Abstract
Patients with major depressive disorder (MDD) have clinically relevant, significant decreases in bone mineral density (BMD). We sought to determine if predictive markers of bone inflammation—the osteoprotegerin (OPG)-RANK-RANKL system or osteopontin (OPN)—play a role in the bone abnormalities associated with MDD and, if so, whether ketamine treatment corrected the abnormalities. The OPG-RANK-RANKL system plays the principal role in determining the balance between bone resorption and bone formation. RANKL is the osteoclast differentiating factor and diminishes BMD. OPG is a decoy receptor for RANKL, thereby increasing BMD. OPN is the bone glue that acts as a scaffold between bone tissues matrix composition to bind them together and is an important component of bone strength and fracture resistance. Twenty-eight medication-free inpatients with treatment-resistant MDD and 16 healthy controls (HCs) participated in the study. Peripheral bone marker levels and their responses to IV ketamine infusion in MDD patients and HCs were measured at four time points: at baseline, and post-infusion at 230 minutes, Day 1, and Day 3. Patients with MDD had significant decreases in baseline OPG/RANKL ratio and in plasma OPN levels. Ketamine significantly increased both the OPG/RANKL ratio and plasma OPN levels and significantly decreased RANKL levels. Bone marker levels in HCs remained unaltered. We conclude that the OPG-RANK-RANKL system and the OPN system play important roles in the serious bone abnormalities associated with MDD. These data suggest that in addition to its antidepressant effects, ketamine also has a salutary effect on a major medical complication of depressive illness.
INTRODUCTION
More than 20 studies have found that patients with major depressive disorder (MDD) have a clinically significant loss of trabecular bone mineral density (BMD) at the hip and spine (1–23). Older women with depression experience increased fracture rates, and our group previously reported that even premenopausal women with depression (age 40–50 years old) had osteopenia or osteoporosis (1). These women had an unusual pattern of loss, with greater losses of BMD at the hip than at the spine. This pattern is found more frequently when inflammatory mediators are thought to be a particularly important etiologic factor in bone loss (1, 24).
Bone formation is a dynamic, ever-evolving process in which bone production and bone resorption are taking place all the time (25, 26). If production is the predominant mode, bone tissue increases. If resorption is the predominant mode, bone tissue and BMD are lost. Bone production is mediated by osteoblasts, while bone resorption is mediated by osteoclasts (27). One function of osteoblast and osteoclast-mediated bone turnover is to allow the skeleton to be as light as possible while meeting biomechanical needs, with a safety margin sufficient to avoid most fractures. Another reason is to accommodate changes in the pattern of activity that might benefit from a shift in BMD from one spot to another. A third would be to correct as many cracks or micro-fractures that accumulate over time as possible.
Receptor activator of nuclear factor-κB ligand (RANKL) is the osteoclast differentiating factor and thus causes bone loss (28–30). Osteoprotegerin (OPG) is a decoy for RANKL and thus preserves BMD (31, 32). The OPG/RANKL ratio is a widely accepted index of the balance between bone resorption and bone formation (29, 32–35). Osteopontin (OPN) is secreted by osteoblasts in the early stages of osteogenesis. It is thought to act as a scaffold between bone tissues with different matrix compositions and to provide cohesion between them (36–38). It has been suggested that OPN is the bone ‘glue’ that promotes fiber matrix bonding as well as crack bridging in the case of micro-crack formation. Our bones are full of microscopic cracks, but they are highly resistant to fracture. OPN knockout mice show a 40% increase in bone fractures, independent of changes in whole bone mass, structure, or matrix porosity (39). This is important because BMD cannot always predict fracture risk in humans or laboratory animals. While OPN also helps anchor osteoclasts, data clearly show that OPN is crucial to the maintenance of bone toughness and resistance to fracture (37, 39, 40). Thus, the effect of osteoclast binding is not enough to materially influence its important role in bone strength. OPN may play a particularly important role as bone ages and becomes more fragile and subject to fracture.
This study addressed three questions: 1) is the OPG/RANKL ratio altered in MDD patients compared to healthy controls (HCs), and if so, does ketamine correct the deficit?; 2) are OPN plasma levels reduced in patients with MDD compared to HCs, and if so, does ketamine correct the deficit?; and 3) what are the potential clinical implications of these findings? As part of the study, we also measured other relevant bone markers involved in bone remodeling, including osteocalcin (involved in bone metabolism and mineralization (41)), eotaxin-1 (originally associated with inflammatory chemotaxis in mood disorders (42)), and fibroblast growth factor 23 (FGF-23, a key regulator of phosphorus and vitamin D metabolism (43)).
MATERIALS AND METHODS
Patient selection, study design, and outcome measures
Forty-four subjects (females (n=25) and males (n=19), ages 18–65 years) were included in the study; 28 had treatment-resistant MDD and 16 were HCs. Patients with MDD were currently experiencing a major depressive episode lasting at least four weeks. Data were drawn from previous studies exploring ketamine’s mechanism of action (NCT00088699); results of these studies have been previously published (44, 45). Briefly, each study was a double-blind, randomized, placebo-controlled, crossover trial assessing the antidepressant efficacy of ketamine for treatment-resistant depression. Treatment-resistance was defined as a current or past history of lack of response to at least two adequate antidepressant or neuromodulatory (including electroconvulsive therapy) trials as assessed by our modified version of the Antidepressant Treatment History Form (46). Participants enrolled in this protocol had to meet DSM-IV-TR criteria for MDD without having a comorbid diagnosis of alcohol or substance abuse or dependence in the past 90 days, as determined by the Structured Clinical Interview for DSM-IV-TR (47). All participants had a Montgomery-Asberg Depression Rating Scale (MADRS) score of at least 20 at baseline, were unmedicated for at least two weeks (five weeks for fluoxetine) before their first ketamine/placebo IV infusion, and were in good medical health, as determined by medical history, physical examination, and routine blood and urine laboratory tests. All patients had similar diets during the studies. HCs had no DSM-IV-TR Axis I disorders nor any medical comorbidities. The studies were approved by the NIH Combined Neuroscience institutional review board and written informed consent was provided by all participants before study entry.
Subjects received a single infusion of ketamine hydrochloride (0.5 mg/ kg) over 40 minutes. Here we report results from 60 minutes prior to infusion (baseline) as well as 230 minutes, Day 1, and Day 3 post-infusion. Ratings included the MADRS and the 17-item Hamilton Depression Rating Scale (HAM-D), both of which were administered at the same time points as those used for peripheral blood collection. Bone marker levels were examined using samples only through Day 3, given that maximum antidepressant response to ketamine generally reaches its maximum by that point (48).
Bone Marker Measurements
Whole-blood samples were collected using the Vacutainer system. Baseline samples were obtained at 0800 hours for all patients. Samples were centrifuged at 3000 r.p.m. at 4°C for 10 minutes and stored at −80°C until assay performance. We measured the four most critical mediators in bone turnover—circulating plasma levels of RANKL and OPG as well as the OPG/RANKL and OPN/RANKL ratios—using the high-sensitivity multiplex Luminex immunoassay (xMAP Technology, Austin, TX, USA) and fluorescently color-coded magnetic microsphere beads (R&D Systems; Minneapolis, MN, USA) according to the manufacturer’s instructions; we used the same method to measure the three other bone markers of interest (osteocalcin, eotaxin-1, and FGF-23). Samples were diluted 1:2, measured in duplicate, and blinded to clinical information. The standard cocktail was created as a fourfold dilution series to concentrations ranging from 4.81–10,510 pg/ml for RANKL, 13.4–29,310 pg/ml for eotaxin, 0.46–7500 pg/ml for OPG, 9.16–150000 pg/ml for osteocalcin, 6.1–100000 pg/ml for OPN, and 2.29–37500 pg/ml for FGF-23. After the addition of a biotinylated antibody cocktail and streptavidin-PE, levels of all analytes were determined with a Bio-Plex Magpix Multiplex Reader (Bio-Rad; Hercules, CA, USA). Concentration values were calculated automatically with Bio-Plex Manager MP Software (Philadelphia, PA, USA) by generating a five-parameter logistic curve-fit standard curve for each analysis. Bone marker measurements were measured at baseline (60 minutes prior to IV ketamine infusion) and at 230 minutes, Day 1, and Day 3 post-IV ketamine for both MDD patients and for HCs at the same time points except for Day 3, due to lack of HC data.
Statistical Analysis
Data for RANKL, OPN, and eotaxin were used in their original form. Osteocalcin, OPG, and FGF-23 values were transformed using a natural log to make their distributions closer to normal. Fisher’s exact tests were used for categorical variables and independent t-tests were used with continuous variables to compare MDD patients and HCs on demographic and clinical characteristics and on bone markers.
Linear mixed models with time as fixed factor and a compound symmetry covariance structure were used to examine changes over time in MDD patients. Bonferroni adjusted post-hoc tests were used to examine change from baseline to each point. Observations at 60 minutes pre-infusion and 230 minutes, Day 1, and Day 3 post-infusion were included. An additional series of models included diagnosis as a second fixed factor as well as the interaction between diagnosis and time. Baseline was used as a covariate, and post-hoc tests compared diagnostic groups at each time point.
Bivariate associations were assessed with Pearson correlations. Correlations examined baseline demographic factors, baseline bone marker levels, and antidepressant response measured as a 50% decrease in MADRS scores at any time point as well as changes in bone marker levels in response to ketamine infusion at 230 minutes, Day 1, and Day 3 (see Figure 1).
Figure 1.
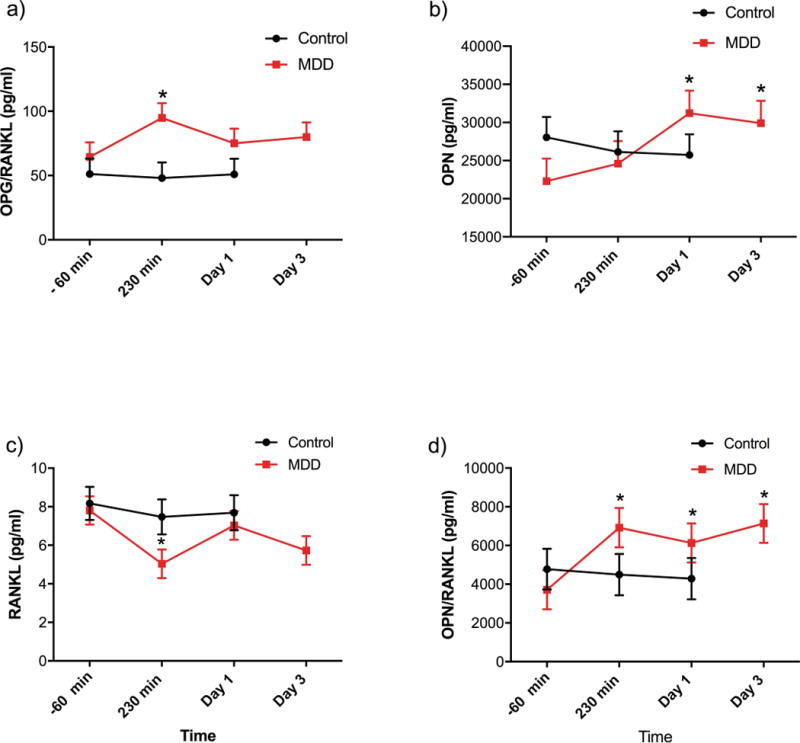
(a) Osteoprotegerin (OPG)/receptor activator of nuclear factor kappa-B ligand (RANKL) ratio, (b) osteopontin (OPN), (c) RANKL, and (d) OPN/RANKL ratio plasma levels of healthy control (HC) and major depressive disorder (MDD) subjects at baseline (pre-treatment), 230 minutes post-ketamine infusion, Day 1, and Day 3 post-ketamine infusion. *P<0.001, **P<0.001.
All tests were two-tailed with significance set at P<0.05. A Bonferroni correction was applied for the number of bone markers examined with each type of analysis, so a cutoff of P<0.01 was used. Data are presented as mean±standard deviation. All statistical analyses were completed using IBM SPSS Version 21 (Armonk, NY, USA).
RESULTS
Clinical and demographic characteristics for all 44 subjects are summarized in Table 1. The OPG/RANKL ratio, which indicates a process of positive bone balance between resorption and formation, was strikingly increased after acute ketamine infusion in MDD patients (230 min (p>0.004)); it maintained an increased trend but did not reach significance at Day 1 (p=0.736) or Day 3 (p=0.27) (Fig 1A). Interestingly, ketamine infusion had no effect on bone markers for HCs, and there were no significant changes from baseline. Plasma OPN, a factor in bone strength, was also significantly reduced in MDD patients (22296.8 ± 2953.4 pg/ml) compared to HCs (28062.5 ± 2673.8 pg/ml). While OPN was relatively unchanged in HCs, MDD patients showed a significant increase in response to ketamine infusion at Day 1 (31219.7 ± 2953.5 pg/ml; P<0.001) and Day 3 (29912 ± 2953.5 pg/ml; P<0.001) (Fig 1B).
Table 1.
| Demographics. | Total | HCs | MDD | |||
|---|---|---|---|---|---|---|
|
| ||||||
| (N =44) | (n = 16) | (n = 28) | ||||
|
| ||||||
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 35.34 | 10.19 | 33.67 | 10.38 | 37 | 10 |
| Age of Onset (Years) | 16.40 | 6.75 | 16.4 | 6.75 | ||
| Duration of Current Episode (Months) | 29.46 | 47.87 | 29.46 | 47.87 | ||
| Duration of Illness (Years) | 20.96 | 11.31 | 20.96 | 11.31 | ||
| BMI (kg/m2) | 28.25 | 5.9 | 27.21 | 4.72 | 29.29 | 7.08 |
| HAM-D17 (Baseline) | 11.17 | 2.635 | 0.8 | 0.86 | 21.54 | 4.41 |
| MADRS (Baseline) | 17.18 | 2.67 | 0.67 | 0.72 | 33.68 | 4.62 |
|
| ||||||
| n | % | n | % | n | % | |
|
| ||||||
| Gender (Female) | 25 | 58.1 | 7 | 46.7 | 18 | 64.3 |
| Ethnicity (Caucasian) | 39 | 97.5 | 11 | 91.7 | 28 | 100 |
| Education (Completed College) | 19 | 59.4 | 5 | 100 | 14 | 51.9 |
BMI: body mass index; HAM-D17: Hamilton Depression Rating Scale, 17-item; MADRS: Montgomery-Asberg Depression Rating Scale; MDD: major depressive disorder; HCs: healthy controls.
Notably, patients with MDD who had a lower OPN/RANKL ratio compared to HCs (indicating a bone loss state) had a significant increase in the OPN/RANKL ratio in response to ketamine infusion at 230 minutes (p<0.005), Day 1 (p<0.043), and Day 3 (p<0.002). HCs had no significant change in OPN/RANKL ratio to ketamine administration (Fig 1C). Similarly, while RANKL (a key peripheral and central osteoclastogenenic cytokine) levels were relatively comparable at baseline among MDD patients (7.8 ± 0.74 pg/ml) and HCs (8.17 ± 0.86 pg/ml), MDD patients had significant decreases in RANKL levels in response to ketamine infusion at 230 minutes (p<0.0007) and Day 3 (p<0.003) but not at Day 1 (p<0.622); no such changes were noted in HCs (Fig 1D). Thus, in patients with MDD, ketamine corrected an adverse bone metabolic state, returning it to normal.
Table 2 summarizes the estimated marginal means for the peripheral blood levels of bone markers involved in bone remodeling, including osteocalcin, eotaxin-1, and FGF-23. These factors were largely not associated with significant changes.
Table 2.
Estimated Marginal Means
| Bone Marker | HCs (n=16) | MDD (n=28) | |||
|---|---|---|---|---|---|
|
| |||||
| Mean | S.E.M. | Mean | S.E.M. | P-value | |
|
|
|||||
| OPN | |||||
| Baseline | 24028.24 | 24028.24 | |||
| 230 min | 22202.92 | 2184.84 | 26395.68 | 1564.93 | 0.128 |
| Day 1 | 21802.01 | 2180.01 | 32886.39 | 1539.29 | 0.000 |
|
| |||||
| RANKL | |||||
| Baseline | 7.77 | 7.77 | |||
| 230 min | 7.23 | 0.82 | 4.96 | 0.59 | 0.03 |
| Day 1 | 7.56 | 0.82 | 7.01 | 0.59 | 0.58 |
|
| |||||
| OPG (natural log) | |||||
| Baseline | 5.72 | 5.72 | |||
| 230 min | 5.65 | 0.03 | 5.70 | 0.03 | 0.242 |
| Day 1 | 5.71 | 0.02 | 5.72 | 0.02 | 0.820 |
|
| |||||
| Eotaxin | |||||
| Baseline | 345.42 | 345.42 | |||
| 230 min | 317.19 | 19.59 | 327.56 | 14.15 | 0.670 |
| Day 1 | 359.07 | 19.58 | 362.72 | 13.97 | 0.880 |
|
| |||||
| OC (natural log) | |||||
| Baseline | 9.51 | 9.51 | |||
| 230 min | 9.30 | 0.04 | 9.39 | 0.03 | 0.106 |
| Day 1 | 9.37 | 0.04 | 9.45 | 0.03 | 0.125 |
|
| |||||
| FGF-23 (natural log) | |||||
| Baseline | 3.17 | 3.17 | |||
| 230 min | 3.04 | 0.05 | 3.03 | 0.03 | 0.941 |
| Day 1 | 3.06 | 0.05 | 3.09 | 0.03 | 0.650 |
|
| |||||
| OPN/RANKL ratio | |||||
| Baseline | 4175.24 | 4175.24 | |||
| 230 min | 3997.20 | 1088.44 | 7336.81 | 782.79 | 0.015 |
| Day 1 | 3834.11 | 1087.90 | 6509.51 | 769.48 | 0.049 |
|
| |||||
| OPG/RANKL ratio | |||||
| Baseline | 61.71 | 61.71 | |||
| 230 min | 56.83 | 9.55 | 92.91 | 6.89 | 0.003 |
| Day 1 | 59.61 | 9.55 | 72.80 | 6.80 | 0.265 |
Covariates appearing in the model are evaluated based on the estimated marginal means of same baseline. The difference between HCs and MDD patients was significant at the 0.05 level.
P<0.001,
P<0.001.
MDD: major depressive disorder; HCs: healthy controls; RANKL: receptor activator of nuclear factor kappa-B ligand; OPN: osteopontin; OPG: osteoprotegerin; OC: osteocalcin; FGF-23: fibroblast growth factor 23
DISCUSSION
MDD patients have clinically significant decreases in BMD (1, 5, 7, 8, 10, 11, 13, 15, 16, 19). A previous study from our laboratory found that reduced BMD was a function of both decreased production and increased resorption (1). The present study found that the OPG/RANKL ratio, a recognized index of bone formation/resorption, was altered in MDD patients and increased significantly after ketamine infusion, thus suggesting that ketamine may have mediated a possible increase in BMD. OPN, a critical factor in maintaining bone strength, was also significantly reduced in MDD patients compared to HCs and increased significantly in response to ketamine both at Day 1 and Day 3. Although RANKL, the osteoclastic differentiating factor, was not altered at baseline in MDD patients compared to HCs, its levels fell significantly after ketamine infusion. Ketamine had no impact on any of these indices in HCs. To our knowledge, this is the first study to examine levels of the OPG-RANK-RANKL system and of OPN in MDD patients, and the first to study their response to ketamine. We also found that plasma levels of osteocalcin, eotaxin-1 and FGF-23 were normal at baseline in MDD patients and not affected by ketamine infusion.
Interestingly, circulating proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6), are involved in the pathophysiology of depression for a subgroup of MDD patients known to be in a heightened proinflammatory state (49, 50). Both TNF-α and IL-6 alone can directly stimulate osteoclastogenesis and bone resorption (51, 52) and both can also affect RANKL production by osteoblastic cells and act synergistically with RANKL (30, 53). While IL-6 and other cytokines impair BMD, RANKL can promote osteoclast bone resorption even in the absence of IL-6 (54). Thus, though not obligatory to bone resorption, IL-6 is one of the stimuli that promotes the production of RANKL (55). IL-6 and other cytokines may also work in synergy with RANKL. This suggests that proinflammatory cytokines likely contribute to the bone pathology associated with depression and to the pattern of greater loss at the hip than the spine. Relatedly, estrogens and androgens promote the maintenance of BMD, in part because their receptors can bind to transcription factors that prevent RANK and DNA binding. Estrogens and androgens also repress IL-6-mediated BMD loss, thus further promoting the integrity of BMD in MDD patients (52, 55, 56).
Another possible mechanism underlying ketamine’s salutary effects on bone could be its ability to inhibit inducible nitric oxide synthase (iNOS)-mediated inflammation, which is known to trigger cytokine effects on bone, potentially in synergy with RANKL (57, 58). Ketamine-induced inhibition of iNOS partly occurs via its inhibition of TNF-α, which acts as an autocrine stimulatory factor for iNOS (58), but not via the N-methyl-D-aspartate (NMDA) receptor, which is involved in neuronal nitric oxide production. Under this scenario, ketamine-induced inhibition of iNOS could promote bone growth.
Ketamine also antagonizes neuronal nitric oxide synthase (nNOS) release from cerebral cortex after ischemia-induced middle cerebral artery ligation (59), a process associated with ketamine-induced amelioration of ischemic injury. However, the relationship between this ketamine-induced amelioration of ischemia-induced tissue damage in the brain and ketamine’s antidepressant efficacy is unknown. Whether inhibiting nNOS in the periphery would impact ketamine’s antidepressant effect is also unknown. It should be noted that total nNOS knockout mice were found to have increased BMD, suggesting that nNOS antagonizes bone growth regardless of whether it acts in the brain or on bone (60). In contrast to iNOS and nNOS, endothelial nitric oxide synthase (eNOS) is necessary for normal osteoblast functioning. To the best of our knowledge, ketamine’s putative ability to modulate eNOS is unknown, as a study of the role of eNOS on BMD mediators has not yet been performed.
It is interesting to note that selective serotonin reuptake inhibitors (SSRIs) significantly increase the yearly loss of BMD in children (61), adults (61), and elderly subjects (6, 62) with MDD. An elegant recent animal study with a mechanistic, molecular approach found that three weeks of fluoxetine administration resulted in a local antiresorptive response by impairing the maturation of osteoclastic cells, and that after six weeks of treatment, fluoxetine mediated a centrally-triggered increase in sympathetic nervous system activity (63), which is known to cause substantial bone loss (64). To further validate this premise, the authors showed that the administration of a beta blocker antagonized fluoxetine’s effect on lowering bone mass at six weeks, thereby resulting in no loss of bone mass overall (63).
Relatedly, in vitro and in vivo experimental animal data also indicate a functional role for the serotonergic system on bone formation (65–67). Functional receptors for serotonin and the serotonin transporter have been identified in osteoblasts, osteoclasts, and osteocytes (68). Genetic disruption of the serotonin transporter produces a phenotype of decreased bone mass, altered architecture, and decreased mechanical properties (69). Serotonin transporter inhibition results in a significant loss in bone accrual (70). Thus, in the periphery, serotonin seems to have proinflammatory effects and a detrimental outcome on BMD. This could further contribute to fluoxetine-mediated loss of BMD and a possible increased fracture rate.
Another issue of potential interest is that many systems influenced by ketamine may affect RANKL levels. For example, sympathetic nervous system activation reduces BMD and also plays a role in SSRI-induced decrements in BMD (71, 72). Ketamine stereospecifically stimulates noradrenergic neurons and inhibits catecholamine uptake, leading to an increased hyperadrenergic state (71). Under these circumstances, ketamine would be expected to lower BMD. Nevertheless, we observed that ketamine ultimately reduced RANKL plasma levels, regardless of its impact on noradrenergic function, underscoring its overall ability to increase BMD in individuals with depression.
Finally, several clinical trials using a recombinant fusion protein of OPG, the endogenous inhibitor of RANKL, provided strong proof-of-principle evidence that RANKL was critical for bone resorption in humans (73, 74). Denosumab is a targeted antibody that binds and inhibits RANKL to reduce bone resorption. A large trial of denosumab was conducted in 7808 women aged 60 to 90 years with postmenopausal osteoporosis and baseline BMD T scores between −2.5 and −4.0 at the lumbar spine or total hip. After three years of treatment, denosumab significantly reduced the risk of new radiographic vertebral fractures by 68% compared with placebo. Reductions in nonvertebral and hip fracture risk were also observed (20% (P = 0.01) and 40% (P = 0.04), respectively) (75). Denosumab also proved superior to the bisphosphonates in treating osteoporosis, but its effect is chronic as opposed to the acute effects of ketamine (76, 77).
Our study has several clinical implications. First, it adds to the growing literature showing that decreases in the OPG-RANK-RANKL system are an important contributing factor to osteoporosis (29, 34) and also affect the final common pathways for proinflammatory cytokines (33, 54). Thus, the ability of acute ketamine administration to restore the OPG/RANKL ratio could potentially protect bone and reduce inflammation. In addition, the ability of ketamine to normalize decreased OPN levels and the OPN/RANKL ratio and, inversely, to decrease RANKL levels in MDD patients, indicates that ketamine or other ketamine-like drugs with a more favorable side effect profile—in addition to having potent mood effects—may also help ameliorate a serious medical complication of depressive illness. Although we studied 28 medication-free MDD patients in this study, studies in a larger group of patients would help to substantiate these findings.
As noted above, ketamine influences many mediators that could have either positive or negative effects on BMD, including multiple proinflammatory cytokines and the central and peripheral serotonergic system. The present study measured compounds, such as RANKL, that serve as the final common mediator for many of the effects that cytokines and other compounds have on reducing BMD (28, 29, 35, 53). The fact that RANKL decreased significantly in MDD patients after acute ketamine administration suggests that the impact of ketamine on BMD will be positive. The singular importance of RANKL is demonstrated by the fact that studies have shown that an antibody to RANKL increases BMD when given over long periods of time (75, 78–80). However, only studies of long-term ketamine administration over multiple cycles of bone formation and resorption can definitively answer this question.
Acknowledgments
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA MH002927), by a NARSAD Independent Investigator Award to Dr. Zarate, and by a Brain and Behavior Mood Disorders Research Award to Dr. Zarate. The authors thank the 7SE research unit and staff for their support.
Footnotes
Conflict of Interest
Dr. Zarate is listed as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain. Dr. Zarate is listed as co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation and post-traumatic stress disorders; he has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. All other authors have no conflict of interest to disclose, financial or otherwise.
References
- 1.Michelson D, Stratakis C, Hill L, Reynolds J, Galliven E, Chrousos G, et al. Bone mineral density in women with depression. N Engl J Med. 1996 Oct 17;335:1176–1181. doi: 10.1056/NEJM199610173351602. [DOI] [PubMed] [Google Scholar]
- 2.Altindag O, Altindag A, Asoglu M, Gunes M, Soran N, Deveci Z. Relation of cortisol levels and bone mineral density among premenopausal women with major depression. Int J Clin Pract. 2007 Mar;61:416–420. doi: 10.1111/j.1742-1241.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 3.Cizza G, Mistry S, Nguyen VT, Eskandari F, Martinez P, Torvik S, et al. Do premenopausal women with major depression have low bone mineral density? A 36-month prospective study. PLoS One. 2012;7:e40894. doi: 10.1371/journal.pone.0040894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cizza G, Ronsaville DS, Kleitz H, Eskandari F, Mistry S, Torvik S, et al. Clinical subtypes of depression are associated with specific metabolic parameters and circadian endocrine profiles in women: the power study. PLoS One. 2012;7:e28912. doi: 10.1371/journal.pone.0028912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coelho R, Silva C, Maia A, Prata J, Barros H. Bone mineral density and depression: a community study in women. J Psychosom Res. 1999 Jan;46:29–35. doi: 10.1016/s0022-3999(98)00064-6. [DOI] [PubMed] [Google Scholar]
- 6.Diem SJ, Harrison SL, Haney E, Cauley JA, Stone KL, Orwoll E, et al. Depressive symptoms and rates of bone loss at the hip in older men. Osteoporos Int. 2013 Jan;24:111–119. doi: 10.1007/s00198-012-1975-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eskandari F, Martinez PE, Torvik S, Phillips TM, Sternberg EM, Mistry S, et al. Low bone mass in premenopausal women with depression. Arch Intern Med. 2007 Nov 26;167:2329–2336. doi: 10.1001/archinte.167.21.2329. [DOI] [PubMed] [Google Scholar]
- 8.Furlan PM, Ten Have T, Cary M, Zemel B, Wehrli F, Katz IR, et al. The role of stress-induced cortisol in the relationship between depression and decreased bone mineral density. Biol Psychiatry. 2005 Apr 15;57:911–917. doi: 10.1016/j.biopsych.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 9.Hsiao MC, Liu CY, Wang CJ. Factors associated with low bone density among women with major depressive disorder. Int J Psychiatry Med. 2012;44:77–90. doi: 10.2190/PM.44.1.f. [DOI] [PubMed] [Google Scholar]
- 10.Jacka FN, Pasco JA, Henry MJ, Kotowicz MA, Dodd S, Nicholson GC, et al. Depression and bone mineral density in a community sample of perimenopausal women: Geelong Osteoporosis Study. Menopause. 2005 Jan-Feb;12:88–91. doi: 10.1097/00042192-200512010-00015. [DOI] [PubMed] [Google Scholar]
- 11.Kurmanji JM, Sulaiman SA, Kah LK, Chandrasekaran PK. Depression and low bone mineral density: The correlation among Chinese. Asian J Psychiatr. 2010 Sep;3:134–137. doi: 10.1016/j.ajp.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Laudisio A, Marzetti E, Cocchi A, Bernabei R, Zuccala G. Association of depressive symptoms with bone mineral density in older men: a population-based study. Int J Geriatr Psychiatry. 2008 Nov;23:1119–1126. doi: 10.1002/gps.2037. [DOI] [PubMed] [Google Scholar]
- 13.Mussolino ME, Jonas BS, Looker AC. Depression and bone mineral density in young adults: results from NHANES III. Psychosom Med. 2004 Jul-Aug;66:533–537. doi: 10.1097/01.psy.0000132873.50734.7d. [DOI] [PubMed] [Google Scholar]
- 14.Oh SM, Kim HC, Ahn SV, Rhee Y, Suh I. Association between depression and bone mineral density in community-dwelling older men and women in Korea. Maturitas. 2012 Feb;71:142–146. doi: 10.1016/j.maturitas.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Petronijevic M, Petronijevic N, Ivkovic M, Stefanovic D, Radonjic N, Glisic B, et al. Low bone mineral density and high bone metabolism turnover in premenopausal women with unipolar depression. Bone. 2008 Mar;42:582–590. doi: 10.1016/j.bone.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Robbins J, Hirsch C, Whitmer R, Cauley J, Harris T. The association of bone mineral density and depression in an older population. J Am Geriatr Soc. 2001 Jun;49:732–736. doi: 10.1046/j.1532-5415.2001.49149.x. [DOI] [PubMed] [Google Scholar]
- 17.Schweiger U, Deuschle M, Korner A, Lammers CH, Schmider J, Gotthardt U, et al. Low lumbar bone mineral density in patients with major depression. Am J Psychiatry. 1994 Nov;151:1691–1693. doi: 10.1176/ajp.151.11.1691. [DOI] [PubMed] [Google Scholar]
- 18.Schweiger U, Weber B, Deuschle M, Heuser I. Lumbar bone mineral density in patients with major depression: evidence of increased bone loss at follow-up. Am J Psychiatry. 2000 Jan;157:118–120. doi: 10.1176/ajp.157.1.118. [DOI] [PubMed] [Google Scholar]
- 19.Spangler L, Scholes D, Brunner RL, Robbins J, Reed SD, Newton KM, et al. Depressive symptoms, bone loss, and fractures in postmenopausal women. J Gen Intern Med. 2008 May;23:567–574. doi: 10.1007/s11606-008-0525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whooley MA, Kip KE, Cauley JA, Ensrud KE, Nevitt MC, Browner WS. Depression, falls, and risk of fracture in older women. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999 Mar 8;159:484–490. doi: 10.1001/archinte.159.5.484. [DOI] [PubMed] [Google Scholar]
- 21.Williams LJ, Bjerkeset O, Langhammer A, Berk M, Pasco JA, Henry MJ, et al. The association between depressive and anxiety symptoms and bone mineral density in the general population: the HUNT Study. J Affect Disord. 2011 Jun;131:164–171. doi: 10.1016/j.jad.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Wong SY, Lau EM, Lynn H, Leung PC, Woo J, Cummings SR, et al. Depression and bone mineral density: is there a relationship in elderly Asian men? Results from Mr. Os (Hong Kong) Osteoporos Int. 2005 Jun;16:610–615. doi: 10.1007/s00198-004-1730-2. [DOI] [PubMed] [Google Scholar]
- 23.Yazici KM, Akinci A, Sutcu A, Ozcakar L. Bone mineral density in premenopausal women with major depressive disorder. Psychiatry Res. 2003 Mar 25;117:271–275. doi: 10.1016/s0165-1781(03)00017-9. [DOI] [PubMed] [Google Scholar]
- 24.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009 May 1;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seibel MJ. Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem Rev. 2005 Nov;26:97–122. [PMC free article] [PubMed] [Google Scholar]
- 26.Seibel MJ. Biochemical markers of bone turnover part II: clinical applications in the management of osteoporosis. Clin Biochem Rev. 2006 Aug;27:123–138. [PMC free article] [PubMed] [Google Scholar]
- 27.Raisz LG. Physiology and pathophysiology of bone remodeling. Clin Chem. 1999 Aug;45:1353–1358. [PubMed] [Google Scholar]
- 28.Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9(Suppl 1):S1. doi: 10.1186/ar2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008 May 15;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giuliani N, Colla S, Morandi F, Rizzoli V. The RANK/RANK ligand system is involved in interleukin-6 and interleukin-11 up-regulation by human myeloma cells in the bone marrow microenvironment. Haematologica. 2004 Sep;89:1118–1123. [PubMed] [Google Scholar]
- 31.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998 May 01;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tyrovola JB. The ‘Mechanostat’ Principle and the Osteoprotegerin -OPG/RANKL/RANK System PART II. The Role of the Hypothalamic – Pituitary Axis. J Cell Biochem. 2016 Nov 15; doi: 10.1002/jcb.25793. [DOI] [PubMed] [Google Scholar]
- 33.Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012 May;11:401–419. doi: 10.1038/nrd3705. [DOI] [PubMed] [Google Scholar]
- 34.Liu JZ, Ji ZL, Chen SM. The OPG/RANKL/RANK system and bone resorptive disease. Sheng Wu Gong Cheng Xue Bao. 2003 Nov;19:655–660. [PubMed] [Google Scholar]
- 35.Tyrovola JB. The “Mechanostat Theory” of Frost and the OPG/RANKL/RANK System. J Cell Biochem. 2015 Dec;116:2724–2729. doi: 10.1002/jcb.25265. [DOI] [PubMed] [Google Scholar]
- 36.Butler WT. The nature and significance of osteopontin. Connect Tissue Res. 1989;23:123–136. doi: 10.3109/03008208909002412. [DOI] [PubMed] [Google Scholar]
- 37.McKee MD, Nanci A. Osteopontin deposition in remodeling bone: an osteoblast mediated event. J Bone Miner Res. 1996 Jun;11:873–875. doi: 10.1002/jbmr.5650110620. [DOI] [PubMed] [Google Scholar]
- 38.Pagel CN, Wasgewatte Wijesinghe DK, Taghavi Esfandouni N, Mackie EJ. Osteopontin, inflammation and myogenesis: influencing regeneration, fibrosis and size of skeletal muscle. J Cell Commun Signal. 2014 Jun;8:95–103. doi: 10.1007/s12079-013-0217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thurner PJ, Chen CG, Ionova-Martin S, Sun L, Harman A, Porter A, et al. Osteopontin deficiency increases bone fragility but preserves bone mass. Bone. 2010 Jun;46:1564–1573. doi: 10.1016/j.bone.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- 41.Hoang QQ, Sicheri F, Howard AJ, Yang DS. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature. 2003 Oct 30;425:977–980. doi: 10.1038/nature02079. [DOI] [PubMed] [Google Scholar]
- 42.Grassi-Oliveira R, Brieztke E, Teixeira A, Pezzi JC, Zanini M, Lopes RP, et al. Peripheral chemokine levels in women with recurrent major depression with suicidal ideation. Rev Bras Psiquiatr. 2012 Mar;34:71–75. doi: 10.1590/s1516-44462012000100013. [DOI] [PubMed] [Google Scholar]
- 43.Wesseling-Perry K. FGF-23 in bone biology. Pediatr Nephrol. 2010 Apr;25:603–608. doi: 10.1007/s00467-009-1384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010 Dec;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006 Aug;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 46.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
- 47.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version, Patient Edition. New York State Psychiatric Institute, Biometrics Research; New York: 2001. [Google Scholar]
- 48.Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, et al. Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry. 2009 Dec;70:1662–1666. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshimura R, Hori H, Ikenouchi-Sugita A, Umene-Nakano W, Ueda N, Nakamura J. Higher plasma interleukin-6 (IL-6) level is associated with SSRI- or SNRI-refractory depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Jun 15;33:722–726. doi: 10.1016/j.pnpbp.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 50.Lotrich FE. Inflammatory cytokine-associated depression. Brain Res. 2015 Aug 18;1617:113–125. doi: 10.1016/j.brainres.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hughes AE, Ralston SH, Marken J, Bell C, MacPherson H, Wallace RG, et al. Mutations in TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis. Nat Genet. 2000 Jan;24:45–48. doi: 10.1038/71667. [DOI] [PubMed] [Google Scholar]
- 52.Axmann R, Bohm C, Kronke G, Zwerina J, Smolen J, Schett G. Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum. 2009 Sep;60:2747–2756. doi: 10.1002/art.24781. [DOI] [PubMed] [Google Scholar]
- 53.Weitzmann MN. The Role of Inflammatory Cytokines, the RANKL/OPG Axis, and the Immunoskeletal Interface in Physiological Bone Turnover and Osteoporosis. Scientifica (Cairo) 2013;2013:125705. doi: 10.1155/2013/125705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwan Tat S, Padrines M, Theoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004 Feb;15:49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 55.Stein B, Yang MX. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol Cell Biol. 1995 Sep;15:4971–4979. doi: 10.1128/mcb.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, Listwak SJ, et al. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J Clin Endocrinol Metab. 2005 May;90:2522–2530. doi: 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- 57.van’t Hof RJ, Ralston SH. Nitric oxide and bone. Immunology. 2001 Jul;103:255–261. doi: 10.1046/j.1365-2567.2001.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li CY, Chou TC, Wong CS, Ho ST, Wu CC, Yen MH, et al. Ketamine inhibits nitric oxide synthase in lipopolysaccharide-treated rat alveolar macrophages. Can J Anaesth. 1997 Sep;44:989–995. doi: 10.1007/BF03011971. [DOI] [PubMed] [Google Scholar]
- 59.Lin SZ, Chiou AL, Wang Y. Ketamine antagonizes nitric oxide release from cerebral cortex after middle cerebral artery ligation in rats. Stroke. 1996 Apr;27:747–752. doi: 10.1161/01.str.27.4.747. [DOI] [PubMed] [Google Scholar]
- 60.Rahnert J, Fan X, Case N, Murphy TC, Grassi F, Sen B, et al. The role of nitric oxide in the mechanical repression of RANKL in bone stromal cells. Bone. 2008 Jul;43:48–54. doi: 10.1016/j.bone.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calarge CA, Burns TL, Schlechte JA, Zemel BS. Longitudinal examination of the skeletal effects of selective serotonin reuptake inhibitors and risperidone in boys. J Clin Psychiatry. 2015 May;76:607–613. doi: 10.4088/JCP.14m09195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diem SJ, Blackwell TL, Stone KL, Yaffe K, Haney EM, Bliziotes MM, et al. Use of antidepressants and rates of hip bone loss in older women: the study of osteoporotic fractures. Arch Intern Med. 2007 Jun 25;167:1240–1245. doi: 10.1001/archinte.167.12.1240. [DOI] [PubMed] [Google Scholar]
- 63.Ortuno MJ, Robinson ST, Subramanyam P, Paone R, Huang YY, Guo XE, et al. Serotonin-reuptake inhibitors act centrally to cause bone loss in mice by counteracting a local anti-resorptive effect. Nat Med. 2016 Oct;22:1170–1179. doi: 10.1038/nm.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yirmiya R, Goshen I, Bajayo A, Kreisel T, Feldman S, Tam J, et al. Depression induces bone loss through stimulation of the sympathetic nervous system. Proc Natl Acad Sci U S A. 2006 Nov 07;103:16876–16881. doi: 10.1073/pnas.0604234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hodge JM, Wang Y, Berk M, Collier FM, Fernandes TJ, Constable MJ, et al. Selective serotonin reuptake inhibitors inhibit human osteoclast and osteoblast formation and function. Biol Psychiatry. 2013 Jul 01;74:32–39. doi: 10.1016/j.biopsych.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 66.Warden SJ, Nelson IR, Fuchs RK, Bliziotes MM, Turner CH. Serotonin (5-hydroxytryptamine) transporter inhibition causes bone loss in adult mice independently of estrogen deficiency. Menopause. 2008 Nov-Dec;15:1176–1183. doi: 10.1097/gme.0b013e318173566b. [DOI] [PubMed] [Google Scholar]
- 67.Sansone RA, Sansone LA. SSRIs: bad to the bone? Innov Clin Neurosci. 2012 Jul;9:42–47. [PMC free article] [PubMed] [Google Scholar]
- 68.Bliziotes MM, Eshleman AJ, Zhang XW, Wiren KM. Neurotransmitter action in osteoblasts: expression of a functional system for serotonin receptor activation and reuptake. Bone. 2001 Nov;29:477–486. doi: 10.1016/s8756-3282(01)00593-2. [DOI] [PubMed] [Google Scholar]
- 69.Kerbage H, Bahadori S, Leger J, Carel JC, Purper Ouakil D. Effect of SSRIs on bone metabolism. Encephale. 2014 Feb;40:56–61. doi: 10.1016/j.encep.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 70.Warden SJ, Robling AG, Sanders MS, Bliziotes MM, Turner CH. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology. 2005 Feb;146:685–693. doi: 10.1210/en.2004-1259. [DOI] [PubMed] [Google Scholar]
- 71.Mion G, Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings) CNS Neurosci Ther. 2013 Jun;19:370–380. doi: 10.1111/cns.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Can A, Zanos P, Moaddel R, Kang HJ, Dossou KS, Wainer IW, et al. Effects of Ketamine and Ketamine Metabolites on Evoked Striatal Dopamine Release, Dopamine Receptors, and Monoamine Transporters. J Pharmacol Exp Ther. 2016 Oct;359:159–170. doi: 10.1124/jpet.116.235838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tanaka R, Kumagai Y. Pharmacokinetics of anti-RANKL antibody drugs:Denosumab. Clin Calcium. 2016;26:1597–1603. [PubMed] [Google Scholar]
- 74.Varenna M, Gatti D. The role of rank-ligand inhibition in the treatment of postmenopausal osteoporosis. Reumatismo. 2010 Jul-Sep;62:163–171. doi: 10.4081/reumatismo.2010.163. [DOI] [PubMed] [Google Scholar]
- 75.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009 Aug 20;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 76.Simon JA, Recknor C, Moffett AH, Jr, Adachi JD, Franek E, Lewiecki EM, et al. Impact of denosumab on the peripheral skeleton of postmenopausal women with osteoporosis: bone density, mass, and strength of the radius, and wrist fracture. Menopause. 2013 Feb;20:130–137. doi: 10.1097/gme.0b013e318267f909. [DOI] [PubMed] [Google Scholar]
- 77.Torring O. Denosumab efficient against osteoporosis. A biological drug gives new possibilities to treat a public disease. Lakartidningen. 2010 Mar 3–9;107:574–575. [PubMed] [Google Scholar]
- 78.Boonen S, Adachi JD, Man Z, Cummings SR, Lippuner K, Torring O, et al. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab. 2011 Jun;96:1727–1736. doi: 10.1210/jc.2010-2784. [DOI] [PubMed] [Google Scholar]
- 79.Dempster DW, Lambing CL, Kostenuik PJ, Grauer A. Role of RANK ligand and denosumab, a targeted RANK ligand inhibitor, in bone health and osteoporosis: a review of preclinical and clinical data. Clin Ther. 2012 Mar;34:521–536. doi: 10.1016/j.clinthera.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 80.Ferrari S, Adachi JD, Lippuner K, Zapalowski C, Miller PD, Reginster JY, et al. Further reductions in nonvertebral fracture rate with long-term denosumab treatment in the FREEDOM open-label extension and influence of hip bone mineral density after 3 years. Osteoporos Int. 2015 Dec;26:2763–2771. doi: 10.1007/s00198-015-3179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


