Abstract
Adolescence is a unique developmental period when the salience of social and emotional information becomes particularly pronounced. Although this increased sensitivity to social and emotional information has frequently been considered with respect to risk behaviors and psychopathology, evidence suggests that increased adolescent sensitivity to social and emotional cues may confer advantages. For example, greater sensitivity to shifts in the emotions of others is likely to promote flexible and adaptive social behavior. In this study, a sample of 54 children and adolescents (age 8–19 years) performed a delayed match-to-sample task for emotional faces while undergoing fMRI scanning. Recruitment of the anterior cingulate and anterior insula when the emotion of the probe face did not match the emotion held in memory followed a quadratic developmental pattern that peaked during early adolescence. These findings indicate meaningful developmental variation in the neural mechanisms underlying sensitivity to changes in the emotional expressions. Across all participants, greater activation of this network for changes in emotional expression was associated with less social anxiety and fewer social problems. These results suggest that the heightened salience of social and emotional information during adolescence may confer important advantages for social behavior, providing sensitivity to others' emotions that facilitates flexible social responding.
1 | Introduction
The ability to encode, interpret, and hold in mind facial expressions is a critical aspect of non-verbal social interaction that is necessary for planning and guiding behavior for successful interactions with others (Neta & Whalen, 2011). For instance, an individual may adjust their behavior when conversing with one who at first appears neutral but whose facial expression suddenly becomes angry.
Adolescence is a unique period of development in which emotional responses to social information are particularly intense, suggesting that certain types of social information are more salient during adolescence. This has been shown in a variety of different contexts, including reward and threat processing, self-conscious emotion, and social reinforcement (Casey, Jones, & Hare, 2008; Galvan, Hare, Voss, Glover, & Casey, 2007; Jones et al., 2014; Somerville, Jones, & Casey, 2010; Spielberg, Olino, Forbes, & Dahl, 2014). In addition, adolescence is a period of development in which peer relationships become increasingly important (Blakemore, 2008; Steinberg, 2005). During this period, fears and anxieties about social interactions, social rejection, and social status begin to emerge (Haller, Cohen Kadosh, & Lau, 2013).
A rich literature highlights the adverse effects of the imbalance of heightened social, affective, and reward sensitivities with immature cognitive control in adolescence (Steinberg 2005; Casey, Jones, & Somerville, 2011), including risk-taking, sensitivity to peer-pressure, and sensation seeking behavior (Dreyfuss et al., 2014; Heller & Casey, 2016). However, recent work has suggested that certain emotional sensitivities may also confer advantages during adolescence. It has been suggested that the increase in social and affective sensitivity that emerges during adolescence facilitates the ability to flexibly update behavior and adapt to one's environment (Crone & Dahl, 2012; Pfeifer & Allen, 2012). Recent studies have suggested that neural and behavioral sensitivity to reward in adolescence is associated with better response inhibition and lower risk-seeking and susceptibility to peer pressure in certain contexts (Pfeifer et al., 2011; Telzer, Ichien, & Qu, 2015). In a similar vein, it is possible that sensitivity to the emotions of others during adolescence is protective against the development of social difficulties. If changes in the emotional expressions of others are salient and capture attention, this may result in being better able to update and modify behavior accordingly for more successful social outcomes in the increasingly complex social environment of adolescence (Crone & Dahl, 2012; Nelson & Guyer, 2011; Pfeifer & Allen, 2012).
In the present study, youths performed a delayed match-to-sample task using emotional faces while undergoing fMRI scanning. To determine the developmental pattern of neural activation in response to changes in emotional facial expression, we tested three potential models of age-related change (Somerville et al., 2013). These included a linear model testing a stable change in activation from childhood into late adolescence, a logarithmic model, reflecting change in activation from childhood to early adolescence followed by a leveling off in late adolescence (adolescent-emergent), and a quadratic model indicating activation occurring uniquely during a particular point in development (e.g., an adolescent-specific response). Given that sensitivity to social and affective information peaks during early adolescence (McLaughlin, Garrad, & Somerville, 2015; Nelson, Lau, & Jarcho, 2014), we hypothesized that early adolescents would be particularly sensitive to changes in emotional facial expression from encoding to probe. Adult studies have highlighted a role of the salience network, including the anterior cingulate (ACC) and anterior insula in detecting changes in the emotional expression of others (Luo et al., 2014). Therefore, we anticipated that recruitment of a network of regions involved in salience processing, including the ACC and anterior insula (Seeley et al., 2007), would emerge when processing changes in emotional facial expression. We expected that recruitment of this network of regions would follow a quadratic pattern across development (i.e., adolescent-specific) when the emotional expression changed, given the heightened salience of social and emotional information in adolescence (McLaughlin et al., 2015; Nelson et al., 2014), but not when the identity of the person changed. Finally, we hypothesized that greater activity in the salience network during changes in emotional expression would be associated with lower levels of social problems and social anxiety because greater sensitivity to shifts in the emotions of others is likely to promote flexible and adaptive social behavior.
2 | Materials and Methods
2.1 | Participants
A sample of 66 participants aged 6 to 19 years (M = 13.68 years, SD = 3.23 years; 35 male) without MRI contraindications (e.g., orthodontic braces) participated. The sample was recruited in Seattle, WA between February 2014 and February 2015. Youths were recruited at schools, after-school and prevention programs, medical clinics, and in the general community. The sample size was determined prior to any data collection and was based on the largest number of participants possible given the grant funding that supported the study. This sample size is moderate to large for a developmental cognitive neuroscience study. Half of the sample was recruited based on exposure to interpersonal violence in order to test additional questions about how environmental experiences are associated with neural processes involved in memory for emotional information (Lambert et al., 2017). We controlled for violence exposure by including it as a binary covariate of non-interest in all models of fMRI data in the present analyses. Results were identical with and without this covariate, but we retain it in all final models. There were no effects of violence exposure on the contrast of interest in this study (Emotion Mismatch > Match).
The study sample was racially and ethnically diverse (53.5% White, 6.25% Black, 14.55% Hispanic, 2.1% Asian, 23.6% Multiracial or Other) and varied with regard to parental socioeconomic status (Maximum parental educational attainment: Less than high school: 9.1%, High school degree: 18.2%, Some college: 12.12%, College degree or higher: 21.2%, Graduate degree: 33.3%, No report: 6.1%). The Institutional Review Board at the University of Washington approved all procedures. Participants were compensated and written informed consent was obtained from legal guardians, while youths provided written assent.
Eight participants (6 female, mean age: 10.23±3.26) were excluded from analyses due to below-chance performance. One participant (female, 15 years) was excluded due to an incidental neurological finding, and one participant (female, 8 years) did not complete the tasks in the scanner. The excluded participants were significantly younger than the final analytic sample (t(63) = 4.239, p < .0001). The final analytic sample included 54 participants (8-19 years, M = 14.26±2.83, see Supplemental Table 1 for details).
2.2 | Delayed- match- to- sample task
Participants completed an emotional face working memory task (Figure 1a) using a delayed-match-to-sample design. All stimuli were faces drawn from a standardized stimulus set (Tottenham et al., 2009). Stimuli were neutral, happy, and angry faces, distributed evenly across trials and presented in a counter-balanced order across participants. Participants were instructed to attend and respond to the faces and their emotional expressions. The delayed-match-to-sample (DMS) task consisted of two runs of 50 trials. Each trial involved a Cue (2000 ms), Delay (1000 or 5000 ms), and Probe (2000 ms) and an inter-trial interval (ITI) of 500 ms (67% of trials) or 2000 ms (33% of trials). Each actor was presented 6-7 times for each facial expression. During the Cue phase, facial stimuli were embedded in realistic background scenes. This made encoding more similar to real-world facial encoding. During the Probe phase, an image of a face without a background scene was presented, and participants were asked to indicate whether the Probe face matched the Cue face. On one-third of trials, the Probe face presented matched the Cue (i.e., was the same person showing the same emotion) and on the other two-thirds of trials, the Probe did not match the Cue. There were two types of mismatches: Emotion Mismatch (same person, different emotion), Identity Mismatch (different person). Each subject had 30 trials of each probe type (16-17 per run, for a total of 100 trials; Figure 1a). All trial types were interspersed throughout the two task runs. Subjects had the same instructions for all trial types and were not aware ahead of time whether the trial would be a Match, Emotion Mismatch, or Identity Mismatch. Subjects completed two runs of the working memory task, with the exception of one subject that completed only one run. The data from the subject who only completed one run was still included in analyses to maximize the sample size.
Figure 1.
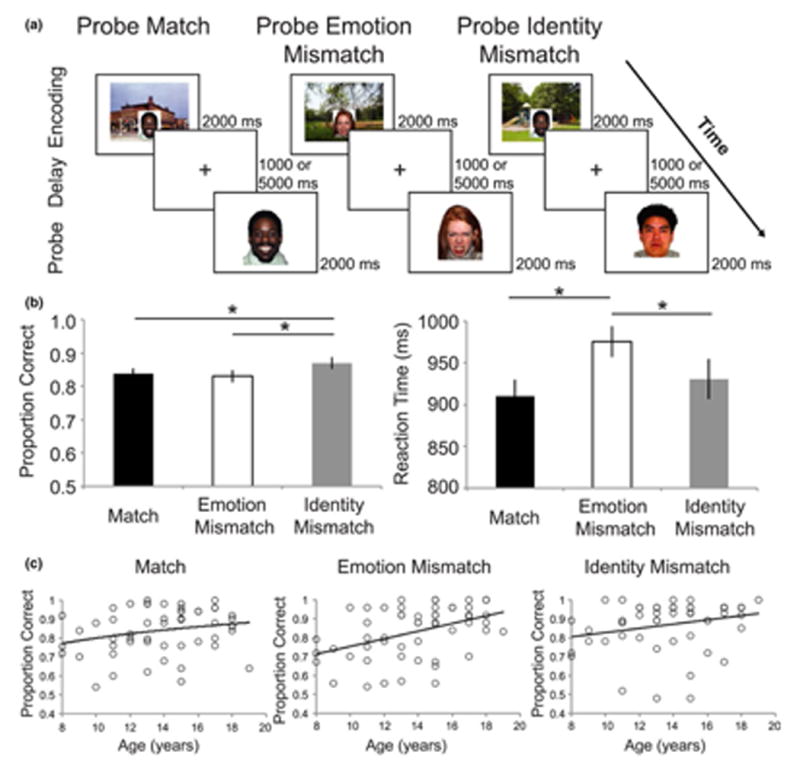
Delayed-Match-to-Sample Task and Performance. (a) Subjects were presented with a face embedded in a scene and instructed to hold the face in memory over a variable delay period. On one-third of trials, the probe face matched the identity and emotion of the cue face (Match). On one-third of trials, the probe face matched the identity but was a mismatch on emotion (Emotion Mismatch). On one-third the face was a mismatch on identity (Identity Mismatch). The Identity Mismatch trials contained trials where the probe face portrayed the same emotion as the cue as well as trials where the probe face portrayed a different emotion than the cue. (b) Accuracy and reaction time varied as a function of probe type such that accuracy was highest for Identity Mismatch trials and response time was highest for Emotion Mismatch trials. * represents post-hoc tests with p < .05, FDR corrected C) Accuracy varied as a function of age (linearly) most strongly for Emotion Mismatch trials
2.3 | Social functioning
To assess social problems, children and adolescents completed the Youth Self Report (YSR) of the Child Behavior Check List (CBCL; Achenbach, Howell, Quay, & Conners, 1991). The social problems subscale of the YSR is a psychometrically sound measure of social behavior and has been validated and shown to have internal consistency, high test-retest reliability, and stability (Achenbach et al., 1991). It consists of 11 items for which participants are presented with a statement (e.g., ‘I'm too dependent on adults’, ‘I feel lonely’, ‘I feel that others are out to get me’) and are asked to rate the truth of the statement on a 3-point Likert scale. One participant (13-year-old female) failed to complete the YSR due to time constraints and was removed from analyses that included the YSR.
As a measure of social anxiety, participants completed the Screen for Child Anxiety and Related Disorders (SCARED; Birmaher et al., 1997, 1999), a psychometrically sound measure of anxiety symptoms. This measure has 41 items for which participants are presented with a statement (e.g., ‘I don't like to be with people I don't know well’) and are asked to rate how much they agree with the statement on a 3-point Likert scale. We used the social phobia subscale, which is commonly used in both children and adolescence (Lewis-Morrarty et al., 2012; Silverman & Ollendick, 2005) and demonstrates good test-retest reliability and convergent and discriminant validity (Birmaher et al., 1997, 1999). The scale includes seven items, for a maximum possible score of 21. We also used the SCARED sub-scale assessing panic disorder as a non-social control measure of anxiety to determine whether associations of neural function with anxiety were specific to anxiety surrounding social interactions.
Participants exposed to violence had significantly higher levels of social problems (57.44±6.32) compared to those not exposed to violence (53.84±5.75, t(51) = 2.164, p = .03), as well as higher levels of social anxiety (5.43±3.56 and 3.65±3.21, respectively,, t(52) = 2.208, p = .03).
2.4 | Image acquisition and processing
Before undergoing scanning, children 12 years and younger and any older children exhibiting anxiety about the scan were trained to minimize head movements in a mock scanner. They watched a movie with a head-mounted motion tracker that stopped playing if a movement of over 2 mm occurred. This method has been shown to significantly reduce head motion once children are in the scanner (Raschle et al., 2012). In addition, in the scanner, we used a head-stabilizing pillow to further restrict movement.
Scanning was performed on a 3T Phillips Achieva scanner at the University of Washington Integrated Brain Imaging Center using a 32-channel head coil. T1-weighted multi-echo MPRAGE volumes were acquired (TR= 2530 ms, TE = 1.64-7.04 ms, flip angle = 7°, FOV = 256mm2, 176 slices, in-plane voxel size = 1 mm3). Blood oxygenation level dependent (BOLD) signal during functional runs was acquired using a gradient-echo T2*-weighted EPI sequence. Thirty-two 3-mm thick slices were acquired parallel to the AC-PC line (TR = 2000 ms, TE = 30ms, flip angle = 90°, bandwidth = 2300, echo spacing = 0.5, FOV = 256 × 256, matrix size = 64 × 64). Prior to each scan, four images were acquired and discarded to allow longitudinal magnetization to reach equilibrium.
2.4.1 | fMRI pre- processing
Pre-processing and statistical analysis of fMRI data was performed in a pipeline using Make, a software development tool designed for describing how to build executables from source files that can be used to create neuroimaging workflows that rely on multiple software packages (Askren et al., 2016). Simultaneous motion and slice-time correction was performed in NiPy (Roche, 2011). Spatial smoothing with a Gaussian kernel (6-mm full width at half maximum [FWHM]) was performed in FSL (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012). Data were inspected for artifacts, and volumes with motion >2-mm or >3-SD change in signal intensity were excluded from analysis using volume-specific covariates of non-interest. All but two subjects (one male, 12 years and one female, 9 years) had very little motion; those with the highest motion had fewer than 10% of volumes with framewise displacement outliers across both runs, with the next highest being 3.6% of volumes with framewise displacement outliers. Six rigid-body motion regressors were included in person-level models. A component-based anatomical noise correction method (Behzadi, Restom, Liau, & Liu, 2007) was used to reduce noise associated with physiological fluctuations. Person- and group-level models were estimated in FSL. Following estimation of person-level models, the resulting contrast images were normalized into standard space, and anatomical co-registration of the functional data with each participant's T1-weighted image was performed using surface-based registration in FreeSurfer version 5.3 (Dale, Fischl, & Sereno, 1999), which provides better alignment than other methods in children (Ghosh et al., 2010). Normalization was implemented in Advanced Normalization Tools (ANTs) software, version 2.1.0 (Avants et al., 2011).
2.5 | Statistical analysis
2.5.1 | Behavioral data
Accuracy and response time (RT) on the working memory task were examined as a function of age separately by probe status (Match, Emotion Mismatch, Identity Mismatch). Variation in accuracy and response time (RT) by probe status was examined with repeated-measures ANOVA with probe status (Match, Emotion Mismatch, Identity Mismatch) as a within-subjects factor. We additionally investigated linear, logarithmic, and quadratic models of age to investigate the association between age and accuracy and RT for each probe type. A false-discovery rate (FDR) correction was applied to all behavioral analysis and brain-behavior association analysis.
2.5.2 | fMRI
FMRI data processing was performed using FEAT (FMRI Expert Analysis Tool) Version 6.00, part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). Regressors were created by convolving a boxcar function of phase duration with the standard double-gamma hemodynamic response function for each of the Cue and Delay periods and the Probe period by probe type (Match, Emotion Mismatch, Identity Mismatch). A general linear model (GLM) was constructed for each participant. Higher-level analysis was carried out using FLAME (FMRIB's Local Analysis of Mixed Effects) in FSL (Woolrich, Behrens, Beckmann, Jenkinson, & Smith, 2004). Individual-level estimates of BOLD activity were submitted to group-level random effects models of probe Match, Emotion Mismatch, and Identity Mismatch periods using correct trials only, each compared to Baseline (ITI). We constructed additional contrasts for Emotion Mismatch > Match, Identity Mismatch > Match, and Emotion Mismatch > Identity Mismatch.
We apply a cluster-level correction approach that is associated with low risk of both false positive and false negative findings in recent simulations (see Eklund, Nichols, & Knutsson, 2016, Figure 1). Specifically, we apply cluster-level correction in FSL (z>2.3, cluster-defining threshold p<.01, minimum cluster size/k of 10 voxels) to our models run in FSL FLAME, which is not associated with elevated false positive or false negative findings in event-related designs. All analyses included a covariate of non-interest for violence exposure. Results were then projected onto the cortical surface for visualization purposes using Connectome Workbench (Washington University, St Louis; Marcus et al., 2013).
To investigate age-related effects, we created three separate models. In the first model, we included a mean-centered covariate for age to investigate linear age-related changes during all three probe types: Match, Emotion Mismatch and Identity Mismatch. In a separate model, we added a covariate for age2 in addition to age in order to investigate quadratic age effects. We created a third model in which we included a covariate for age by taking the log age of each participant and them mean-centering those values. These models allow us to probe for early adolescent-specific (quadratic) and early adolescent-emergent (logarithmic) effects (Somerville et al., 2013).
2.5.3 | Region of interest analysis
ROI analyses were conducted to relate activation during Emotion Mismatch trials with measures of social problems and social anxiety obtained from the YSR and SCARED. ROIs were created by masking functional activation from the model including the best-fitting age term for Emotion Mismatch > Match for regions with significant whole-brain developmental differences. This resulted in a region in the right anterior insula and bilateral anterior cingulate. Because the cluster in anterior cingulate cortex spanned both hemispheres, we divided the cluster into a left and right region of interest. Parameter estimates for the contrast of Emotion Mismatch > Match were extracted for these ROIs for each participant. Linear regression was performed using variation in activation in these ROIs with mental health and behavioral outcomes separately.
3 | Results
3.1 | Behavioral results
Overall mean accuracy was 0.843±0.015 SEM. A repeated measures ANOVA on accuracy was performed with probe type (Match, Emotion Mismatch, or Identity Mismatch, Figure 1b) as a within-subjects factor with lower-bound correction as Mauchly's test for sphericity was not met (p = .029). This revealed variation in accuracy based on probe type: F(1, 53) = 5.15 p = .027; Figure 1b). Post-hoc t-tests revealed significantly higher accuracy in the Identity Mismatch condition (Mean = 0.869±0.016) than both Emotion Mismatch (Mean = 0.830±0.019, t(53) = 2.92, p = .005, Cohen's d = 0.80), and Match (Mean = 0.837±0.016, t(53) = 2.15, p = .036, Cohen's d = 0.59), consistent with previous findings in an adult sample (Neta & Whalen, 2011). There was no difference in accuracy between the Match and Emotion Mismatch trials (p > .250). A repeated measures ANOVA on RT revealed a main effect of probe type: F(2, 106) = 9.72, p < .0001) Post-hoc t-tests revealed significantly slower RT for Emotion Mismatch trials (Mean = 952.5±25.1 ms) compared to both Identity Mismatch (Mean = 897.1±29.1 ms, t(53) = 2.67, p = .002, FDR corrected, Cohen's d = .734) and Match trials (Mean = 888.47±23.44 ms, t(53) = 5.01, p < .0001, FDR corrected, Cohen's d = 1.38) and no significant RT difference for Identity Mismatch and Match trials (p > .250, FDR corrected).
In addition, we investigated linear, logarithmic, and quadratic effects of age on accuracy and RT for each probe contrast (Figure 1c). There was a non-significant logarithmic effect of age on accuracy for Match trials (β = .24, p = .079) and a non-significant linear association between age and accuracy on Identity Mismatch trials (β = .25, p = .079). Accuracy for Emotion Mismatch trials was related to age and the best-fitting model was linear (β = 0.43, p = .003). In contrast, age and RT showed a negative logarithmic association for Match and Identity Mismatch (β = −0.44, p = .003 and β = −0.41, p = .003, respectively), but a non-significant negative linear association for Emotion Mismatch (β = .24, p = .078). Together, these findings suggest that the Emotion Mismatch trials contain the most conflict overall, as evident by the longer RT, and accuracy increases into adolescence. All p-values are FDR corrected.
3.2 | Social functioning
The mean score for the SCARED social anxiety scale was 4.44±0.48 SEM (range 0-12), with a positive linear association with age (β = .27, p = .047, Cohen's f2 = 0.080), while quadratic and logarithmic models were not significant (β = .27, p = .051 and β = .24, p = .078, respectively). The mean score for the YSR social problems scale was 55.67±0.87 SEM (range 50-70), with non-significant association with age (linear association: β = .24, p = .083, logarithmic: logarithmic: β = .21, p = .129, quadratic: β = .28, p = .126).
3.3 | Neural response to probe mismatches
Neural response to Emotion Mismatches and Identity Mismatches compared to Matches in the entire sample are shown in Figure 2. The pattern of activation observed here is consistent with prior findings in adults from a similar task (Lopresti et al., 2008; Neta & Whalen, 2011). Emotion Mismatches elicited greater BOLD response in the right postcentral sulcus and superior parietal lobule, bilateral middle frontal gyrus and inferior frontal gyrus, bilateral medial superior frontal sulcus and ACC, and bilateral superior temporal sulcus than matches (Figure 2a, Table 1). The contrast of Identity Mismatch > Match resulted in some overlapping regions as those for Emotion Mismatches including bilateral middle frontal gyrus, and right postcentral sulcus (Figure 2b, Table 2). This contrast also revealed activation in the precuneus, angular gyrus and temporal pole.
Figure 2.
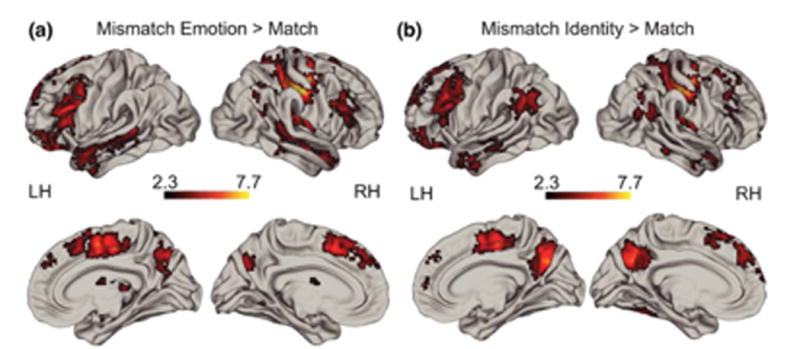
Whole group whole brain effects of Emotion Mismatch > Match (a) and Identity Mismatch > Match (b). See Tables 1 and 2 for details
Table 1. Whole group results. MNI coordinates reflect the peak of each cluster.
| Emotion Mismatch > Match | ||||||
|---|---|---|---|---|---|---|
| Anatomical region | x | y | Z | voxels | z- max | p- value |
| Left inferior frontal gyrus Middle frontal gyrus Frontal pole Posterior superior temporal gyrus |
−48 | 22 | 24 | 3578 | 5.61 | <.0001 |
| Right postcentral gyrus Precentral gyrus Superior parietal lobule |
36 | −26 | 54 | 3124 | 9.45 | <.0001 |
| Right thalamus Putamen Central opercular cortex Insula |
16 | −20 | 6 | 2272 | 6.09 | <.0001 |
| Bilateral supplemental motor cortex Superior frontal gyrus Paracingulate cortex Anterior cingulate cortex |
6 | −4 | 48 | 2222 | 6.60 | <.0001 |
| Cerebellum | −16 | −54 | 20 | 1170 | 9.42 | <.0001 |
| Right middle frontal gyrus Inferior frontal gyrus |
44 | 18 | 28 | 667 | 4.36 | <.0001 |
| Right superior temporal gyrus/sulcus Middle temporal gyrus Supramarginal gyrus Temporal pole |
52 | −36 | 4 | 501 | 4.49 | <.0001 |
| Bilateral precuneus Cuneus |
2 | −68 | 40 | 586 | 4.60 | <.0001 |
| Left superior parietal lobule Lateral occipital cortex Angular gyrus Supramarginal gyrus |
−34 | −58 | 42 | 274 | 4.87 | <.0001 |
Table 2. Whole group results. MNI coordinates reflect the peak of each cluster.
| Identity Mismatch > Match | ||||||
|---|---|---|---|---|---|---|
| Anatomical region | x | y | z | voxels | z- max | p- value |
| Right postcentral gyrus Precentral gyrus Superior frontal gyrus Superior parietal lobule |
42 | −24 | 52 | 2914 | 9.85 | <.0001 |
| Bilateral medial precentral gyrus Superior frontal Precentral gyrus Frontal pole Anterior cingulate |
8 | −16 | 48 | 1738 | 6.32 | <.0001 |
| Bilateral precuneus Posterior cingulate cortex |
2 | −64 | 30 | 1631 | 7.52 | <.0001 |
| Left middle frontal gyrus Inferior frontal gyrus |
−46 | 26 | 26 | 1584 | 5.02 | <.0001 |
| Cerebellum Fusiform cortex |
−14 | −52 | 18 | 1548 | 8.87 | <.0001 |
| Right putamen Central operculum cortex Insula |
32 | −8 | 0 | 1132 | 6.69 | <.0001 |
| Left lateral occipital cortex Angular gyrus Supramarginal gyrus |
−42 | −66 | 42 | 990 | 5.14 | <.0001 |
| Right angular gyrus Lateral occipital cortex |
50 | −58 | 24 | 505 | 4.81 | <.0001 |
| Right cerebellum | 36 | −76 | −30 | 491 | 3.70 | <.0001 |
| Right thalamus | 16 | −20 | 4 | 344 | 6.23 | <.0001 |
| Left orbital frontal cortex | −48 | 30 | −10 | 313 | 3.90 | <.0001 |
| Left anterior middle temporal gyrus Superior temporal gyrus/sulcus |
−62 | −4 | −18 | 260 | 3.95 | <.0001 |
| Right middle temporal gyrus Superior temporal gyrus/sulcus |
−64 | −26 | −4 | 222 | 3.87 | .0001 |
| Right middle frontal gyrus Inferior frontal gyrus |
44 | 30 | 34 | 204 | 3.96 | .0003 |
| Right temporal pole Middle temporal gyrus Superior temporal gyrus |
46 | 12 | −26 | 159 | 3.43 | .0023 |
| Right posterior middle temporal gyrus | 60 | −38 | −8 | 107 | 3.50 | .0367 |
The direct contrast between Emotion Mismatch and Identity Mismatch revealed a significant cluster in the left anterior intraparietal sulcus (x = −50, y = −28, z = 46, max z-statistic = 4.04, p < .0001, 322 voxels), bilateral dorsal anterior cingulate / supplementary motor area (x = 0, y = 14, z = 58, max z-statistic = 3.6, p = .0002, 220 voxels; Supplemental Figure 1).
3.4 | Age- related associations for probe mismatches
We next examined whether age-related variation was present during different types of mismatches. MNI coordinates reflect the peak of each cluster.
3.4.1 | Age associations during Emotion Mismatches
There were no significant positive or negative linear age effects or logarithmic age effects for Emotion Mismatch vs. Match. Using a quadratic predictor of age, while controlling for linear age effects, revealed a significant early adolescent-specific effect for Emotion Mismatch > Match in right anterior insula (x = 48, y = 14, z = −2, max z-statistic = 3.41, p = .0003, 167 voxels) and bilateral ACC (x = −8, y = 38, z = 20; max z-statistic = 3.23, p = .0064,148 voxels; see Figure 3).
Figure 3.
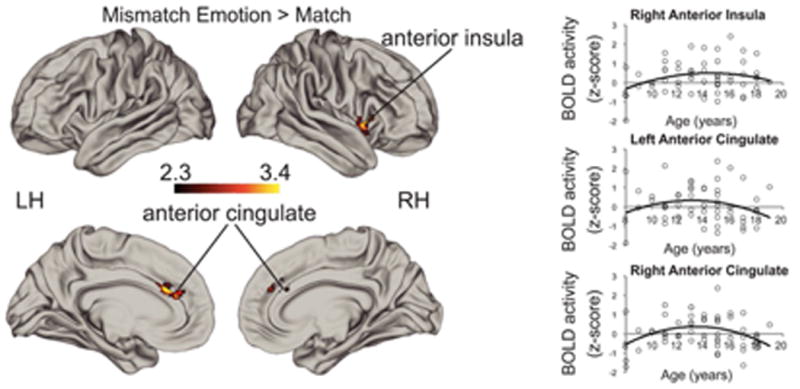
Heightened adolescent recruitment of the ACC and right anterior insula for Emotion Mismatch > Match. Scatterplots show activation in regions with significant early adolescent-specific effects in the whole sample as a function of age simply for illustrative purposes in order to show the direction of the quadratic effect
We further examined whether the quadratic age effect varied by condition in an ROI analysis using anatomical definitions (Harvard-Oxford Cortical Atlas, 20% threshold) of ACC and insula. This analysis revealed a significant quadratic age × condition interaction for the right insula and ACC (see Supplemental Materials for details).
3.4.2 | Age associations during Identity Mismatches
In contrast to quadratic age effects for Emotion Mismatches, a positive linear age effect of Identity Mismatch > Match emerged in the left cerebellum (x = −14, y = −62, z = −30, max z-statistic = 3.87, p < .0001, 835 voxels), bilateral posterior cingulate cortex (x = 10, y = −44, z = 36, max z-statistic = 3.74, p < .0001, 353 voxels), bilateral cuneus (x = −8, y = −80, z = 28, max z-statistic = 3.54, p = .0004,191 voxels; x = 12, y = −78, z = 30, max z-statistic = 2.55, 153 voxels), right superior temporal sulcus and angular gyrus (x = 44, y = −50, z = 22, max z-statistic = 3.36, p = .030, 112 voxels), and right medial PFC (x = 10, y = 44, z = −8, max z-statistic = 3.62, p = .040, 102 voxels). See Figure 4 for details. No significant logarithmic or quadratic age effects emerged.
Figure 4.
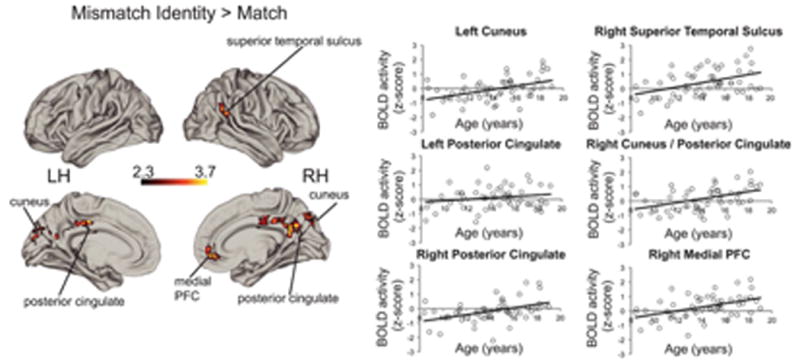
Linear age associations for Identity Mistmach > Match. Scatterplots show activation in regions with significant linear age-related increases in the whole sample as a function of age simply for illustrative purposes
There were no significant associations with age for the direct contrasts of Emotion Mismatch and Identity Mismatch.
3.5 | Brain-social functioning associations
Activation during Emotion Mismatch trials in the ACC and anterior insula were examined as predictors of social problems and social anxiety to evaluate our hypothesis that greater sensitivity to shifts in emotional expression would be associated with better social functioning (Figure 5). Greater right ACC recruitment to Emotion Mismatch was negatively associated with social functioning, such that higher activity predicted lower social anxiety (β = −.41, p = .012, FDR corrected Cohen's f2 = 0.164) and fewer social problems (β = −.32 p = .046, FDR corrected, Cohen's f2 = 0.116; Figure 3) and these associations remained when controlling for age (β = −.39, p = .006, FDR corrected, Cohen's f2 = 0.295 and β = −.31, p = .021, FDR corrected, Cohen's f2 = 0.179, respectively). Greater recruitment of the right anterior insula to Emotion Mismatch was also negatively associated with social problems (β = −.31, p = .046, FDR corrected, Cohen's f2 = 0.109) and this association remained when controlling for a linear predictor of age (β = −.35, p = .014, FDR corrected Cohen's f2 = 0.217), while there was no significant association between activation of the right anterior insula and social anxiety (p > .250, FDR corrected). There was no significant association between recruitment of the left anterior cingulate cortex and social anxiety (β = −.18, p = .250, FDR corrected) or social problems (p > .250, FDR corrected).
Figure 5.
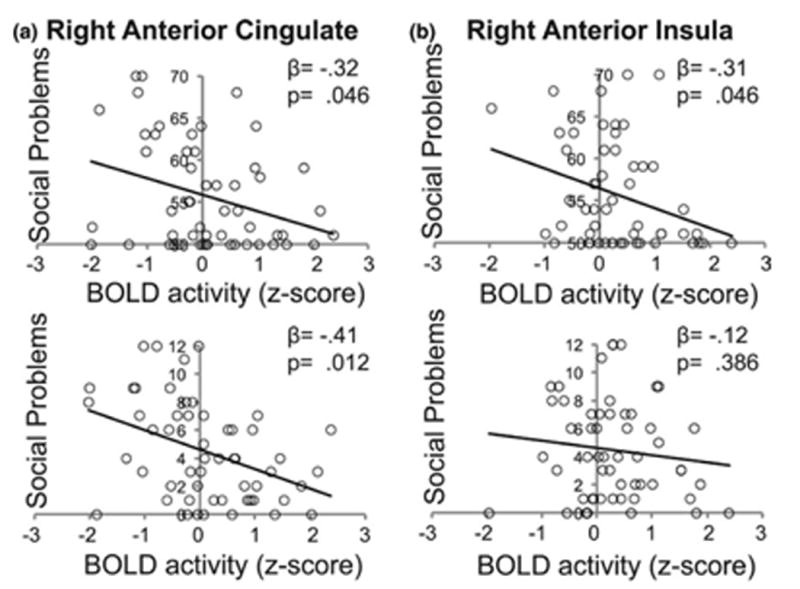
Linear regression revealed significant negative associations between activation in the (a) right anterior cingulate and both social problems and social anxiety and (b) the right anterior insula with social problems. All p-values are FDR corrected
We conducted one additional analysis as a control to determine whether the associations of salience network activation were specific to anxiety surrounding social interactions rather than anxiety more broadly. Specifically, we examined the association of activation within the salience network regions during emotion mismatches with panic disorder symptoms. Like social anxiety symptoms, panic symptoms had a significant positive linear association with age (β = .269, p = .049, Cohen's f2 = 0.078). In contrast to social anxiety symptoms, however, there were no significant associations between activation in any of the salience network regions and panic symptoms (all ps > .250). These findings suggest that the associations seen between salience network activation and social anxiety and social problems are not simply due to their associations with age.
4 | Discussion
We investigated the neural mechanisms underlying the detection of changes in identity and emotional expression of faces across development. First, we documented a quadratic developmental pattern of salience network recruitment when observing changes in the emotional expressions of others that peaked during early adolescence. Increased salience network recruitment in early adolescence in response to changes in emotional expression of others likely reflects the shift in importance of social and emotional cues that occurs during this period of adolescence (Nelson et al., 2014). Second, we found that age effects follow a quadratic pattern in our sample specifically for shifts in emotion, whereas age effects related to changes in identity follow a linear pattern. This suggests that the pattern of salience network recruitment, which peaks in early adolescence, appears uniquely sensitive to changes in the emotional expression of others. Third, greater salience network activation was associated with lower levels of social anxiety and social problems. These findings support the idea that sensitivity to changes in the emotional expression of others, as indexed by salience network activation in the current study, may facilitate flexibility in social interaction and promote adaptive social behavior.
The first goal of the study was to evaluate differences across development in responses to unpredictable changes in emotion. We observed quadratic effects of age in bilateral ACC and right anterior insula for trials where the probe emotion was different from the emotion held in mind, indicating early adolescent-specific recruitment of the salience network when processing an unpredictable change in emotion. These emotion mismatches recruited two nodes of the salience network - a network of brain regions widely documented to be recruited when viewing salient social and emotional information (Luo et al., 2014; Touroutoglou, Lindquist, Dickerson, & Barrett, 2015) - particularly in early adolescents, suggesting that unpredictable changes in the emotions of others might be particularly salient during this developmental period. Indeed, extensive work suggests that early adolescence is a period when social and emotional information is particularly salient (Davey, Yücel, & Allen, 2008; Silvers et al., 2012; Somerville et al., 2010; Spielberg et al., 2014; Stephanou et al., 2016).
Holding in mind and updating another person's emotional state might be particularly important during this developmental period as this information helps to adaptively guide socially effective behavior in the novel and increasingly complex social environment of adolescence (Crone & Dahl, 2012). Previous work has implicated the ACC and anterior insula in working memory of emotional faces (Luo et al., 2014), and found stronger recruitment of the salience network for processing emotional faces in adolescents than adults (Monk et al., 2003; Nelson et al., 2003). We extend these findings by documenting specificity of recruitment of the salience network in response to changes in emotional expression to early adolescence. During early adolescence, social cues become increasingly relevant for mate selection as well as gaining independence to navigate a more complex social world, and becoming less reliant on parental figures (Nelson et al., 2014). Therefore, enhanced activation in the salience network during this period of development in response to shifts of emotional expression of others may help update and guide behavior in this increasingly complex social environment.
Critically, this heighted response pattern of neural activation among early adolescents seen for shifts in emotion was not observed when a shift happened in the identity of the target. No brain regions exhibited a quadratic pattern of activation for shifts in identity. Rather, recruitment of regions that have been previously implicated in facial processing and working memory of faces and their identities (Neta & Whalen, 2011) increased linearly with age when the identity of the person being held in mind changed. These patterns may provide a mechanism to explain the age-related increases in performance on working memory for faces observed here. The specificity of the quadratic age pattern to emotion mismatches was further supported in an ROI analysis examining the interaction of age with mismatch condition. These findings suggest specificity of enhanced neural processing to changes in emotional expression during early adolescence rather than heightened response to any type of novelty or change in target stimuli during this period.
The final goal of the study was to assess whether activation patterns in the salience network were predictive of measures of social problems and social anxiety. Greater activation in the right ACC during Emotion Mismatch trials was associated with both fewer social problems and less social anxiety, and greater activation of the right anterior insula was similarly associated with fewer social problems. A non-social form of anxiety was not associated with activation in the salience network. If changes in emotional facial expressions of others are more salient, this may provide a signal that behavior must be updated accordingly; greater flexibility and responsiveness to the cues of others is likely to facilitate adaptive social behavior, resulting in fewer social problems and less anxiety surrounding social interactions. These findings are consistent with recent work documenting that some features of adolescence that have been conceptualized as risk factors and are associated with risk behavior, such as reward sensitivity (Casey et al., 2008; Steinberg, 2005), also confer some social advantages (Crone & Dahl, 2012; Nelson et al., 2014; Pfeifer & Allen, 2012). The present study provides support for the idea that social and affective sensitivity in adolescence, as indexed by heightened activation in salience networks to social stimuli, may also provide some advantages.
Social anxiety is characterized by attentional biases toward the self and heightened self-monitoring (Bögels & Mansell, 2004) as well as biased negative interpretation of ambiguous social cues (Haller et al., 2013) which can in turn negatively impact social performance (Schultz & Heimberg, 2008). Studies of individuals with social anxiety have found increased activity in the amygdala and ACC during passive processing of emotional faces (Amir et al., 2005; Battaglia et al., 2012). Furthermore, several studies in adults with social anxiety have shown increased coupling between the salience network and the default mode network, which is involved in self-referential processing (Pannekoek et al., 2013), and greater connectivity of the salience network and the default mode network has been shown to be associated with greater behavioral inhibition, a precursor for social anxiety, in children (Taber-Thomas, Morales, Hillary, & Pérez-Edgar, 2016). Thus, prior findings suggest altered processing in the salience network among individuals with social anxiety as well as a bias toward self-referential processing and away from social cues displayed by others.
Therefore, a possible alternative explanation of the present findings is that as a result of developing social anxiety, individuals become biased toward negative interpretation of external cues and towards internal self-focused processing in the presence of social information; together, this results in less attention to the external cues themselves, making them less salient. Indeed, high internal self-focused attention in the context of social interactions has been well documented among individuals with social anxiety (Bögels & Mansell, 2004). Future studies will be needed to disentangle these hypotheses, perhaps by directly assessing changes in attention to the emotional expressions of oneself compared to the expression of others.
One limitation of this study is that we did not have enough statistical power to investigate changes from one particular emotion to another. It is possible that neural and behavioral responses to changes from, for example, a happy face to an angry face might have more developmental specificity than other emotion combinations. Furthermore, a more nuanced look into these particular emotional changes might provide further insight into the link with social anxiety, given the bias toward negative interpretation associated with social anxiety. In addition, given the salience of peer relationships in particular in early adolescence (Nelson et al., 2014; Steinberg, 2005), future studies should investigate neural processing in response to shifts in the emotional expression of peers compared to non-peers across development. Finally, given the cross-sectional nature of this study and work that demonstrates that the age range of the sample can impact the inflection point of quadratic models (Fjell et al., 2010), the peak found in the present study in early adolescence should be interpreted with caution. Future longitudinal studies would provide a more nuanced understanding of where this peak lies and insight into whether neural sensitivity to the emotional expression of others indeed buffers against risk for social problems and social anxiety.
In sum, these findings indicate developmental variation in the neural mechanisms underlying sensitivity to changes in emotional expressions and have implications for understanding the neural systems that support social functioning. Early adolescence is associated with a developmentally specific pattern of recruitment of the salience network in response to unpredictable changes in the emotional expression of others. Moreover, activation in the salience network during shifts in emotional expression was associated with fewer social problems and less social anxiety. The current findings highlight early adolescence as a period of heightened neural sensitivity to changes in social cues, potentially identifying this period of development as an optimal window for treating children with signs of social anxiety.
Supplementary Material
Research Highlights.
We provide evidence for increased activation in the salience network in response to changes in emotional expressions of others during early adolescence.
Moreover, greater activation in these regions was associated with lower social anxiety and fewer social problems.
These findings add to the growing literature that adolescent-specific sensitivities to social and affective information may confer advantages that promote adaptive behavior.
Acknowledgments
Funding information: This work was supported by the National Institute of Child Health and Human Development at the National Institute of Health (F32 HD089514-01A1), National Institute of Mental Health at the National Institutes of Health (R01-MH103291), the Brain and Behavior Foundation NARSAD Early Investigator Award, an Early Career Research Fellowship from the Jacobs Foundation, and the IMHRO Rising Star Award.
Footnotes
Supporting Information: Additional Supporting Information may be found online in the supporting information tab for this article.
References
- Achenbach TM, Howell CT, Quay HC, Conners CK. National survey of problems and competencies among four- to sixteen-year-olds: Parents' reports for normative and clinical samples. Monographs of the Society for Research in Child Development. 1991;56:1–131. [PubMed] [Google Scholar]
- Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biological Psychiatry. 2005;57:975–981. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Askren MK, McAllister-Day TK, Koh N, Mestre Z, Dines JN, Korman BA, Madhyastha TM. Using Make for reproducible and parallel neuroimaging workflow and quality-assurance. Frontiers in Neuroinformatics. 2016;10:2. doi: 10.3389/fninf.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M, Zanoni A, Taddei M, Giorda R, Bertoletti E, Lampis V, Tettamanti M. Cerebral responses to emotional expressions and the development of social anxiety disorder: A preliminary longitudinal study. Depression and Anxiety. 2012;29:54–61. doi: 10.1002/da.20896. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): A replication study. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1230–1236. doi: 10.1097/00004583-199910000-00011. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Bögels SM, Mansell W. Attention processes in the maintenance and treatment of social phobia: Hypervigilance, avoidance and self-focused attention. Clinical Psychology Review. 2004;24:827–856. doi: 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones BM, Somerville LH. Braking and accelerating of the adolescent brain. Journal of Research on Adolescence. 2011;21:21–33. doi: 10.1111/j.1532-7795.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davey CG, Yücel M, Allen NB. The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neuroscience and Biobehavioral Reviews. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Dreyfuss M, Caudle K, Drysdale AT, Johnston NE, Cohen AO, Somerville LH, Casey BJ. Teens impulsively react rather than retreat from threat. Developmental Neuroscience. 2014;36:220–227. doi: 10.1159/000357755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences, USA. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Westlye LT, Østby Y, Tamnes CK, Jernigan TL, Dale AM. When does brain aging accelerate? Dangers of quadratic fits in cross-sectional studies. NeuroImage. 2010;50:1376–1383. doi: 10.1016/j.neuroimage.2010.01.061. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: Who is at risk? Developmental Science. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Ghosh SS, Kakunoori S, Augustinack J, Nieto-Castanon A, Kovelman I, Gaab N, Fischi B. Evaluating the validity of volume-based and surface-based brain image registration for developmental cognitive neuroscience studies in children 4 to 11 years of age. NeuroImage. 2010;53:85–93. doi: 10.1016/j.neuroimage.2010.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller SPW, Cohen Kadosh K, Lau JYF. A developmental angle to understanding the mechanisms of biased cognitions in social anxiety. Frontiers in Human Neuroscience. 2013;7:846. doi: 10.3389/fnhum.2013.00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Casey BJ. The neurodynamics of emotion: Delineating typical and atypical emotional processes during adolescence. Developmental Science. 2016;19:3–18. doi: 10.1111/desc.12373. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jones RM, Somerville LH, Li J, Ruberry EJ, Powers A, Mehta N, Casey BJ. Adolescent-specific patterns of behavior and neural activity during social reinforcement learning. Cognitive, Affective, and Behavioral Neuroscience. 2014;14:683–697. doi: 10.3758/s13415-014-0257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert HK, Sheridan MA, Sambrook K, Rosen ML, Askren MK, McLaughlin KA. Hippocampal contribution to context encoding across development is disrupted following early-life adversity. Journal of Neuroscience. 2017;37:1925–1934. doi: 10.1523/JNEUROSCI.2618-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Morrarty E, Degnan KA, Chronis-Tuscano A, Rubin KH, Cheah CSL, Pine DS, Fox NA. Maternal over-control moderates the association between early childhood behavioral inhibition and adolescent social anxiety symptoms. Journal of Abnormal Child Psychology. 2012;40:1363–1373. doi: 10.1007/s10802-012-9663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopresti ML, Schon K, Tricarico MD, Swisher JD, Celone KA, Stern CE. Working memory for social cues recruits orbitofrontal cortex and amygdala: A functional magnetic resonance imaging study of delayed matching to sample for emotional expressions. Journal of Neuroscience. 2008;28:3718–3728. doi: 10.1523/JNEUROSCI.0464-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Qin S, Fernández G, Zhang Y, Klumpers F, Li H. Emotion perception and executive control interact in the salience network during emotionally charged working memory processing. Human Brain Mapping. 2014;35:5606–5616. doi: 10.1002/hbm.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DS, Harms MP, Snyder AZ, Jenkinson M, Wilson JA, Glasser MF, Van Essen DC. Human Connectome Project informatics: Quality control, database services, and data visualization. NeuroImage. 2013;80:202–219. doi: 10.1016/j.neuroimage.2013.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Garrad MC, Somerville LH. What develops during emotional development? A component process approach to identifying sources of psychopathology risk in adolescence. Dialogues in Clinical Neuroscience. 2015;17:403–410. doi: 10.31887/DCNS.2015.17.4/kmclaughlin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Guyer AE. The development of the ventral prefrontal cortex and social flexibility. Developmental Cognitive Neuroscience. 2011;1:233–245. doi: 10.1016/j.dcn.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Lau JYF, Jarcho JM. Growing pains and pleasures: How emotional learning guides development. Trends in Cognitive Sciences. 2014;18:99–108. doi: 10.1016/j.tics.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, McClure EB, Monk CS, Zarahn E, Leibenluft E, Pine DS, Ernst M. Developmental differences in neuronal engagement during implicit encoding of emotional faces: An event-related fMRI study. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2003;44:1015–1024. doi: 10.1111/1469-7610.00186. [DOI] [PubMed] [Google Scholar]
- Neta M, Whalen PJ. Individual differences in neural activity during a facial expression vs. identity working memory task. NeuroImage. 2011;56:1685–1692. doi: 10.1016/j.neuroimage.2011.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek JN, Veer IM, van Tol MJ, dervan Werff SJA, Demenescu LR, Aleman A, der van Wee NJ. Resting-state functional connectivity abnormalities in limbic and salience networks in social anxiety disorder without comorbidity. European Neuropsychopharmacology. 2013;23:186–195. doi: 10.1016/j.euroneuro.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends in Cognitive Sciences. 2012;16:322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Moore WE, Oswald TM, Mazziotta JC, Iacoboni M, Dapretto M. Entering adolescence: Resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69:1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle N, Zuk J, Ortiz-Mantilla S, Sliva DD, Franceschi A, Grant PE, Gaab N. Pediatric neuroimaging in early childhood and infancy: Challenges and practical guidelines. Annals of the New York Academy of Sciences. 2012;1252:43–50. doi: 10.1111/j.1749-6632.2012.06457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche A. A four-dimensional registration algorithm with application to joint correction of motion and slice timing in fMRI. IEEE Transactions on Medical Imaging. 2011;30:1546–1554. doi: 10.1109/TMI.2011.2131152. [DOI] [PubMed] [Google Scholar]
- Schultz LT, Heimberg RG. Attentional focus in social anxiety disorder: Potential for interactive processes. Clinical Psychology Review. 2008;28:1206–1221. doi: 10.1016/j.cpr.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman WK, Ollendick TH. Evidence-based assessment of anxiety and its disorders in children and adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34:380–411. doi: 10.1207/s15374424jccp3403_2. [DOI] [PubMed] [Google Scholar]
- Silvers JA, McRae K, Gabrieli JDE, Gross JJ, Remy KA, Ochsner KN. Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion. 2012;12:1235–1247. doi: 10.1037/a0028297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Ruberry EJ, Dyke JP, Glover G, Casey BJ. The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychological Science. 2013;24:1554–1562. doi: 10.1177/0956797613475633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Olino TM, Forbes EE, Dahl RE. Exciting fear in adolescence: Does pubertal development alter threat processing? Developmental Cognitive Neuroscience. 2014;8:86–95. doi: 10.1016/j.dcn.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Stephanou K, Davey CG, Kerestes R, Whittle S, Pujol J, Yücel M, Harrison BJ. Brain functional correlates of emotion regulation across adolescence and young adulthood. Human Brain Mapping. 2016;37:7–19. doi: 10.1002/hbm.22905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber-Thomas BC, Morales S, Hillary FG, Pérez-Edgar KE. Altered topography of intrinsic functional connectivity in childhood risk for social anxiety. Depression and Anxiety. 2016;33:995–1004. doi: 10.1002/da.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Ichien NT, Qu Y. Mothers know best: Redirecting adolescent reward sensitivity toward safe behavior during risk taking. Social Cognitive and Affective Neuroscience. 2015;10:1383–1391. doi: 10.1093/scan/nsv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Lindquist KA, Dickerson BC, Barrett LF. Intrinsic connectivity in the human brain does not reveal networks for ‘basic’ emotions. Social Cognitive and Affective Neuroscience. 2015;10:1257–1265. doi: 10.1093/scan/nsv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


