. 2012;15(1):27–35. doi: 10.1007/s10071-011-0428-z. [DOI] [PubMed] [Google Scholar]
- Hampton RR. Rhesus monkeys know when they remember. P Natl Acad Sci USA. 2003;98(3):5359–5362. doi: 10.1073/pnas.071600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RR. Metacognition as evidence for explicit representation in nonhumans. Behav Brain Sci. 2003;26(3):346–347. doi: 10.1017/S0140525X03300081. [DOI] [PubMed] [Google Scholar]
- Hampton RR, Zivin A, Murray EA. Rhesus monkeys (Macaca mulatta) discriminate between knowing and not knowing and collect information as needed before acting. Anim Cogn. 2004;7:239–254. doi: 10.1007/s10071-004-0215-1. [DOI] [PubMed] [Google Scholar]
- Hampton RR, Hampstead BM. Spontaneous behavior of rhesus monkeys (Macaca mulatta) during memory tests suggests memory awareness. Behav Process. 2006;72:184–189. doi: 10.1016/j.beproc.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Hampton RR. Multiple demonstrations of metacognition in nonhumans: converging evidence or multiple mechanisms? Comp Cogn Behav Rev. 2009;4:17–28. doi: 10.3819/ccbr.2009.40002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman A, Shettleworth SJ. Detecting metamemory in nonverbal subjects: A test with pigeons. J Exp Psychol Anim B. 1999;25:389–395. doi: 10.1037/0097-7403.25.3.389. [DOI] [Google Scholar]
- Iwasaki S, Watanabe S, Fujita K. Do pigeons (Columba livia) seek information when they have insufficient knowledge? Anim Cogn. 2013;16:211–221. doi: 10.1007/s10071-012-0566-y. [DOI] [PubMed] [Google Scholar]
- Jozefowiez KJ, Staddon JER, Cerutti DT. Metacognition in animals: how do we know that they know? Comp Cogn Behav Rev. 2009;4:29–39. [Google Scholar]
- Keppel G, Wickens TD. Design and analysis, a researchers handbook. 4. Upper Saddle Rober, NJ: Pearson; 2004. [Google Scholar]
- Kirk CR, McMillan N, Roberts WA. Rats Respond for Information: Metacognition in a Rodent? J Exp Psychol Anim B. 2014;40(2):249–259. doi: 10.1037/xan0000018. [DOI] [PubMed] [Google Scholar]
- Kornell N, Son LK, Terrace HS. Transfer of metacognitive skills and hint seeking in monkeys. Psychol Sci. 2007;18(1):64–71. doi: 10.1111/j.1467-9280.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- Kornell N. Metacognition in humans and animals. Curr Dir Psychol Sci. 2009;18:11–15. [Google Scholar]
- Le Pelley Metacognitive monkeys or associative animals? Simple reinforcement learning explains uncertainty in nonhuman animals. J Exp Psychol: Learn, Mem, and Cogn. 2012;38:686–708. doi: 10.1037/a0026478. [DOI] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16(9):795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- Marsh HL, MacDonald SE. Information seeking by orangutans: A generalized search strategy? Anim Cogn. 2012;15:293–304. doi: 10.1007/s10071-011-0453-y. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Kober H. Self-reflective consciousness and the projectable self. In: Terrace HS, Metcalfe J, editors. The missing link in cognition: Origins of self-reflective consciousness. New York, NY: Oxford University Press; 2005. pp. 57–83. [Google Scholar]
- Mazur JE. Choice between small certain and large uncertain reinforcers. Anim Learn Behav. 1988;16:199–205. [Google Scholar]
- Nakamura N, Watanabe S, Betsuyaku T, Fujita K. Do birds (pigeons and bantams) know how confident they are of their perceptual decisions? Anim Cogn. 2011;14(1):83–93. doi: 10.1007/s10071-010-0345-6. [DOI] [PubMed] [Google Scholar]
- Nelson TO, editor. Metacognition: Core readings. Toronto, Canada: Allyn & Bacon; 1992. [Google Scholar]
- Panoz-Brown D, Corbin HE, Dalecki SJ, Gentry M, Brotheridge S, Sluka CM, … Crystal JD. Rats Remember Items in Context Using Episodic Memory. Curr Biol. 2016;26(20):2821–2826. doi: 10.1016/j.cub.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukner A, Anderson JR, Fujita K. Redundant food searches by capuchin monkeys (Cebus apelia): a failure of metacognition? Anim Cogn. 2006;9(2):110–117. doi: 10.1007/s10071-005-0007-2. [DOI] [PubMed] [Google Scholar]
- Roberts WA. Short-term memory in pigeons-effects of repetition and spacing. J Exp Psychol Gen. 1972;94(1):74. [Google Scholar]
- Roberts WA, Feeney MC, McMillan N, MacPherson K, Musolino E, Petter M. Do pigeons (Columba livia) study for a test? J Exp Psychol Anim Behav Proc. 2009;35:129–142. doi: 10.1037/a0013722. [DOI] [PubMed] [Google Scholar]
- Roberts WA, McMillan N, Musolino E, Cole M. Information seeking in animals: Metacognition? Comp Cogn Behav Rev. 2012;7:85–109. doi: 10.3819/ccbr.2012.70005. [DOI] [Google Scholar]
- Rosati AG, Santos LR. Spontaneous Metacognition in Rhesus Monkeys. Psychol Sci. 2016;27(9):1181–1191. doi: 10.1177/0956797616653737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz BL. Sources of information in metamemory: Judgments of learning and feelings of knowing. Psychon B Rev. 1994;1:357–375. doi: 10.3758/BF03213977. [DOI] [PubMed] [Google Scholar]
- Shettleworth SJ. Cognition, Evolution, and Behavior. 2. New York: Oxford University Press; 2010. [Google Scholar]
- Smith JD. The study of animal metacognition. Trends Cogn Sci. 2009;13:389–396. doi: 10.1016/j.tics.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Smith JD, Beran MJ, Couchman JJ, Coutinho MVC. The comparative study of metacognition: Sharper paradigms, safer inferences. Psychon B Rev. 2008;15(4):679–691. doi: 10.3758/pbr.15.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Couchman JJ, Beran MJ. Animal Metacognition: A Tale of Two Comparative Psychologies. J Comp Psychol. 2014;128(2):115–131. doi: 10.1037/a0033105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Redford JS, Beran MJ, Washburn DA. Rhesus monkeys (Macaca mulatta) adaptively monitor uncertainty while multi-tasking. Anim Cogn. 2010;13(1):93–101. doi: 10.1007/s10071-009-0249-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Shields WE, Schull J, Washburn DA. The uncertain response in humans and animals. Cognition. 1997;62(1):75–97. doi: 10.1016/s0010-0277(96)00726-3. [DOI] [PubMed] [Google Scholar]
- Smith JD, Shields WE, Washburn DA. Memory monitoring by animals and humans. J Exp Psychol Gen. 1998;127(3):227–250. doi: 10.1037//00963445.127.3.227. [DOI] [PubMed] [Google Scholar]
- Suda-King C. Do orangutans (Pongo pygmaeus) know when they do not remember? Anim Cogn. 2008;11(1):21–42. doi: 10.1007/s10071-007-0082-7. [DOI] [PubMed] [Google Scholar]
- Suda-King C, Bania AE, Stromberg EE, Subiaul F. Gorillas’ use of the escape response in object choice memory tests. Anim Cogn. 2013;16(1):65–84. doi: 10.1007/s10071-012-0551-5. [DOI] [PubMed] [Google Scholar]
- Sutton JE, Shettleworth SJ. Memory without awareness: Pigeons do not show metamemory in delayed matching to sample. J Exp Psychol Anim B. 2008;34(2):266–282. doi: 10.1037/00977403.34.2.266. [DOI] [PubMed] [Google Scholar]
- Templer VL, Hampton RR. Rhesus monkeys (Macaca mulatta) show robust evidence for memory awareness across multiple generalization tests. Anim Cogn. 2012;15(3):409–419. doi: 10.1007/S10071-011-0468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrace HS, Son LK. Comparative metacognition. Curr Opin in Neurobiol. 2009;19(1):67–74. doi: 10.1016/j.conb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Vining AQ, Marsh HL. Information seeking in capuchins (Cebus apella): A rudimentary form of metacognition? Anim Cogn. 2015;18(3):667–681. doi: 10.1007/s10071-015-0835-7. [DOI] [PubMed] [Google Scholar]
- Zentall TR, Stagner JP. Pigeons prefer conditional stimuli over their absence: A comment on Roberts et al. J Exp Psychol Anim B. 2010;36:506–509. doi: 10.1037/a0020202. [DOI] [PubMed] [Google Scholar]