Abstract
Research is scant regarding differential effects of specific types of recreational drugs use on antiretroviral therapy adherence among women, particularly to single-tablet regimens (STR). This is increasingly important in the context of marijuana legalization. We examined the effects of self-reported substance use on suboptimal (<95%) adherence in the Women’s Interagency HIV Study, 2003–2014. Among 1,799 HIV-infected women, the most prevalent substance used was marijuana. In multivariable Poisson GEE regression, substance use overall was significantly associated with suboptimal adherence (adjusted prevalence ratio, aPR=1.20, 95% CI: 1.10–1.32), adjusting for STR use, socio-demographic, behavioral, and clinical factors. Among STR users, compared to no drug use, substance use overall remained detrimental to ART adherence (aPR=1.61, 95% CI: 1.24–2.09); specifically, both marijuana (aPR=1.48, 95% CI: 1.11–1.97) and other drug use (aPR=1.87, 95% CI: 1.29–2.70) predicted suboptimal adherence. These findings highlight the need to intervene with drug-using women taking antiretroviral therapy to maintain effective adherence.
Keywords: adherence, substance use, antiretroviral therapy, marijuana, women
INTRODUCTION
Adherence to antiretroviral therapy (ART) is essential among patients living with HIV to maintain satisfactory health outcomes [1,2]. Multiple tablet regimens (MTR) require multiple units or dosing times per day [3]. The advent of once-daily single-tablet ART regimens (STR) in 2006 brought the potential for improvements in ART adherence, due not only to the lower pill burden and simplified dosing schedules, but also to the decreased highly active antiretroviral therapy (HAART) toxicity [4–6].
Four STR co-formulations are currently available: Atripla (Efavirenz + Tenofovir (TDF) + Embricitabine (FTC)); Complera (TDF + FTC + Rilpivirine); Stribild (TDF + FTC + Elvitegravir + Cobicistat); and Triumeq (Dolutegravir + Abacavir + Lamivudine) [7]. Studies of the effects of these STRs on treatment adherence have produced inconsistent findings. A multicenter randomized clinical trial in 2006 found that, patients’ treatment satisfaction improved, while treatment adherence and quality of life were maintained after switching to a STR from multiple tablet ART regimens [8]. More recent observational studies have shown that STRs improve medication adherence and other treatment outcomes [4,9,10]. This includes a longitudinal study within the Women’s Interagency HIV Study (WIHS), which found that STR use was significantly associated with increased ART adherence and virologic suppression [4]. Among homeless and marginally housed people, STR use was associated with increased ART adherence, which further explained the association between STR use and viral suppression [10]. However, the potential therapeutic benefits of STR among active substance users remain unclear. Previous studies have consistently found that active drug use has detrimental impact on the health seeking behaviors among HIV-infected patients, specifically ART adherence [11–16]. In a multicenter longitudinal cohort study (ACTG 362), the use of hard drugs, defined as cocaine, amphetamines, or heroin was significantly associated with increased odds of non-adherence to ART [12]. Another cohort study of 150 HIV-seropositive adults found that active drug users had significantly worse ART adherence than non-drug users, and individuals using stimulants were at greatest risk for suboptimal adherence [13]. Specifically, among injection drug users, the use of cocaine and multiple substances were significantly associated with suboptimal adherence [10]. Cognitive impairment, inconsistent lifestyles and other acute effects of intoxication have been posited to explain the suboptimal adherence among substance users [13,17,18]. Other behavioral factors such as cigarette smoking [19,20], and alcohol use [21,22], as well as demographic and clinical profiles including race/ethnicity [19,23], depression [21,24], CD4+ cell count [19,21], and HIV RNA viral load [21] have been found to be associated with ART adherence level as well. The generalizability of these findings to the context of STR regimens has not been elucidated. As of May 2017, twenty-six states and the District of Columbia have legalized marijuana, either for medical or recreational use [25]. The most commonly reported reasons among HIV patients for marijuana use were relaxation, appetite stimulation, for social situation, and for reductions of HIV symptoms [26,27]. Despite increasing legal access to marijuana, the impact of marijuana use on medication adherence has not been well characterized. A previous WIHS study from 1994 to 2010 found that current marijuana use was significantly associated with 40% lower odds of ART adherence among HIV-infected women [27]. In another longitudinal prospective cohort of HIV-infected drug users in Canada, there was no association between daily marijuana use and suboptimal ART adherence [28]. Studies have shown that marijuana and other drugs are differentially associated with brain functioning and decision making performance [29]. It remains unclear whether these differences will attribute to a disproportionate prevalence of suboptimal ART adherence, by marijuana and other specific drugs.
There has been a gap in the HIV care continuum for women living with HIV; specifically, women are more vulnerable to suboptimal ART adherence than men, which may be explained by different psychological and behavioral profiles [30–32]. The objective of this study was to examine the overall and drug-specific impact of substance use on ART adherence in a cohort of HIV-infected women using single tablet and multiple tablet ART regimens. We hypothesized that substance use overall would negatively impact ART adherence in this cohort of women, and further examined whether this effect differed by specific drug used. Moreover, we investigated whether any association between drug use and ART adherence would differ by regimen (i.e., STR versus MTR), adopting a 2-tailed hypothesis about the relationship between STR and adherence.
METHODS
Study design
Data were collected semiannually from participants in the Women’s Interagency HIV Study between April 1, 2003 and March 31, 2014. The WIHS is the largest ongoing prospective cohort study of HIV among women in the United States. Participants for this analysis were enrolled during three recruitment waves: 1994–95, 2001–02, 2011–12 from six clinical consortia: Brooklyn NY, Bronx NY, Chicago IL, Los Angeles CA, San Francisco CA, and Washington DC (Supplementary Figure 1). The WIHS collects data in semiannual study visits, which includes standardized interviews, specimen collection, and clinical examination. Details of the WIHS have been reported previously [33,34]. In this analysis, the baseline visit was considered as each participant’s initial visit during the study period from 2003 to 2014.
Study Population
In this analysis, we included HIV-infected women who had at least 2 consecutive follow-up visits and reported having been prescribed antiretroviral therapy at any study visit, and who provided information on substance use at their first visit between April 1, 2003 and March 31, 2014. Women who did not have at least 2 consecutive follow-up visits during the study period were excluded.
Substance Use
Women reported substance use over the past 6 months at WIHS study visit. We considered this time-varying exposure in multiple ways. For our first analysis, a binary variable for any substance use was defined as reporting either injection or non-injection of any of the following drugs: marijuana, cocaine (including both crack and powder forms), heroin, methamphetamines (including crank, crystal, tina), club drugs (e.g., ecstasy, ketamine, Gamma Hydroxybutyrate), or non-prescribed prescription drugs (e.g., amphetamines, narcotics, hallucinogens). For our second analysis, we examined the exposure in terms of specific drug used. In order to ensure sufficient sample size within each exposure category, we assessed the type of specific drug categorized as: no substance use, marijuana only, or use of other drugs.
Adherence to Antiretroviral Therapy
Participants self-reported use of ART by indicating the proportion of time ART was taken as prescribed over the previous 6 months, using the following categorical responses: <75% of the time, 75–94%, 95–99%, or 100% of the time. We dichotomized participants’ responses, defining suboptimal adherence as <95%, to be consistent with the literature [1,2,4,28,35]. The construct validity for this outcome definition is supported by a WIHS study by Wilson et al., which found a significant association between suboptimal adherence and subsequent detectable HIV viral load [35].
Covariates
Variables hypothesized a priori to be associated with substance use or with suboptimal ART adherence were examined in the analysis. Continuous calendar time was used as the time scale to adjust for any potential secular trends.
Time-invariant covariates
Fixed covariates assessed at baseline included: race/ethnicity (non-Hispanic Black, non-Hispanic White/other, and Hispanic), education ( high school and >high school), and enrollment cohort (1994–1995, 2001–2002, and 2011–2012).
Time-varying covariates
Before the introduction of STR in 2006, WIHS participants reported their MTR use. Since 2006, WIHS participants have also been asked about their STR use; a binary covariate was constructed to indicate STR use over the past 6 months. All WIHS participants on STR were using one of the three available single tablet formulations during the study period: Atripla, Complera, or Stribild. Symptoms of depression were assessed semiannually using the Center for Epidemiologic Studies Depression scale (CES-D) [36]. Because of the known correlation with somatic symptoms and substance use, CES-D scores ≥23 have been used to indicate depressive symptoms among drug-using populations [37–39]. We therefore used this more stringent cutoff in this analysis. CD4+ cell count was measured semiannually and was categorized as follows: <350, 350–499, and ≥500 cells/mL. The lower limit of quantification (LLQ) for HIV viral load changed from 80 copies/mL in 2003 to 20 copies/mL in 2014. For our analyses, a binary covariate was generated to indicate whether HIV RNA was detectable at each study visit, using LLQ cutoff that was imposed at that time. Other time-varying covariates included: employment status, annual income (≤ $12,000 and >$12,000), alcohol use, and current smoking status. Alcohol use was categorized as abstainer (0 drink/week), moderate (1–7 drinks/week) and heavy (>7 drinks/week) alcohol use [40].
Statistical Analyses
Descriptive statistics were generated to summarize the data. Bivariate associations between substance use and key covariates were evaluated using Chi-square tests for categorical variables and Wilcoxon rank-sum (Mann-Whitney) tests for continuous variables.
Bivariate and multivariable Poisson regression models were constructed, applying generalized estimating equations (GEE) methods with a log link function and a working exchangeable correlation structure, to estimate the relative prevalence over time of suboptimal adherence among those with no reported substance use compared to (a) any substance use and to (b) type of specific drug used. For each analysis, we also analyzed STR and MTR users separately, and tested the interaction between STR use and exposures on suboptimal adherence. Logistic regression models with GEE methods were initially constructed to estimate odds ratios over time, but the model failed to converge to provider parameter estimates. Poisson regression with a robust variance estimator and GEE methods was then applied to estimate prevalence ratios [41]. In each multivariable model, we included covariates found to be significantly (p<0.25) associated with both suboptimal adherence and substance use in bivariate analysis. Variables hypothesized a priori to be potential confounders included age, race, CD4+ cell count, and detectable viral load; these were included in the multivariable models regardless of the bivariate results. GEE methods were chosen to estimate population means and to account for correlations in measurements from repeated observations for each participant [42,43]. The within-subject correlation structure was selected based on the QIC statistics [44,45]. A robust variance estimator was used to obtain a valid standard error of estimates through incorporating both information about selected working within-subject correlation structures and empirical information from the data.
In both bivariate and multivariable analyses, suboptimal adherence was paired with exposure data from the visit immediately prior (Supplementary Figure 2), and observations only from these consecutive visit pairs were included in the analysis. We hypothesized an acute effect of substance use on ART adherence; therefore this analytic design enabled us not only to ensure temporality, but also to assess a more immediate effect of exposure on outcome. In the case of a missed visit, observations from the visit immediately prior were excluded from the analysis. For women who switched ART regimen type (STR versus MTR), observations from the visit immediately prior to the switch were excluded. For women who did not have at least two consecutive follow-up visits, none of the observations from the participant were included in the analysis and therefore these women were excluded from the analysis. Statistical analyses were performed using Stata version 12 software [46].
RESULTS
Study Population Characteristics
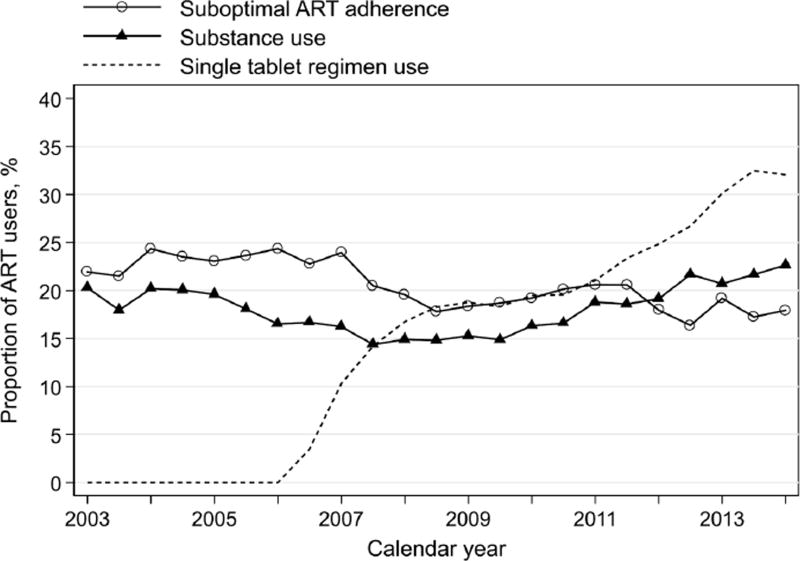
Of 1,911 women who met other eligibility criteria, 112 (6%) were excluded from the analysis because they did not have at least 2 consecutive follow-up visits during the study period. A total of 1,799 ART-treated women included in this analysis contributed 23,787 person-visits between April 2003 and March 2014. At the baseline visit, median age was 42 years (interquartile range, IQR: 36–48), 57% were Black or African-American, and 25% had depressive symptoms. Substance use was reported by 396 (22%) women (Table 1). Those identify as White, non-Hispanic as compared to Black or African-American (30% vs 24%, p<0.001), and to be unemployed (27% vs 12%, p<0.001), current smokers (37% vs 11%, p<0.001), and have depressive symptoms (32% vs 18%, p<0.001), and CD4+ cell count <350 as compared to ≥500 (25% vs 18%, p=0.02; Table 1) were more likely to be drug users. Women who reported moderate (28% vs 13%) or heavy (62% vs 13%) alcohol use were also significantly (p<0.001) more likely to be drug users than women who did not drink (Table 1). At baseline, active drug users most frequently used marijuana (77%) or crack (30%). There were 58% of drug users who reported using marijuana only, and 19% reported using marijuana in addition to other drugs. During the study period, participants reported substance use at 4,036 person-visits (17% of 23,787 person-visits), suboptimal ART adherence at 4,869 (20%) person-visits, and single tablet ART regimen use at 3,525 (15%) person-visits. Participants reported changing ART regimens at 570 (2%) person-visits. The prevalence of suboptimal adherence decreased significantly (p<0.001) over the study period, from 22% in 2003 to 18% in 2014 (Figure 1). The prevalence of substance use in 2013 was 20%. Despite some fluctuations over time, by 2014 the prevalence was 23% (p=0.27). The prevalence of STR use increased significantly (p<0.001), from 2% when it first became available in 2006 to 32% by 2014.
Table 1.
Baseline characteristics of HIV-infected WIHS participants, by substance use, 2003–2014
| Characteristic Med (IQR) or N (row %) |
Total (N=1,799) |
Substance usea (N=396) |
No substance use (N=1,403) |
p-valueb |
|---|---|---|---|---|
| Age at baseline visit (years) | 42 (36–48) | 43 (37–49) | 41 (35–47) | 0.001 |
| Race/Ethnicity | ||||
| Black, non-Hispanic | 1020 | 244 (24) | 776 (76) | <0.001 |
| White, non-Hispanic/Other c | 304 | 92 (30) | 212 (70) | |
| Hispanic | 475 | 60 (13) | 415 (87) | |
| Education | ||||
| ≤ High school | 1202 | 282 (23) | 920 (77) | 0.04 |
| > High school | 595 | 114 (19) | 481 (81) | |
| Employment status | ||||
| Unemployed | 1196 | 324 (27) | 872 (73) | <0.001 |
| Employed | 599 | 69 (12) | 530 (88) | |
| Annual income | ||||
| ≤$12,000 | 931 | 247 (27) | 684 (73) | <0.001 |
| > $12,000 | 756 | 124 (16) | 632 (84) | |
| Depressive symptoms | ||||
| No | 1330 | 243 (18) | 1087 (82) | <0.001 |
| Yes | 446 | 144 (32) | 302 (68) | |
| Alcohol use (drinks/week)a | ||||
| Abstainer (0) | 984 | 125 (13) | 859 (87) | <0.001 |
| Moderate (1–7) | 683 | 189 (28) | 494 (72) | |
| Heavy (>7) | 132 | 82 (62) | 50 (38) | |
| Currently smoking | ||||
| No | 1015 | 108 (11) | 907 (89) | <0.001 |
| Yes | 783 | 288 (37) | 495 (63) | |
| CD4+ cell count | ||||
| <350 | 689 | 170 (25) | 519 (75) | 0.02 |
| 350–499 | 392 | 89 (23) | 303 (77) | |
| ≥500 | 687 | 127 (18) | 560 (82) | |
| Detectable HIV viral load | ||||
| No | 978 | 204 (21) | 774 (79) | 0.20 |
| Yes | 799 | 187 (23) | 612 (77) | |
| Study site | ||||
| Bronx, NY | 319 | 72 (23) | 247 (77) | <0.001 |
| Brooklyn, NY | 319 | 47 (15) | 272 (85) | |
| Washington, DC | 261 | 39 (15) | 222 (85) | |
| Los Angeles, CA | 342 | 46 (13) | 296 (87) | |
| San Francisco, CA | 279 | 126 (45) | 153 (55) | |
| Chicago, IL | 279 | 66 (22) | 213 (78) | |
|
| ||||
| Enrollment cohort | ||||
| 1994–1995 | 992 | 226 (23) | 766 (77) | <0.001 |
| 2001–2002 | 586 | 97 (17) | 489 (83) | |
| 2011–2012 | 221 | 73 (33) | 148 (67) | |
| Specific drug typea, N (col %) | ||||
| Marijuana only | – | 231 (58) | – | N/A |
| Crack, no marijuana | 71 (18) | |||
| Crack and marijuana | 45 (11) | |||
| Other | 49 (12) | |||
IQR: interquartile range
In the 6 months prior to the study visit
Chi-square test for categorical variables and Wilcoxon rank-sum (Mann-Whitney) test for continuous variables
Included 24 Asian/Pacific Islander, 9 native American/Alaska, and 31 other race.
Figure 1.
Trends in substance use, suboptimal ART adherence, and single tablet ART regimen use among ART users, WIHS, 2003–2014
Adherence by Substance Use
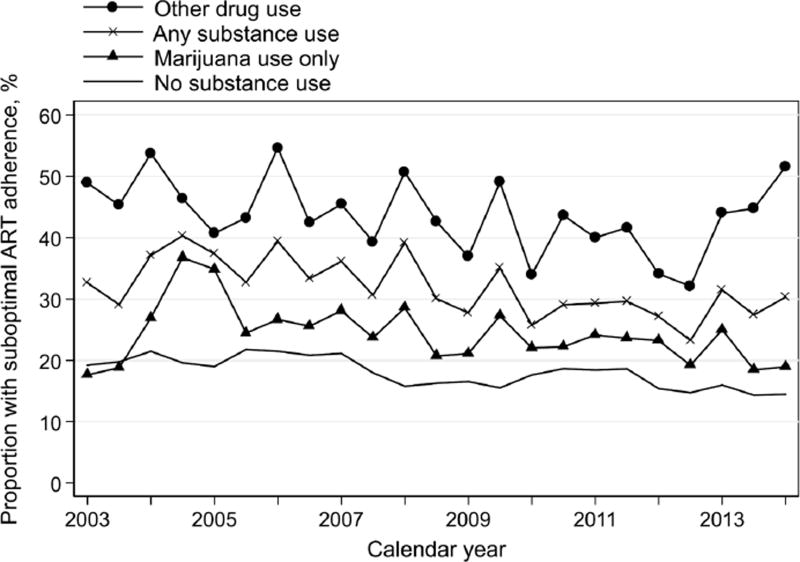
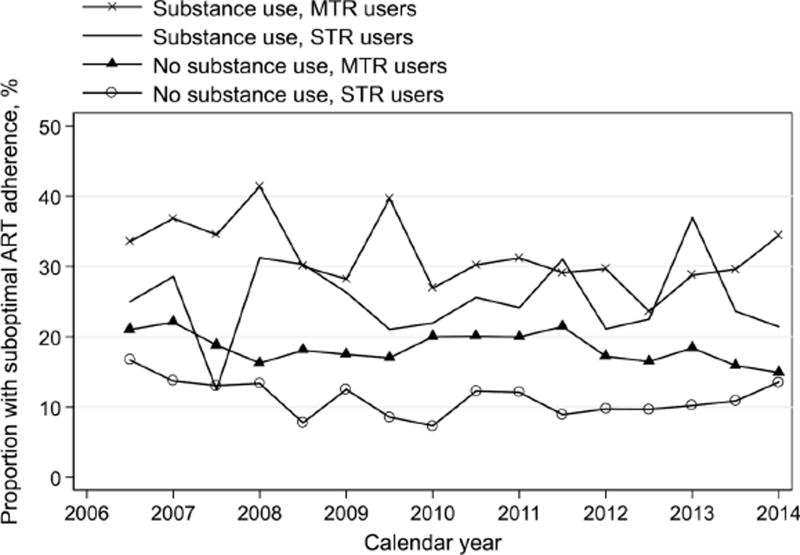
Throughout the study period, the prevalence of suboptimal adherence to ART among drug users was consistently higher than that among non-drug users (Figure 2). This observed difference between drug users to non-drug users appeared consistent among STR users and MTR users separately (Figure 3). As compared to no use, active substance use was associated with a 20% higher prevalence of suboptimal adherence (adjusted Prevalence Ratio, aPR=1.20, 95% CI: 1.10–1.32; Table 2). Other significant predictors included younger age, Black or African-American race, having depressives symptoms, moderate and heavy alcohol use, current smoking, no single tablet ART regimen use, lower CD4+ cell count, detectable HIV viral load, and being in the original 1994–1995 enrollment cohort (Table 2). This association between substance use and suboptimal adherence appeared similar when restricted to the MRA users only (aPR=1.17, 95% CI: 1.06–1.29; Table 3). When restricting to STR users only, substance use was associated with 61% higher prevalence of suboptimal adherence, after adjusting for calendar year, age, race, employment, depressive symptoms, CD4+ cell count, detectable HIV viral load, and enrollment cohort (aPR=1.61, 95% CI: 1.24–2.09; Table 3). To examine if this impact of substance use on suboptimal ART adherence differed by STR use, an interaction between substance use and STR use was explored, but was non-significant (pinteraction, pi=0.47).
Figure 2.
Trends in suboptimal ART adherence by any substance use (used in the first analysis) and by specific drug type (used in the second analysis), WIHS, 2003–2014.
Categories by any substance use included (a) any substance use, and (b) no substance use. Categories by specific drug type included (a) marijuana use only, (b) other drug use, and (c) no substance use. Categories as any substance use, marijuana use only, and other drug use are not mutually exclusive.
Figure 3.
Trends in suboptimal ART adherence by any substance use and by ART regimen type (single tablet regimen, STR; multiple tablet regimen, MTR), WIHS, 2006–2014
Table 2.
Bivariate and multivariable associations between substance use with suboptimal ART adherence, WIHS, 2003–2014
| Covariate | Unadjusted PR (95% CI) |
Adjusted PR a,b (95% CI) |
|---|---|---|
| Substance usec | 1.32 (1.21–1.45) | 1.20 (1.10–1.32) |
| Specific drug typec | ||
| None | 1 | 1 |
| Marijuana use only | 1.10 (0.98–1.24) | 1.03 (0.93–1.15) |
| Other | 1.64 (1.46–1.84) | 1.46 (1.30–1.65) |
| Calendar year | 0.97 (0.96–0.98) | 0.99 (0.98–1.00) |
| Age at baseline visit | 0.98 (0.98–0.99) | 0.98 (0.97–0.98) |
| Race/Ethnicity | ||
| Black, non-Hispanic | 1 | 1 |
| White, non-Hispanic/Other | 0.78 (0.66–0.93) | 0.80 (0.68–0.93) |
| Hispanic | 0.73 (0.63–0.83) | 0.71 (0.63–0.81) |
| Education > high school | 0.98 (0.86–1.10) | – |
| Currently employed | 1.00 (0.93–1.08) | – |
| Annual income > $12,000 | 0.97 (0.90–1.03) | – |
| Depressive symptoms | 1.16 (1.08–1.25) | 1.11 (1.03–1.20) |
| Alcohol use (drinks/week)c | ||
| Abstainer (0) | 1 | 1 |
| Moderate (1–7) | 1.20 (1.12–1.29) | 1.14 (1.06–1.23) |
| Heavy (>7) | 1.37 (1.20–1.55) | 1.26 (1.12–1.42) |
| Current smoking | 1.30 (1.19–1.42) | 1.18 (1.09–1.29) |
| Single tablet ART regimen usec | 0.64 (0.56–0.73) | 0.69 (0.60–0.79) |
| CD4+ cell count | ||
| <350 | 1 | 1 |
| 350–499 | 0.79 (0.73–0.86) | 0.86 (0.79–0.93) |
| ≥500 | 0.72 (0.65–0.78) | 0.80 (0.73–0.87) |
| Detectable HIV viral load | 1.36 (1.28–1.45) | 1.27 (1.19–1.36) |
PR, prevalence ratio; CI, confidence interval
Estimates were adjusted for enrollment cohort.
Adjusted estimates for the exposure were shown by (a) any substance use and (b) specific drug type. Adjusted estimates for all other covariates in the table were derived from a model that included the dichotomous exposure variable.
In the 6 months prior to the study visit
Table 3.
Multivariable associations between substance use with suboptimal ART adherence, among multiple and single tablet users, WIHS, 2003–2014
| Adjusted PR (95% CI)a
|
||
|---|---|---|
| Covariate | Multiple tablet users | Single tablet users |
| Substance useb | 1.17 (1.06–1.29) | 1.61 (1.24–2.09) |
| Race/Ethnicity | ||
| Black, non-Hispanic | 1 | 1 |
| White, non-Hispanic/Other | 0.80 (0.68–0.94) | 0.51 (0.28–0.91) |
| Hispanic | 0.73 (0.64–0.84) | 0.55 (0.37–0.83) |
| Depressive symptoms | 1.09 (1.01–1.18) | 1.53 (1.23–1.91) |
| Currently employed | – | 0.97 (0.74–1.27) |
| Annual income > $12,000 | 0.99 (0.92–1.07) | – |
| Alcohol use (drinks/week)b | ||
| Abstainer (0) | 1 | – |
| Moderate (1–7) | 1.17 (1.09–1.27) | |
| Heavy (>7) | 1.29 (1.14–1.46) | |
| Current smoking | 1.22 (1.12–1.34) | – |
| CD4+ cell count | ||
| <350 | 1 | 1 |
| 350–499 | 0.87 (0.80–0.95) | 0.73 (0.55–0.97) |
| ≥500 | 0.79 (0.72–0.87) | 0.75 (0.56–1.01) |
| Detectable HIV viral load | 1.28 (1.19–1.37) | 1.22 (0.98–1.53) |
PR, prevalence ratio; CI, confidence interval
Estimates were adjusted for age at baseline visit, calendar year and enrollment cohort.
In the 6 months prior to the study visit
Adherence by Specific Drug Type
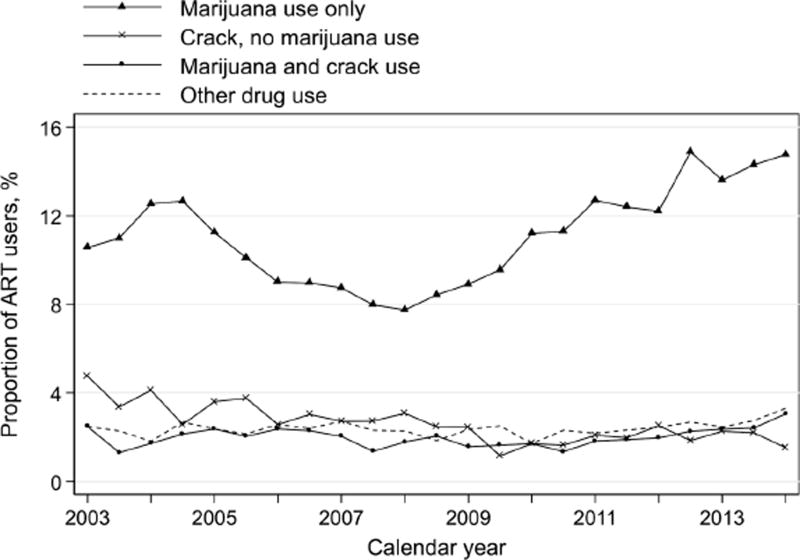
As access to legal marijuana expanded during the study period, we explored the trend and distribution of marijuana use and other specific drugs over time. The prevalence of marijuana use alone fluctuated from 11% in 2003 to 14% in 2014 (Figure 4). The prevalence of crack use and other specific drugs remained relatively stable, and were consistently lower than 8% from 2003 to 2014 (Figure 4). Given that marijuana was the most prevalent substance used (over 61% of total person-visits among active drug users), we analyzed the substance use type as marijuana use only and other drugs as compared to none.
Figure 4.
Trends in substance use by specific drug type among ART users, WIHS, 2003–2014
When we examined the frequency of specific drugs used, we found collinearity between drug type and drug use frequency. Women who used marijuana only were more likely to be daily users (47%; Table 4) than women who used all other types of drugs (26%; p<0.001). Therefore we did not include drug use frequency in our statistical models, in which drug type was our main exposure of interest.
Table 4.
Frequency of use by specific drug type among substance users, WIHS, 2003–2014
| Specific drug typea,b | Less than weekly |
Weekly, less than daily |
At least daily |
p-valuec |
|---|---|---|---|---|
| Marijuana use only (N=2,482) | 616 (25%) | 695 (28%) | 1,171 (47%) | <0.001 |
| Crack, no marijuana use (N=575) | 211 (37%) | 219 (38%) | 145 (25%) | |
| Crack and marijuana use (N=441) | 175 (40%) | 169 (38%) | 97 (22%) | |
| Other (N=519) | 217 (42%) | 148 (29%) | 154 (30%) |
Drug use in previous six months
Data shown are N (row %) person-visits.
Chi-square test
The prevalence of suboptimal ART adherence was consistently higher among marijuana users than non-drug users; women using other drugs had worst adherence throughout the study period (Figure 2). After adjusting for calendar year, age, race, depressive symptoms, alcohol use and current smoking, STR, CD4+ cell count, detectable HIV viral load, and enrollment cohort, there was no significant association between marijuana use and suboptimal adherence (aPR=1.03, 95% CI: 0.93–1.15; Table 2). The use of other drugs was significantly associated with 46% higher prevalence of suboptimal adherence (aPR=1.46, 95% CI: 1.30–1.65), as compared to no drug use (Table 2). When restricted to MTR users, results remained similar to that from overall study population (Table 5). On the contrary, when restricted to STR users, women who used marijuana only (aPR=1.48, 95% CI: 1.11–1.97; Table 5) had significantly higher prevalence of suboptimal ART adherence than non-drug users. Meanwhile, the use of other drugs was significantly associated with 87% higher prevalence of suboptimal adherence (aPR=1.87, 95% CI: 1.29–2.70; Table 5). To examine if these impacts of drug-specific substance use on suboptimal ART adherence differed by STR use, interactions between specific drug types (pi marijuana vs none=0.35, pi others vs none=0.86) and STR use were explored, but were both non-significant. In the subanalysis restricted to marijuana users, we did not observe significant differences in the prevalence of suboptimal ART adherence by the frequency of drug use (results not shown).
Table 5.
Multivariable associations between specific drug type with suboptimal ART adherence, among multiple and single tablet users, WIHS, 2003–2014
| Adjusted PR (95% CI)a
|
||
|---|---|---|
| Covariate | Multiple tablet users | Single tablet users |
| Specific drug typeb | ||
| None | 1 | 1 |
| Marijuana use only | 1.02 (0.91–1.14) | 1.48 (1.11–1.97) |
| Other | 1.40 (1.23–1.60) | 1.87 (1.29–2.70) |
| Race/Ethnicity | ||
| Black, non-Hispanic | 1 | 1 |
| White, non-Hispanic/Other | 0.80 (0.68–0.94) | 0.49 (0.27–0.89) |
| Hispanic | 0.73 (0.64–0.83) | 0.55 (0.37–0.83) |
| Depressive symptoms | 1.08 (1.00–1.17) | 1.52 (1.22–1.89) |
| Currently employed | – | 0.97 (0.74–1.27) |
| Annual income > $12,000 | 0.99 (0.92–1.08) | – |
| Alcohol use (drinks/week)b | ||
| Abstainer (0) | 1 | – |
| Moderate (1–7) | 1.17 (1.09–1.27) | |
| Heavy (>7) | 1.26 (1.11–1.42) | |
| Current smoking | 1.22 (1.11–1.33) | – |
| CD4+ cell count | ||
| <350 | 1 | 1 |
| 350–499 | 0.87 (0.80–0.95) | 0.74 (0.55–0.98) |
| ≥500 | 0.79 (0.72–0.87) | 0.76 (0.57–1.02) |
| Detectable HIV viral load | 1.28 (1.19–1.37) | 1.22 (0.98–1.53) |
PR, prevalence ratio; CI, confidence interval
Estimates were adjusted for age at baseline visit, calendar year, and enrollment cohort.
In the 6 months prior to the study visit
DISCUSSION
In this cohort of HIV-infected women, substance use other than marijuana only was associated with suboptimal adherence to ART, among both STR and MTR users. Marijuana use was significantly associated with suboptimal ART adherence only among STR users. Other specific drugs were significantly associated with suboptimal ART adherence among both STR and MTR users.
The prevalence of single tablet ART regimen use in this study population was slightly lower than the concurrent prevalence (24%) among Medicaid enrollees in the U.S, 2006–2009 [9]. Meanwhile, higher ART adherence with STR use in our study is consistent with the recent evidence suggesting that simplified ART regimens improve adherence [4–6,9,47]. Factors such as perceived ease of the regimen, patient preference, reported quality of life, and HIV symptoms might explain the observed difference in ART adherence between STR users and MTR users [8,48].
The overall detrimental effect of substance use observed in this study is consistent with findings from previous studies [11–13,15,16,18,49–52]. In our study, other behavioral factors including moderate and heavy alcohol use, as well as current smoking, were significantly associated with suboptimal ART adherence, consistent with prior research findings [19–22]. Independent of these behavioral factors, we observed a significant association between substance use and suboptimal ART adherence. Importantly, our study is the first to demonstrate that substance use is detrimental to ART adherence after adjusting for STR use. When we assessed potential effect modification, we did not observe a significant interaction between substance use and STR use on ART adherence. According to the current ART use guidelines, providers may prioritize STR to drug users who are deemed to have low likelihood of ART adherence [53]. Our data suggest that, in practice, substance use may continue to have a strong detrimental impact on women’s health seeking behavior, whether they are prescribed STR or MTR regimens.
We found that, among STR users, marijuana use only was significantly associated with 48% higher prevalence of suboptimal adherence as compared with no drug use. However, such detrimental effect was not observed among MTR users. The existing evidence regarding the longitudinal impact of marijuana on medication adherence is limited and inconsistent. In a previous WIHS study, current marijuana use was found to be associated with 40% lower odds of ART adherence, although this observed association was not accounted for other specific drug use [27]. In another longitudinal cohort study, daily marijuana use was not associated with suboptimal ART adherence [28]. These differences might be explained by different exposure definitions, e.g., classification by diagnostic criteria (e.g., dependence). More specifically, another study found that marijuana dependent individuals have significantly lower ART adherence than non-dependent marijuana users [54]. Of the existing studies of the association between marijuana use and ART adherence, few have compared whether this association varies by the purpose of marijuana use. A study of 252 HIV-infected patients found that marijuana use was beneficial for ART adherence among those with moderate to severe nausea, while no significant association was observed among those with mild or no nausea. In the current study, we were unable to differentiate between medical and recreational marijuana use. With increasing legal access to marijuana, special attention should be given to the therapeutic value of marijuana use, and how that may be offset by potential adverse effects on ART adherence. Empirical data are needed in the context of health outcomes and health seeking behaviors in order to elucidate these relationships.
Interpretation of these findings warrants consideration of several limitations. Although it has been suggested that self-reported adherence is consistently higher than estimates obtained via other approaches (e.g., electronic monitoring devices), the validity of self-reported adherence data has been widely established [55–58]. There was a limited sample size when we restricted to the STR users only. In addition, we did not have the data to evaluate whether the observed association was mediated or confounded by participation in drug treatment programs. We were also unable to determine the temporality between exposure and outcome data because of the semiannual data collection structure, and therefore we conducted the analysis on paired consecutive visits to ensure outcome data were collected after the exposure data (Supplementary Figure 2). Consistent with other published papers, WIHS as an interval cohort, has data from the structured semiannual visits, and it is not feasible to have more frequent data collection [4,21,27]. Finally, less than 95% adherence as a cutoff defining suboptimal adherence was based on older studies of unboosted protease inhibitor therapy [2]. It might be a conservative cutoff in the current ART era, as adherence levels as low as 80%–84% may be sufficient for newer regimens to achieve viral load suppression [59]. Our study period began in 2003, thus a conservative cutoff is preferred with the assumption of non-differential measurement error of adherence, resulting in a conservative study finding.
Despite these limitations, there are several strengths of our study. Our detailed individual-level data on drug type and ART regimen type enabled us to investigate differential effects of substance use on adherence among US women taking STR and MTR. Drug use behavior differs between men and women, hence our findings extend the existing knowledge and inference to female drug users, a population with limited resources available [60]. Moreover, a study population of 1,799 over an 11-year study period provides robust evidence for possible causal interpretation of study findings.
CONCLUSIONS
Current guidelines for the use of ART among drug users recommend regimens with simple dosing schedules in order to enhance medication adherence [53]. Drug users, however, might continue to face adherence challenges when prescribed single tablet regimens as compared to multiple tablet regimens. Our study findings thus highlight the continuing need to monitor active drug use among HIV-infected patients, particularly with increasing access to legal marijuana, to maintain effective ART adherence. Future research should focus on recreational and medical marijuana use, among HIV-infected individuals, particularly those prescribed single tablet ART regimens.
Supplementary Material
Supplementary Figure 1. Flowchart of the study visits, WIHS, 2003-2014
Semiannual study visits from 2003 to 2014 in the dashed box were included in the analysis. Calendar year was listed as last two digits (i.e. 1994: 94).
Supplementary Figure 2. Study visit of exposure, covariate, and outcome data included in the analysis
Exposure and covariate data (i.e., from visit X) were paired with outcome data (i.e., from visit X+1 in the analysis.
Acknowledgments
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Michael Saag, Mirjam-Colette Kempf, and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Mary Young and Seble Kassaye), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I – WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA).
The authors would like to thank Chiung-Yu Huang, PhD and Gayle Springer, MLA for statistical advice and data management support.
Funding: This study was funded by the National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Mental Health.
Footnotes
Oral presentation at the 48th Annual Meeting of the Society for Epidemiologic Research, Denver, CO, June 18th, 2015.
Conflict of Interest: Dr. Merenstein has been an expert witness on probiotic cases for General Mills, Nestle, Procter and Gamble and Bayer Health. All the other authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Lima VD, Harrigan R, Bangsberg DR, Hogg RS, Gross R, Yip B, et al. The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. J Acquir Immune Defic Syndr. 2009;50(5):529–536. doi: 10.1097/QAI.0b013e31819675e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 3.Clay PG, Nag S, Graham CM, Narayanan S. Meta-analysis of studies comparing single and multi-tablet fixed dose combination HIV treatment regimens. Medicine (Baltimore) 2015;94(42):e1677. doi: 10.1097/MD.0000000000001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanna DB, Hessol NA, Golub ET, Cocohoba JM, Cohen MH, Levine AM, et al. Increase in single-tablet regimen use and associated improvements in adherence-related outcomes in HIV-infected women. J Acquir Immune Defic Syndr. 2014;65(5):587–596. doi: 10.1097/QAI.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nachega JB, Parienti JJ, Uthman OA, Gross R, Dowdy DW, Sax PE, et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: A meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;58(9):1297–1307. doi: 10.1093/cid/ciu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sax PE, Meyers JL, Mugavero M, Davis KL. Adherence to antiretroviral treatment and correlation with risk of hospitalization among commercially insured HIV patients in the United States. PLoS One. 2012;7(2):e31591. doi: 10.1371/journal.pone.0031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Truong WR, Schafer JJ, Short WR. Once-daily, single-tablet regimens for the treatment of HIV-1 infection. Pharm Ther. 2015;40(1):44–55. [PMC free article] [PubMed] [Google Scholar]
- 8.Hodder SL, Mounzer K, Dejesus E, Ebrahimi R, Grimm K, Esker S, et al. Patient-reported outcomes in virologically suppressed, HIV-1-infected subjects after switching to a simplified, single-tablet regimen of Efavirenz, Emtricitabine, and Tenofovir DF. AIDS Patient Care STDS. 2010;24(2):87–96. doi: 10.1089/apc.2009.0259. [DOI] [PubMed] [Google Scholar]
- 9.Cohen CJ, Meyers JL, Davis KL. Association between daily antiretroviral pill burden and treatment adherence, hospitalisation risk, and other healthcare utilisation and costs in a US medicaid population with HIV. BMJ Open. 2013;3:e003028. doi: 10.1136/bmjopen-2013-003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bangsberg DR, Ragland K, Monk A, Deeks SG. A single tablet regimen is associated with higher adherence and viral suppression than multiple tablet regimens in HIV+ homeless and marginally housed people. AIDS. 2010;24(18):2835–2840. doi: 10.1097/QAD.0b013e328340a209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez A, Mimiaga MJ, Israel J, Andres Bedoya C, Safren SA. Substance use predictors of poor medication adherence: the role of substance use coping among HIV-infected patients in opioid dependence treatment. AIDS Behav. 2013;17:168–173. doi: 10.1007/s10461-012-0319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohn SE, Jiang H, McCutchan JA, Koletar SL, Murphy RL, Robertson KR, et al. Association of ongoing drug and alcohol use with non-adherence to antiretroviral therapy and higher risk of AIDS and death: results from ACTG 362. AIDS Care. 2011;23(6):775–785. doi: 10.1080/09540121.2010.525617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SS, et al. Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav. 2007;11(2):185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen MH, Cook JA, Grey D, Young M, Hanau LH, Tien P, et al. Medically eligible women who do not use HAART: the importance of abuse, drug use, and race. Am J Public Health. 2004;94(7):1147–1151. doi: 10.2105/ajph.94.7.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. J Acquir Immune Defic Syndr. 2001;27(3):251–259. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- 16.Stein MD, Rich JD, Maksad J, Chen MH, Hu P, Sobota M, et al. Adherence to antiretroviral therapy among HIV-infected methadone patients: effect of ongoing illicit drug use. Am J Drug Alcohol Abuse. 2000;26(2):195–205. doi: 10.1081/ada-100100600. [DOI] [PubMed] [Google Scholar]
- 17.Tucker JS, Orlando M, Burnam MA, Sherbourne CD, Kung F-Y, Gifford AL. Psychosocial mediators of antiretroviral nonadherence in HIV-positive adults with substance use and mental health problems. Heal Psychol. 2004;23(4):363–370. doi: 10.1037/0278-6133.23.4.363. [DOI] [PubMed] [Google Scholar]
- 18.Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, et al. Impact of active drug use on atiretorviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17(5):377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connor JL, Gardner EM, Mannheimer SB, Lifson AR, Esser S, Telzak EE, et al. Factors associated with adherence amongst 5295 people receiving antiretroviral therapy as part of an international trial. J Infect Dis. 2013;208(1):40–49. doi: 10.1093/infdis/jis731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shuter J, Bernstein SL. Cigarette smoking is an independent predictor of nonadherence in HIV-infected individuals receiving highly active antiretroviral therapy. Nicotine Tob Res. 2008;10(4):731–736. doi: 10.1080/14622200801908190. [DOI] [PubMed] [Google Scholar]
- 21.Lazo M, Gange SJ, Wilson TE, Anastos K, Ostrow DG, Witt MD, et al. Patterns and predictors of changes in adherence to highly active antiretroviral therapy: longitudinal study of men and women. Clin Infect Dis. 2007;45(10):1377–1385. doi: 10.1086/522762. [DOI] [PubMed] [Google Scholar]
- 22.Chander G, Lau B, Moore RD. Hazardous alcohol use: a risk factor for non-adherence and lack of suppression in HIV infection. J Acquir Immune Defic Syndr. 2006;43(4):411–417. doi: 10.1097/01.qai.0000243121.44659.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simoni JM, Huh D, Wilson IB, Shen J, Goggin K, Reynolds N, et al. Racial/ethnic disparities in ART adherence in the United States: findings from the MACH14 study. J Acquir Immune Defic Syndr. 2012;60(5):466–472. doi: 10.1097/QAI.0b013e31825db0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58(2) doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.State Marijuana Laws in 2017 Map. [Accessed May 14];Governing magazine. 2017 Available at: http://www.governing.com/gov-data/state-marijuana-laws-map-medical-recreational.html.
- 26.Prentiss D, Power R, Balmas G, Tzuang G, Israelski DM. Patterns of marijuana use among patients with HIV/AIDS followed in a public health care setting. J Acquir Immune Defic Syndr. 2004;35(1):38–45. doi: 10.1097/00126334-200401010-00005. [DOI] [PubMed] [Google Scholar]
- 27.D’Souza G, Matson P, Grady CD, Nahvi S, Merenstein D, Weber K, et al. Medical and recreational marijunana use among HIV-infected women in the Women’s Interagency HIV Cohort (WIHS), 1994–2010. J Acquir Immune Defic Syndr. 2012;61(5):618–626. doi: 10.1097/QAI.0b013e318273ab3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slawson G, Milloy M-J, Balneaves L, Simo A, Guillemi S, Hogg R, et al. High-intensity cannabis use and adherence to antiretroviral therapy among people who use illicit drugs in a Canadian setting. AIDS Behav. 2014;19(1):120–127. doi: 10.1007/s10461-014-0847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet JL, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend. 2007;90(1):2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puskas CM, Forrest JI, Parashar S, Salters KA, Cescon AM, Kaida A, et al. Women and vulnerability to HAART non-adherence: A literature review of treatment adherence by gender from 2000 to 2011. Curr HIV/AIDS Rep. 2011;8(4):277–287. doi: 10.1007/s11904-011-0098-0. [DOI] [PubMed] [Google Scholar]
- 31.Ubbiali A, Donati D, Chiorri C, Bregani V, Cattaneo E, Maffei C, et al. Prediction of adherence to antiretroviral therapy: can patients’ gender play some role? An Italian pilot study. AIDS Care. 2008;20(5):571–575. doi: 10.1080/09540120701867172. [DOI] [PubMed] [Google Scholar]
- 32.Kuyper LM, Wood E, Montaner JSG, Yip B, O’connell JM, Hogg RS. Gender differences in HIV-1 RNA rebound attributed to incomplete antiretroviral adherence among HIV-infected patients in a population-based cohort. J Acquir Immune Defic Syndr. 2004;37(4):1470–1476. doi: 10.1097/01.qai.0000138379.39317.62. [DOI] [PubMed] [Google Scholar]
- 33.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women’s Interagency HIV Study. Epidemiology. 1998;9(2):117–125. [PubMed] [Google Scholar]
- 35.Wilson TE, Barrón Y, Cohen M, Richardson J, Greenblatt R, Sacks HS, et al. Adherence to antiretroviral therapy and its association with sexual behavior in a national sample of women with human immunodeficiency virus. Clin Infect Dis. 2002;34(4):529–534. doi: 10.1086/338397. [DOI] [PubMed] [Google Scholar]
- 36.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 37.Perdue T, Hagan H, Thiede H, Valleroy L. Depression and HIV risk behavior among Seattle-area injection drug users and young men who have sex with men. AIDS Educ Prev. 2003;15(1):81–92. doi: 10.1521/aeap.15.1.81.23842. [DOI] [PubMed] [Google Scholar]
- 38.Johnson ME, Fisher DG, Fenaughty A, Theno S. Hepatitis C virus and depression in drug users. Am J Gastroenterol. 1998;93(5):785–789. doi: 10.1111/j.1572-0241.1998.225_a.x. [DOI] [PubMed] [Google Scholar]
- 39.Weissman MM, Sholomskas D, Pottenger M, Prusoff B, Locke B. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106(3):203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 40.Moyer VA. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. preventive services task force recommendation statement. Ann Intern Med. 2013;159(3):210–218. doi: 10.7326/0003-4819-159-3-201308060-00652. [DOI] [PubMed] [Google Scholar]
- 41.Cummings P. Methods for estimating adjusted risk ratios. Stata J. 2009;9(2):175–196. [Google Scholar]
- 42.Hardin JW. Generalized Estimating Equations (GEE) John Wiley & Sons, Ltd; 2005. [Google Scholar]
- 43.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 44.Cui J. QIC program and model selection in GEE analyses. Stata J. 2007;7(2):209–220. [Google Scholar]
- 45.Pan W. Akaike’s Information Criterion in Generalized Estimating Equations. Biometrics. 2004;57(1):120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 46.StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 47.Raffi F, Yazdanpanah Y, Fagnani F, Laurendeau C, Lafuma A, Gourmelen J. Persistence and adherence to single-tablet regimens in HIV treatment: a cohort study from the French national healthcare insurance database. J Antimicrob Chemother. 2015;70:2121–2128. doi: 10.1093/jac/dkv083. [DOI] [PubMed] [Google Scholar]
- 48.Airoldi M, Zaccarelli M, Bisi L, Bini T, Antinori A, Mussini C, et al. One-pill once-a-day HAART: a simplification strategy that improves adherence and quality of life of HIV-infected subjects. Patient Prefer Adherence. 2010;4:115–125. doi: 10.2147/ppa.s10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joseph B, Kerr T, Puskas CM, Montaner J, Wood E, Milloy M-J. Factors linked to transitions in adherence to antiretroviral therapy among HIV-infected illicit drug users in a Canadian setting. AIDS Care. 2015;27(9):1128–1136. doi: 10.1080/09540121.2015.1032205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber R, Huber M, Rickenbach M, Furrer H, Elzi L, Hirschel B, et al. Uptake of and virological response to antiretroviral therapy among HIV-infected former and current injecting drug users and persons in an opiate substitution treatment programme: the Swiss HIV cohort study. HIV Med. 2009;10(7):407–416. doi: 10.1111/j.1468-1293.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 51.Palepu A, Tyndall M, Yip B, O’Shaughnessy MV, Hogg RS, Montaner JSG. Impaired virologic response to highly active antiretroviral therapy associated with ongoing injection drug use. J Acquir Immune Defic Syndr. 2003;32(5):522–526. doi: 10.1097/00126334-200304150-00009. [DOI] [PubMed] [Google Scholar]
- 52.Lucas GM, Gebo K, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16(5):767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- 53.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [Accessed May 14];Department of Health and Human Services. 2017 Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdoles-centGL.pdf.
- 54.Bonn-Miller MO, Oser ML, Bucossi MM, Trafton Ja. Cannabis use and HIV antiretroviral therapy adherence and HIV-related symptoms. J Behav Med. 2014;37:1–10. doi: 10.1007/s10865-012-9458-5. [DOI] [PubMed] [Google Scholar]
- 55.Bulgiba A, Mohammed UY, Chik Z, Lee C, Peramalah D. How well does self-reported adherence fare compared to therapeutic drug monitoring in HAART? Prev Med (Baltim) 2013;57(SUPPL):S34–S36. doi: 10.1016/j.ypmed.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Thompson MA, Mugavero MJ, Amico KR, Cargill VA. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an international association of physicians in AIDS care panel. Ann Intern Med. 2012;156(11):817–833. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buscher A, Hartman C, Kallen MA, Giordano TP. Validity of self-report measures in assessing antiretroviral adherence of newly diagnosed, HAART-Naïve, HIV patients. HIV Clin Trials. 2011;12(5):244–254. doi: 10.1310/hct1205-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10(3):227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Viswanathan S, Detels R, Mehta SH, Macatangay BJC, Kirk GD, Jacobson LP. Level of adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy (HAART) AIDS Behav. 2015;19(4):601–611. doi: 10.1007/s10461-014-0927-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnston LD, O’Malley PM, Bachman JG, Schulenberg J. Monitoring the Future national survey results on drug use, 1975–2008. Bethesda, MD: National Institute on Drug Abuse; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Flowchart of the study visits, WIHS, 2003-2014
Semiannual study visits from 2003 to 2014 in the dashed box were included in the analysis. Calendar year was listed as last two digits (i.e. 1994: 94).
Supplementary Figure 2. Study visit of exposure, covariate, and outcome data included in the analysis
Exposure and covariate data (i.e., from visit X) were paired with outcome data (i.e., from visit X+1 in the analysis.