Abstract
DNA methyltransferase inhibitors sensitize leukemia cells to chemotherapeutics. We therefore conducted a phase 1/2 study of mitoxantrone, etoposide, and cytarabine following “priming” with 5-10 days of decitabine (dec/MEC) in 52 adults (median age 55 [range: 19-72] years) with relapsed/refractory acute myeloid leukemia (AML) or other high-grade myeloid neoplasms. During dose escalation in cohorts of 6-12 patients, all dose levels were well-tolerated. As response rates appeared similar with 7 and 10-days of decitabine, a 7-day course was defined as the recommended phase 2 dose (RP2D). Among 46 patients treated at/above the RP2D, 10 (22%) achieved a complete remission (CR), 8 without measurable residual disease; five additional patients achieved CR with incomplete platelet recovery, for an overall response rate of 33%. Seven patients (15%) died within 28 days of treatment initiation. Infection/neutropenic fever, nausea, and mucositis were the most common adverse events. While the CR rate compared favorably to a matched historic control population (observed/expected CR ratio=1.77), CR rate and survival were similar to two contemporary salvage regimens used at our institution (G-CLAC and G-CLAM). Thus, while meeting the pre-specified efficacy goal, we found no evidence that dec/MEC is substantially better than other cytarabine-based regimens currently used for relapsed/refractory AML.
Keywords: Acute myeloid leukemia, chemotherapy, decitabine, MEC, priming, salvage
Introduction
The majority of adults with acute myeloid leukemia (AML) will need re-induction (“salvage”) treatment because of failure of initial chemotherapy or relapse from complete remission (CR).1,2 Although numerous regimens containing cytarabine ≥1 gram/m2 per dose (e.g. MEC [mitoxantrone, etoposide, cytarabine],3 FLAG [fludarabine, cytarabine, G-CSF]) have been tested for this purpose, response rates have been low, particularly if the prior CR was short (<6-12 months) or the disease was refractory to prior therapy.4-6
Increasing evidence highlights the importance of epigenetic modification in the pathogenesis of myeloid neoplasms.7-11 Unlike DNA mutations, epigenetic changes can be pharmacologically reversed. Pre-clinical studies with the DNA methyltransferase (DNMT) inhibitors decitabine and azacitidine demonstrate dose-dependent apoptosis in myeloid leukemia cells and synergistic cytotoxicity or chemosensitization when combined with conventional chemotherapeutics.12-14 These effects appear greater if the DNMT inhibitors are used before (“priming”), rather than together with, the other chemotherapeutics.13,15 These observations, and studies showing the tolerability of decitabine combined with conventional chemotherapy,16,17 prompted a dose-escalation study of decitabine followed by MEC (dec/MEC). Our primary objectives were to determine the decitabine dose with the most favorable efficacy and toxicity profile when used as priming for MEC in adults receiving salvage therapy for AML or analogous malignancies and then to estimate the efficacy and toxicity profile of that dose in an expansion cohort.
Patients and Methods
Study population
Adults aged ≥18 years with relapsed/refractory AML (acute promyelocytic leukemia excepted) or other myeloid neoplasms with ≥10% blasts in either peripheral blood and/or bone marrow were eligible if they had relapsed or refractory disease according to standard International Working Group Criteria18 and a treatment-related mortality (TRM) score of ≤9.2. This score, composed of weighted information from 8 covariates (age, performance status, white blood cell [WBC] count, peripheral blood blast percentage, type of AML [de novo vs. secondary], platelet count, serum albumin, and serum creatinine), corresponds to a ≤9.2% probability of death within 28 days (“TRM”) of receipt of intensive chemotherapy for newly diagnosed AML.19 Patients were also required to have a left ventricular ejection fraction ≥40%, creatinine ≤1.5 times the institutional upper limit of normal, and bilirubin ≤2.5 times the institutional upper limit of normal. An expected survival of <1 year from another illness, uncontrolled infection, or treatment with other investigational agents (including treatment with tyrosine kinase [“FLT3”] inhibitors) were exclusions. Prior autologous or allogeneic hematopoietic cell transplantation (HCT) was permitted provided any graft-versus-host disease was well controlled with stable use of immunosuppressive agents, as was prior use of DNMT inhibitors or MEC (but not in combination). Cytogenetic risk was assessed according to the modified United Kingdom Medical Research Council/National Cancer Research Institute (MRC/NCRI) criteria.20 Treatment responses were defined according to standard criteria.18 Measurable (“minimal”) residual disease (MRD) was assessed by multiparametric flow cytometry, with any level of MRD considered positive (MRDpos).21,22 Relapse after study treatment was defined by standard morphologic criteria18 or emergence of MRD after MRD negativity was achieved if this finding led to therapeutic intervention. The protocol (registered as NCT01729845) was approved by the Fred Hutchinson Cancer Research Center (Fred Hutch) Institutional Review Board (IRB), and patients gave written informed consent in accordance with the Declaration of Helsinki. Six patients who were otherwise eligible (TRM scores ≤9.2) received dec/MEC outside of this clinical trial during the study period.
Treatment plan
In phase 1, patients were assigned to intravenous (IV) decitabine (20 mg/m2/day) for 5, 7, or 10 days. Following a 5-day break, patients then received standard dose MEC (mitoxantrone 8 mg/m2/day IV on days 1-5; etoposide 100 mg/m2/day IV on days 1-5; cytarabine 1 g/m2/day IV on days 1-5). In phase 2, patients received the recommended phase 2 dose (RP2D) of decitabine identified in phase 1. No dose adjustments were made for age or any other baseline characteristic. Patients who did not achieve a CR or CR with incomplete platelet recovery (CRp) after the first course of dec/MEC were eligible to receive a second identical induction course. Patients achieving a CR/CRp with 1-2 cycles of dec/MEC could receive up to 2 post-remission courses of dec/MEC given at the same doses as the induction cycle(s). Patients were taken off study for lack of CR/CRp achievement after 2 cycles of therapy, consolidation with HCT, excess toxicity including persistent aplasia without evidence of leukemia after day 45 of treatment, or relapse on therapy. Toxicities were evaluated based on the CTCAE (NCI Common Terminology Criteria for Adverse Events) Version 4.03 (http://ctep.cancer.gov).
Comparison of dec/MEC with other intensive re-induction chemotherapy regimens
Two commonly used salvage regimens at our institution include G-CLAC (G-CSF 5 μg/kg subcutaneously [SC] from day 0 until neutrophil recovery; clofarabine 15-25 mg/m2/day IV on days 1-5; cytarabine 2 g/m2/day IV on days 1-5)23 and G-CLAM (G-CSF 300 or 480 μg SC on days 0-5; cladribine 5 mg/m2/day IV on days 1-5; cytarabine 2 g/m2/day IV on days 1-5; mitoxantrone 10 mg/m2/day IV on days 1-3).24,25 Patients received G-CLAC either as part of a phase 1/2 study (NCT00602225) or off-protocol; all patients given G-CLAM at this dose level were treated outside a clinical study. Covariates collected included age, sex, cytogenetic risk, primary vs. secondary disease, prior HCT, duration of first CR, performance status, TRM score, WBC and platelet count, peripheral blood blast percentage, FLT3 and NPM1 mutational status, and whether or not treatment occurred as part of a clinical trial. This retrospective analysis was approved by the Fred Hutch IRB.
Statistical considerations
Phase 1
Cohorts of 6 patients were assigned to increasing days of decitabine therapy. Dose-limiting toxicity (DLT) was defined as: 1) any grade 3 non-hematologic toxicity lasting >48 hours that resulted in a > 7-day delay of the subsequent treatment cycle, with the exception of febrile neutropenia or infection; 2) any grade ≥4 non-hematologic toxicity, with the exception of febrile neutropenia or infection or constitutional symptoms, if recovery to grade ≤2 within 14 days. Maximum tolerated dose (MTD) was defined as the highest dose studied in which the incidence of DLTs was <33% (2/6 of each patient cohort). To better define safety and initial evidence of anti-leukemic activity, any dose level cohort could be expanded up to 12 patients, provided that ≤2/6 patients had DLT at that dose level.
Phase 2
We considered dec/MEC of no further interest if the true CR/CRp (ORR) rate was ≤15% (null hypothesis)26 while an ORR ≥30% would spur further investigation (alternative hypothesis). A Simon minimax 2-stage design was used, with 80% power and a 1-sided alpha of 7%, for enrollment of 20 patients each in the first and second stage. Patients enrolled at/above the RP2D in phase 1 were included in the phase 2 analyses. A multivariable logistic regression model was used to compare outcomes across different treatment regimens. Data cut-off date for analysis was December 23, 2016.
Results
Study cohort and treatment
Between January 2013 and June 2016, 52 adults (median age 55 [range: 19-72] years; median TRM score 3.15 [0.07-9.05]) were enrolled, 87% of whom had AML (Table 1). Nineteen had primary refractory and 33 relapsed disease, with a median first CR duration of 5 (range: 1-19) months, and 25% had previously undergone allogeneic HCT; 54% had intermediate-risk and 40% had adverse-risk cytogenetics. Patients received a median of 2 (1-7) therapies prior to study enrollment. All patients received at least one course of decitabine. Two patients (4%) died before receiving MEC (one from intracranial hemorrhage, one from infection). The other 50 patients (96%) completed at least 1 course of dec/MEC, with 35 receiving 1, 13 receiving 2, and 2 receiving 3 courses.
Table 1. Characteristics of the entire and RP2D study cohort.
| Parameter | Entire cohort, n= 52 | At/above R2PD cohort, n=46 |
|---|---|---|
|
| ||
| Median age (range), years | 55 (19-72) | 55 (19-72) |
|
| ||
| Male gender, n (%) | 29 (55.8%) | 24 (52.2%) |
|
| ||
| Disease-type | ||
| AML | 45 (86.5%) | 41 (89.1%) |
| With recurrent genetic abnormalities | 9 (17.3%) | 7 (15.2%) |
| With mutated NPM1 | 5 (9.6%) | 5 (10.9%) |
| With myelodysplasia-related changes | 19 (36.5%) | 17 (36.9%) |
| Treatment-related myeloid neoplasms | 1 (1.9%) | 1 (2.2%) |
| AML, not otherwise specified | 11 (21.2%) | 11 (23.9%) |
| MDS-RAEB2 | 7 (13.5%) | 5 (10.9%) |
|
| ||
| Secondary disease* | 9 (17.3%) | 7 (15.2%) |
|
| ||
| Disease status, n (%) | ||
| Primary refractory | 19 (36.6%) | 18 (39.1%) |
| Relapse | 33 (63.4%) | 28 (60.9%) |
| Median CR1 duration (range), months | 5 (1-19) | 5 (1-19) |
| Prior HCT | 13 (25.0%) | 13 (28.3%) |
| Median number of prior therapies (range) | 2 (1-7) | 2 (1-7) |
| Prior receipt of intermediate/high-dose cytarabine** | 39 (75.0%) | 35 (76.1%) |
|
| ||
| Median TRM score (range) | 3.15 (0.07-9.05) | 3.17 (0.07-9.05) |
|
| ||
| Performance status, n (%) | ||
| 0 | 7 (13.5%) | 6 (13.0%) |
| 1 | 39 (75.0%) | 34 (73.9%) |
| 2 | 6 (11.5%) | 6 (13.0%) |
|
| ||
| Cytogenetic risk, n (%) | ||
| Favorable | 3 (5.6%) | 1 (2.2%) |
| Intermediate | 28 (53.8%) | 25 (54.3%) |
| Adverse | 21 (40.4%) | 20 (43.5%) |
|
| ||
| Mutational status, n (%) | ||
| FLT3-ITD | ||
| No | 32 (61.5%) | 30 (65.2%) |
| Yes | 5 (9.6%) | 5 (10.9%) |
| Unknown | 15 (28.8%) | 11 (23.9%) |
| NPM1 | ||
| No | 31 (59.6%) | 29 (63.0%) |
| Yes | 5 (9.6%) | 5 (10.9%) |
| Unknown | 16 (30.8%) | 12 (26.1%) |
|
| ||
| Laboratory findings at baseline, median (range) | ||
| WBC (x 109L) | 2.12 (0.05-59.4) | 1.90 (0.05-59.4) |
| Peripheral blood blasts (%) | 8 (0-83) | 8 (0-83) |
| Hemoglobin (g/dL) | 9.4 (5.5-14.0) | 9.3 (5.5-14.0) |
| Platelets (x 109L) | 30 (4-457) | 34 (4-447) |
| Creatinine (mg/dL) | 0.80 (0.41-1.33) | 0.79 (0.41-1.33) |
| Total bilirubin (mg/dL) | 0.5 (0.2-2.7) | 0.6 (0.2-2.7) |
| AST (U/L) | 26 (11-191) | 25 (11-117) |
| ALT (U/L) | 27 (10-440) | 27 (10-161) |
AML transformed from antecedent hematologic disorder or AML/MDS after prior cytotoxic therapy
Regimen containing ≥500 mg/m2 cytarabine/day: most common prior regimens included HiDAC (high-dose cytarabine), G-CLAM, G-CLAC, IAP (idarubicin, high-dose cytarabine, pravastatin), and FLAG-Ida
Abbreviations: CR1, first complete remission; RP2D, recommended phase 2 dose; TRM, treatment-related mortality
Phase 1: determination of safety and tolerability of dec/MEC
Thirty patients were enrolled in the dose escalation portion of this study and received a median of 1 (range: 1-2) cycles of therapy (Supplemental Table 1). As summarized in Table 2, 1 DLT occurred at each the 2nd and 3rd dose level (respiratory failure with shock and multisystem organ failure in both). Thus, all dose levels had acceptable toxicity based on our DLT/MTD definition. Adverse events by dose levels for the phase 1 patient cohort are summarized in Supplemental Table 2. Nine of the 30 patients achieved a CR (30%, 95% exact confidence interval [CI]: 15-49%), of which 7 were negative for MRD (MRDneg). Five additional patients achieved a CRp (3 MRDneg) for an ORR of 47% (95% CI: 28-66%). One other patient achieved an MRDpos CR with incomplete neutrophil recovery (CRi), 3 achieved a morphologic leukemia free state (MLFS), 4 died early from indeterminate cause (i.e. died before a treatment response was assessed), and 8 had resistant disease. Six of the 30 patients died within 28 days of treatment initiation due to respiratory failure in the setting of pneumonia (n=2), sepsis and multisystem organ failure (n=3), or intracranial hemorrhage (n=1). Because of the relatively high TRM and the lack of obvious differences in response rates between the 7 and 10-day course of decitabine, 7-day priming with decitabine before MEC was declared the recommended phase 2 dose (RP2D).
Table 2. Dose escalation scheme, best responses, and dose-limiting toxicities during phase 1.
| Dose Level | Decitabine | Patients (n) | Best Response | Dose-limiting toxicities |
|---|---|---|---|---|
| 1 | 5 days | 6 | 1 CR, 2 CRp, 1 RD, 2 DI | --- |
| 2 | 7 days | 12 | 5 CR, 1 CRp, 1 CRi, 3 RD, 2 DI* | Respiratory failure/shock (1) |
| 3 | 10 days | 12 | 3 CR, 2 CRp, 3 MLFS, 4 RD | Respiratory failure/shock (1) |
1 patient died before receiving MEC
Abbreviations: CR, complete remission; CRi, complete remission with incomplete blood count recovery; CRp, complete remission with incomplete platelet recovery; DI: death from indeterminate cause; MLFS, morphologic-leukemia free state; RD, resistant disease
Phase 2: estimation of anti-leukemia efficacy of dec/MEC
Overall, we treated 46 patients at/above the RP2D, including 34 and 12 patients who received priming with 7 days and 10 days of decitabine prior to MEC, respectively (Table 1). Best responses after 1-2 cycles of induction chemotherapy for the entire study population as well as those treated at/above the RP2D are summarized in Table 3. Ten of the 46 (22%, 95% CI: 11-36%) achieved a CR (8 MRDneg), and 5 obtained a CRp (11%, 95% CI: 4-24%), for an ORR of 33% (95% CI: 20-48%). Eleven of the 15 responders (73%) were negative for MRD, for an MRDneg ORR of 24% (95% CI: 13-39%). One further patient achieved an MRDpos CRi (2%, 95% CI: 0-12%), 7 patients obtained a MLFS (3 MRDneg), 20 patients had resistant disease, and 3 patients died from indeterminate cause/in aplasia. Restriction to the 34 patients treated at the 7-day dose level of decitabine yielded very similar results.
Table 3. Best response and outcomes after 1-2 cycles of study therapy.
| Response | All patients (n=52) | Patients treated at/above RP2D (n=46) |
|---|---|---|
|
| ||
| CR, n (%) | 11 (21.2%) | 10 (21.7%) |
| MRDneg | 9 (17.3%) | 8 (17.4%) |
| MRDpos | 2 (3.8%) | 2 (4.3%) |
|
| ||
| CRp, n (%) | 7 (13.5%) | 5 (10.9%) |
| MRDneg | 4 (7.7%) | 3 (6.5%) |
| MRDpos | 3 (5.8%) | 2 (4.3%) |
|
| ||
| Overall remission rate (CR+CRp), n (%) | 18 (34.6%) | 15 (32.6%) |
|
| ||
| CRi, n (%) | 1 (1.9%) | 1 (2.2%) |
| MRDneg | 0 (0%) | 0 (0%) |
| MRDpos | 1 (1.9%) | 1 (2.2%) |
|
| ||
| Morphologic leukemia-free state, n (%) | 7 (13.5%) | 7 (15.2%) |
| MRDneg | 3 (5.8%) | 3 (6.5%) |
| MRDpos | 4 (7.7%) | 4 (8.7%) |
|
| ||
| Resistant disease, n (%) | 21 (40.3%) | 20 (43.5%) |
|
| ||
| Death from indeterminate cause, n (%) | 5 (9.6%) | 3 (6.5%) |
|
| ||
| Early death**, n (5) | 9 (17.3%) | 7 (15.2%) |
|
| ||
| 8-week mortality, n (%) | 11 (21.2%) | 9 (19.6%) |
|
| ||
| Median overall survival, months | 5 | 5 |
|
| ||
| Median relapse-free survival, months | 5 | 5 |
2 patients died prior to receiving MEC;
Death within 28 days of initiation of study therapy
Abbreviation: CR, complete remission; CRi, complete remission with incomplete blood count recovery; CRp, complete remission with incomplete platelet recovery; MRD, measurable residual disease; RP2D, recommended phase 2 dose
Table 4 summarizes associations between baseline characteristics and induction response for the patients treated at/above the RP2D. While limited by the small sizes of individual patient subsets, we found higher response rates in patients with longer durations of their first CR, patients receiving dec/MEC as first salvage therapy, patients with favorable/intermediate-risk cytogenetics, and possibly those who did not receive prior therapy with intermediate/high-dose cytarabine. In contrast, we found no strong evidence that response rates differed depending on patient age or history of prior allogeneic HCT. Response rates were not lower, and perhaps even higher, in patients with secondary disease.
Table 4. Associations between baseline characteristics and response in patients treated at/above the RP2D, n=46.
| Baseline characteristic | Response rate, n (%) |
|---|---|
|
| |
| Age | |
| <40 years | 5/12 (41.7%) |
| <50 years | 6/18 (33.3%) |
| <60 years | 8/32 (25.0%) |
| ≥60 years | 7/14 (50.0%) |
|
| |
| Secondary disease* | |
| Yes | 3/7 (42.9%) |
| No | 12/39 (30.8%) |
|
| |
| Duration of CR1 | |
| 0 months (primary refractory) | 4/18 (22.2%) |
| 1-6 months | 5/20 (25.0%) |
| 7-12 months | 3/4 (75.0%) |
| >12 months | 3/4 (75.0%) |
|
| |
| Salvage number | |
| 1 | 11/29 (37.9%) |
| 2 | 3/12 (25.0%) |
| ≥3 | 1/5 (20.0%) |
|
| |
| Prior intermediate/high-dose cytarabine | |
| Yes | 10/35 (28.6%) |
| No | 5/11 (45.5%) |
|
| |
| Prior allogeneic HCT | |
| Yes | 5/13 (38.5%) |
| No | 10/33 (30.3%) |
|
| |
| Cytogenetic risk | |
| Favorable/intermediate | 12/26 (46.2%) |
| Adverse | 3/20 (15.0%) |
AML transformed from antecedent hematologic disorder or AML/MDS after prior cytotoxic therapy
Abbreviations: CR, complete remission; HCT, hematopoietic cell transplantation; RP2D, recommended phase 2 dose
Seven patients treated at/above the RP2D died within 28 days of treatment initiation due to respiratory failure in the setting of pneumonia (n=1), sepsis and multisystem organ failure (n=4), intracranial hemorrhage (n=1), and progressive disease (n=1), for a TRM rate of 15% (95% CI: 6-29%). Cytopenias, infections, and neutropenic fever were the most common grade 3-5 toxicities. Grade 3-4 mucositis, gastrointestinal problems, and hypoxia/respiratory failure were also common, the latter usually associated with pneumonia (Table 5 and Supplementary Table 3).
Table 5. Tolerability and safety of study therapy at/above the RP2D.
| Parameter | Grade 3-4 n (% cycles) |
Grade 5 n (% cycles) |
|---|---|---|
|
| ||
| Bacteremia | 18 (30.0%) | - |
| Catheter-related infection | 2 (3.3%) | - |
| Disseminated fungal infection | - | 1 (1.7%) |
| Pneumonia, sinusitis | 19 (31.7%) | 1 (1.7%) |
| Neutropenic fever | 47 (78.3%) | - |
| Sepsis | 17 (28.3%) | - |
| Soft-tissue infection | 4 (6.7%) | - |
| Other infection | 4 (6.7%) | - |
|
| ||
| Atrial tachycardia | 2 (3.3%) | - |
| Cardiac arrest | - | 1 (1.7%) |
| Cardiomyopathy | 2 (3.3%) | - |
| Hypotension | 4 (6.7%) | - |
|
| ||
| Multi-system organ failure | 1 (1.7%) | 2 (3.3%) |
| Hypoxia | 10 (16.7%) | - |
| Respiratory failure | 7 (11.7%) | 4 (6.7%) |
|
| ||
| Nausea/vomiting | 4 (6.7%) | - |
| Mucositis | 14 (23.3%) | - |
| Esophagitis | 3 (5.0%) | - |
| Abdominal pain | 2 (3.3%) | - |
| Diarrhea | 5 (8.3%) | - |
| Anorexia | 6 (10.0%) | - |
|
| ||
| Acute kidney injury | 1 (1.7%) | - |
| ALT or AST elevation | 4 (6.7%) | - |
| Bilirubin elevation | 2 (3.3%) | - |
| Cardiac troponin elevation | 2 (3.3%) | - |
| Hypokalemia, hyponatremia, hypophosphatemia | 14 (23.3%) | - |
| Tumor lysis | 2 (3.3%) | - |
|
| ||
| Intracranial hemorrhage | - | 1 (1.7%) |
| Syncope | 2 (3.3%) | - |
|
| ||
| Fall | 2 (3.3%) | - |
| Rash | 2 (3.3%) | - |
Table summarizing grade 3-5 non-hematologic adverse effects considered as definitively, probably, or possibly related to study treatment by the investigator that were experienced by the 46 patients treated at/above the R2PD over 60 treatment cycles
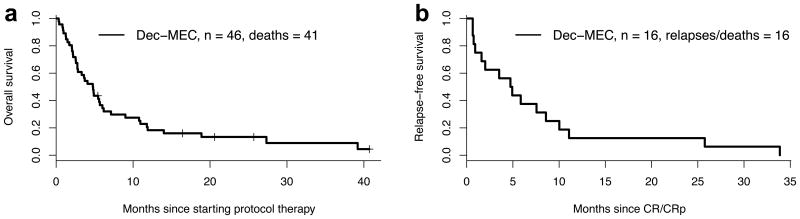
Eleven of the 15 patients achieving a CR/CRp at doses at/above the RP2D received alternate post-remission therapy (allogeneic HCT in 7). The other four discontinued study therapy due to relapse (n=1) or death while in remission (n=3, with deaths attributed to infection during post-remission therapy with dec/MEC, infection in the setting of high doses of steroids used to treat GVHD, and an unrelated malignancy). Relapses occurred in 10 of the 15 patients (median CR duration: 246 days, range 28 -1031 days) and death in CR in 5 (after CR durations of 20, 20, 23, 61 and 107 days), including the 3 patients noted above and 2 patients who died from septic shock and unknown cause after completion of study therapy. For the cohort of 46 patients treated at/above the RP2D, these results translated into a median overall and relapse-free survival of 148 and 144 days, respectively (Figure 1). For the patients alive at day 110 from study therapy (the maximum time to best response among all patients), the median survival for responders (n=12) was 403 days compared to 190 days in the patients who failed to achieve a CR/CRp but did not experience early death (n=17; p=.096). In total, 13 of the 46 patients (28%) treated at/above the RP2D underwent allogeneic HCT after receipt of dec/MEC, including 6 in CR/CRp at the time of transplant, 4 with persistent disease, and 3 with MLFS. Median time from start of dec/MEC therapy to transplant was 100 (range: 67-207) days. For these 13 patients, the median overall survival after transplant was 8 months (1-year survival: 26.5%). Among the 6 patients in CR/CRp at the time of transplant, median overall and relapse-free survival after transplant was 9 months and 6 months (1-year survival: 33%; 1-year relapse-free survival: 33%), respectively. Among the 7 patients not in CR/CRp at the time of transplant, median survival post-transplant was only 1 month (1-year survival: 21%).
Figure 1.
Kaplan-Meier estimates of (a) overall survival and (b) relapse-free survival of the 46 patients treated with dec/MEC at/above the RP2D.
Given specific concern about increased treatment-related toxicity in older patients and those who previously did or did not receive intermediate/high-dose cytarabine-containing regimens, we performed additional exploratory subset analyses. For the 32 patients age <60 treated with dec/MEC at/above the RP2D, the CR/CRp rate was 8/32 (25%), the 8-week mortality was 25%, and the median survival was 4 months (1-year survival: 10%). These outcomes did not appear worse for the 14 patients ≥60 years, with a CR/CRp rate of 7/14 (50%), an 8-week mortality of 1/14 (7%), and a median survival of 9 months (1-year survival: 36%). However, for the 10 patients age <60 who underwent subsequent allogeneic HCT, the median survival after transplant was 7 months (1-year survival: 36%), whereas for the 3 patients ≥60 years who underwent HCT median post-transplant survival was 3 months (1-year survival: 0%). Survival appeared slightly worse in the 25 patients who received prior intermediate/high-dose cytarabine-based treatments vs. the 11 who did not, with a median survival of 4 months vs. 5 months (1-year survival: 15% vs. 27%), and the 8-week mortality was higher (7/35 [20%] vs. 2/11 [11%]).
Duration of cytopenias
Data on duration of neutropenia and thrombocytopenia may be most informative (and least confounded by residual leukemia) in patients who achieved a CR. Among the 10 patients who achieved a CR after receipt of dec/MEC at/above the RP2D, the median time to recovery of ANC >1000 and platelets >100,000 was 29 (range: 27-66) and 29 (range: 20-66) days, respectively. When restricting this analysis to the 8 patients who achieved an MRDneg CR, median time to recovery of ANC >1000 and platelets >100,000 was 30 (range: 27-66) and 29 (range: 26-66) days, respectively.
Comparison to other salvage regimens
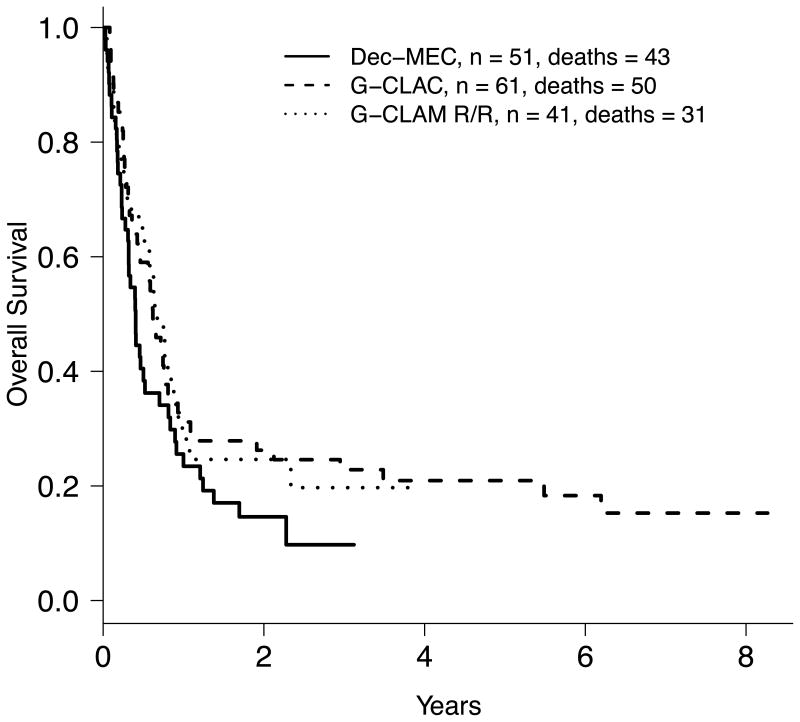
As this was a single-arm trial, we compared the outcomes observed on this trial to those obtained with other salvage regimens, controlling for prognostic factors. First, for each patient given dec/MEC we calculated a probability of CR based on prior CR duration (primary refractory =0) and number of salvage regimens. Summing these probabilities indicated that 5.6 of the 46 patients would have been expected to enter CR with the salvage regimens used at MD Anderson in the 1990s (mainly including high-dose cytarabine).27 Since we observed 10 CRs in these 46 patients, the observed/expected (O/E) CR ratio was 1.77. This O/E method is not amenable to multivariable statistical analysis. Hence, we also compared study results with outcomes from two contemporary patient cohorts, one receiving G-CLAC23 and the other receiving G-CLAM.24,25 For these comparisons, we included the dec/MEC patients treated at/above the RP2D as well as 6 additional patients who received dec/MEC outside this clinical trial for logistical reasons but met all study inclusion/exclusion criteria, and restricted the G-CLAC and G-CLAM cohorts to patients with relapsed/refractory AML and TRM scores ≤9.2. Basic characteristics of these three study cohorts are summarized in Table 6. In univariate analysis, patients treated with G-CLAC and G-CLAM were more likely to achieve a CR/CRp (odds ratio [OR] of 2.26 and 2.30; p=0.04 and 0.06, respectively). After controlling for various prognostic factors via multivariable analysis, treatment with G-CLAC or G-CLAM was associated with higher CR/CRp rates (for G-CLAC: OR=2.40 [95% CI: 0.87-6.64], p=0.09; for G-CLAM: OR=2.80 [95% CI: 0.96-8.17], p=0.06). Survival was 4.9 months for dec/MEC, 5.2 months for G-CLAC and 8.1 months for G-CLAM (Figure 2). In multivariable analysis, the hazard ratio for death was 0.69 for G-CLAC (95% CI: 0.42-1.14, p=0.15) and 0.9 for G-CLAM (95% CI: 0.53-1.52, p=0.69) compared to dec/MEC.
Table 6. Comparison of baseline characteristics and outcomes across salvage regimens.
| Regimen | Dec/MEC at/above RP2D (n=51) | G-CLAM (n=41) | G-CLAC (n=61) |
|---|---|---|---|
|
| |||
| Median age (range), years | 55 (19-75) | 51 (19-69) | 51 (19-91) |
|
| |||
| Male gender, n (%) | 26 (51.0%) | 23 (44.2%) | 41 (67.2%) |
|
| |||
| Disease, n (%) | |||
| AML | 45 (88.2%) | 31 (75.6%) | 61 (100%) |
| MDS-RAEB2 | 6 (11.8%) | 10 (24.4%) | 0 (0%) |
|
| |||
| Secondary disease*, n (%) | 7 (13.7%) | 12 (29.3%) | 15 (24.6%) |
|
| |||
| Disease status, n (%) | |||
| Primary refractory | 19 (37.3%) | 17 (41.5%) | 32 (52.5%) |
| Relapse | 32 (62.7%) | 24 (58.5%) | 29 (47.5%) |
| CR1 duration median (range), months | 5 (1-63) | 6 (1-81) | 4 (1-60) |
|
| |||
| Prior allogeneic HCT | 16 (31.4%) | 15 (36.6%) | 9 (14.8%) |
|
| |||
| Median number of prior therapies (range) | 2 (1-7) | 2 (1-7) | 1 (1-5) |
|
| |||
| Prior receipt of treatment with intermediate/high-dose cytarabine, n (%) | 38 (74.5%) | 22 (53.7%) | 17 (27.9%) |
|
| |||
| Median TRM score (range) | 3.17 (0.07-9.05) | 3.46 (0.14-9.08) | 3.52 (0.22-9.17) |
|
| |||
| Performance status, n. (%) | |||
| 0-1 | 46 (90.2%) | 38 (92.7%) | 53 (86.8%) |
| 2 | 5 (9.8%) | 3 (7.3%) | 7 (11.5%) |
| 3 | 0 (0%) | 0 (0%) | 1 (1.6%) |
|
| |||
| Cytogenetic risk, n (%) | |||
| Favorable | 1 (2.0%) | 3 (7.3%) | 2 (3.3%) |
| Intermediate | 28 (54.9%) | 22 (53.7%) | 35 (57.4%) |
| Adverse | 22 (44.2%) | 10 (24.4%) | 22 (36.1%) |
| Unknown | 0 (0%) | 6 (14.6%) | 2 (3.3%) |
|
| |||
| Mutational status, n (%) | |||
| FLT3-ITD | |||
| No | 30 (58.9%) | 25 (61.0%) | 24 (39.4%) |
| Yes | 5 (9.8%) | 4 (9.7%) | 11 (18.0%) |
| Unknown | 16 (31.4%) | 12 (29.3%) | 26 (42.6%) |
| NPM1 | |||
| No | 26 (51.0%) | 21 (51.2%) | 20 (32.8%) |
| Yes | 7 (13.7%) | 4 (9.8%) | 7 (11.5%) |
| Unknown | 16 (36.5%) | 16 (39.0%) | 34 (55.7%) |
|
| |||
| Overall response, n (%) | 16 (31.4%) | 21 (51.2%) | 32 (52.5%) |
| Prior intermediate/high-dose cytarabine | 10/38 (26.3%) | 13/22 (59.1%) | 8/17 (47.1%) |
| No prior intermediate/high-dose cytarabine | 6/13 (46.2%) | 8/19 (42.1%) | 24/44 (54.5%) |
| CR | 11 (21.6%) | 16 (39.0%) | 30 (49.2%) |
| CRp | 5 (9.8%) | 5 (12.1%) | 2 (3.3%) |
|
| |||
| Subsequent allogeneic HCT | 13 (25.0%) | 15 (36.6%) | 26 (42.6%) |
|
| |||
| Median overall survival, months | 5 | 8 | 5 |
| Prior intermediate/high-dose cytarabine | 5 | 8 | 9 |
| No prior intermediate/high-dose cytarabine | 5 | 10 | 7 |
|
| |||
| Early death**, n (%) | 8 (15.4%) | 3 (7.3%) | 0 (0%) |
|
| |||
| 8-week mortality | 10/51 (19.6%) | 7/41 (17.1%) | 6/61 (9.8%) |
| Prior intermediate/high-dose cytarabine | 8/38 (21.1%) | 2/22 (9.1%) | 2/17 (11.8%) |
| No prior intermediate/high-dose cytarabine | 2/13 (15.4%) | 5/19 (26.3%) | 4/44 (9.1%) |
AML transformed from antecedent hematologic disorder or AML/MDS after prior cytotoxic therapy
Death within 28 days of starting study treatment
Abbreviation: CR, complete remission; CRp, complete remission with incomplete platelet recovery; HCT, hematopoietic cell transplantation; RP2D, recommended phase 2 dose
Figure 2.
Kaplan-Meier estimates of overall survival of 51 patients treated with dec/MEC at/above the RP2D, 61 patients treated with G-CLAC, and 41 patients treated with G-CLAM.
Discussion
In this study, we explored the sequential use of decitabine and MEC based on the premise that priming with a DNA methyltransferase inhibitor sensitizes AML cells to conventional chemotherapeutics. Our limited ex vivo experiments indicated this sensitizing effect is greater with delayed use of chemotherapy agents (D.L.S.: unpublished observation). Consistent with this observation are Kantarjian et al.'s findings from correlative analyses of specimens from patients with MDS participating in a phase 3 trial demonstrating extended demethylation in peripheral blood cells after decitabine treatment.28 With a CR/CRp rate of 33% for patients treated at/above the R2PD, the dec/MEC regimen met our pre-specified efficacy goal of “being worthy of further investigation”. Many of the responders were subsequently able to undergo allogeneic HCT, the preferred curative-intent treatment option for relapsed or refractory disease. Still, although we selected a medically fit subset of patients for dec/MEC therapy, we noted a relatively high early mortality (TRM) rate, highlighting the difficulties in administering intense cytotoxic therapy in previously treated AML/MDS patients.
Our study was limited in that it followed a traditional single-arm design and did not include a control group. To address this, we compared outcomes with dec/MEC with those of a historical control population that we matched based on duration of prior remission and number of prior salvage therapies,27 and obtained an observed/expected CR ratio that favored dec/MEC, indeed suggesting value of this combination regimen. However, this approach can be criticized for the “historic” nature of the control patient population, which was treated between 1991 and 1994.27 We therefore additionally compared study results with outcomes from two contemporary patient cohorts that received high-dose cytarabine-based salvage chemotherapy, G-CLAC or G-CLAM, salvage regimens commonly used at our institution since 2008. After controlling for various prognostic factors via multivariable analysis, we found the response rates with dec/MEC to be no better (and, in fact, to be likely slightly worse) than with G-CLAC or G-CLAM, whereas survival estimates were similar for all three patient cohorts after multivariable adjustments. This may be partly due to the higher TRM rate with dec/MEC than G-CLAC or G-CLAM in our patient cohorts. Specifically, despite very similar TRM scores in all three cohorts (median [range] for dec/MEC: 3.8 [0.1-9.1]; for G-CLAM: 3.5 [0.1-9.1]; for G-CLAC: 3.5 [0.2-9.2]), early death rates varied substantially (dec/MEC: 15%; for G-CLAM: 7%; for G-CLAC: 0%; see Table 6). We were unable to identify particular patient- or disease-specific characteristics that would account for these differences between treatment cohorts, and we can only speculate that they may be due to comorbidities not accurately captured in the TRM score, which was originally developed in patients with newly diagnosed (not relapsed/refractory) AML. Still, these data suggest no obvious advantage of dec/MEC over other contemporary high-dose cytarabine-based salvage regimens and highlight the need for controlled assessments of anti-leukemia efficacies of experimental treatment regimens.29 Also, the difference in conclusion one would draw from the historic patient comparison and the comparison with contemporary patients stresses the importance of including appropriate control patients in the design of trials testing new therapies for AML.29
The clinical value of combining DNA methyltransferase inhibitors with conventional chemotherapeutics remains uncertain. So far, several studies have examined combinations of decitabine and cytotoxic chemotherapy for AML. In a phase 1 trial, decitabine priming followed by daunorubicin and cytarabine (“3+7”) for patients with de novo, unfavorable risk AML was well tolerated, and no DLTs were reached when doses of decitabine were escalated.16 More recently, decitabine was used both sequentially with G-CSF, idarubicin, and low-dose cytarabine30 or G-CSF, aclarubicin, and low-dose cytarabine31 as well as concurrently with aclacinomycin and cytarabine.17 In these studies, it was felt that outcomes were better than what would have been expected without the use of decitabine. In contrast, a study that investigated the value of adding azacitidine before 3+7 chemotherapy in a randomized fashion in 214 older adults (median age: 70 years) with newly diagnosed AML found no differences in efficacy but increased toxicity in the combination arm.32 Since we did not have a contemporary cohort of patients with relapsed/refractory AML receiving MEC as salvage therapy, we were unable to determine whether dec/MEC provided any benefit over MEC.
Dec/MEC showed anti-leukemia activity that was comparable to other contemporary salvage regimens in this challenging patient population which continues to have a poor prognosis with currently used treatments. A follow-up study at our institution will explore decitabine together with G-CLAM in newly diagnosed as well as relapsed/refractory AML (NCT02921061), with non-randomized comparison of trial results to outcomes with G-CLAM alone. This study will assess the importance of decitabine timing relative to G-CLAM by randomizing patients to receive decitabine sequentially or concurrently with G-CLAM.
Supplementary Material
Acknowledgments
The conduct of the clinical trial was partially funded by Protocol Specific Research Support from the Fred Hutchinson/University of Washington Cancer Consortium Cancer Center Support Grant of the National Institutes of Health (P30-CA015704). A.B.H. and S.A.B. are supported by a fellowship training grant from the National Heart, Lung, and Blood Institute/National Institutes of Health (NHLBI/NIH: T32-HL007093). R.B.W. is a Leukemia & Lymphoma Society Scholar in Clinical Research.
Footnotes
Conflict of interest: The authors declare no competing financial interests.
Supplementary information is available at Leukemia's website.
References
- 1.Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. Lancet. 2013;381(9865):484–495. doi: 10.1016/S0140-6736(12)61727-9. [DOI] [PubMed] [Google Scholar]
- 2.Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 3.Amadori S, Arcese W, Isacchi G, Meloni G, Petti MC, Monarca B, et al. Mitoxantrone, etoposide, and intermediate-dose cytarabine: an effective and tolerable regimen for the treatment of refractory acute myeloid leukemia. J Clin Oncol. 1991;9(7):1210–1214. doi: 10.1200/JCO.1991.9.7.1210. [DOI] [PubMed] [Google Scholar]
- 4.Leopold LH, Willemze R. The treatment of acute myeloid leukemia in first relapse: a comprehensive review of the literature. Leuk Lymphoma. 2002;43(9):1715–1727. doi: 10.1080/1042819021000006529. [DOI] [PubMed] [Google Scholar]
- 5.Ravandi F. Relapsed acute myeloid leukemia: why is there no standard of care? Best Pract Res Clin Haematol. 2013;26(3):253–259. doi: 10.1016/j.beha.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thol F, Schlenk RF, Heuser M, Ganser A. How I treat refractory and early relapsed acute myeloid leukemia. Blood. 2015;126(3):319–327. doi: 10.1182/blood-2014-10-551911. [DOI] [PubMed] [Google Scholar]
- 7.Shih AH, Abdel-Wahab O, Patel JP, Levine RL. The role of mutations in epigenetic regulators in myeloid malignancies. Nat Rev Cancer. 2012;12(9):599–612. doi: 10.1038/nrc3343. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Wahab O, Levine RL. Mutations in epigenetic modifiers in the pathogenesis and therapy of acute myeloid leukemia. Blood. 2013;121(18):3563–3572. doi: 10.1182/blood-2013-01-451781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estey EH. Epigenetics in clinical practice: the examples of azacitidine and decitabine in myelodysplasia and acute myeloid leukemia. Leukemia. 2013;27(9):1803–1812. doi: 10.1038/leu.2013.173. [DOI] [PubMed] [Google Scholar]
- 10.Roboz GJ. Epigenetic targeting and personalized approaches for AML. Hematology Am Soc Hematol Educ Program. 2014;2014(1):44–51. doi: 10.1182/asheducation-2014.1.44. [DOI] [PubMed] [Google Scholar]
- 11.Wouters BJ, Delwel R. Epigenetics and approaches to targeted epigenetic therapy in acute myeloid leukemia. Blood. 2016;127(1):42–52. doi: 10.1182/blood-2015-07-604512. [DOI] [PubMed] [Google Scholar]
- 12.Qin T, Youssef EM, Jelinek J, Chen R, Yang AS, Garcia-Manero G, et al. Effect of cytarabine and decitabine in combination in human leukemic cell lines. Clin Cancer Res. 2007;13(14):4225–4232. doi: 10.1158/1078-0432.CCR-06-2762. [DOI] [PubMed] [Google Scholar]
- 13.Hollenbach PW, Nguyen AN, Brady H, Williams M, Ning Y, Richard N, et al. A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines. PLoS One. 2010;5(2):e9001. doi: 10.1371/journal.pone.0009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurimoto M, Matsuoka H, Hanaoka N, Uneda S, Murayama T, Sonoki T, et al. Pretreatment of leukemic cells with low-dose decitabine markedly enhances the cytotoxicity of gemtuzumab ozogamicin. Leukemia. 2013;27(1):233–235. doi: 10.1038/leu.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonard SM, Perry T, Woodman CB, Kearns P. Sequential treatment with cytarabine and decitabine has an increased anti-leukemia effect compared to cytarabine alone in xenograft models of childhood acute myeloid leukemia. PLoS One. 2014;9(1):e87475. doi: 10.1371/journal.pone.0087475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scandura JM, Roboz GJ, Moh M, Morawa E, Brenet F, Bose JR, et al. Phase 1 study of epigenetic priming with decitabine prior to standard induction chemotherapy for patients with AML. Blood. 2011;118(6):1472–1480. doi: 10.1182/blood-2010-11-320093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song LX, Xu L, Li X, Chang CK, Zhang Y, Wu LY, et al. Clinical outcome of treatment with a combined regimen of decitabine and aclacinomycin/cytarabine for patients with refractory acute myeloid leukemia. Ann Hematol. 2012;91(12):1879–1886. doi: 10.1007/s00277-012-1550-y. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 19.Walter RB, Othus M, Borthakur G, Ravandi F, Cortes JE, Pierce SA, et al. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: a novel paradigm for treatment assignment. J Clin Oncol. 2011;29(33):4417–4423. doi: 10.1200/JCO.2011.35.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 21.Walter RB, Gooley TA, Wood BL, Milano F, Fang M, Sorror ML, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29(9):1190–1197. doi: 10.1200/JCO.2010.31.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y, et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: time to move toward a minimal residual disease-based definition of complete remission? J Clin Oncol. 2016;34(4):329–336. doi: 10.1200/JCO.2015.63.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker PS, Kantarjian HM, Appelbaum FR, Petersdorf SH, Storer B, Pierce S, et al. Clofarabine with high dose cytarabine and granulocyte colony-stimulating factor (G-CSF) priming for relapsed and refractory acute myeloid leukaemia. Br J Haematol. 2011;155(2):182–189. doi: 10.1111/j.1365-2141.2011.08831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wierzbowska A, Robak T, Pluta A, Wawrzyniak E, Cebula B, Holowiecki J, et al. Cladribine combined with high doses of arabinoside cytosine, mitoxantrone, and G-CSF (CLAG-M) is a highly effective salvage regimen in patients with refractory and relapsed acute myeloid leukemia of the poor risk: a final report of the Polish Adult Leukemia Group. Eur J Haematol. 2008;80(2):115–126. doi: 10.1111/j.1600-0609.2007.00988.x. [DOI] [PubMed] [Google Scholar]
- 25.Jaglal MV, Duong VH, Bello CM, Al Ali NH, Padron E, Fernandez HF, et al. Cladribine, cytarabine, filgrastim, and mitoxantrone (CLAG-M) compared to standard induction in acute myeloid leukemia from myelodysplastic syndrome after azanucleoside failure. Leuk Res. 2014;38(4):443–446. doi: 10.1016/j.leukres.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Ravandi F, Ritchie EK, Sayar H, Lancet JE, Craig MD, Vey N, et al. Vosaroxin plus cytarabine versus placebo plus cytarabine in patients with first relapsed or refractory acute myeloid leukaemia (VALOR): a randomised, controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 2015;16(9):1025–1036. doi: 10.1016/S1470-2045(15)00201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estey E, Kornblau S, Pierce S, Kantarjian H, Beran M, Keating M. A stratification system for evaluating and selecting therapies in patients with relapsed or primary refractory acute myelogenous leukemia. Blood. 1996;88(2):756. [PubMed] [Google Scholar]
- 28.Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O'Brien S, Cortes J, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109(1):52–57. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 29.Walter RB, Appelbaum FR, Tallman MS, Weiss NS, Larson RA, Estey EH. Shortcomings in the clinical evaluation of new drugs: acute myeloid leukemia as paradigm. Blood. 2010;116(14):2420–2428. doi: 10.1182/blood-2010-05-285387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye XN, Zhou XP, Wei JY, Xu GX, Li Y, Mao LP, et al. Epigenetic priming with decitabine followed by low-dose idarubicin/cytarabine has an increased anti-leukemic effect compared to traditional chemotherapy in high-risk myeloid neoplasms. Leuk Lymphoma. 2016;57(6):1311–1318. doi: 10.3109/10428194.2015.1091931. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Chen Y, Zhu Y, Zhou J, Xu Y, Li Y, et al. Efficacy and safety of decitabine in combination with G-CSF, low-dose cytarabine and aclarubicin in newly diagnosed elderly patients with acute myeloid leukemia. Oncotarget. 2015;6(8):6448–6458. doi: 10.18632/oncotarget.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Müller-Tidow C, Tschanter P, Röllig C, Thiede C, Koschmieder A, Stelljes M, et al. Azacitidine in combination with intensive induction chemotherapy in older patients with acute myeloid leukemia: The AML-AZA trial of the Study Alliance Leukemia. Leukemia. 2016;30(3):555–561. doi: 10.1038/leu.2015.306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.