Abstract
Speech sound disorder (SSD) is common, yet its neurobiology is poorly understood. Recent studies indicate atypical structural and functional anomalies either in one hemisphere or both hemispheres, which might be accompanied by alterations in inter-hemispheric connectivity. Indeed, abnormalities of the corpus callosum – the main fiber tract connecting the two hemispheres – have been linked to speech and language deficits in associated disorders, such as stuttering, dyslexia, aphasia, etc. However, there is a dearth of studies examining the corpus callosum in SSD. Here, we investigated whether a sample of 18 children with SSD differed in callosal morphology from 18 typically developing children carefully matched for age. Significantly reduced dimensions of the corpus callosum, particularly in the callosal anterior third, were observed in children with SSD. These findings indicating pronounced callosal aberrations in SSD make an important contribution to an understudied field of research and may suggest that SSD is accompanied by atypical lateralization of speech and language function.
Keywords: anterior third, brain, corpus callosum, development, language, magnetic resonance imaging
Introduction
Speech sound disorder (SSD) is a pervasive speech production deficit occurring in the absence of a frank neurological lesion or known cause (Morgan et al., 2016). SSD is common, occurring in 3.5% – 5% of pre-school children (Eadie et al., 2015, Reilly et al., 2015), and encompasses articulation and phonological impairments, which can further be categorized into disordered or delayed profiles (Eadie et al., 2015, Reilly et al., 2015, Dodd et al., 2017). Articulation impairments are phonetic-level errors (i.e., they affect motor planning and execution), such as a lisp on/s/or distortion of/r/(Gunther and Hautvast, 2010, Dodd et al., 2017, Morgan et al., 2017). Phonological impairments manifest as errors in the phonemic rules of one’s language, where incorrect rules (phonological processes) are applied, such as producing “t” for ‘k’, “tat” for ‘cat’, or “gog” for ‘dog’ (Dodd et al., 2017, Morgan et al., 2017). SSDs may be associated with literacy difficulties and, in the longer term, with restricted educational and vocational attainment (Mann and Foy, 2007, Johnson et al., 2010a, Lewis et al., 2011). There is heterogeneity of the SSD phenotype, where individuals may present with any combination of articulation or phonological errors, and this symptomatology has been well characterized in early and middle childhood, adolescence, and even adulthood (Beitchman et al., 2001, Johnson et al., 2010b, Wren et al., 2012, Eadie et al., 2015, Dodd et al., 2017, Morgan et al., 2017). Despite this thorough phenotypic characterization, causation for SSD is poorly understood. While multifactorial genetic and environmental influences are indicated (Graham and Fisher, 2015), explicit etiological pathways remain unknown.
From a neurobiological perspective, there have been only a limited number of imaging studies examining structural and functional correlates of SSD and associated speech pathologies (for review, see Liegeois et al., 2014, Morgan et al., 2016). The few existing findings seem to suggest structural or functional anomalies in several cortical and subcortical areas, such as the pre- and post-central gyrus, the supramarginal gyrus, the middle and superior temporal gyrus, the fusiform gyrus, the cingulate, the insula, the basal ganglia including globus pallidus, as well as several white matter tracts and the cerebellum (Preston et al., 2012, Liegeois et al., 2013, Morgan et al., 2013, Kadis et al., 2014, Preston et al., 2014, Redle et al., 2015, Silveri et al., 2016). In terms of laterality, some of the aforementioned studies report aberrations in both hemispheres, while others point to the left hemisphere in particular. The question arises whether communication channels between hemispheres are also affected. It is possible, for example, that SSD is accompanied by aberrations of cerebral commissures. The corpus callosum is the main commissure system of the human brain. It connects the right and left hemisphere through more than 200 million fibers, not only facilitating inter-hemispheric communication but also modulating hemispheric specialization, including language-dominance. Thus, the corpus callosum appears to be a plausible brain structure and attractive candidate when exploring the underlying anatomical substrates of SSD.
To our knowledge, there is only one study noting atypicality of the corpus callosum in SSD, where significant effects were evident within the splenium and anterior body of the corpus callosum (Preston et al., 2014). Furthermore, callosal aberrations have been observed (among other brain abnormalities) in other speech- and language-related conditions, such as childhood or adult stuttering (Choo et al., 2011, Connally et al., 2014, Chang et al., 2015, Civier et al., 2015, Chow and Chang, 2017) and dyslexia (Hynd et al., 1995). With respect to the specific callosal subregions affected, these latter non-SSD studies revealed mixed findings implicating the splenium, the (anterior) midbody, and the callosal anterior third, including genu and rostrum1, in addition to the corpus callosum as a whole. Moreover, a recent stroke study in adults with aphasia linking interhemispheric connectivity to speech fluency seems to point to the callosal midbody as well as the rostral body (Pani et al., 2016). Results are similarly inconsistent with respect to the direction of the effect: Some studies revealed larger measures of the corpus callosum (as well as other cerebral features) in healthy controls, while others reported enlarged features in affected individuals (Hynd et al., 1995, Choo et al., 2011, Connally et al., 2014, Chang et al., 2015, Civier et al., 2015, Pani et al., 2016, Chow and Chang, 2017).
The goal of the current study was thus three-fold: (1) to examine whether SSD is characterized by significant deviations in callosal morphology; (2) to determine the exact location of the possible aberration; and (3) to elucidate the direction of the SSD-related effect (i.e., callosal reduction versus enlargement). For this purpose, we applied a refined computational method to calculate the thickness of the corpus callosum with a high regional specificity (i.e., at 100 equidistant points across the callosal surface) in a sample of children with SSD and age-matched typically developing children. In addition, we conducted an exploratory analysis using tensor-based morphometry to complement (and possibly extend) the outcomes of the aforementioned ‘callosal thickness’ approach.
Experimental Procedures
Early Language in Victoria Study (ELVS)
The children included in the present study were selected from a large pool of participants recruited for the Early Language in Victoria Study; ELVS (Reilly et al., 2007, Reilly et al., 2010). ELVS is a longitudinal epidemiological community cohort study of 1,910 children who were enrolled at 8 months of age in 2003/2004. With language development being tracked almost annually, language trajectories in those children were well-known from infancy to time of scanning when children were between 9;3 years and 11;3 years old. Ethics approval (HREC31225) was obtained from the Human Research Ethics Committee at the Royal Children’s Hospital, Melbourne (Australia). At least one parent provided informed consent and children provided oral assent. Further information on ELVS, including inclusion and exclusion criteria, is detailed elsewhere (Reilly et al., 2007, Reilly et al., 2010). However, information as relevant to the current study (i.e., pertaining to children with SSD as well as typically developing children) is provided below.
ELVS Eligibility Criteria
Inclusion criteria for children with SSD as well as typically developing children were a non-verbal IQ of ≥ 80 on the Kaufman Brief Intelligence Test; KBIT (Kaufman and Kaufman, 2004) at age 4, and the Wechsler Abbreviated Scales of Intelligence; WASI (Wechsler, 1999) at age 7. Moreover, children in both groups were required to be English native speakers and, aside from SSD, to be free of any neurodevelopmental disorders (e.g., attention-deficit/hyperactivity disorder, autism spectrum disorder, developmental coordination disorder) and any other significant medical or developmental issues. Importantly, children in both groups were also required to have normal language scores (≥85) as per the Clinical Evaluation of Language Fundamentals (CELF) assessment tool using CELF Preschool-II (Wiig et al., 2004) at age 4, and CELF-IV (Semel et al., 2003) at ages 5 and 7. SSD children showed impaired speech, defined as a score of ≤ 85 on the Goldman-Fristoe Test of Articulation; GFTA-II (Goldman and Fristoe, 2005) at age 4 and/or as per diagnosis of articulation or phonological errors (Dodd et al., 2017, Morgan et al., 2017). Typically developing children were required to have normal speech as based on the GFTA-II and in conversation.
Study-specific Speech Assessments
To assess speech performance at age 9–11, the GFTA-II was administered as a single-word test that elicits all the speech sounds of English language in initial, medial and final positions. All sounds were transcribed and assessed for the presence of articulation and phonological errors to confirm a diagnosis of SSD (Dodd et al., 2017, Morgan et al., 2017). In addition, conversational samples were rated to confirm the presence of errors noted in single-word stimuli in connected speech. Articulation disorder was denoted as a phonetic-based distortion (e.g., interdental and lateral lisps, de-rhoticism), with the distortion occurring more frequently than the correct production of that phone. Articulation disorder could also include an omission error, with the phone absent in the child’s phonetic inventory but present in >90% of peers (Dodd et al., 2002, Dodd et al., 2017). Phonological disorder was defined as use of a phonological process that is atypical and seen in <10% of the normative sample population at any age (Dodd et al., 2003). A phonological delay was denoted as use of a phonological process that occurs in typically developing speech, but that is used beyond an age where it is typically resolved in >90% of peers (Dodd et al., 2003).
Sample Characteristics
The current study included 18 children with SSD and 18 typically developing (TD) children, closely matched for age (SSD [mean ± SD]: 123.22 ± 3.90 months; TD: 122.44 ± 3.71 months). The groups did not differ significantly with respect to sex (SSD: 7 boys/11 girls; TD: 10 boys/8 girls), handedness (SSD: 17 right-/1 non-right; TD: 15 right-/3 non-right), the CELF-IV total language scores (SSD: 101.39 ± 10.36; TD: 107.28 ± 8.55), the CELF-IV receptive language scores (SSD: 100.83 ± 8.47; TD: 105.83 ± 8.39), and the CELF-IV expressive language scores (SSD: 102.78 ± 10.66; TD: 109.00 ± 9.88). However, as expected, SSD children had significantly lower GFTA-II scores than typically developing children (SSD: 99.72 ± 4.39; TD: 103 ± 2.54; p=0.011). Comparing the GFTA-II scores within the SSD group between age 4 and age 9–11 (i.e., when image data were acquired) revealed a significant change over time (93.00 ± 9.13 versus 99.72 ± 4.39; p=0.0013). Moreover, as shown in Table 1, the speech diagnostic profiles changed from 4 years to 9–11 years. Nevertheless, the vast majority of the SSD group (16/18) had persistent speech errors at age 9–11.
Table 1.
Speech-diagnostics at age 4 and age 9–11 (n=18)
| number of children affected | ||
|---|---|---|
| at age 4 | at age 9–11 | |
| Articulation Disorder | 6 | 12 |
| Phonological Disorder | 2 | 0 |
| Phonological Delay | 4 | 3 |
| Articulation Disorder + Phonological Disorder | 2 | 0 |
| Articulation Disorder + Phonological Delay | 1 | 1 |
| Articulation Disorder + Phonological Disorder + Phonological Delay | 1 | 0 |
| Phonological Disorder + Phonological Delay | 2 | 0 |
| Resolved | 0 | 2* |
One child presents with inconsistent sub-clinical phonetic distortions.
Image Data
Participants were scanned at the Florey Institute of Neuroscience and Mental Health in Melbourne, Australia (https://www.florey.edu.au/). All brain images were acquired on a Siemens 3 Tesla Skyra system with a 20-channel head coil using the following parameters: TR = 1900 ms, TE = 2.49 ms, flip angle = 9°, matrix size = 256 × 256, field of view: 240 × 240 mm2, voxel size = 0.9 × 0.9 × 0.9 mm3. For each participant, the acquired brain images were immediately inspected for motion artifacts and the scan was repeated if necessary. All images were corrected for magnetic field inhomogeneities and spatially normalized using 6-parameter (rigid-body) transformations in SPM12 (http://www.fil.ion.ucl.ac.uk/spm) using the CAT12 toolbox (http://www.neuro.uni-jena.de/cat/). In addition, the total intracranial volume was estimated for each brain (in cm3) to be included as a covariate in the statistical model (SSD [mean ± SD]: 1,609.11 ± 126.64; TD: 1,595.00 ± 128.63).
Callosal Thickness
Using the preprocessed images, the corpus callosum was outlined manually and blind to group status in each brain’s midsagittal section (Luders et al., 2003, Luders et al., 2007a). Inter-rater reliability was assessed by comparing callosal traces produced by two experienced operators (E.L. and F.K.) using the Jaccard index (Jaccard 1901), as previous described (Luders et al., 2016). The Jaccard index across duplicate traces in ten subjects was 0.94 indicating a high inter-rater reliability. Nevertheless, all callosal outlines were carefully checked (and corrected if indicated) to ensure that they precisely reflected the size and shape of each individual corpus callosum. Subsequently, callosal thickness was established in a number of successive steps, as illustrated (Luders et al., 2006, Luders et al., 2018) and further described in detail elsewhere (Luders et al., 2011, Luders et al., 2014). Briefly, the upper and lower callosal boundaries were first separated into 100 nodes and re-sampled at regular intervals rendering the discrete points comprising the two boundaries spatially uniform. Then, a new midline curve was created by calculating the 2D average from the 100 equidistant nodes representing the upper and the lower callosal boundaries. Finally, the distances between the 100 nodes of the upper as well as the lower callosal boundaries to the 100 nodes of the midline curve were calculated (in mm). These distances – indicating callosal thickness at 100 locations distributed evenly over the callosal surface – were entered as the dependent variables into the statistical analysis.
Statistical Analysis
Differences in point-wise callosal thickness between children with SSD and typically developing children were assessed using a general linear model, while removing the variance associated with age and total intracranial volume. The resulting point-wise significance values (p) were projected onto the mean callosal surface created from all participants included in this study (n=36). Alpha was set at 0.05. To control for multiple comparisons, a Monte Carlo simulation using 10,000 permutations was employed, as previously established (Thompson et al., 2004, Luders et al., 2009, Anastasopoulou et al., 2016). Last but not least, effect sizes (Cohen’s d) were calculated and projected onto the mean callosal surface.
Exploratory Analysis
To complement the main analysis directed at investigating point-wise callosal thickness, we used tensor-based morphometry to examine voxel-wise information reflecting the shape and size of the corpus callosum. For this purpose, all brain images were corrected for magnetic field inhomogeneities and spatially normalized to the DARTEL template provided by the CAT12 toolbox using 12-parameter (affine) transformations and high-dimensional warping (Ashburner, 2007). The Jacobian determinants – which encode the local expansions/contractions necessary to match the individual brains to the DARTEL template – were then derived from the resulting normalization matrices and smoothed using an 8 mm FWHM Gaussian kernel. Closely following the statistical approach described above, voxel-wise group differences were assessed using a general linear model, while removing the variance associated with age and total intracranial volume. Since we were only interested in callosal effects (as opposed to whole-brain effects), a mask was created to restrict the outcomes of the statistical analysis to the corpus callosum. The resulting point-wise significance values (p) were projected onto the mean brain created from all participants (n=36). Due to the exploratory nature of this analysis, corrections for multiple comparisons were not applied. This entire analysis was conducted in SPM12 (http://www.fil.ion.ucl.ac.uk/spm) using the CAT12 toolbox (http://www.neuro.uni-jena.de/cat/); the mask was created in MRIcron (http://www.mccauslandcenter.sc.edu/crnl/mricron/).
Results
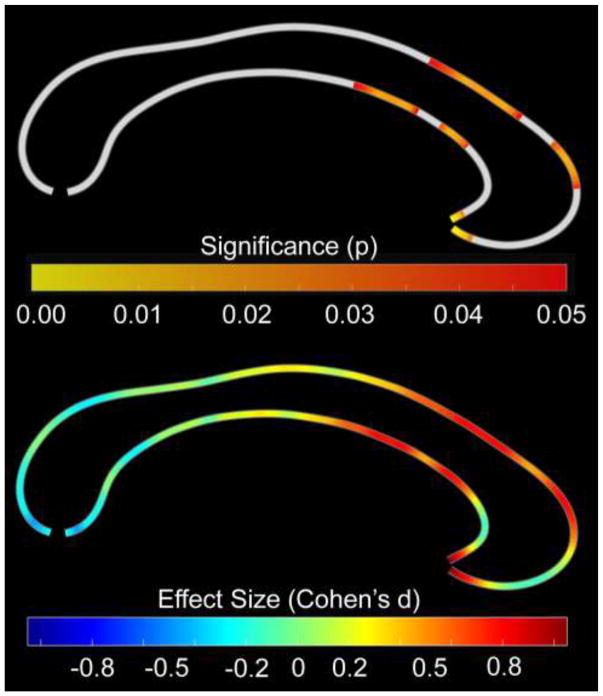
As shown in Figure 1 (top panel), applying the ‘callosal thickness’ approach, we observed significantly thinner corpora callosa in children with SSD compared to typically developing children. More specifically, such effects were evident within the anterior third of the corpus callosum – especially in the rostral body but also partly within genu and rostrum – as well as the most rostral part of the anterior midbody (Witelson, 1989). These findings were confirmed by permutation testing (p=0.048) and substantiated by Cohen’s d indicating moderate to large effect sizes (Figure 1, bottom panel). No region of the corpus callosum was significantly thicker in children with SSD compared to typically developing children, even if we abstained from applying corrections for multiple comparisons.
Figure 1. Group Differences in Point-wise Callosal Dimensions (Callosal Thickness).
Thinner callosal regions in children with SSD compared to typically developing children within the callosal anterior third, extending into the anterior midbody. The posterior part of the corpus callosum points to the left; the anterior part points to the right. Top Panel: Statistical significance, with the color bar encoding uncorrected significance (p); the significance profile is confirmed by permutation testing (p=0.048). Bottom Panel: Effect size, with the color bar encoding Cohen’s d (effect sizes: <0.2 trivial; 0.2–0.5 small; 0.5–0.8 moderate; >0.8 large).
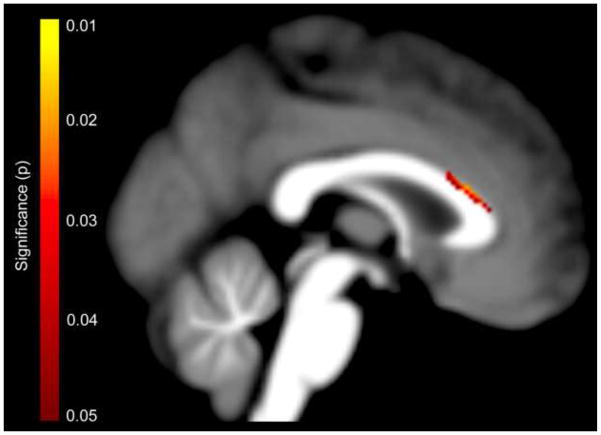
As shown in Figure 2, applying tensor-based morphometry, we also observed significant effects at the dorsal border of the callosal anterior third, specifically the rostral body, indicating volumetric contractions in children with SSD compared to typically developing children. Effect sizes were moderate, with Cohen’s d ranging between 0.60 and 0.76. No region of the corpus callosum was significantly expanded in children with SSD compared to typically developing children.
Figure 2. Group Differences in Voxel-wise Callosal Dimensions (Tensor-based Morphometry).
Significant callosal contractions in children with SSD compared to typically developing children within the dorsal callosal anterior third. The color bar encodes the uncorrected statistical significance (p). The posterior part of the corpus callosum points to the left; the anterior part points to the right.
Discussion
The outcomes of our study suggest that SSD is accompanied by regional aberrations in callosal morphology in affected children, specifically a reduced thickness as well as volumetric contractions (the latter only on a trend level; p≤0.05 uncorrected). Comparable data are extremely sparse, but our findings seem to corroborate other scientific reports proposing the corpus callosum to be involved in the pathogenesis of related speech disorders (Hynd et al., 1995, Choo et al., 2011, Connally et al., 2014, Chang et al., 2015, Pani et al., 2016, Chow and Chang, 2017).
Effect Location and Direction
In terms of the exact spatial location, SSD-related aberrations in the current study were confined to the anterior third/anterior midbody, callosal regions heavily connected to various (pre)frontal, premotor and supplemental motor areas of the cortex (Witelson, 1989, Hofer and Frahm, 2006, Zarei et al., 2006), some of them involved in speech and language production. Given that SSD is defined as a pervasive speech deficit, the spatial location of the observed effect seems plausible and also is in agreement with outcomes of other studies, where significant links have been detected between speech-related functions and the callosal anterior third and/or anterior midbody (Hynd et al., 1995, Choo et al., 2011, Preston et al., 2014, Chang et al., 2015, Civier et al., 2015, Pani et al., 2016). The only existing corpus callosum-related study in SSD also revealed significant effects within the callosal anterior body but additionally within the splenium (Preston et al., 2014). Moreover, in contrast to our findings, affected children had greater (rather than smaller) white matter volumes than children with typical speech (Preston et al., 2014). This opposite direction is intriguing and warrants an explanation. However, given the lack of other callosal data in SSD, a solid frame of reference is missing and we can only speculate that the discrepant findings across studies are due, at least in part, to the differing nature of the cohorts assessed, the speech phenotyping procedures used, and/or the morphometric approach applied. While it is impossible to arrive at a definite (and justified) answer at this point, it seems worth pointing out that callosal findings in other speech disorders are similarly inconsistent: some studies revealed larger measures in healthy controls than in affected individuals, other studies report the opposite effect (Hynd et al., 1995, Choo et al., 2011, Connally et al., 2014, Chang et al., 2015, Civier et al., 2015, Pani et al., 2016, Chow and Chang, 2017). Clearly, additional studies are required to further develop this under-investigated field of research.
Possible Links to Functional Lateralization
As discussed elsewhere (Luders et al., 2007b, Luders et al., 2016), a thinner corpus callosum (or smaller callosal areas) might indicate fewer axons and/or reduced axonal diameters. Thus, smaller callosal dimensions, as currently observed, are likely to reflect a decreased inter-hemispheric connectivity and signal conduction in speech/language-related channels overall. Impaired inter-hemispheric signal conduction, in turn, might be associated with an atypical lateralization of speech and language function. More specifically, in the typically developing brain, the language perception/speech production network shows a leftward asymmetry, at least for some structures (Dubois et al., 2009). Such leftward asymmetry seems to be maintained by exerting inter-hemispheric inhibition – from the left to the right hemisphere – across the corpus callosum (Tzourio-Mazoyer et al., 2016). Indeed, analyses in normative cohorts revealed a positive correlation between callosal size and the degree of left-lateralization for language (Josse et al., 2008). Pathological conditions, on the other hand, were suggested to be accompanied by decreased inter-hemispheric inhibition altering hemispheric dominance for language (Tzourio-Mazoyer et al., 2016), as also supported by reports of atypical functional lateralization in developmental communication disorders (Njiokiktjien, 1990, Fabbro et al., 2002, Mayes et al., 2015). Thus, the detected aberrations – i.e., reduced anterior callosal dimensions in SSD – might point to a reduced lateralization of speech and language functions in affected children, perhaps due to a disadvantageous recruitment of the right-hemispheric (frontal) cortex during speech production, similar as has been argued with respect to stuttering (Civier et al., 2015).
Etiology and the Quest for Causation
Given the complex genetics of SSD, the heterogeneity of the phenotype, and the relatively subtle neural involvement in this condition compared to most cerebral malformation syndromes, identifying specific genetic contributions remains challenging. Nevertheless, a possible reason for aberrations of the anterior third (over any other callosal region) might be deduced from the order of precedence during neurodevelopment: That is, the corpus callosum originates at 10–12 weeks of gestation and follows an anterior-to-posterior gradient (Rakic and Yakovlev, 1968, Achiron and Achiron, 2001). It is possible that maturation of the corpus callosum – perhaps of the anterior third, in particular – is for some reason delayed or accelerated in children with SSD prepartum and/or negatively impacted postpartum, potentially due to disruption of genes important for callosal development (Parrini et al., 2016). That being said though, it remains an open question whether callosal aberrations would be identifiable at birth in children who later present with SSD. In other words, callosal aberrations might have caused (or contributed) to the speech disorder, but could also be a consequence thereof. More specifically, according to our understanding of brain plasticity, it is possible that SSD is initiated by other factors (e.g., environmental or endogenous). As a consequence, aberrations in callosal morphology might arise (e.g., due to atypical brain functioning, unusual stimulation/deprivation, or use of compensatory mechanisms), possibly further enhancing speech impairments and ultimately leading to a clinical symptomatology. Alternatively, although perhaps less likely, links between callosal dimensions and functional speech/language lateralization in SSD might be devoid of any causal relationship with each other, but simply underlie the same developmental mechanisms, similarly as has been argued, for example, for callosal morphology and handedness (Habib et al., 1991).
Conclusion and Implications for Follow-up Studies
The present findings indicating pronounced aberrations in the brain’s largest white matter tract significantly contribute to an understudied field of research and may support that SSD is accompanied by atypical lateralization of speech and language function. Future studies, ideally longitudinal in nature, may further expand this line of research by complementing indicators of callosal macro-structure with descriptors of callosal micro-structure, such as based on callosal fiber tracking using diffusion tensor imaging. Moreover, follow-up research combining callosal measures with other cortical, subcortical, perhaps even cerebellar measures of brain structure (e.g., gray matter density, cortical thickness, or via shape/size estimates) as well as measures of brain function (e.g., behavioral, electrophysiological, or oxygenation/perfusion parameters) are indicated to provide a more comprehensive account of the neurobiology of SSD. Last but not least, genetic studies are required to foster our understanding of the mechanistic pathways leading to SSD.
Highlights.
There is a link between callosal morphology and speech sound disorder (SSD).
The corpus callosum is thinner in children with SSD than in typically developing children.
The group difference was particularly evident within the callosal anterior third.
Acknowledgments
EL is funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (R01HD081720) and further supported by the Cousins Center for Psychoneuroimmunology at the University of California, Los Angeles (UCLA). AM is supported by National Health and Medical Research Council Practitioner Fellowship #1105008; NHMRC Centre of Research Excellence (CRE) in Child Language #1023493; NHMRC CRE in Speech and Language Neurobiology #1116976; NHMRC Project grant #1127144; NHMRC CRE Moving Ahead #1023043; and HEARing Collaborative Research Centre. This work is also supported by the Victorian Government’s Operational Infrastructure Support Programme.
Footnotes
For clarity, study outcomes (ours and others) are described by referring to well-known vertical callosal segments (Witelson, 1989). There, the splenium represents the posterior fifth, the isthmus two fifteenths, the posterior midbody and anterior midbody each one sixth. The remaining anterior third may be further subdivided in rostral body, genu, and rostrum.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achiron R, Achiron A. Development of the human fetal corpus callosum: a high-resolution, cross-sectional sonographic study. Ultrasound Obstet Gynecol. 2001;18:343–347. doi: 10.1046/j.0960-7692.2001.00512.x. [DOI] [PubMed] [Google Scholar]
- Anastasopoulou S, Kurth F, Luders E, Savic I. Generalized epilepsy syndromes and callosal thickness: Differential effects between patients with juvenile myoclonic epilepsy and those with generalized tonic-clonic seizures alone. Epilepsy Res. 2016;129:74–78. doi: 10.1016/j.eplepsyres.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Beitchman JH, Wilson B, Johnson CJ, Atkinson L, Young A, Adlaf E, Escobar M, Douglas L. Fourteen-year follow-up of speech/language-impaired and control children: psychiatric outcome. J Am Acad Child Adolesc Psychiatry. 2001;40:75–82. doi: 10.1097/00004583-200101000-00019. [DOI] [PubMed] [Google Scholar]
- Chang SE, Zhu DC, Choo AL, Angstadt M. White matter neuroanatomical differences in young children who stutter. Brain: a journal of neurology. 2015;138:694–711. doi: 10.1093/brain/awu400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo AL, Kraft SJ, Olivero W, Ambrose NG, Sharma H, Chang SE, Loucks TM. Corpus callosum differences associated with persistent stuttering in adults. J Commun Disord. 2011;44:470–477. doi: 10.1016/j.jcomdis.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow HM, Chang SE. White matter developmental trajectories associated with persistence and recovery of childhood stuttering. Hum Brain Mapp. 2017 doi: 10.1002/hbm.23590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civier O, Kronfeld-Duenias V, Amir O, Ezrati-Vinacour R, Ben-Shachar M. Reduced fractional anisotropy in the anterior corpus callosum is associated with reduced speech fluency in persistent developmental stuttering. Brain and language. 2015;143:20–31. doi: 10.1016/j.bandl.2015.01.012. [DOI] [PubMed] [Google Scholar]
- Connally EL, Ward D, Howell P, Watkins KE. Disrupted white matter in language and motor tracts in developmental stuttering. Brain and language. 2014;131:25–35. doi: 10.1016/j.bandl.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Dodd B, Holm A, Hua Z, Crosbie S. Phonological development: a normative study of British English-speaking children. Clin Linguist Phon. 2003;17:617–643. doi: 10.1080/0269920031000111348. [DOI] [PubMed] [Google Scholar]
- Dodd B, Ttoffari-Eecen T, Brommeyer K, Reilly S, Morgan A. Delayed and Disordered Development of Articulation and Phonology between Four and Seven Years. Child Language Teaching and Therapy. 2017 doi: 10.1177/0265659017735958. E-pub ahead of print. [DOI] [Google Scholar]
- Dodd B, Zhu H, Crosbie C, Holm A, Hua Z, Ozanne A. Diagnostic Evaluation of Articulation and Phonology. Pearson; Australia: 2002. [Google Scholar]
- Dubois J, Hertz-Pannier L, Cachia A, Mangin JF, Le Bihan D, Dehaene-Lambertz G. Structural asymmetries in the infant language and sensori-motor networks. Cerebral cortex. 2009;19:414–423. doi: 10.1093/cercor/bhn097. [DOI] [PubMed] [Google Scholar]
- Eadie P, Morgan A, Ukoumunne OC, Ttofari Eecen K, Wake M, Reilly S. Speech sound disorder at 4 years: prevalence, comorbidities, and predictors in a community cohort of children. Developmental medicine and child neurology. 2015;57:578–584. doi: 10.1111/dmcn.12635. [DOI] [PubMed] [Google Scholar]
- Fabbro F, Libera L, Tavano A. A callosal transfer deficit in children with developmental language disorder. Neuropsychologia. 2002;40:1541–1546. doi: 10.1016/s0028-3932(02)00026-x. [DOI] [PubMed] [Google Scholar]
- Goldman R, Fristoe M. Goldman–Fristoe Test of Articulation. 2. Circle Pines, MN: American Guidance Service; 2005. (GFTA-II) [Google Scholar]
- Graham SA, Fisher SE. Understanding Language from a Genomic Perspective. Annu Rev Genet. 2015;49:131–160. doi: 10.1146/annurev-genet-120213-092236. [DOI] [PubMed] [Google Scholar]
- Gunther T, Hautvast S. Addition of contingency management to increase home practice in young children with a speech sound disorder. Int J Lang Commun Disord. 2010;45:345–353. doi: 10.1080/13682820903026762. [DOI] [PubMed] [Google Scholar]
- Habib M, Gayraud D, Oliva A, Regis J, Salamon G, Khalil R. Effects of handedness and sex on the morphology of the corpus callosum: a study with brain magnetic resonance imaging. Brain Cogn. 1991;16:41–61. doi: 10.1016/0278-2626(91)90084-l. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hynd GW, Hall J, Novey ES, Eliopulos D, Black K, Gonzalez JJ, Edmonds JE, Riccio C, Cohen M. Dyslexia and corpus callosum morphology. Arch Neurol. 1995;52:32–38. doi: 10.1001/archneur.1995.00540250036010. [DOI] [PubMed] [Google Scholar]
- Jaccard P. Distribution de la flore alpine dans le bassin des Dranses et dans quelques régions voisines. Bulletin de la Société Vaudoise des Sciences Naturelles. 1901;37:241–272. [Google Scholar]
- Johnson CJ, Beitchman JH, Brownlie EB. Twenty-year follow-up of children with and without speech-language impairments: family, educational, occupational, and quality of life outcomes. Am J Speech Lang Pathol. 2010a;19:51–65. doi: 10.1044/1058-0360(2009/08-0083). [DOI] [PubMed] [Google Scholar]
- Johnson CJ, Beitchman JH, Brownlie EB. Twenty-year follow-up of children with and without speech-language impairments: family, educational, occupational, and quality of life outcomes. Am J Speech Lang Pathol. 2010b;19:51–65. doi: 10.1044/1058-0360(2009/08-0083). [DOI] [PubMed] [Google Scholar]
- Josse G, Seghier ML, Kherif F, Price CJ. Explaining function with anatomy: language lateralization and corpus callosum size. JNeurosci. 2008;28:14132–14139. doi: 10.1523/JNEUROSCI.4383-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadis DS, Goshulak D, Namasivayam A, Pukonen M, Kroll R, De Nil LF, Pang EW, Lerch JP. Cortical thickness in children receiving intensive therapy for idiopathic apraxia of speech. Brain Topogr. 2014;27:240–247. doi: 10.1007/s10548-013-0308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. 2. Bloomington, MN: Pearson; 2004. (KBIT-II) [Google Scholar]
- Lewis BA, Avrich AA, Freebairn LA, Hansen AJ, Sucheston LE, Kuo I, Taylor HG, Iyengar SK, Stein CM. Literacy outcomes of children with early childhood speech sound disorders: impact of endophenotypes. J Speech Lang Hear Res. 2011;54:1628–1643. doi: 10.1044/1092-4388(2011/10-0124). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegeois F, Mayes A, Morgan A. Neural Correlates of Developmental Speech and Language Disorders: Evidence from Neuroimaging. Curr Dev Disord Rep. 2014;1:215–227. doi: 10.1007/s40474-014-0019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegeois F, Tournier JD, Pigdon L, Connelly A, Morgan AT. Corticobulbar tract changes as predictors of dysarthria in childhood brain injury. Neurology. 2013;80:926–932. doi: 10.1212/WNL.0b013e3182840c6d. [DOI] [PubMed] [Google Scholar]
- Luders E, Di Paola M, Tomaiuolo F, Thompson PM, Toga AW, Vicari S, Petrides M, Caltagirone C. Callosal morphology in Williams syndrome: a new evaluation of shape and thickness. Neuroreport. 2007a;18:203–207. doi: 10.1097/WNR.0b013e3280115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Kurth F, Das D, Oyarce DE, Shaw ME, Sachdev P, Easteal S, Anstey KJ, Cherbuin N. Associations between corpus callosum size and ADHD symptoms in older adults: The PATH through life study. Psychiatry Res. 2016;256:8–14. doi: 10.1016/j.pscychresns.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Bilder RM, Thompson PM, Szeszko PR, Hamilton L, Toga AW. Positive correlations between corpus callosum thickness and intelligence. Neuroimage. 2007b;37:1457–1464. doi: 10.1016/j.neuroimage.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Hamilton LS, Phillips OR, Thompson PM, Valle JS, Del’Homme M, Strickland T, McCracken JT, Toga AW, Levitt JG. Decreased callosal thickness in attention-deficit/hyperactivity disorder. BiolPsychiatry. 2009;65:84–88. doi: 10.1016/j.biopsych.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Zaidel E, Thompson PM, Jancke L, Toga AW. Parasagittal asymmetries of the corpus callosum. CerebCortex. 2006;16:346–354. doi: 10.1093/cercor/bhi112. [DOI] [PubMed] [Google Scholar]
- Luders E, Rex DE, Narr KL, Woods RP, Jancke L, Thompson PM, Mazziotta JC, Toga AW. Relationships between sulcal asymmetries and corpus callosum size: gender and handedness effects. CerebCortex. 2003;13:1084–1093. doi: 10.1093/cercor/13.10.1084. [DOI] [PubMed] [Google Scholar]
- Luders E, Thompson PM, Kurth F. Morphometry of the Corpus Callosum. In: Spalletta G, Gili T, Piras F, editors. Brain Morphometry: Methods and Clinical Applications. Springer; 2018. [Google Scholar]
- Luders E, Thompson PM, Narr KL, Zamanyan A, Chou YY, Gutman B, Dinov ID, Toga AW. The link between callosal thickness and intelligence in healthy children and adolescents. Neuroimage. 2011;54:1823–1830. doi: 10.1016/j.neuroimage.2010.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Toga AW, Thompson PM. Why size matters: differences in brain volume account for apparent sex differences in callosal anatomy. NeuroImage. 2014;84:820–824. doi: 10.1016/j.neuroimage.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann VA, Foy JG. Speech development patterns and phonological awareness in preschool children. Ann Dyslexia. 2007;57:51–74. doi: 10.1007/s11881-007-0002-1. [DOI] [PubMed] [Google Scholar]
- Mayes AK, Reilly S, Morgan AT. Neural correlates of childhood language disorder: a systematic review. Developmental medicine and child neurology. 2015;57:706–717. doi: 10.1111/dmcn.12714. [DOI] [PubMed] [Google Scholar]
- Morgan A, Bonthrone A, Liegeois FJ. Brain basis of childhood speech and language disorders: are we closer to clinically meaningful MRI markers? Curr Opin Pediatr. 2016;28:725–730. doi: 10.1097/MOP.0000000000000420. [DOI] [PubMed] [Google Scholar]
- Morgan A, Ttofari Eecen K, Pezic A, Brommeyer K, Mei C, Eadie P, Reilly S, Dodd B. Who to Refer for Speech Therapy at 4 Years of Age Versus Who to “Watch and Wait”? J Pediatr. 2017 doi: 10.1016/j.jpeds.2017.02.059. [DOI] [PubMed] [Google Scholar]
- Morgan AT, Masterton R, Pigdon L, Connelly A, Liegeois FJ. Functional magnetic resonance imaging of chronic dysarthric speech after childhood brain injury: reliance on a left-hemisphere compensatory network. Brain: a journal of neurology. 2013;136:646–657. doi: 10.1093/brain/aws355. [DOI] [PubMed] [Google Scholar]
- Njiokiktjien C. Developmental dysphasia: clinical importance and underlying neurological causes. Acta Paedopsychiatr. 1990;53:126–137. [PubMed] [Google Scholar]
- Pani E, Zheng X, Wang J, Norton A, Schlaug G. Right hemisphere structures predict poststroke speech fluency. Neurology. 2016;86:1574–1581. doi: 10.1212/WNL.0000000000002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrini E, Conti V, Dobyns WB, Guerrini R. Genetic Basis of Brain Malformations. Mol Syndromol. 2016;7:220–233. doi: 10.1159/000448639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston JL, Felsenfeld S, Frost SJ, Mencl WE, Fulbright RK, Grigorenko EL, Landi N, Seki A, Pugh KR. Functional brain activation differences in school-age children with speech sound errors: speech and print processing. J Speech Lang Hear Res. 2012;55:1068–1082. doi: 10.1044/1092-4388(2011/11-0056). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston JL, Molfese PJ, Mencl WE, Frost SJ, Hoeft F, Fulbright RK, Landi N, Grigorenko EL, Seki A, Felsenfeld S, Pugh KR. Structural brain differences in school-age children with residual speech sound errors. Brain and language. 2014;128:25–33. doi: 10.1016/j.bandl.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Yakovlev PI. Development of the corpus callosum and cavum septi in man. JComp Neurol. 1968;132:45–72. doi: 10.1002/cne.901320103. [DOI] [PubMed] [Google Scholar]
- Redle E, Vannest J, Maloney T, Tsevat RK, Eikenberry S, Lewis B, Shriberg LD, Tkach J, Holland SK. Functional MRI evidence for fine motor praxis dysfunction in children with persistent speech disorders. Brain Res. 2015;1597:47–56. doi: 10.1016/j.brainres.2014.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly S, McKean C, Morgan A, Wake M. Identifying and managing common childhood language and speech impairments. BMJ. 2015;350:h2318. doi: 10.1136/bmj.h2318. [DOI] [PubMed] [Google Scholar]
- Reilly S, Wake M, Bavin EL, Prior M, Williams J, Bretherton L, Eadie P, Barrett Y, Ukoumunne OC. Predicting language at 2 years of age: a prospective community study. Pediatrics. 2007;120:e1441–1449. doi: 10.1542/peds.2007-0045. [DOI] [PubMed] [Google Scholar]
- Reilly S, Wake M, Ukoumunne OC, Bavin E, Prior M, Cini E, Conway L, Eadie P, Bretherton L. Predicting language outcomes at 4 years of age: findings from Early Language in Victoria Study. Pediatrics. 2010;126:e1530–1537. doi: 10.1542/peds.2010-0254. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical evaluation of language fundamentals. 4. Toronto, Canada: The Psychological Corporation/A Harcourt Assessment Company; 2003. (CELF-IV) [Google Scholar]
- Silveri MC, Incordino F, Lo Monaco R, Bizzarro A, Masullo C, Piludu F, Colosimo C. Neural substrates of the ‘low-level’ system for speech articulation: Evidence from primary opercular syndrome. J Neuropsychol. 2016 doi: 10.1111/jnp.12099. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Sowell ER, Gogtay N, Giedd JN, Rapoport JL, de Zubicaray GI, Janke AL, Rose SE, Semple J, Doddrell DM, Wang Y, van Erp TG, Cannon TD, Toga AW. Mapping cortical change in Alzheimer’s disease, brain development, and schizophrenia. Neuroimage. 2004;23(Suppl 1):S2–18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Perrone-Bertolotti M, Jobard G, Mazoyer B, Baciu M. Multi-factorial modulation of hemispheric specialization and plasticity for language in healthy and pathological conditions: A review. Cortex; a journal devoted to the study of the nervous system and behavior. 2016 doi: 10.1016/j.cortex.2016.05.013. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation, Harcourt Brace and Company; San Antonio, TX: 1999. [Google Scholar]
- Wiig EH, Secord WA, Semel E. Clinical evaluation of language fundamentals—Preschool, second edition (CELF Preschool-2) Toronto, Canada: The Psychological Corporation/A Harcourt Assessment Company; 2004. [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112(Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Wren YE, Roulstone SE, Miller LL. Distinguishing groups of children with persistent speech disorder: findings from a prospective population study. Logoped Phoniatr Vocol. 2012;37:1–10. doi: 10.3109/14015439.2011.625973. [DOI] [PubMed] [Google Scholar]
- Zarei M, Johansen-Berg H, Smith S, Ciccarelli O, Thompson AJ, Matthews PM. Functional anatomy of interhemispheric cortical connections in the human brain. JAnat. 2006;209:311–320. doi: 10.1111/j.1469-7580.2006.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]