Abstract
Trastuzumab is the first-line drug to treat breast cancer with high Her2 expression. However, many cancers failed to respond, largely due to their resistance to NK cell-triggered antibody-dependent cellular cytotoxicity (ADCC). Poliovirus receptor (PVR)-like molecules are known to be important for lymphocyte functions. We found that all PVR-like receptors are expressed on human NK cells, and only TIGIT is preferentially expressed on the CD16+ NK cell subset. Disrupting the interactions of PVR-like receptors with their ligands on cancer cells regulates NK cell activity. More importantly, TIGIT is upregulated upon NK cell activation via ADCC. Blockade of TIGIT or CD112R, separately or together, enhances trastuzumab-triggered antitumor response by human NK cells. Thus, our findings suggest that PVR-like receptors regulate NK cell functions and can be targeted for improving trastuzumab therapy for breast cancer.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-2031-x) contains supplementary material, which is available to authorized users.
Keywords: ADCC, CD112R, NK cell, PVR-like, TIGIT, Trastuzumab
Introduction
HER2/neu overexpression or amplification occurs in about 20% of invasive breast cancers and is strongly associated with poor prognosis [1, 2]. Trastuzumab, a recombinant humanized anti-HER2 monoclonal antibody, is the first therapeutic option for patients with HER2-positive breast cancer [3, 4]. The major antitumor mechanisms of trastuzumab are inhibition of HER2-mediated signal transduction and induction of ADCC [5–7]. Despite improvements in patient outcome for this poor-prognostic group of patients, response rates in metastatic breast cancer to trastuzumab as monotherapy are limited, consisting of approximately 10–15% [8]. Trastuzumab-triggered ADCC is dependent upon immune effector cells, mainly NK cells, binding via their Fc receptor (FcγRIII, CD16) to the IgG1 Fc, the heavy-chain portion of trastuzumab [9]. This leads to the activation of NK cells, release of their cytotoxic granules, and lysis of the trastuzumab-bound breast cancer cells [10]. Therefore, new strategies are needed to augment NK cell activities, so as to increase the clinical efficacy of trastuzumab therapy.
The poliovirus receptor (PVR)-like molecules are a newly emerging group of the immunoglobulin superfamily (IgSF) that play important regulatory roles in NK cell functions [11]. These groups of surface molecules share PVR-signature motifs in the first immunoglobulin variable-like (IgV) domain, and are originally known to mediate epithelial cell–cell contact [12, 13]. CD155 (also known as PVR or Necl-5) and CD112 (also known as Pvrl2 or Nectin-2) are two major ligands that are heavily expressed on many cancer cells. They both interact with CD226 (DNAM-1) to stimulate T and NK cells. They also inhibit T/NK cell functions through another coinhibitory receptor, T cell immunoglobulin and ITIM domain (TIGIT) [13]. CD226−/− mice are susceptible to 3-methylcholanthrene (MCA)-induced sarcoma and resistant to LPS-induced inflammation, and both of the disease models involve NK cells [14]. CD155, but not CD112, interacts with CD96, another PVR-like co-receptor present on T cells and NK cells, though the function of this interaction is still unclear [14–16]. Adding to the complexity of this network, we recently identified CD112R as a new inhibitory receptor of the PVR-like family, which interacts with CD112, but not CD155 [17]. CD112R is expressed on human NK cells, though its function on this cell type is unclear.
Here we examine the role of PVR-like receptors on NK cell functions against human breast cancer, with the aim to enhance the antitumor activity of trastuzumab via NK cell-triggered ADCC.
Materials and methods
Cell lines and culture
The human breast cancer cell lines SK-BR3, MDA-MB-453, and MCF7 cell lines were obtained from the Cell Line Repository at the University of Colorado Cancer Center. Daudi, a human CD20-positive B lymphoblast cell line, was maintained in the laboratory. The SK-BR3 cell line was cultured in McCoy’s 5A modified medium supplemented with 10% heat-inactivated fetal bovine serum (Gemini), 100 µg/ml streptomycin, and 100 U/ml penicillin (Life Technologies). MDA MB453 and MCF7 were grown in DMEM/F12 Ham liquid media (Sigma D8437), Daudi cell line was cultured in RPMI, and all supplemented as above. Cells were incubated at 37 °C in 5% CO2. Adherent cells were treated with trypsin to detach cells before passaging.
Antibodies
Anti-human TIGIT mAb (Clone MBSA43, mouse IgG1) was purchased from eBioscience. Antibodies for human 4-1BB (CD137), CD96, CD112, CD155, CD107a, and IFN-γ were obtained from Biolegend. Anti-human CD226 mAb (clone DX11, mouse IgG1) was purchased from Abcam. Mouse anti-human CD112R mAb (clone 2H6, IgG1) was generated from a hybridoma derived from the fusion of SP2 myeloma with B cells from a mouse immunized with human CD112R-Fc [17]. LEAF™ (Low Endotoxin, Azide-Free) purified mouse IgG1 (clone MG1-45), used for isotype control in culture, was obtained from Biolegend. All other antibodies used in flow cytometry were purchased from BD Bioscience, Biolegend, or eBioscience. Trastuzumab and Rituximab were obtained from the Pharmacy of the University of Colorado Hospital.
Human NK cells purification and activation
Peripheral blood mononuclear cells (PBMCs), which were obtained from the Bonfils Blood Center in Denver, CO with institutional approval, were isolated from healthy donors by density gradient separation using Ficoll. NK cells were isolated by either positive magnetic cell sorting using NK cell isolation beads CD56, or by negative magnetic cell sorting to remove T cell (CD3), monocyte (CD14), and B cell (CD20). NK cells were activated with cytokines IL-2 (1000 U/ml) plus IL-12 (20 ng/ml) or trastuzumab-coated SK-BR3 tumors at the ratio of 2.5:1 for 16 h.
Flow cytometry
Purified NK cells or cultured cell mixture was blocked with human serum 100 μl for 15 min at 4 °C. The cells were stained with PE-conjugated anti-CD56 antibodies (BD Pharmingen) and APC-conjugated anti-mAb antibody (BD Pharmingen) for 30 min at 4 °C. Cells were washed with the staining buffer before analyzing with FACSCalibur cytometer (Becton–Dickinson). Data analysis was performed using FlowJo version 10.1 software.
Intracellular cytokine staining assay for IFN-γ
Purified NK cells (2.5 × 105) were cultured alone or with breast cancer cell lines in 96-well plates in a total volume of 200 μl. Monensin (1 μM, eBioscience) was added to cultures. Cells were stimulated with tumor and trastuzumab with the presence of different mAbs for 16 h at 37 °C with 5% CO2. Cells stimulated with phorbol 12-myristate 13-acetate (PMA) (100 ng/ml; Sigma) and ionomycin (1 μM, Sigma) were used as positive controls. After incubation, cells were collected and blocked with human serum 100 μl for 15 min at 4 °C. Then cells were labeled with PE-conjugated anti-CD56 (BD Pharmingen) antibodies for 30 min at 4 °C. After cell surface staining, cells were fixed and permeabilized with Fixation and Permeabilization Buffer (BD Pharmingen) and stained with APC-conjugated anti-IFN-γ antibody (BD Pharmingen). After washing with the staining buffer, the cells were resuspended in 300 μl cold staining buffer, followed by analysis with flow cytometry.
CD107a degranulation assay
Purified NK cells (2.5 × 105) were cultured alone or with breast cancer cell lines in 96-well plates in a 200 μl volume. Cells were stimulated with tumor cells and trastuzumab, with the presence of different mAbs for PVR-like receptors. APC-conjugated CD107a antibody (BD Pharmingen) and Monensin were directly added to the culture medium and co-cultured with cells for 16 h at 37 °C with 5% CO2. After culture, cell mixture was blocked with human serum for 15 min at 4 °C, and stained with PE-conjugated anti-CD56 antibodies (BD Pharmingen) for 30 min at 4 °C. The cells were washed with the staining buffer and analyzed with flow cytometry.
Enzyme-linked immunosorbent assay (ELISA) for IFN-γ
Purified NK cells (2.5 × 105) were cultured alone or with breast cancer cell lines in 96-well plates in a 200 μl volume. NK cells were co-cultured with tumor cells, trastuzumab (2 µg/mL) for 16 h with the presence of different mAbs. After incubation, the supernatants were harvested by centrifugation at 1500 rpm for 20 min. The IFN-γ level in supernatants was determined by ELISA, according to manufacturer’s instructions.
Human breast cancer cell killing assay by human NK cells
Human breast cancer cells were harvested and incubated with 0.25 µM CFSE at room temperature for 10 min. After washing, labeled breast cancer cells were cultured in 96-well low-attachment plate overnight with human NK cells, with the presence or absence of trastuzumab. Neutralizing mAbs for PVR-like receptors or isotype-matching mIgG1 were added at the beginning of culture. After culture, cells were collected for flow cytometry. Tumor cells were distinguished from NK cells by CFSE staining, and the death of tumor cells was determined by propidium iodide (PI) staining. The killing was calculated by: Killing% = The percentage of PI + Tumor cells in sample – The percentage of PI + Tumor cells in culture alone.
Statistics
Prism software (GraphPad) was used to analyze data and determine statistical significance of differences [including mean ± standard error of measurement (SEM)] between groups by applying a 2-tailed, unpaired Student’s t test or 2-way ANOVA with Bonferroni’s correction for multiple comparisons. P < 0.05 was considered significant.
Results
PVR-like receptors are broadly expressed on human NK cells
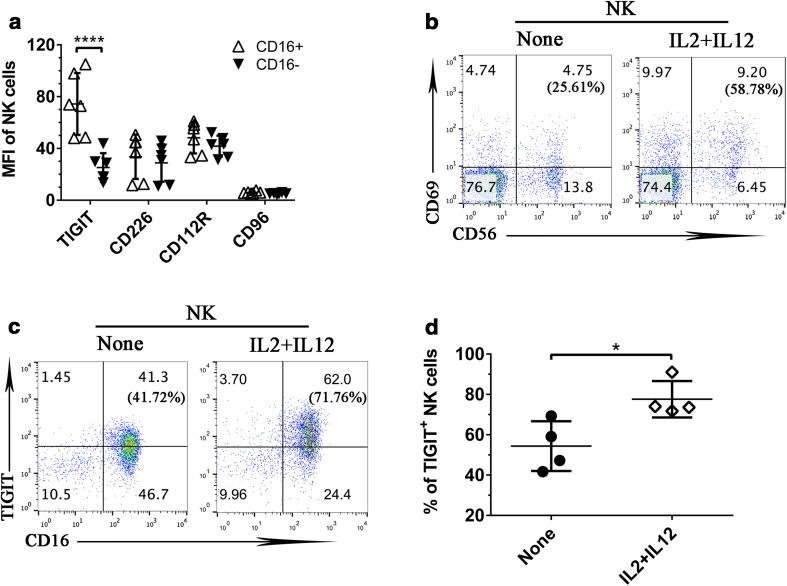
Many of the PVR-like receptors are known to be important for NK cell functions. Here we examined how PVR-like receptors are expressed respectively on human circulating NK cells from peripheral blood mononuclear cells (PBMCs) of healthy donors. NK cells were identified as CD56+CD3− lymphocytes, and further divided into two subsets based on CD16 expression. We found that TIGIT was preferentially expressed on CD16-positive NK cell subset. All other PVR-like receptors, including CD226, CD112R, and CD96, were evenly distributed on CD16+ and CD16− NK cells (Fig. 1a), though in different expression levels.
Fig. 1.
PVR-like receptor expression on human NK cells. a The expression of PVR-like receptors on CD16+ and CD16− NK cells from human PBMCs, based on CD3–CD56+ expression. Data shown are from six healthy donors and were analyzed by Student’s t test. ****P < 0.0001. b Human PBMCs stimulated overnight by cytokines IL-2 plus IL-12 were stained for CD69 expression on NK cells (CD56+). c TIGIT expression was examined on NK cells (CD3−CD56+) upon IL-2 and IL-12 stimulation. The numbers in each bracket mean the percentage of CD69-positive expression on NK cells. d The percentage of TIGIT-positive NK cells (CD56+) upon overnight IL-2 plus IL-12 stimulation. Data were collected from four independent experiments. Data showed mean ± SD and were analyzed by two-way ANOVA test. *P < 0.05
Next we examined expression changes of PVR-like receptors on NK cells upon activation. Freshly isolated human NK cells were stimulated by cytokines IL-2 plus IL-12 overnight. The activation of NK cells (CD56+) was confirmed by the upregulation of CD69, a classical activation marker for NK cells (Fig. 1b). Upon stimulation, we found that TIGIT expression was significantly upregulated on NK cells in multiple healthy donors (Fig. 1c, d). In contrast, the same treatment had minimal effect on the expression of other PVR-like receptors, including CD96, CD226, and CD112R (data not shown). Thus, our results indicated that NK cells activated via cytokines selectively upregulated TIGIT, but not other PVR-like receptors.
PVR-like receptors are involved in NK cell functions against breast cancer cells
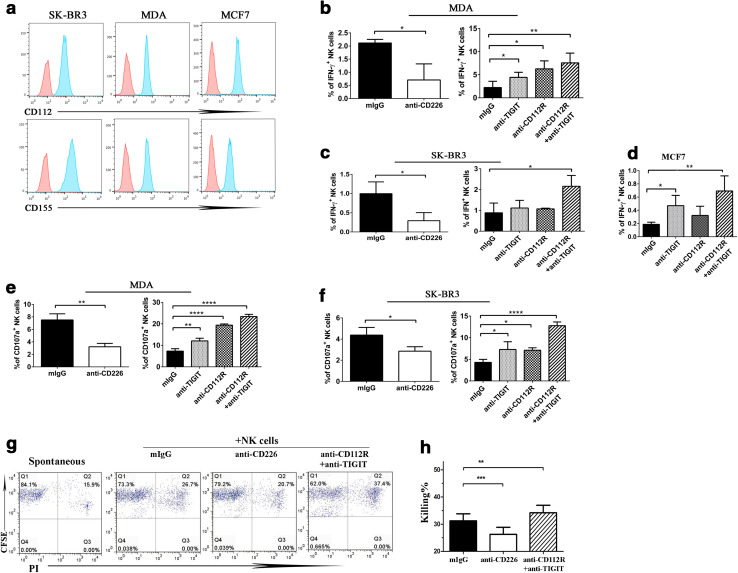
CD112 and CD155 are the two main ligands of the PVR-like receptors, which are broadly expressed on tumor cells [11, 18]. Here we examined the respective roles of the PVR-like receptors on NK cell activities against tumor cells. We cultured purified human NK cells with MDA-MB-453 (MDA), SK-BR3, and MCF7 respectively; three human breast cancer cell lines expressing high levels of both CD112 and CD155 (Fig. 2a). Individual blocking mAbs against PVR-like receptors were added from the beginning of culture. We found that a small percentage of human NK cells (CD56+) expresses IFN-γ upon culturing with MDA cancer cells (Supplemental Figure 1). CD226 signal seems to be involved, because inclusion of CD226 neutralizing mAb nearly eliminated the effect (Fig. 2b, left). On the other hand, TIGIT or CD112R blockade resulted in a significant increase of IFN-γ-producing NK cells. The addition of both TIGIT and CD112R mAbs further increased the percentages of IFN-γ-positive NK cells (Fig. 2b, right). Similarly, human NK cells produce little IFN-γ upon culturing with SK-BR3 cells, and the stimulating effect seems to rely on CD226 signal (Fig. 2c, left). Inclusion of both CD112R and TIGIT neutralizing antibodies sensitized NK cells to secrete IFN-γ (Fig. 2c, right). Although NK cells responded poorly to MCF7 cells, the addition of CD112R and TIGIT blocking mAbs was able to induce IFN-γ production in NK cells (Fig. 2d). Thus our findings suggest that PVR-like receptors are important for NK cell cytokine production against human breast cancer cells.
Fig. 2.
PVR-like receptors are involved in NK cell functions against human breast cancer cells. a The expression of CD112 and CD155 ligands on breast cancer cell lines MDA, SK-BR3, and MCF7 were detected by staining with isotype control (red), CD112 or CD155 mAbs. b–f The involvement of PVR-like receptors in NK cell activities against human breast cancer cells. Purified NK cells from healthy donors were cultured with MDA (b, e), SK-BR3 (c, f), or MCF7 (d) cancer cell lines at a ratio of 2.5:1. Different neutralizing antibodies as indicated were added from the beginning of culture. The percentages of IFN-γ-positive and CD107a-expressing NK cells were determined by flow cytometry. g, h CFSE-labeled MDA cancer cells were cultured with human NK cells overnight, with the presence of different neutralizing mAbs as indicated. MDA tumor cells were identified by CFSE staining, and the killing of tumor cells was analyzed by PI staining. All data shown are representative of at least three independent experiments with different donor cells. Data showed mean ± SD and were analyzed by Student’s t test. *P < 0.05, **P < 0.005, ****P < 0.0001
Consistently, when we examined NK cell cytotoxicity, blockade of CD226 signal significantly reduced human NK cells to express CD107a, with the presence of either MDA or SK-BR3 tumor cells. On the other hand, TIGIT or CD112R blockade alone increased the percentages of CD107a-expressing NK cells, and inclusion of both TIGIT and CD112R mAbs was able to further enhance this effect (Fig. 2e, f). To directly measure tumor killing by NK cells, we labeled tumor cells with CFSE before co-culturing with NK cells. Tumor cells were distinguished from NK cells by CFSE staining and tumor killing was revealed by PI staining of the dead cells. As shown in Fig. 2g, h inclusion of the TIGIT and CD112R neutralizing antibodies was able to promote NK cell killing against MDA cells. Taken together, our results suggest that blockade of CD112R and/or TIGIT cooperatively enhanced NK cell activities in response to tumor cells.
PVR-like ligands on breast cancer cells induce TIGIT internalization on NK cells
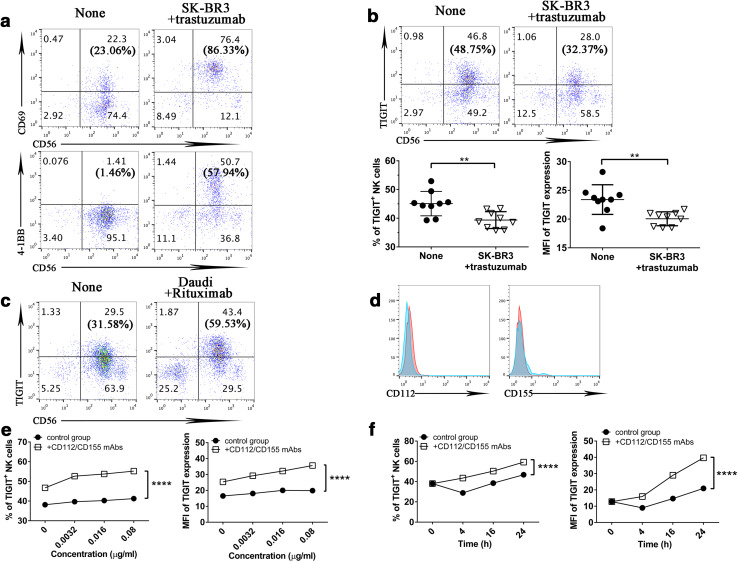
ADCC is one of the therapeutic mechanisms for trastuzumab, and NK cells are the major cell type involved in this process. We reasoned that blockade of the PVR-like inhibitory receptors on NK cells can promote trastuzumab-triggered ADCC by human NK cells. First, we examined PVR-like receptor expression on NK cells upon trastuzumab stimulation. We incubated purified NK cells with trastuzumab-coated SK-BR3 cells overnight. This incubation resulted in dramatic CD69 and 4-1BB upregulation [6], demonstrating that trastuzumab-coated SK-BR3 activated NK cells via ADCC (Fig. 3a). When we examined the expression of PVR-like receptors, to our surprise, we did not find any TIGIT upregulation on NK cells in response to trastuzumab-triggered ADCC (Fig. 3b). This contrasted greatly with what we saw in cytokine-activated NK cells (Fig. 1c). This phenomenon does not seem to be unique to ADCC-triggered NK cell activation, because we saw clear TIGIT upregulation on NK cells in response to Rituximab (anti-CD20)-coated Daudi cells (Fig. 3c), a CD20-positive human B cell lymphoma [19].
Fig. 3.
PVR-like ligands on breast cancer cells induce TIGIT internalization on NK cells. a, b NK cells were incubated alone or with trastuzumab and SK-BR3 cells overnight. a The expression of CD69 and 4-1BB on NK cells (CD56+) were revealed by flow cytometry. b The expression of TIGIT on NK cells (CD56+), the percentage, and MFI of TIGIT on NK cells were determined by flow cytometry. Data were collected from nine independent experiments. The numbers in each bracket mean the percentage of CD69- or 4-1BB-positive expression on NK cells (CD56+). c NK cells were incubated alone or with Rituximab and Daudi cells overnight. The expression of TIGIT on NK cells (CD56+) was determined by flow cytometry. The numbers in each bracket mean the percentage of TIGIT-positive expression on NK cells (CD56+). d The expression of CD112 and CD155 on Daudi cells were detected by flow cytometry. e Purified NK cells were incubated for 16 h with different doses of trastuzumab and SK-BR3 cells. Neutralizing mAbs for ligands CD112 and CD155 were included at the beginning of the culture, and the percentage of TIGIT-positive and MFI of NK cells were analyzed by flow cytometry. Data were collected from three independent experiments. f Purified NK cells were incubated with 0.016 µg/ml trastuzumab and SK-BR3 cells for 4, 16, or 24 h. The percentage of TIGIT-positive and MFI of NK cells were determined by flow cytometry. Data were collected from three independent experiments. Data showed mean ± SD and were analyzed by two-way ANOVA test. **P < 0.005, ****P < 0.0001
We hypothesized that the presence of TIGIT ligands on breast cancer cells can affect the expression of TIGIT on NK cells. TIGIT ligands, CD155 and CD112, were highly expressed on SK-BR3 cells (Fig. 2a), but not on Daudi cells (Fig. 3d). The interaction between TIGIT ligands and TIGIT could lead to the internalization of TIGIT on NK cells. To confirm this, we added CD155- and CD112-blocking mAbs to the culture media from the beginning. When we evaluated TIGIT expression upon treatment with CD155 and CD112 mAbs, we consistently observed TIGIT upregulation on NK cells from different donors in response to trastuzumab stimulation. The presence of these mAbs could prevent TIGIT from internalization (Fig. 3e). With the presence of both CD155- and CD112-blocking mAbs, TIGIT expression was upregulated steadily in terms of either percentage or MFI of TIGIT-positive NK cells, with a peak at 24 h (Fig. 3f). Therefore, without blocking mAbs for TIGIT ligands, TIGIT upregulation on human NK cells was masked by internalization. Taken together, the data demonstrate that TIGIT is upregulated on activated NK cells, and can be affected by ligand-induced internalization.
Blockade of TIGIT and CD112R promotes NK cell functions triggered by trastuzumab
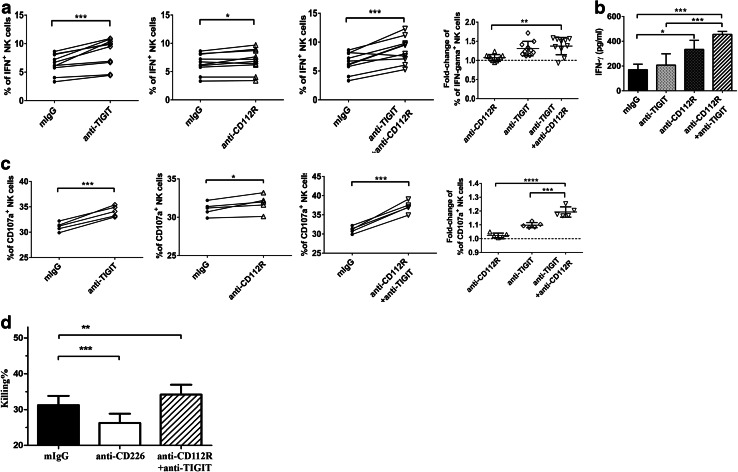
The TIGIT upregulation as well as internalization led us to further evaluate the function of the TIGIT/CD112R pathway on trastuzumab-triggered ADCC by NK cells. Purified NK cells were incubated with trastuzumab-coated SK-BR3 cells, and neutralizing mAbs against TIGIT, CD112R, or control mouse IgG at the beginning of the culture. We found that TIGIT or CD112R blockade alone resulted in significant increase in the percentages of IFN-γ-positive NK cells, comparing with those of NK cells blockaded by control mAb. Dual TIGIT and CD112R blockade further enhanced the percentages of IFN-γ-positive NK cells comparing to CD112R blockade alone (Fig. 4a). Consistently, when we measured IFN-γ secretion in the supernatant, we also found that blockade of CD112R and TIGIT was able to synergistically promote IFN-γ secretion by human NK cells in response to trastuzumab-triggered ADCC (Fig. 4b). These findings support that CD112R and/or TIGIT blockade increased NK cell cytokine production, in response to stimulus from trastuzumab-coated breast cancer cells.
Fig. 4.
Blockade of TIGIT and CD112R promotes NK cell functions triggered by trastuzumab. a Purified NK cells were cultured with trastuzumab and SK-BR3 cancer cells in the presence of different neutralizing antibodies as indicated. The percentages of IFN-γ-producing NK cells were examined by flow cytometry. Data were collected from 10 independent experiments. b Purified NK cells were cultured at the same condition as (a), and supernatant was collected to determine the level of IFN-γ by ELISA. Data shown are representative of three independent experiments. c Purified NK cells were cultured with trastuzumab and SK-BR3 cancer cell with the presence of different neutralizing mAbs as indicated. The percentages of CD107a-producing cells were determined by flow cytometry. d CFSE-labeled MDA cells were incubated with trastuzumab before being cultured with human NK cells overnight, with the presence of different neutralizing mAbs as indicated. MDA tumor cells were identified by CFSE staining, and the death of tumor cells was analyzed by PI staining. Data shown are representative of five independent experiments. Data showed mean ± SD and were analyzed by Student’s t test. *P < 0.05, ***P < 0.0005, ****P < 0.0001
The enhanced NK cell activation by TIGIT or CD112R blockade implies an enhanced antitumor effect. We examined the cytotoxicity activity of NK cells based on the expression of CD107a. TIGIT or CD112R blockade increases the percentages of CD107a-expressing NK cells, and inclusion of both TIGIT and CD112R mAbs was able to further enhance this effect (Fig. 4c). Consistently, when we analyzed tumor killing by NK cells directly, the inclusion of TIGIT and CD112R mAbs together was able to enhance trastuzumab-triggered tumor killing (Fig. 4d). Taken together, our results suggest that blockade of CD112R and TIGIT cooperatively enhanced NK cell activities in response to trastuzumab-triggered ADCC.
Discussion
Trastuzumab has been the standard therapy for patients with HER2/neu-positive breast cancer, but it is not effective against a large proportion of these patients due to resistances during the course of treatment [20]. Many strategies have been investigated to enhance the antitumor activity of trastuzumab. ADCC is a major mechanism of action for trastuzumab, and targeting stimulation of NK cells can enhance trastuzumab-mediated ADCC [6]. PVR-like receptors are a group of surface receptors that are known to be important for NK cell functions. We found that many PVR-like receptors are involved in trastuzumab-mediated ADCC by NK cells, and blockade of TIGIT and CD112R is able to enhance trastuzumab-triggered anti-breast cancer response. Thus, our findings imply a novel approach to improve trastuzumab efficacy in human breast cancer.
Our study found that TIGIT is preferentially expressed on CD16-positive NK cells, while CD112R, CD226, and CD96 are evenly expressed on CD16-positive and CD16-negative NK cells. Furthermore, cytokine-activated NK cells upregulate surface TIGIT, but not other PVR-like receptors. Interestingly, TIGIT upregulation can be masked by ligand internalization when human NK cells are activated by trastuzumab-coated human breast cancer, which implies a role of TIGIT in trastuzumab resistance. Consistent with that, blockade of TIGIT is able to further promote trastuzumab therapy against SK-BR3 and MDA, which are two HER2-positive human breast cancer cell lines. The addition of CD112R blocking mAb also enhances trastuzumab-triggered ADCC, and has a synergistic effect with TIGIT blockade. The addition of F(ab)2 forms of TIGIT and CD112R neutralizing antibodies was still able to promote NK cell cytotoxicity against MDA cells, excluding possible effects of FcR crosslinking (Supplemental Figure 2). Based on our knowledge, this is the first study that demonstrates a suppressive function for CD112R on NK cells.
Our studies also suggest that CD226 is one of the major stimulatory receptors for NK cells against human breast cancer with high expression levels of ligands, CD112 and CD155. Disrupting CD226 signal significantly reduces NK cell activities against both SK-BR3 and MDA tumor cells, regardless of the presence or absence of trastuzumab. On the other hand, blockage of TIGIT and/or CD112R increased NK cell cytokine production when NK cells were incubated with trastuzumab-coated breast cancer cells. It is unclear whether the mechanism of TIGIT/CD112R mAbs is to neutralize their own negative signal, or to negate their competition for ligand binding with CD226. Besides CD226, human NK cells utilize multiple NK cell receptors to kill breast cancer cells, including NKG2D (Natural Killer Group 2D), NKp30 (NK p30 receptor), and NKp46 (NK p46 receptor), [21]. Supporting that, our preliminary experiment supported that NKG2D could be involved in this aspect (Supplemental Figure 3). Therefore, it would be interesting to further evaluate the role of other NK receptors in antibody-triggered ADCC by NK cells [22].
Currently trastuzumab therapy is limited for breast cancer patients with high-level HER2 expression. It would be interesting to see whether blockade of PVR-like inhibitors can sensitize breast cancer with low-level HER2 expression for trastuzumab therapy. In this case, the application of PVR-like checkpoint inhibitors will expand the application of trastuzumab in cancer therapy. It is also unclear how effective the combinatory therapy of TIGIT/CD112R blockade and trastuzumab will be in human breast cancer therapy in vivo. Adaptive immunity, mainly mediated by T cells, is known to be essential for trastuzumab therapy of breast cancer [23, 24]. Besides NK cells, TIGIT and CD112R are also known to be key checkpoints for T cells [17, 25]. Although our studies here only address the role of NK cell-mediated antitumor response in trastuzumab therapy, the effect of PVR-like checkpoint inhibitor on promoting T cell-mediated adaptive antitumor immunity remains to be investigated.
Taken together, our studies demonstrate that PVR-like receptors are involved in NK cell activity against human breast cancers. More importantly, our findings advocate the application of TIGIT and CD112R blockade in combination with trastuzumab therapy to treat breast cancer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work is partly supported by American Cancer Society Institutional Research Grant Number 57-001-53 and Cancer League of Colorado 163479.
Abbreviations
- ADCC
antibody-dependent cellular cytotoxicity
- APC
allophycocyanin
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- IgSF
immunoglobulin superfamily
- LEAF
low endotoxin, azide-free
- MDA
MDA-MB-453
- NKG2D
Natural Killer Group 2D
- NKp30
NK p30 receptor
- NKp46
NK p46 receptor
- PBMCs
peripheral blood mononuclear cells
- PI
propidium iodide
- PVR
poliovirus receptor
- SEM
standard error of measurement
- TIGIT
T cell Ig and ITIM domain
Compliance with ethical standard
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Sjogren S, Inganas M, Lindgren A, Holmberg L, Bergh J. Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol. 1998;16(2):462–469. doi: 10.1200/JCO.1998.16.2.462. [DOI] [PubMed] [Google Scholar]
- 2.Andrulis IL, Bull SB, Blackstein ME, et al. Neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. Toronto Breast Cancer Study Group. J Clin Oncol. 1998;16(4):1340–1349. doi: 10.1200/JCO.1998.16.4.1340. [DOI] [PubMed] [Google Scholar]
- 3.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 4.Madarnas Y, Trudeau M, Franek JA, McCready D, Pritchard KI, Messersmith H. Adjuvant/neoadjuvant trastuzumab therapy in women with HER-2/neu-overexpressing breast cancer: a systematic review. Cancer Treat Rev. 2008;34(6):539–557. doi: 10.1016/j.ctrv.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the treatment of breast cancer. Annu Rev Med. 2015;66:111–128. doi: 10.1146/annurev-med-042513-015127. [DOI] [PubMed] [Google Scholar]
- 6.Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, Colevas AD, Weng WK, Clarke MF, Carlson RW, Stockdale FE, Mollick JA, Chen L, Levy R. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest. 2012;122(3):1066–1075. doi: 10.1172/JCI61226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Petricevic B, Laengle J, Singer J, Sachet M, Fazekas J, Steger G, Bartsch R, Jensen-Jarolim E, Bergmann M. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J Transl Med. 2013;11:307. doi: 10.1186/1479-5876-11-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall PS, Cameron DA. Current perspective—trastuzumab. Eur J Cancer. 2009;45(1):12–18. doi: 10.1016/j.ejca.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 10.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan CJ, Andrews DM, Smyth MJ. Receptors that interact with nectin and nectin-like proteins in the immunosurveillance and immunotherapy of cancer. Curr Opin Immunol. 2012;24(2):246–251. doi: 10.1016/j.coi.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9(8):603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- 13.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, Eaton D, Grogan JL. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 14.Chan CJ, Martinet L, Gilfillan S, Souza-Fonseca-Guimaraes F, Chow MT, Town L, Ritchie DS, Colonna M, Andrews DM, Smyth MJ. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat Immunol. 2014;15(5):431–438. doi: 10.1038/ni.2850. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs A, Cella M, Giurisato E, Shaw AS, Colonna M. Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155) J Immunol. 2004;172(7):3994–3998. doi: 10.4049/jimmunol.172.7.3994. [DOI] [PubMed] [Google Scholar]
- 16.Seth S, Maier MK, Qiu Q, Ravens I, Kremmer E, Forster R, Bernhardt G. The murine pan T cell marker CD96 is an adhesion receptor for CD155 and nectin-1. Biochem Biophys Res Commun. 2007;364(4):959–965. doi: 10.1016/j.bbrc.2007.10.102. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Y, Paniccia A, Schulick AC, Chen W, Koenig MR, Byers JT, Yao S, Bevers S, Edil BH. Identification of CD112R as a novel checkpoint for human T cells. J Exp Med. 2016;213(2):167–176. doi: 10.1084/jem.20150785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N, Vitale M, Moretta L, Lopez M, Moretta A. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198(4):557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tokunaga T, Tomita A, Sugimoto K, Shimada K, Iriyama C, Hirose T, Shirahata-Adachi M, Suzuki Y, Mizuno H, Kiyoi H, Asano N, Nakamura S, Kinoshita T, Naoe T. De novo diffuse large B-cell lymphoma with a CD20 immunohistochemistry-positive and flow cytometry-negative phenotype: molecular mechanisms and correlation with rituximab sensitivity. Cancer Sci. 2014;105(1):35–43. doi: 10.1111/cas.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad S, Gupta S, Kumar R, Varshney GC, Raghava GP. Herceptin resistance database for understanding mechanism of resistance in breast cancer patients. Sci Rep. 2014;4:4483. doi: 10.1038/srep04483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mamessier E, Sylvain A, Bertucci F, Castellano R, Finetti P, Houvenaeghel G, Charaffe-Jaufret E, Birnbaum D, Moretta A, Olive D. Human breast tumor cells induce self-tolerance mechanisms to avoid NKG2D-mediated and DNAM-mediated NK cell recognition. Cancer Res. 2011;71(21):6621–6632. doi: 10.1158/0008-5472.CAN-11-0792. [DOI] [PubMed] [Google Scholar]
- 22.Ochoa MC, Minute L, Rodriguez I, Garasa S, Perez-Ruiz E, Inoges S, Melero I, Berraondo P. Antibody-dependent cell cytotoxicity: immunotherapy strategies enhancing effector NK cells. Immunol Cell Biol. 2017;95(4):347–355. doi: 10.1038/icb.2017.6. [DOI] [PubMed] [Google Scholar]
- 23.Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, Sattar H, Wang Y, Brown NK, Greene M, Liu Y, Tang J, Wang S, Fu YX. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18(2):160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaganty BK, Lu Y, Qiu S, Somanchi SS, Lee DA, Fan Z. Trastuzumab upregulates expression of HLA-ABC and T cell costimulatory molecules through engagement of natural killer cells and stimulation of IFNgamma secretion. Oncoimmunology. 2016;5(4):e1100790. doi: 10.1080/2162402X.2015.1100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston RJ, Yu X, Grogan JL. The checkpoint inhibitor TIGIT limits antitumor and antiviral CD8+ T cell responses. Oncoimmunology. 2015;4(9):e1036214. doi: 10.1080/2162402X.2015.1036214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.