Abstract
Background and Aims
Celiac disease (CD) is a widespread condition triggered by dietary gluten and treated with a lifelong gluten-free diet (GFD); however, inadvertent exposure to gluten can result in episodic symptoms. A previous trial of latiglutenase (clinicaltrials.gov; NCT01917630), an orally administered mixture of two recombinant gluten-specific proteases, was undertaken in symptomatic subjects with persistent injury. The primary endpoint for histologic improvement was not met, presumably due to a trial effect. In this post hoc analysis we investigated the efficacy of latiglutenase for reducing symptoms in subgroups of the study participants based on their seropositivity.
Methods
The study involved symptomatic CD patients following a GFD for at least one year prior to randomization. Patients were treated for 12 weeks with latiglutenase or placebo. Of 398 completed patients 173 (43%) were seropositive at baseline. Symptoms were recorded daily and weekly symptom scores were compiled. P-values were calculated by analysis of covariance (ANCOVA).
Results
A statistically significant, dose-dependent reduction was detected in the severity and frequency of symptoms in seropositive but not seronegative patients. The severity of abdominal pain and bloating was reduced by 58% and 44%, respectively, in the cohort receiving the highest latiglutenase dose (900 mg, n=14) relative to placebo (n=54). Symptom improvement increased from week 6 to week 12. There was also a trend toward greater symptom improvement with greater baseline symptom severity.
Conclusions
Seropositive CD patients show symptomatic improvement from latiglutenase taken with meals and would benefit from the availability of this treatment.
Keywords: Celiac disease, latiglutenase, therapy, symptoms
INTRODUCTION
Celiac disease (CD) is a lifelong immune disorder of the small intestine that affects 0.5–1% of most populations [1]. In genetically susceptible individuals, intestinal inflammation is triggered by the ingestion of gluten derived from cereals such as wheat, rye, or barley, leading to crypt hyperplasia and villous atrophy. Although patients want additional therapies [2,3], the only treatment currently available consists of a gluten-free diet (GFD).
Although several experimental therapies for CD have entered clinical trials [4], randomized clinical trials to evaluate drug efficacy have only been conducted on two modes of action – enzyme supplementation therapy [5,6] and modulation of tight junctions in the small intestine [7]. Latiglutenase (formerly ALV003) is a promising example of enzyme supplementation therapy comprised of two recombinant proteins. The pharmacological rationale for this experimental therapeutic agent is based on enzymatic degradation of the poorly digested immunotoxic gluten peptides in the stomach preventing exposure of these peptides to the small intestinal mucosal immune system [8].
Two key issues that must be addressed for latiglutenase (as well as other non-dietary therapies for CD) are the gluten dose that can be effectively detoxified in vivo by a given enzyme dose and whether this prevents the symptoms associated with inadvertent gluten ingestion. A lower limit on the former variable was identified by an earlier double-blind study involving CD patients in remission [3]. Specifically, it was shown that 900 mg latiglutenase protected most patients from up to 2 g gluten, approximately one-tenth of the average daily gluten consumption in a normal western diet and about 100-fold higher than the safe threshold of gluten in a celiac diet [9]. To address the latter question, a double-blind, placebo controlled study was performed to evaluate the effects of varying doses of latiglutenase in symptomatic CD patients who reported following a gluten-free diet for at least one year prior to randomization. In all study groups, including the placebo group, a comparable improvement in histological scores was observed and therefore the primary endpoint for histologic improvement was not met [10]. The published manuscript focused on the reasons for the failed endpoint, but did note indication of statistically significant improvement for certain symptoms that were particularly pronounced in seropositive patients.
Here we report analysis that reveals significant symptom improvements for seropositive, but not seronegative, patients in response to latiglutenase administration. These new findings provide important insights for the future clinical development of latiglutenase and similar approaches to treating celiac disease. The 3rd Gastroenterology Regulatory Endpoints and Advancement of Therapeutics (GREAT-3) conference sponsored by the U.S. Food and Drug Administration in 2015 made a clear case for the need of measurement outcome tools relevant to the suffering of CD patients and specifically cited the need to develop treatments that address symptoms due to inadvertent gluten ingestion [11].
METHODS
Subjects and clinical study design
The clinical trial (www.clinicaltrials.gov: NCT01917630) was a multi-center, multinational, randomized, double-blind, placebo-controlled, dose-ranging study in symptomatic patients with CD. Both seropositive and seronegative CD patients were enrolled in this study. Details of the trial are reported elsewhere [8]. Briefly, symptoms of each subject were monitored over a 4-week baseline period. This was followed by a randomization period (1–4 weeks) during which patients underwent serological and endoscopic analysis. The primary inclusion criterion in the study was histological evidence for active disease. Subjects who qualified under this criterion underwent an additional 12 weeks of study during which either a placebo or a defined dose of latiglutenase (100, 300, 450, 600, 900 mg) was administered orally TID. The baseline values for villous height to crypt depth ratio (Vh:Cd) were ≤ 2.5.
Celiac Disease Symptom Diary (CDSD©) patient reported outcome (PRO) instrument
The CDSD is a daily diary administered across a seven-day period that assesses common celiac symptoms (abdominal pain, bloating, tiredness, nausea, diarrhea, and constipation). Patients were asked to respond to the presence or absence of individual symptoms in each prior 24-hour period. If a given symptom was present on a given day, follow-up questions were asked to establish its severity on that occasion. Further detail is given elsewhere [12,13]. Briefly, for all symptoms except constipation, each patient’s daily severity score was normalized from 0 to 10 where 0 represents no symptom. The weekly score therefore ranged from 0 to 70. The frequency value was the number of non-zero events, irrespective of severity. The measure of constipation required several days of data, and therefore a daily score was not recorded other than to measure the number of complete spontaneous bowel movements (CSBMs) per day. A constipation event was defined when fewer than three bowel events occurred for the week. The severity of constipation was calculated from the number of bowel movements for the week, and ranged from 0 to 70. There was no measure of constipation frequency; instead, this value was represented by the number of bowel movements per week.
Symptom statistical analysis
The reduction in symptom value relative to placebo at each dose (RISdose) was quantified using the following equation:
| (1) |
where Bdose is the baseline value (i.e., the total severity score of the symptom in the week prior to randomization), and ΔBdose is the change in baseline value cumulative for all patients for a particular dose in week 6 or week 12 of drug dosing. The subscript PBO represents the placebo dose population. The (1−(ΔBPBO/BPBO)) term in the denominator accounted for the improvement in a symptom due to latiglutenase activity relative to the placebo effect; as a result, RISdose could assume values between 0% (corresponding to the placebo effect) and 100% (full recovery of the symptom). P-values for ΔBdose/Bdose (including dose = PBO) and (ΔBdose/Bdose) − (ΔBPBO/BPBO) were calculated by analysis of covariance (ANCOVA).
Trends in individual patients’ symptoms were analyzed using the daily data from the CDSD instrument. This data was divided into three periods: days 1–56 (weeks −8 to 0) used for baseline calculations; days 57–98 (weeks 1–6) of the trial and days 99–140 (weeks 7–12) of the trial.
Serological analysis
Serum levels of IgA autoantibodies to transglutaminase 2 (TG2) and IgA and IgG autoantibodies to deamidated gliadin peptides (DGP) were measured by enzyme linked immunosorbent assay (ELISA). If any of these three measurements were positive, the patient was labeled seropositive.
RESULTS
Among the 398 patients that completed the study and maintained compliance with the CDSD, 173 (43%) were seropositive. The number of seropositive and seronegative patients, respectively, for each arm of the study was PBO (n=54, n=68), 100 mg (n=20, n=27), 300 mg (n=35, n=40), 450 mg (n=15, n=23), 600 mg (n=35, n=45), 900 mg (n=14, n=22).
Dependence of symptom severity and frequency on latiglutenase dose
A dose-dependent symptomatic benefit of latiglutenase was observed in seropositive but not seronegative patients. Symptoms showing the greatest benefit were abdominal pain and bloating. At week 12, the RISdose values for abdominal pain severity relative to placebo were 100 mg (25%), 300 mg (24%), 450 mg (29%), 600 mg (48%), and 900 mg (58%) as plotted in Figure 1A. The difference in abdominal pain severity between the combined 600 mg and 900 mg dose cohorts and the placebo cohort was 51% (p = 0.008). Dose dependence was also observed in abdominal pain frequency (Figure 1B). Similarly, the RISdose values for bloating severity relative to placebo were 100 mg (3%), 300 mg (−8%), 450 mg (3%), 600 mg (31%), and 900 mg (44%) as plotted in Figure 1C. The difference in bloating severity between the combined 600 mg and 900 mg dose cohorts and the placebo cohort was 35% (p = 0.007). Dose dependence was also observed for bloating frequency (Figure 1D). Improvements in abdominal pain and bloating severity and frequency were also observed as a function of duration of treatment (Figure 2 for severity). While not evident for abdominal pain, a statistically non-significant trend was also observed in the improvement of bloating symptoms in seronegative patients on latiglutenase relative to placebo (Figures 1C and 1D).
Figure 1.
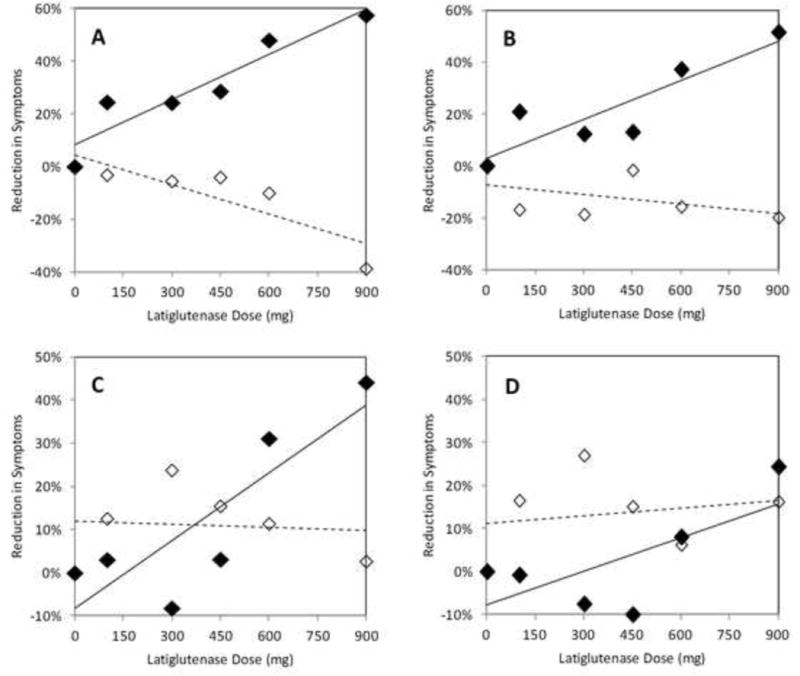
Dose dependence of latiglutenase treatment for week 12 on the severity (A & C) and frequency (B & D) of abdominal pain (A & B) and bloating (C & D) in seropositive (solid diamonds) and seronegative (open diamonds) celiac disease patients with evidence of ongoing mucosal injury. Reduction in symptoms (RIS) relative to placebo was quantified according to equation 1.
Figure 2.
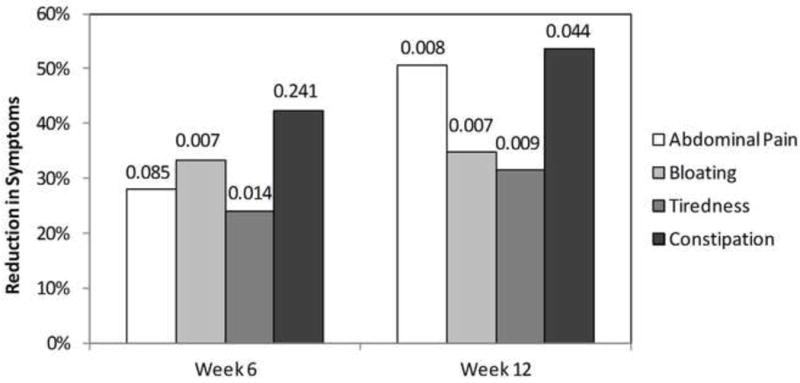
Time dependence of latiglutenase treatment (combined 600 and 900 mg doses) on the severity of abdominal pain, bloating, tiredness, and constipation in seropositive celiac disease patients. Reduction in symptoms (RIS) relative to placebo was quantified according to equation 1. P-values are shown on top of bars.
Statistically significant effects were also observed for tiredness and constipation. At week 12, the RISdose values for severity for the combined 600 mg and 900 mg dose cohorts relative to placebo were 32% (p = 0.009) and 54% (p = 0.044), respectively. Improvements in tiredness and constipation severity and frequency were generally observed as a function of duration of treatment (Figure 2 for severity). Within the statistical accuracy of the results there was no discernable difference in any of these symptom responses for positive serologic readings for any of the three antibody titers.
Of the two other symptom domains monitored by the CDSD, neither nausea nor diarrhea showed significant improvement in the latiglutenase-treated cohorts, although these two symptoms did not worsen either as a result of latiglutenase administration.
RISdose dependence on baseline symptom severity
The magnitude of RIS for the 600 and 900 mg seropositive latiglutenase arms correlates with the baseline symptom severity for abdominal pain and bloating (results not shown). A similar but less distinct trend was observed for tiredness as well as for all symptoms in the 600 mg arm. These observations indicate that the benefits of latiglutenase are greatest for the most symptomatic CD patients.
CONCLUSIONS
Patients both in the United States [2] and England [3] have indicated that they are dissatisfied with the gluten-free diet and would like the availability of additional therapies. A glutenase preparation (latiglutenase) was tested in a large trial that failed to fulfil the primary endpoint of mucosal healing.
In this study, further analysis of the symptom data reveals and provides compelling evidence for latiglutenase-induced reduction of several symptoms associated with likely gluten ingestion in a subset of CD patients. Abdominal pain and bloating showed the greatest improvement relative to placebo in seropositive patients, who were seropositive despite attempting to adhere to a gluten-free diet. The lack of a significant response in seronegative patients reflects the fact that patients with CD when evaluated for persistent symptoms often have etiologies such as small intestinal bacterial overgrowth, fructose and lactose intolerance, microscopic colitis, refractory CD and irritable bowel syndrome [14,15]. These conditions are not related to ongoing gluten exposure and would not be expected to respond to therapy with a glutenase preparation. Although the study was not primarily powered to establish the benefit of latiglutenase specifically in seropositive CD patients, our data demonstrate that such patients show significant symptomatic benefit from the intake of latiglutenase with meals. As to why the study showed significant differentiation in symptom improvement, but not histologic improvement for high dose vs. placebo arms may be attributable to the different time scales forrequired for histologic improvement, compared to symptom improvement as well as the poor coorelation beteen symptoms severity and same degree of villous atrophy and gluten ingestion [16].
Acknowledgments
Grant Support: Clinical trial NCT01917630 was sponsored by Alvine Pharmaceuticals; all data from this trial is presently owned by ImmunogenX. The data analysis reported here was supported in part by a grant from the National Institutes of Health (R01 DK063158 to C.K.).
Disclosure of Financial Arrangements:
JAS is a founder of and owns stock in ImmunogenX.
JAM has received grant support from the National Institutes of Health, Alvine Pharmaceuticals, and Alba Therapeutics; receives ongoing support from Oberkotter Foundation and Broad Medical Research Program at CCFA; serves on the advisory board of Celimmune, LLC and ImmunogenX; was a consultant to, BioLineRx, GlaxoSmithKline (GSK), Genentech, and Glenmark Pharmaceuticals Ltd; and is a consultant to ImmunosanT, Institute for Protein Design (PvP Biologics), Takeda Pharmaceutical Company, Ltd., Innovate Biopharmaceuticals, Inc., and Intrexon.
PHRG is an advisor to ImmusanT and ImmunogenX.
CK is a director of Protagonist Pharmaceuticals and an advisor to Sitari Pharmaceuticals, and holds stock in both companies.
Footnotes
Specific author contributions:
JAS, JAM, PHRG, CK; acquisition of data
JAS, CK; analysis and interpretation of data
JAS, CK; technical and material support
JAS, PHRG, CK; drafting of manuscript
JAS, JAM, PHRG, CK; critical revision of the manuscript and important intellectual content.
References
- 1.Rubio-Tapia A, Murray JA. Celiac disease. Curr Opin Gastroenterol. 2010;26:116–122. doi: 10.1097/MOG.0b013e3283365263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tennyson CA, Simpson S, Lebwohl B, Lewis S, Green PH. Interest in medical therapy for celiac disease. Therap Adv Gastroenterol. 2013;6:358–364. doi: 10.1177/1756283X13492580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aziz I, Evans KE, Papageorgiou V, Sanders DS. Are patients with coeliac disease seeking alternative therapies to a gluten-free diet? J Gastrointestin Liver Dis. 2011;20:27–31. [PubMed] [Google Scholar]
- 4.Gottlieb K, Dawson J, Hussain F, Murray JA. Development of drugs for celiac disease: Review of endpoints for phase 2 and 3 trials. Gastroenterol Rep (Oxf) 2015;3:91–102. doi: 10.1093/gastro/gov006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lahdeaho ML, Kaukinen K, Laurila K, et al. Glutenase alv003 attenuates gluten-induced mucosal injury in patients with celiac disease. Gastroenterology. 2014;146:1649–1658. doi: 10.1053/j.gastro.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Wolf C, Siegel JB, Tinberg C, et al. Engineering of kuma030: A gliadin peptidase that rapidly degrades immunogenic gliadin peptides in gastric conditions. J Am Chem Soc. 2015;137:13106–13113. doi: 10.1021/jacs.5b08325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leffler DA, Kelly CP, Green PH, et al. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: A randomized controlled trial. Gastroenterology. 2015;148:1311–1319 e1316. doi: 10.1053/j.gastro.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gass J, Bethune MT, Siegel M, Spencer A, Khosla C. Combination enzyme therapy for gastric digestion of dietary gluten in patients with celiac sprue. Gastroenterology. 2007;133:472–480. doi: 10.1053/j.gastro.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Catassi C, Fabiani E, Iacono G, et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr. 2007;85:160–166. doi: 10.1093/ajcn/85.1.160. [DOI] [PubMed] [Google Scholar]
- 10.Murray JA, Kelly CP, Green PH, et al. No difference between latiglutenase and placebo in reducing villous atrophy or improving symptoms in patients with symptomatic celiac disease. Gastroenterology. 2017;152:787–798 e782. doi: 10.1053/j.gastro.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Leffler D, Kupfer SS, Lebwohl B, Bugin K, Griebel D, et al. Development of Celiac Disease Therapeutics: Report of the Third Gastroenterology Regulatory Endpoints and Advancement of Therapeutics Workshop. Gastroenterology. 2016;151:407–411. doi: 10.1053/j.gastro.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Leffler DA, Acaster S, Gallop K, et al. A novel patient-derived conceptual model of the impact of celiac disease in adults: Implications for patient-reported outcome and hela-related quality-of-life instrument development. Value in Health. 2017 doi: 10.1016/j.jval.2016.12.016. http://dxdoiorog/101016/jjval201612016. [DOI] [PubMed]
- 13.Hindryckx P, Levesque BG, Holvoet T, et al. Disease activity indices in coeliac disease: Systematic review and recommendations for clinical trials. Gut. 2016 doi: 10.1136/gutjnl-2016-312762. [DOI] [PubMed] [Google Scholar]
- 14.Leffler DA, Dennis M, Hyett B, Kelly E, Schuppan D, Kelly CP. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol. 2007;5:445–50. doi: 10.1016/j.cgh.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Abdulkarim AS, Burgart LJ, See J, Murray JA. Etiology of nonresponsive celiac disease: results of a systematic approach. The American journal of gastroenterology. 2002;97:2016–21. doi: 10.1111/j.1572-0241.2002.05917.x. [DOI] [PubMed] [Google Scholar]
- 16.Brar P, Kwon GY, Egbuna I, et al. Lack of correlation of degree of villous atrophy with severity of clinical presentation of coeliac disease. Dig Liver Dis. 2007;39:26–9. doi: 10.1016/j.dld.2006.07.014. [DOI] [PubMed] [Google Scholar]


