Abstract
Injectable nanomaterials that interact with the host immune system without surgical intervention present spatially anchored complements to cell transplantation and could offer improved pharmacokinetics compared to systemic cytokine therapy. Here we demonstrate fabrication of high aspect ratio polycaprolactone nanowires coupled with cytokine-binding antibodies that assemble into porous matrices when injected into the subcutaneous space. These structures are fabricated using a nanotemplating technique that allows for tunability of particle dimensions and utilize a straightforward maleimide conjugation chemistry to allow site-specific coupling to proteins. Nanowires are well tolerated in vivo and incite minimal inflammatory infiltrate. Nanowires conjugated with antibodies were designed to capture and potentiate endogenous interleukin-2 (IL-2), an important leukocyte activating cytokine. Together these nanowire-antibody matrices were capable of localizing endogenous IL-2 in the skin and activated targeted specific natural killer and T cell subsets, demonstrating both tissue- and cell-specific immune activation. These self-assembling nanowire matrices show promise as scaffolds to present engineered, local receptor–ligand interactions for cytokine-mediated disease.
Keywords: nanotechnology, immunomodulation, cytokines, interleukin-2, S4B6-1, polycaprolactone
Graphical abstract
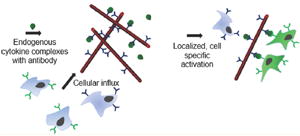
Three-dimensional biomaterials offer a promising platform for modulation of host cell populations.1-3 In particular, injectable, self-organizing matrices of asymmetric structures that form focal points for immune regulation are of great interest, as a result of increased cell infiltration owing to large interparticle pores.4 Protein conjugated nanowires (NWs) present a straightforward strategy to generate a self-organizing matrix. They present a form factor that retains the high surface area to volume ratio of a nanoparticle but also confer resistance to phagocytic clearance given their large size relative to macrophages in the longitudinal axis. Previous attempts to fabricate discrete, high aspect ratio polymeric structures has proved difficult without the exploitation of exotic chemical properties such as conductivity, which may give rise to undesirable side effects in vivo.5-8
Recent advances in receptor ligand engineering for cytokine and cellular therapy to treat immune disorders have further necessitated the fabrication of versatile matrices for targeted presentation of biological species. Cytokine therapy, both as standalone9,10 and in combination with adoptively transferred immune cells,11-13 has been of great interest in clinical immunology to treat conditions ranging from cancer to an expanding set of autoimmune disorders including psoriasis, lupus, and rheumatoid arthritis.14 However, systemic delivery of cytokines such as interleukin-2 (IL-2)9 and IL-1215 has remained a challenge, mainly due to short half-lives, off-target effects on other tissues, and increased risk of cytokine release syndrome, hypotension, and life-threatening tachycardia.9,10
IL-2 is particularly interesting as it has pleiotropic effects on immune cell homeostasis.16 It promotes activation of both stimulatory effector cells and suppressive T regulatory cells (Tregs). Its critical role in immune regulation makes it a valuable therapeutic target both in cancer where heightened cytotoxic immune responses from effector cells are desired as well as in autoimmune disorders where Treg-mediated inflammatory suppression is beneficial.17-19
IL-2 most efficiently signals via a noncovalently linked trimeric receptor complex composed of the IL2Rα chain (CD25), the IL2Rβ chain (CD122), and the common gamma chain, IL-2Rγ (CD132). Activated effector cells and Tregs express the full receptor complex, which binds IL-2 with high affinity, while resting memory T cells and natural killer (NK) cells express only the β and γ chains, which retain signaling capacity but only bind IL-2 with intermediate affinity. Several strategies have been employed to promote selectivity toward the receptor complex and thus cell type of interest, such as mutating the cytokine itself to produce so-called “superkines”. 18-21 An alternative strategy has been to use antibodies which selectively sequester the ligand binding region of IL-2 similarly to influence receptor avidity.22-24 These have been widely characterized25,26 and have garnered recent clinical interest with the development of humanized equivalents.27
Antibody-cytokine presentation could be potentiated and offtarget effects minimized by localizing to the tissue of interest. Currently, local half-lives for antibody therapeutics dosed subcutaneously are on the order of a few hours,28 which makes them unsuitable for sustained local presentation of a preferred cytokine. We hypothesized that immobilizing an anti-IL-2 antibody on an injectable substrate with sufficiently high surface area, even without the use of exogenous cytokine, could result in a potent tissue-specific immunomodulatory platform (Scheme 1) by passive accumulation of endogenous IL-2 passing through the matrix.
Scheme 1.

(a) Nanowires are injected subcutaneously and form a nodule, which forms a loose matrix as saline leaves the injection site (b). (c) Endogenous cytokine of interest in subcutaneous tissue is captured by the functionalized nanowire matrix, and cytokine is presented to relevant immune microenvironment. (d) Specific cell subpopulations (CD25- CD122+) cells are activated, while CD25+ cells remain unaffected.
We employed a wafer scale nanotemplating technique that can fabricate these high aspect ratio structures in great numbers from a degradable and FDA approved polyester, polycaprolactone (PCL). We utilized anodized alumina as a template material, which is a commercially available and has anisotropic pores that run orthogonal to the surface, are tunable in size, and are impervious to organic solvents but easily dissolved in base or acid.29 These have been used in the past for templating nanowires onto various polymeric features on a planar substrate.30-32 We also developed a broadly applicable, inexpensive conjugation chemistry to attach the desired antibody or other sequestering species of interest and demonstrated the utility of the platform in a murine in vivo subcutaneous administration model. Together these nanowireantibody matrices were capable of localizing endogenous IL-2 in the skin and stimulating NK cells as well as CD8+ CD122+ T cell subsets, demonstrating both tissue- and cell-specific immune activation.
RESULTS AND DISCUSSION
Nanowire Fabrication
We first explored how to reliably manufacture polymeric nanowires that could be fabricated in scalable, high throughput fashion. Unfortunately, typical solution-phase methods to synthesize nanoparticles are not suitable for generating asymmetric structures, as emulsion and nanoprecipitation techniques typically form spherical structures to minimize surface energy between phases.
To generate these nanostructures, we adapted a wafer scale nanotemplating technique using anodized alumina as a mold material (Figure 1a). A film of polymer is first spun cast onto a clean substrate and subsequently brought above the melting temperature of the polymer while in contact with the inorganic porous membrane. Nanowire formation is rapid, as capillary force is very strong with small pores.31,33 We allowed the templating to “complete” such that when finished, the inorganic alumina membrane is in contact with the underlying substrate with no remaining underlying polymer layer. The membrane can then be physically detached from the surface and selectively etched with sodium hydroxide. Once released, the nanowires are then sonicated gently to aid in dispersion, filtered, and purified for biological use.
Figure 1.

(a) Nanowire fabrication process. Nanowires are templated in anodized alumina mold with 200 nm pore size. Once templating is complete, the molds are etched to expose nanowires, which are then purified and conjugated to IL-2 binding antibody. Bottom: scanning electron micrographs of (b) long (18 ± 4.3 μm) and (c) short (6 ± 2.1 μm) nanowires, demonstrating tunability of this fabrication scheme. Dotted lines surround typical nanowire size. Scale bars 10 μm.
Nanowire length can be tuned by changing the thickness of the polymer film that is templated, with lengths ranging from as short as 6 ± 2.1 μm and as long as 18 ± 4.3 μm (Figure 1b, c). Wires were approximately 200 nm across, the same diameter as the pores in the mold. We observed no degradation of PCL nanowires over the course of several months in the form of aggregation. Over the course of several days, the nanowires would settle and form a loose floc due to gravity which is easily resuspended by gentle shaking.
Conjugation to Antibody Species
In order to facilitate conjugation to an antibody or other protein with the nanowires, we synthesized a maleimide functionalized PCL derivative to incorporate into the polymeric nanowires during casting. Maleimide groups are desirable because they are stable at relatively high temperatures and readily undergo Michael addition with thiol groups over a broad pH range.34 Since PCL does not have accessible groups to functionalize, our strategy was to couple a very short chain PCL diol (2 kDa) to a maleimide via a phenyl isocyanate linker, resulting in a short PCL chain containing a maleimide group at each end coupled by a stable carbamate bond (Figure 2a).35,36 Mass spectrometry and NMR show complete coupling with no urea or other byproducts and no remaining PCL diol parent compound (Figure 2b, c).
Figure 2.

(a) General synthetic scheme to generate MP-PCL. (b) Relevant NMR spectrum demonstrating complete coupling via isocyanate linkage to PCL molecule indicated by peak shift of aryl protons. (c) MALDI mass spectrometry of PCL diol parent compared to MP-PCL product, where product appears at +428 g/mol with no unmodified PCL detected.
The resulting product, herein referred to as MP-PCL, was blended with the larger 45 kDa PCL when cast into a film and resultant nanowires. Functionalizing these short chains allows for large numbers of maleimide groups per nanowire without the need to modify the polymer backbone as well as faster predicted clearance of the nanowires in vivo as a result of the lower net molecular weight of the blend.37
The MP-PCL nanowires were characterized first on their ability to be surface functionalized with Alexa488-conjugated fluorescent IgG after reduction. When allowed to react with antibodies selectively reduced at the hinge region, we saw that the nanowires were highly fluorescent compared to an unreduced control (Figure 3a–d), which demonstrates sitespecific conjugation to the IgG.
Figure 3.

(a) MP-PCL nanowires conjugated to A488-IgG with selective reduction. (b) Same nanowires reacted without reduction of IgG species. (c) Schematic of conjugated nanowire. (d) Fluorescence intensity of FITC-IgG conjugated nanowires vs an unreduced antibody and nanowire only control, n = 3. (e) Binding assay of 500 pg/mL of IL-2 to S4B6-1 anti-IL-2 conjugated nanowires at various concentrations, n = 6 for each dilution. Scale bar 20 μm.
We then conjugated an IL-2 sequestering antibody, S4B6-1, in the same fashion to evaluate whether the nanowires would interfere with its antigen binding capacity. In a capture assay where nanowires were conjugated to S4B6-1 anti-IL-2, washed, and subsequently challenged with 500 pg/mL of IL-2, the sequestration curve follows a hyperbolic isotherm consistent with specific binding (Figure 3e).38 Given physiological concentrations of IL-2 in the skin range from 1 to 10 pg/mg of tissue,39 this demonstrates these doses of wires have capacity to bind orders of magnitude more cutaneous cytokine than is present in homeostatic conditions. The hinge region-specific reduction used in this protocol ensures that while the antibody is fixed on the underlying nanowire, the Fab binding portions of the antibody are both covalently bound and oriented such that presentation to surrounding infiltrating cells remains intact.
Nanowires as an Injectable Matrix
Biocompatibility, lack of toxicity, and capacity for degradation are important features of any long-residing biomaterial. While this fabrication platform is capable of creating nanowires from any thermoplastic polymer, PCL was chosen because it is an FDA approved biomaterial for use in skin sutures40 and has demonstrated relatively little immunogenicity and local inflammation when used in larger implants such as drug delivery devices.41,42 This is in contrast to polypropylene,43 commonly used in abdominal meshes, and even other polyesters such as polylactide-co-glycolide (PLGA)44,45 which have demonstrated a significant nonspecific inflammatory response in certain tissues upon degradation.
Given that high aspect ratio nanostructured matrices allow for a higher degree of cell infiltrate due to greater pore size,4 and predicted increased residence time due to their respective size relative to professional phagocytes,46-48 the “long” wire formulation from Figure 2b was chosen for all in vivo studies.
In order to evaluate in vivo tolerability and inflammatory capacity of these structures, bare, unmodified PCL nanowires were injected subcutaneously into dorsal skin of B/6 mice with carrier saline. As expected, the saline rapidly drained from the injection site, and the nanowires self-assembled into a stable nodule (Figure 4d). When the skin was harvested and digested for flow cytometry at day 5, we saw no significant myeloid infiltrate when compared to a saline-only sham control (Figure 4a). Nodules were also retrieved, individually sectioned, and stained by immunofluorescence for F4/80-positive macrophages at day 5 with similar results, that is, no qualitative differences were observed when compared to sham injection site (Figure 4b, c).
Figure 4.

(a) Flow cytometry analysis of myeloid cells in dorsal skin of C57BL/6 mice injected with PCL nanowires, (n = 4 mice per group, 10 injections per mouse). (b) Nile Red stained nanowires and (c) saline injected and stained for macrophage infiltrate with anti-F4/80. Dotted line indicates nanowire nodule location. Both analyses done at 5 days post-injection. Scale bars 100 μm. (d) Representative image of nanowire nodules in mouse dorsal skin at 14 days from injection, arrow indicates a single nodule, scale bar 1 cm. (e) Number of wire nodules remaining in each mouse at respective time point, one-way ANOVA, n = 4. (f) A488-IgG nanowire in dorsal skin at 3 and (g) 9 days post-injection compared to equivalent antibody alone (h) 3 days post-injection, scale bars 100 μm. (i) Quantification of fluorescence at injection sites at days 3 and 9 as well as day 3 antibody only control, average of n = 4 sections for each group.
In a subsequent longitudinal study, 10 nanowire injection sites were made per mouse and monitored for 6 weeks. We found on average 5 of 10 nodules per mouse were resorbed by week 6, and no significant dermatitis or inflammation was observed at those sites during harvest at any of the time points (Figure 4d, e), indicating the nanowires were well tolerated. Some groups have suggested that the porosity of biomaterial is a key parameter in shifting the macrophage response to a more anti-inflammatory phenotype.49,50 These nanowire nodules have large internanowire pores which allow cells to penetrate into the resulting matrix,4 which may contribute to the lack of inflammatory response seen with this nanomaterial when injected into the skin.
Next, we wanted to explore the residence time of a conjugated antibody on the PCL nanowires matrix in vivo. Nanowires fabricated with the MP-PCL were conjugated to the same A488 IgG antibody as described above and injected subcutaneously. Mice were sacrificed at days 3 and 9, and we found significant antibody present in the nodule as indicated by A488 staining compared to antibody only control (Figure 4f-i), demonstrating that the nanowires are capable of retaining a biological species in vivo in its respective tissue for an extended period of time, whereas drainage half-lives for comparable molecules injected subcutaneously are approximately 3–6 h.28
Nanowires as an Immunomodulatory Platform
Next, the capability of these PCL nanowires to act as an injectable immunomodulatory platform was examined. We decided to investigate nanowires conjugated with antibodies that bind IL-2 since these antibodies have previously been shown to present different ligand binding regions of bound IL-2 to the surrounding immune environment and thus are able to target different immune subsets based on the respective receptor expression.23,51,52 For this study we chose the S4B6-1 clone of anti-IL-2, which selectively targets cells expressing the dimeric IL-2 receptor complex and should therefore preferentially activate resting effector cells and NK cells over other cell subsets, such as Tregs that express the trimeric complex.22
We set out to determine whether attaching an antibody to the nanowires could enhance its effect in vivo in a target tissue compared to its soluble, nonconjugated counterpart. First, S4B6-1 was conjugated to nanowires containing MP-PCL and washed thoroughly to remove free antibody. Nanowires were then suspended in sterile saline and injected into the subcutaneous space of B/6 mouse dorsal skin at multiple sites. At 72 h post-injection, we observed a marked increase in frequencies of CD8 T cells expressing the dimeric IL-2 receptor (i.e., CD3+CD8+CD122+ cells) in the skin compared to equivalent antibody-only and nanowire-only controls (Figure 5b). We also observed an increase in Ki67 expression, a proliferation marker, in NK cells (CD45+CD3−NK1.1+ cells) in skin. However, we saw no difference in NK cell presence in skin draining lymph nodes and only marginal differences in CD8 T cell numbers. Treg (CD45+ CD3+ CD4+ FOXP3+) and CD4 effector T cell (CD45+ CD3+ CD4+ FOXP3−) populations were unchanged in both skin draining lymph nodes and dorsal skin (Figure S5). The S4B6-1 nanowires were able to not only activate the cell types that are specific for the exposed portion of IL-2 using endogenous cytokine but also specifically target the intended CD8 and NK subsets over regulatory T cells.
Figure 5.

(a) Schematic showing how S4B6-bound IL-2 signals different cell populations. (b) Flow cytometry analysis of whole dorsal skin showing increased proliferation of NK cells as well as an increase in CD122+ CD8+ cells when injected with S4B6-1 conjugated nanowires compared to nanowire- or antibody-only controls. Each data point represents one mouse. (c) Skin draining lymph nodes from the same animals show no such activation in NK cells regardless of experimental group. CD8+ CD122+ cells are significantly reduced in the antibody-only group. Treg activation as measured by CD25+ staining showed no difference between groups in either tissue. One-way ANOVA with multiple comparisons correction for all analysis.
Interestingly, when mice were injected with a soluble antibody dose that matched the total active antibody present in the equivalent nanowire dose, we observed no specific upregulation in the skin. This is likely due to the nanowires being capable of locally concentrating the IL-2 growth factor in the nodule area.
In addition, we saw a marked decrease in the CD8+ T cell populations in the skin draining lymph nodes in mice injected with the equivalent soluble antibody compared to both the conjugated antibody group and the bare nanowire group. It appears that the soluble antibody’s ability to sequester IL-2 and remove it from the lymph node environment is not sufficiently counteracted by its presentation to the relevant surrounding cells. The antibody-conjugated nanowire group, however, seemed to offer some protection from these ‘off-target’ effects. It is possible that the ability to prevent distribution of the antibody to relevant skin draining lymph nodes is responsible for this effect.
CONCLUSION
We have demonstrated a facile, high-throughput fabrication process to generate polymeric nanowires. In addition, we described a conjugation strategy that allows coupling of biological species through a short chain PCL maleimide handle that can be easily incorporated into the polymeric nanowires. Antibodies bound to these nanowires and subsequently injected subcutaneously into mouse dorsal skin demonstrate both celland tissue-specific immunomodulation by amplifying specific immune cell subsets and only in the tissue of interest, without the need to precomplex exogenous cytokines before administration. Furthermore, the nanowires anchor the antibodies in place preventing off-target effects in downstream tissues that are seen with a soluble counterpart. Thus, self-assembling nanowire matrices conjugated to bioactive proteins have the potential to induce a localized, specific immune response.
EXPERIMENTAL METHODS
Synthesis of p-maleimidobenzoyl azide was performed as previously described.53 All reagents and solvents were purchased from commercial sources and used as received without further purification. Reactions were carried out under an inert atmosphere of argon in flame-dried glassware. 1H NMR spectra were obtained on a 400 MHz Bruker AvanceIII HD spectrometer. Matrix-assisted laser desorption/ionization (MALDI) mass spectrometry was performed on an AXIMA Performance MALDI TOF/TOF mass spectrometer. PCL samples were prepared for MALDI as previously described.54
MP-PCL
A solution of p-maleimidobenzoyl azide (2.5 g, 10 mmol) in anhydrous toluene (150 mL) was refluxed for 2 h under argon. Toluene was removed under reduced pressure to afford p-maleimidophenyl isocyanate (PMPI) as a yellow solid. Under argon, polycaprolactone diol (6.7 g, 3.4 mmol, Sigma) was dissolved in anhydrous dimethylformamide (10 mL) warmed to 40 °C. The PCL solution was added to the PMPI, maintained at 40 °C, and stirred under argon for 24 h before quenching with anhydrous ethanol (1 mL) for at least 1 h. The product, MP-PCL, was isolated by precipitation with ice-cold ethanol (200 mL), followed by centrifugation. The mother liquor was discarded, and the precipitate was rinsed with ice-cold ethanol (3 × 50 mL). The product was dried under reduced pressure to yield the desired product as a yellow solid (7.0 g, 2.9 mmol, 85%): 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 8.6 Hz, 4H), 7.29 (d, J = 8.0 Hz, 4H), 6.86 (s, 4H), 4.19 (t, J = 6.6 Hz, 4H), 4.08 (t, J = 6.7 Hz, 32H), 2.32 (t, J = 7.6 Hz, 32H), 1.75–1.36 (m, 108H). Full spectra can be found in Figure S1.
Nanowire Fabrication
A 125 mg/mL solution of either pure 45 kDa PCL or a 30% blend of MP-PCL and 45 kDa PCL in 2,2,2- trifluoroethanol was spun cast onto a clean wafer. The wafers were baked for 5 min at 120 °C to remove any residual solvent and left to cool. Anodized alumina membranes (Whatman, 37 mm diameter) were applied to the polymer films and then heated to 100 °C, applying sparing pressure to the membranes to initiate wicking into the pores. Once the entire film had been taken up into the membrane, they were subsequently cooled and allowed to anneal overnight.
The wire-containing anodized alumina membranes were then removed from the wafer substrate and etched in 5 M NaOH for 15 min, sonicated thoroughly to help disperse the wires, and then washed via centrifugation in distilled water. Nanowires were then dispersed in 1% poly(vinyl alcohol) and were further purified by passing the solution through a 38 μm mesh and subsequent washing with distilled water. Wires were then concentrated and stored in D-PBS in the dark at room temperature until conjugation.
SEM images were taken on a Carl Zeiss Ultra 55 FE-SEM at 5 kV using a secondary electron detector. Nanowire size was determined by measuring 120 sample wires from optical images. All image analysis was performed in ImageJ.
Nanowire Conjugation and Binding Assay
Nanowires were washed and dispersed in reducing buffer (d-PBS with 0.04% EDTA). Antibodies were reduced using freshly dissolved tris(2-carboxyethyl)- phosphine (TCEP) at a 4.5 molar excess for 1 h at 37 °C. Reduced antibody solution at 0.1 mg/mL was added to the nanowires, and thiol-maleimide reaction proceeded at room temperature for 2 h, after which it was washed thrice in d-PBS, resuspended in sterile saline, and subsequently stored at 4 °C until use.
For fluorescent IgG conjugation (Figure 3), the same nanowire batch was exposed to either reduced or unreduced antibody at the same concentration, washed thrice in PBS, and measured for fluorescence, n = 3 replicates per condition.
IL-2 binding assays were performed by taking dilutions from a concentrated stock solution of nanowires conjugated to S4B6-1 antibody and challenging with equal volume of 1000 pg/mL rmIL-2 for 1 h at 37 °C (Abcam). Nanowires were then centrifuged at 12000 rcf, and supernatant was sampled and analyzed by mIL-2 ELISA (Abcam), n = 6 replicates per dilution.
In Vivo Studies
Mouse experiments were performed in compliance with the University of California, San Francisco Institutional Animal Care and Use Committee guidelines with protocols approved for this study (Protocol AN110246).
For T cell activation studies, female C57BL/6 mice (Jackson Laboratories) of 6–8 weeks of age were treated with either nanowires alone (n = 6), nanowires conjugated with anti-IL2 (n = 5) (clone S4B6-1, Bio X Cell), or an equivalent dose of soluble S4B6-1 (n = 6). Each mouse was shaved and injected subcutaneously into the dorsal skin over ten separate sites (50 μL per site). Nanowire dose for each mouse was standardized to 50 ng of active anti-IL2 as measured by binding assay described earlier. Following 3 days, mice were sacrificed, whole mouse dorsal skin was harvested, which included all 10 injection sites for that respective mouse, digested, and stained. Skin was finely minced and digested in RPMI media with collagenase XI (2 mg/mL, Sigma), hyaluronidase (0.5 mg/mL, Sigma), and DNase (0.1 mg/mL, Sigma) for 45 min while being shaken at 37 °C. The skin suspension was vortexed and filtered through a 100 μm filter. Six skin draining lymph nodes (inguinal, axillary, and brachial) were harvested and pooled for each respective mouse. Lymph nodes were mashed through a 100 μm filter. Single cell suspensions from lymph node and skin were then subjected to flow cytometry staining and analysis (see Supporting Information Methods S2 for staining protocol and T cell panel).
For myeloid infiltration studies, nanowires that had incorporated Nile Red dye (Acros Organics) were injected subcutaneously at 10 sites in the dorsal skin of C57BL/6 mice with either nanowires or saline only and subsequently sacrificed at day 5 post-injection, n = 4 mice per group. Whole mouse dorsal skin was harvested, which included all 10 injection sites for that respective mouse. Skin draining lymph nodes were also harvested, and both skin and SDLNs were processed similarly to above, then stained and subjected to flow cytometry for analysis (see Supporting Information Methods S2 for myeloid staining panel).
For degradation studies, nanowires were injected subcutaneously at 10 sites in the dorsal skin of C57L/6 mice and sacrificed at 2, 4, and 6 week time points (n = 4 mice per time point). Nodules that contained nanowires were accounted for visually after removing dorsal skin post-sacrifice.
For macrophage staining, dorsal skin surrounding injection sites of nanowires with Nile Red dye was harvested, placed into OCT, and frozen on isopentane in liquid nitrogen. 20 μm sections cut via cryotome were then fixed in 10% formalin and mounted with ProLong Gold Antifade Reagent with DAPI. Select slides were additionally blocked with 5% goat serum in PBS and stained with Alexa Fluor 647 anti-F4/80 antibody (Life Technologies Inc.). Slides were imaged on a Zeiss Axio Imager M2 microscope using 5× and 10× objectives.
For in vivo antibody tracking, nanowires conjugated to Alexa Fluor 488 - IgG (courtesy of AbbVie Inc.) or equivalent soluble antibody were injected subcutaneously and subsequently sacrificed at 3 and 9 days. Each mouse received five injection sites which were labeled with a dermatological pen. Back skin surrounding injection sites was harvested, placed into OCT, and frozen on isopentane in liquid nitrogen. Twenty μm sections beneath the demarcated injection sites were cut via cryotome and then fixed in 10% formalin and mounted with ProLong Gold Antifade Reagent with DAPI. For analysis, four separate skin sections were averaged for each condition. Averaging was performed in the nanowire groups by calculating the average fluorescence intensity over the ROI defined over the visible injection depot in select sections. A similar area was averaged around the visible injection mark under the injection site for the antibody-only control, with exposure times and settings maintained across samples.
Statistical analysis was performed via one-way ANOVA to determine significance between groups with multiple comparisons correction in Graphpad Prism v7.0.
Supplementary Material
Acknowledgments
This work was partially supported by AbbVie (A123942) and NIH (R01EB018842), and C.R.Z. was partially supported by the Canadian Natural Sciences and Engineering Research Council Postgraduate Scholarship Program (442921-2013). We also are grateful to the Electron Microscopy Facility at SFSU and Clive Owen for help with SEM imaging and acknowledge UCSF Research Resource Program Shared Equipment Award funded by the Chancellor for enabling MALDI-MS measurements. Fluorescence imaging was performed at the Nikon Imaging Center at UCSF Mission Bay. C.R.Z. would also like to thank Dr. Ryan Chang for general experimental discussion and Bahar Zirak for help with tissue processing.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsnano.7b06094.
1H NMR spectra of PCL diol and MP-PCL compounds, flow cytometry staining panels, gating strategies for both myeloid and T cell experiments as well as effector T cell and total NK cell numbers (PDF)
Notes
The authors declare no competing financial interest.
References
- 1.Ali Oa, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-Mimicking Materials to Program Dendritic Cells In Situ. Nat Mater. 2009;8:151–158. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutolf MP, Hubbell Ja. Synthetic Biomaterials as Instructive Extracellular Microenvironments for Morphogenesis in Tissue Engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 3.Ali OA, Emerich D, Dranoff G, Mooney DJ. In Situ Regulation of DC Subsets and T Cells Mediates Tumor Regression in Mice. Sci Transl Med. 2009;1:8ra19. doi: 10.1126/scitranslmed.3000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J, Li WA, Choi Y, Lewin SA, Verbeke CS, Dranoff G, Mooney DJ. Injectable, Spontaneously Assembling, Inorganic Scaffolds Modulate Immune Cells In Vivo and Increase Vaccine Efficacy. Nat Biotechnol. 2014;33:64–72. doi: 10.1038/nbt.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jennings J, Beija M, Richez AP, Cooper SD, Mignot PE, Thurecht KJ, Jack KS, Howdle SM. One-Pot Synthesis of Block Copolymers in Supercritical Carbon Dioxide: A Simple Versatile Route to Nanostructured Microparticles. J Am Chem Soc. 2012;134:4772–4781. doi: 10.1021/ja210577h. [DOI] [PubMed] [Google Scholar]
- 6.George PM, Lyckman AW, LaVan Da, Hegde A, Leung Y, Avasare R, Testa C, Alexander PM, Langer R, Sur M. Fabrication and Biocompatibility of Polypyrrole Implants Suitable for Neural Prosthetics. Biomaterials. 2005;26:3511–3519. doi: 10.1016/j.biomaterials.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 7.Nelson SM, Mahmoud T, Beaux M, Shapiro P, McIlroy DN, Stenkamp DL. Toxic and Teratogenic Silica Nanowires in Developing Vertebrate Embryos. Nanomedicine. 2010;6:93–102. doi: 10.1016/j.nano.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adili A, Crowe S, Beaux MF, Cantrell T, Shapiro PJ, McIlroy DN, Gustin KE. Differential Cytotoxicity Exhibited by Silica Nanowires and Nanoparticles. Nanotoxicology. 2008;2:1–8. [Google Scholar]
- 9.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA. High-Dose Recombinant Interleukin 2 Therapy for Patients with Metastatic Melanoma: Analysis of 270 Patients Treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg SA. IL-2: The First Effective Immunotherapy for Human Cancer. J Immunol. 2014;192:5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito S, Bollard CM, Carlsten M, Melenhorst JJ, Biancotto A, Wang E, Chen J, Kotliarov Y, Cheung F, Xie Z, Marincola F, Tanimoto K, Battiwalla M, Olnes MJ, Perl S, Schum P, Hughes TE, Keyvanfar K, Hensel N, Muranski P, Young NS, et al. Ultra-Low Dose Interleukin-2 Promotes Immune-Modulating Function of Regulatory T Cells and Natural Killer Cells in Healthy Volunteers. Mol Ther. 2014;22:1388–1395. doi: 10.1038/mt.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knorr DA, Bachanova V, Verneris MR, Miller JS. Clinical Utility of Natural Killer Cells in Cancer Therapy and Transplantation. Semin Immunol. 2014;26:161–172. doi: 10.1016/j.smim.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller JS, Soignier Y, Panoskaltsis-Mortari A, Mcnearney SA, Yun GH, Fautsch SK, Mckenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, Mcglave PB. Successful Adoptive Transfer and In Vivo Expansion of Human Haploidentical NK Cells in Patients with Cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 14.Mitra A, Fallen RS, Lima HC. Cytokine-Based Therapy in Psoriasis. Clin Rev Allergy Immunol. 2013;44:173–182. doi: 10.1007/s12016-012-8306-2. [DOI] [PubMed] [Google Scholar]
- 15.Lasek W, Zagożdżon R, Jakobisiak M. Interleukin 12: Still a Promising Candidate for Tumor Immunotherapy? Cancer Immunol Immunother. 2014;63:419–435. doi: 10.1007/s00262-014-1523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busse D, de la Rosa M, Hobiger K, Thurley K, Flossdorf M, Scheffold A, Höfer T. Competing Feedback Loops Shape IL-2 Signaling between Helper and Regulatory T Lymphocytes in Cellular Microenvironments. Proc Natl Acad Sci U S A. 2010;107:3058–3063. doi: 10.1073/pnas.0812851107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bluestone JA, Crellin N, Trotta E. IL-2: Change Structure… Change Function. Immunity. 2015;42:779–781. doi: 10.1016/j.immuni.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Mitra S, Ring AM, Amarnath S, Spangler JB, Li P, Ju W, Fischer S, Oh J, Spolski R, Weiskopf K, Kohrt H, Foley JE, Rajagopalan S, Long EO, Fowler DH, Waldmann TA, Garcia KC, Leonard WJ. Interleukin-2 Activity Can Be Fine Tuned with Engineered Receptor Signaling Clamps. Immunity. 2015;42:826–838. doi: 10.1016/j.immuni.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmenate T, Pacios A, Enamorado M, Moreno E, Garcia-Martínez K, Fuente D, León K. Human IL-2 Mutein with Higher Antitumor Efficacy than Wild Type IL-2. J Immunol. 2013;190:6230–6238. doi: 10.4049/jimmunol.1201895. [DOI] [PubMed] [Google Scholar]
- 20.Levin AM, Bates DL, Ring AM, Krieg C, Lin JT, Su L, Moraga I, Raeber ME, Bowman GR, Novick P, Pande VS, Fathman CG, Boyman O, Garcia KC. Exploiting a Natural Conformational Switch to Engineer an Interleukin-2 “superkine. Nature. 2012;484:529–533. doi: 10.1038/nature10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghasemi R, Lazear E, Wang X, Arefanian S, Zheleznyak A, Carreno BM, Higashikubo R, Gelman AE, Kreisel D, Fremont DH, Krupnick AS. Selective Targeting of IL-2 to NKG2D Bearing Cells for Improved Immunotherapy. Nat Commun. 2016;7:12878. doi: 10.1038/ncomms12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spangler JB, Tomala J, Luca VC, Jude KM, Dong S, Ring AM, Votavova P, Pepper M, Kovar M, Garcia KC. Antibodies to Interleukin-2 Elicit Selective T Cell Subset Potentiation through Distinct Conformational Mechanisms. Immunity. 2015;42:815–825. doi: 10.1016/j.immuni.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyman O, Sprent J. The Role of Interleukin-2 during Homeostasis and Activation of the Immune System. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 24.Létourneau S, van Leeuwen EMM, Krieg C, Martin C, Pantaleo G, Sprent J, Surh CD, Boyman O, Marrack P, Létourneau S, van Leeuwerf EMM, Krieg C, Martin C, Pantaleo G, Sprentdef J, Surhbe CD, Boyman O. IL-2/Anti-IL-2 Antibody Complexes Show Strong Biological Activity by Avoiding Interaction with IL-2 Receptor a Subunit CD25. Proc Natl Acad Sci U S A. 2010;107:2171–2176. doi: 10.1073/pnas.0909384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arenas-ramirez N, Woytschak J, Boyman O. Interleukin-2: Biology, Design and Application. Trends Immunol. 2015;36:763–777. doi: 10.1016/j.it.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Boyman O, Kolios AGA, Raeber ME. Modulation of T Cell Responses by IL-2 and IL-2 Complexes. Clin Exp Rheumatol. 2015;33:54–57. [PubMed] [Google Scholar]
- 27.Arenas-Ramirez N, Zou C, Popp S, Zingg D, Brannetti B, Wirth E, Calzascia T, Kovarik J, Sommer L, Zenke G, Woytschak J, Regnier CH, Katopodis A, Boyman O. Improved Cancer Immunotherapy by a CD25-Mimobody Conferring Selectivity to Human Interleukin-2. Sci Transl Med. 2016;8:367ra166. doi: 10.1126/scitranslmed.aag3187. [DOI] [PubMed] [Google Scholar]
- 28.Wu F, Bhansali SG, Law WC, Bergey EJ, Prasad PN, Morris ME. Fluorescence Imaging of the Lymph Node Uptake of Proteins in Mice after Subcutaneous Injection: Molecular Weight Dependence. Pharm Res. 2012;29:1843–1853. doi: 10.1007/s11095-012-0708-6. [DOI] [PubMed] [Google Scholar]
- 29.Li F, Zhang L, Metzger R. On the Growth of Highly Ordered Pores in Anodized Aluminum Oxide. Chem Mater. 1998;10:2470–2480. [Google Scholar]
- 30.Tao SL, Desai TA. Aligned Arrays of Biodegradable Poly(ε-Caprolactone) Nanowires and Nanofibers by Template Synthesis. Nano Lett. 2007;7:1463–1468. doi: 10.1021/nl0700346. [DOI] [PubMed] [Google Scholar]
- 31.Fox CB, Kim J, Schlesinger EB, Chirra HD, Desai TA. Fabrication of Micropatterned Polymeric Nanowire Arrays for High- Resolution Reagent Localization and Topographical Cellular Control. Nano Lett. 2015;15:1540–1546. doi: 10.1021/nl503872p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang F, Low HY. Ordered Three-Dimensional Hierarchical Nanostructures by Nanoimprint Lithography. Nanotechnology. 2006;17:1884–1890. [Google Scholar]
- 33.Alvine KJ, Shpyrko OG, Pershan PS, Shin K, Russell TP. Capillary Filling of Anodized Alumina Nanopore Arrays. Phys Rev Lett. 2006;97:175503. doi: 10.1103/PhysRevLett.97.175503. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto A, Kubota T, Otsu T. Radical Polymerization of N-(Alkyl-Substituted pheny1)maleimides: Synthesis. Macromolecules. 1990;23:4508–4513. [Google Scholar]
- 35.Manjula BN, Tsai A, Upadhya R, Perumalsamy K, Smith PK, Malavalli A, Vandegriff K, Winslow RM, Intaglietta M, Prabhakaran M, Friedman JM, Acharya AS. Site-Specific PEGylation of Hemoglobin at Cys-93(β): Correlation between the Colligative Properties of the PEGylated Protein and the Length of the Conjugated PEG Chain. Bioconjugate Chem. 2003;14:464–472. doi: 10.1021/bc0200733. [DOI] [PubMed] [Google Scholar]
- 36.Ananda K, Nacharaju P, Smith PK, Acharya SA, Manjula BN. Analysis of Functionalization of Methoxy-PEG as Maleimide-PEG. Anal Biochem. 2008;374:231–242. doi: 10.1016/j.ab.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 37.Cha Y, Pitt CG. The Biodegradability of Polyester Blends. Biomaterials. 1990;11:108–112. doi: 10.1016/0142-9612(90)90124-9. [DOI] [PubMed] [Google Scholar]
- 38.Kuriyan J, Konforti B, Wemmer D. The Molecules of Life: Physical and Chemical Principles. Garland Science; New York: 2009. Molecular Recognition: The Thermodynamics of Binding; pp. 1–58. [Google Scholar]
- 39.Ahlfors EE, Lyberg T. Kinetics of Local Tissue and Regional Lymph Node IL-2 and IFN-γ Responses in Experimental Oral Mucosa and Skin Contact Sensitivity in Mice. Scand J Immunol. 2010;72:8–14. doi: 10.1111/j.1365-3083.2010.02402.x. [DOI] [PubMed] [Google Scholar]
- 40.Woodruff MA, Hutmacher DW. Progress in Polymer Science The Return of a Forgotten Polymer — Polycaprolactone in the 21st Century. Prog Polym Sci. 2010;35:1217–1256. [Google Scholar]
- 41.Li Z, Tan BH. Towards the Development of Polycaprolactone Based Amphiphilic Block Copolymers: Molecular Design, Self-Assembly and Biomedical Applications. Mater Sci Eng C. 2015;45:620–634. doi: 10.1016/j.msec.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Xue J, Feng B, Zheng R, Lu Y, Zhou G, Liu W, Cao Y, Zhang Y, Zhang WJ. Engineering Ear-Shaped Cartilage Using Electrospun Fibrous Membranes of Gelatin/polycaprolactone. Biomaterials. 2013;34:2624–2631. doi: 10.1016/j.biomaterials.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Ayyala RS, Harman LE, Michelini-Norris B, Ondrovic LE, Haller E, Margo CE, Stevens SX. Comparison of Different Biomaterials for Glaucoma Drainage Devices. Arch Ophthalmol. 1999;117:233–236. doi: 10.1001/archopht.117.2.233. [DOI] [PubMed] [Google Scholar]
- 44.Kim MS, Ahn HH, Shin YN, Cho MH, Khang G, Lee HB. An In Vivo Study of the Host Tissue Response to Subcutaneous Implantation of PLGA- And/or Porcine Small Intestinal Submucosa-Based Scaffolds. Biomaterials. 2007;28:5137–5143. doi: 10.1016/j.biomaterials.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Yoon SJ, Kim SH, Ha HJ, Ko YK, So JW, Kim MS, Yang YIl, Khang G, Rhee JM, Lee HB. Reduction of Inflammatory Reaction of Poly(d,l-Lactic-Co-Glycolic Acid) Using Demineralized Bone Particles. Tissue Eng Part A. 2008;14:539–547. doi: 10.1089/tea.2007.0129. [DOI] [PubMed] [Google Scholar]
- 46.Edwards DA, Hanes J, Caponetti G, Hrkach J, BenJebria A, Eskew M, Lou Mintzes J, Deaver D, Lotan N, Langer R. Large Porous Particles for Pulmonary Drug Delivery. Science (Washington DC U S) 1997;276:1868–1872. doi: 10.1126/science.276.5320.1868. [DOI] [PubMed] [Google Scholar]
- 47.Champion JA, Walker A, Mitragotri S. Role of Particle Size in Phagocytosis of Polymeric Microspheres. Pharm Res. 2008;25:1815–1821. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Champion JA, Mitragotri S. Role of Target Geometry in Phagocytosis. Proc Natl Acad Sci U S A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD. Porous Implants Modulate Healing and Induce Shifts in Local Macrophage Polarization in the Foreign Body Reaction. Ann Biomed Eng. 2014;42:1508–1516. doi: 10.1007/s10439-013-0933-0. [DOI] [PubMed] [Google Scholar]
- 50.Sridharan R, Cameron AR, Kelly DJ, Kearney CJ, O’Brien FJ. Biomaterial Based Modulation of Macrophage Polarization: A Review and Suggested Design Principles. Mater Today. 2015;18:313–325. [Google Scholar]
- 51.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective Stimulation of T Cell Subsets with Antibody-Cytokine Immune Complexes. Science (Washington DC U S) 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 52.Williams Ma, Tyznik AJ, Bevan MJ. Interleukin-2 Signals during Priming Are Required for Secondary Expansion of CD8+ Memory T Cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Annunziato ME, Patel US, Ranade M, Palumbo PS. P-Maleimidophenyl Isocyanate: A Novel Heterobifunctional Linker for Hydroxyl To Thiol Coupling. Bioconjugate Chem. 1993;4:212–218. doi: 10.1021/bc00021a005. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Hoskins JN, Sreerama SG, Grayson SM. MALDITOF Mass Spectral Characterization of Polymers Containing an Azide Group: Evidence of Metastable Ions. Macromolecules. 2010;43:6225–6228. doi: 10.1021/ma100599n. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


