Abstract
Objectives
The adverse sexual effects of androgen deprivation therapy (ADT) on men with prostate cancer have been well described. Less well-known is the relative degree of sexual dysfunction and bother associated with ADT compared to other primary treatment modalities such as radical prostatectomy. We sought to describe the trajectory and relative magnitude of changes in sexual function and bother in men on ADT and to examine demographic and clinical predictors of ADT’s adverse sexual effects.
Methods
Prostate cancer patients treated with ADT (n=60) completed assessments of sexual function and sexual bother three times during a one-year period after the initiation of ADT. Prostate cancer patients treated with radical prostatectomy only and not receiving ADT (n=85) and men with no history of cancer (n=86) matched on age and education completed assessments at similar intervals.
Results
ADT recipients reported worsening sexual function and increasing bother over time compared to controls. Effect sizes for the differences in sexual function were large to very large, and for bother were small to very large. Age younger than 83 years predicted relatively poorer sexual function and age younger than 78 years predicted greater sexual bother at 12 months in men on ADT compared to men not on ADT.
Conclusions
Most men on ADT for prostate cancer will never return to baseline levels of sexual function. Interventions focused on sexual bother over function and designed to help couples build and maintain satisfying relationship intimacy are likely to more positively affect men’s psychological well-being while on ADT than medical or sexual aids targeting sexual dysfunction.
Keywords: prostate, cancer, oncology, androgen deprivation therapy, sexual function, sexual bother, sexual health
Introduction
Prostate cancer is the most common cancer in men in the United States. An estimated 181,000 new cases are expected to be diagnosed in 2016.[1] The role of androgens in the pathophysiology of the disease has been well-described.[2] The goal of androgen deprivation therapy (ADT) is to reduce levels of androgens, the hormones responsible for stimulating prostate cancer cells to grow. ADT is widely used as standard of care to treat non-metastatic and biochemically recurrent disease.[3] A recent study indicates that approximately 189,000 men with non-metastatic prostate cancer currently are being managed with ADT during the course of their disease.[4]
ADT is not considered curative and is associated with a number of adverse effects.[5] Rates of erectile dysfunction with ADT are estimated between 70 and 90%.[3] In addition to erectile dysfunction, ADT also is associated with a number of other sexual effects. These include decreased sexual desire, inability to achieve orgasm and decreased penile length and testicular size.[3, 5] Typically, the longer the duration of ADT, the more persistent the effects may be.[6]
Whether men are highly bothered by the sexual effects of ADT in the context of a diagnosis of prostate cancer is not clear. The majority of studies have examined sexual bother in men treated with ADT in combination with radical prostatectomy (RP) or radiotherapy.[7–10] In general, findings indicate a relative moderate effect of ADT on bother that stabilizes in the early period following initiation of treatment. In trying to account for this relative moderate effect, some have suggested that those treated with ADT understand that they have more severe disease and so may be more accepting of side effects.[3, 7] However, studies examining bother in men with prostate cancer on ADT as a primary treatment suggest an increase in bother over time.[11–13]
Radical prostatectomy (RP) is a gold standard treatment for men diagnosed with localized prostate cancer. Erectile dysfunction is a common, well-known side effect of RP. A recent meta-analysis reported rates of erectile dysfunction after RP between 14% and 90%.[14] A number of prospective studies[8, 15–19] have described the course and magnitude of changes in erectile function after RP, demonstrating a decline in function in the initial postsurgical period with improvement over time. Approximately one out of five men can be expected to return to baseline erectile function by two years after surgery.[20, 21] Other adverse sexual effects associated with RP include urinary incontinence and/or lack of ejaculate at orgasm, pain with orgasm and changes in penile length and girth.[22, 23]
Findings as to whether men are highly bothered by these RP-related sexual effects in the context of a diagnosis of prostate cancer are mixed.[12, 24] RP not only has the potential to cure prostate cancer but the adverse effects tend to diminish over time. Thus, men are likely to have relatively low levels of sexual bother in relation to these effects and/or to experience a degree of bother that improves over time as baseline levels of function are recovered.[23, 25, 26] Some have suggested, however, that men treated with RP do not adjust psychologically and that bother may be unrelated to sexual function and/or actually increase over time.[7, 23, 27] These mixed findings highlight the importance of distinguishing sexual function from bother in men with prostate cancer.[28] Different treatment modalities demonstrate distinctive patterns concerning sexual function over time.[7, 12] Whether these same modalities show characteristic or consistent patterns of bother and whether these patterns are more or less indicative of sexual function remains to be determined.
In the current study, we sought to describe the trajectory and relative magnitude of changes in both sexual function and bother in men with prostate cancer during a one-year period after the initiation of ADT. We included two matched groups for comparison: men with prostate cancer treated with RP only and not receiving ADT and men with no history of cancer. We hypothesized that men in the ADT group would report worsening sexual function and increasing bother over time compared to the control groups. We also hypothesized that compared to men on ADT and men with no history of cancer, men treated with RP would demonstrate time-dependent improvements in sexual function and decreasing bother over time. Finally, we sought to build upon prior literature regarding the effects of ADT by conducting exploratory analyses examining potential demographic and clinical moderators of the effect of ADT on sexual function and bother.
Materials and Methods
Participants
Participants were recruited as part of a longitudinal study examining the effect of ADT on quality of life, including cognitive outcomes, on men with prostate cancer. Eligibility criteria, recruitment procedure, and matching criteria have been described in greater detail elsewhere.[29] Briefly, all participants were required to: be at least 18 years of age, be able to speak and read English, have at least a sixth grade education, have no history of cerebrovascular accident, and not demonstrate impaired mental status based on screening (Short Portable Mental Status Exam score less than 3). Men with prostate cancer receiving ADT (ADT+ group) were also required to: be diagnosed with non-metastatic or asymptomatic metastatic prostate cancer, be scheduled to start ADT or have started ADT in the past month, be scheduled to receive ADT for at least 6 months, have not received treatment for any other cancers in the 12 months prior to recruitment, have no history of brain cancer or previous cranial irradiation, and have not been treated with ADT in the 12 months prior to recruitment or an anti-androgen agent in the 6 months prior to recruitment. Men with prostate cancer not treated with ADT (ADT− group) were also required to: be diagnosed with non-metastatic prostate cancer, have no history of other cancers except non-melanoma skin cancer, have undergone RP but no other forms of prostate cancer treatment, have no history of recurrent disease since undergoing RP, and not be receiving testosterone supplementation. Men with no cancer (CA− group) were also required to have no history of any form of cancer except non-melanoma skin cancer and not be receiving testosterone supplementation.
Procedure
Data were collected between September 2008 and June 2013. ADT+ group participants were identified using computerized appointment systems, screened for eligibility via review of the electronic health record and recruited during outpatient appointments at Moffitt Cancer Center (MCC) or James A. Haley Veterans’ Hospital prior to or within one month of initiating ADT treatment. ADT− group participants were identified using the MCC cancer registry, screned for eligibility via electronic health record review, and recruited via mail and telephone following recruitment of each ADT+ group participant. Following recruitment of each ADT+ participant, matching CA− group participants were identified using a commercially available marketing database (Marketing Systems Group, For Washington, PA, USA) and recruited via mail and telephone using procedures mirroring those for the ADT− group. Supplementary figures 1 to 3 show information about participant flow. Written informed consent was obtained prior to initiation of study procedures. Participants were paid $80 at each evaluation. This study was approved by the Institutional Review Board at the University of South Florida (IRB#106381).
ADT− group participants were matched to ADT+ group participants on time since diagnosis (within six months); ADT− and CA− group participants were recruited to be matched to ADT+ participants on age (within five years) and educational level (12 years or less, 13–16 years, or 17 years or more). Baseline assessments were completed by ADT+ participants before or within 21 days of starting ADT and 6 and 12 months later. ADT− and CA− participants were assessed at similar time intervals.
Measures
Age, marital status, education, race, and ethnicity were assessed at baseline via self-report. Medical comorbidities were assessed at baseline using a self-report version of the Charlson Comorbidity Index (CCI).[30] Gleason score was extracted via electronic medical record review.
Self-reported sexual functioning and bother from sexual functioning over the previous four weeks were assessed at each time point using the Sexual Function and Bother From Sexual Function scales of the Expanded Prostate Cancer Index Composite (EPIC),[31] a valid and reliable measure that is widely used in studies of prostate cancer patients.[32] Scores on these scales range from 0 – 100, with higher scores indicating better quality of life (better function and less bother). In addition, EPIC items asking about participants’ level of sexual desire, ability to have an erection, and ability to reach climax were analyzed separately. Scores on these items range from 1 – 5 with higher scores indicating better sexual function.
Statistical analysis
Analyses were restricted to the 60 ADT+, 85 ADT−, and 87 CA− participants who completed the baseline assessment and at least one follow-up assessment. Those deemed ineligible because data were not available from baseline and/or one follow-up assessment (n=53) did not differ from those deemed eligible on demographic or clinical variables (p values ≥ .27). Demographic and clinical variables that were significantly different between the ADT+, ADT− and CA− groups at p < .10 were included as covariates in subsequent analyses.
Mixed model analyses were conducted to compare the rate of change in sexual function and bother over time using PROC MIXED in SAS, Version 9.4 (Cary, NC).[33] Mixed model analyses allow for the use of all available data at each time point without imputing missing data for those missing data at either follow-up assessment.[34] A two-sided alpha value of .05 was used as the criterion for statistical significance. Cohens d[35] was used to calculate effect sizes of group differences. A cutoff of 0.5 standard deviations[36] was used as a cutoff for clinically-significant differences from baseline to 12 months within the ADT+ group and between groups’ 12-month scores. Exploratory linear regression analyses were conducted using the PROCESS macro[37] to identify potential moderators of the effect of ADT on 12-month sexual function and bother while controlling for baseline values. For significant moderators the Johnson-Neyman technique was used to calculate the region of significance: the value of the moderator above which ADT was no longer significantly associated with the outcome.[38] An alpha level of .05 was used.
Results
Demographic and Clinical Characteristics
Demographic and clinical characteristics for the sample (see Footnote 1) are shown in Table 1. The ADT+ group reported more medical comorbidities and had higher Gleason scores than the ADT− group (p values < .01). The ADT+ group also reported fewer years of education, was less likely to be married, and was less likely to be White than the ADT− and CA− groups (p values ≤ .08). Thus, medical comorbidities, education, marital status, and race were included as covariates in analyses comparing groups on outcome measures. Because ADT was prescribed for more advanced disease, Gleason scores were not entered as a covariate in analyses.
Table 1.
Demographic and Clinical Characteristics of the Sample
| ADT+ (n = 60) | ADT− (n = 85) | CA− (n = 87) | ADT+ vs. ADT− | ADT+ vs. CA− | |
|---|---|---|---|---|---|
|
| |||||
| Characteristic | p value | p value | |||
|
| |||||
| Age, years | |||||
| Mean | 68.10 | 67.94 | 69.03 | ||
| SD | 8.75 | 7.37 | 8.03 | .90 | .51 |
|
| |||||
| Years of education | |||||
| Mean | 14.00 | 14.76 | 14.89 | ||
| SD | 2.44 | 2.70 | 2.45 | .08 | .03 |
|
| |||||
| Body mass index, kg/m2 | |||||
| Mean | 28.22 | 29.51 | 29.72 | ||
| SD | 4.21 | 4.26 | 5.24 | .10 | .10 |
|
| |||||
| Comorbidity index score | |||||
| Mean | 2.83 | 2.44 | 2.69 | ||
| SD | 1.04 | 0.89 | 1.07 | .01 | .42 |
|
| |||||
| Characteristic | No. (%) | No. (%) | No. (%) | p value | p value |
|
| |||||
| Marital status | .04 | <.001 | |||
| Married | 37 (62) | 66 (78) | 76 (87) | ||
| Not married | 23 (38) | 19 (22) | 11 (13) | ||
|
| |||||
| Race | .04 | .01 | |||
| White | 51 (85) | 81 (95) | 84 (97) | ||
| Nonwhite | 9 (15) | 4 (5) | 3 (3) | ||
|
| |||||
| Gleason score | <.001 | - | |||
| 4–6 (low) | 8 (13) | 40 (47) | - | ||
| 7 (intermediate) | 22 (37) | 39 (46) | - | ||
| 8 (high) | 19 (32) | 2 (2) | - | ||
| 9–10 (high) | 7 (12) | 0 (0) | - | ||
| Missing | 4 (6) | 4 (5) | - | ||
Note. N = 232. SD = standard deviation. p values calculated using χ2s for categorical variables and t-tests for continuous variables. Missing levels were excluded from calculation of p values.
Change Over Time in Sexual Function and Bother
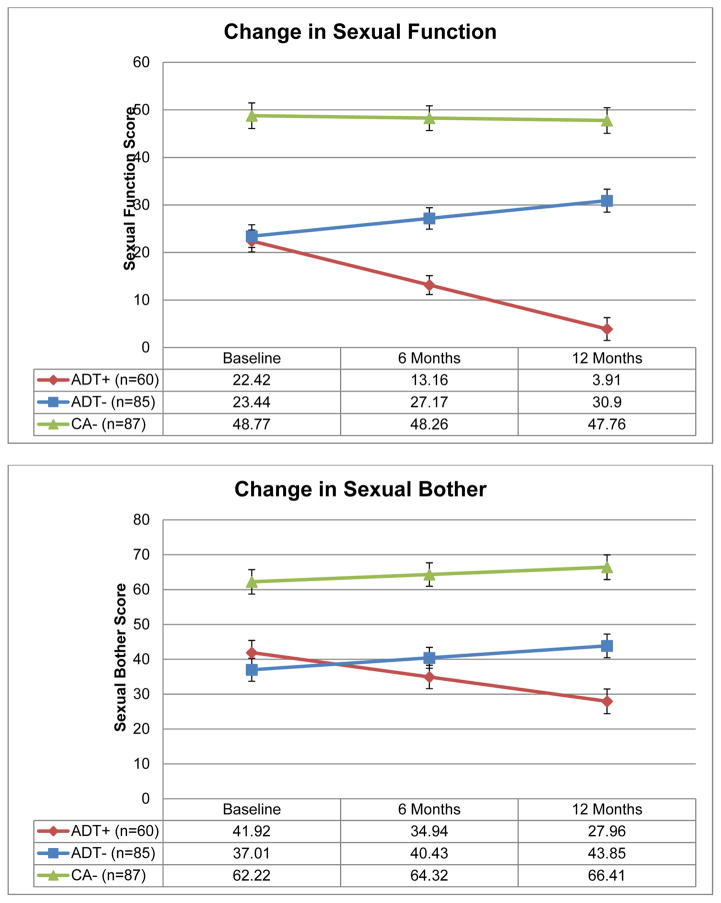
Figure 1 presents estimated means of sexual function and bother by group. Table 2 presents effect sizes for group differences when significant group by time interactions were observed. Significant group by time interactions were observed when comparing the ADT+ group to the ADT− and CA− groups on sexual function (p values < .001). The ADT+ and ADT− groups did not differ on sexual function at baseline (p = .88), but the ADT+ group reported worse sexual functioning at six months and 12 months (p values < .001). The ADT+ group reported worse sexual function than the CA− group at baseline, six months, and 12 months (p values ≤ .001). Similarly, group by time interactions were observed when comparing the ADT+ group to the ADT− and CA− groups on bother from sexual function (p values < .001). The ADT+ and ADT− groups did not differ on bother at baseline or six months (p values ≥ .30), but the ADT+ group reported greater bother at 12 months (p = .01). The ADT+ group reported greater bother than the CA− group at baseline, six months, and 12 months (p values ≤ .001). Examinations of within-group change over time indicated that while the ADT+ group worsened over time on sexual function and bother (p values < .01), the ADT− group improved over time on both measures (p values ≤ .02) and the CA− group did not change over time (p values ≥ .32).
Figure 1.
Adjusted means for Sexual Functioning and Bother From Sexual Function scales.
Table 2.
Effect Sizes for Statistically Significant Group Differences
| ADT+ vs. ADT− Baseline | ADT+ vs. ADT− 6 Months | ADT+ vs. ADT− 12 Months | ADT+ vs. CA− Baseline | ADT+ vs. CA− 6 Months | ADT+ vs. CA− 12 Months | |
|---|---|---|---|---|---|---|
| Outcome Variable | d | d | d | d | d | d |
| Sexual Function | 0.05 | 0.75 | 1.30 | 1.18 | 1.67 | 1.94 |
| Bother From Sexual Function | −0.16 | .20 | .49 | .62 | .97 | 1.15 |
| Level of Sexual Desire | .48 | 1.06 | 1.35 | 1.12 | 1.72 | 1.80 |
| Ability to Have an Erection | −.20 | .44 | .93 | 1.01 | 1.46 | 1.65 |
| Ability to Climax | .23 | .89 | 1.34 | 1.08 | 1.58 | 1.75 |
Note. N = 232. d = effect size.
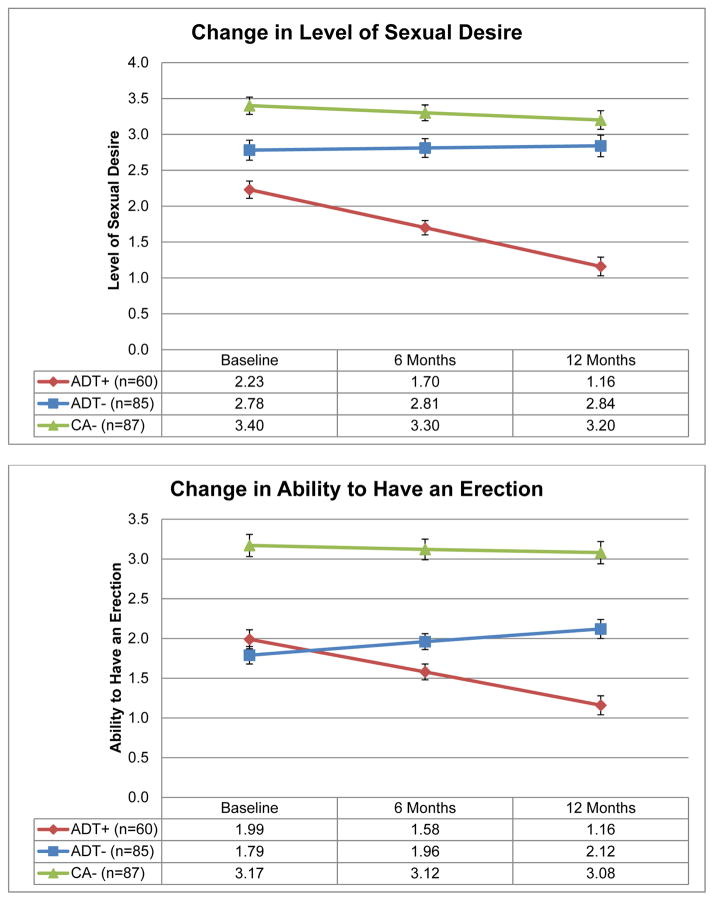
Significant group by time interactions were observed when comparing the ADT+ group to the ADT− and CA− groups on participants’ level of sexual desire, ability to have an erection, and ability to climax (p values < .01; see Figure 2). The ADT+ group reported lower levels of sexual desire than the ADT− and CA− groups at each assessment (p values ≤ .05). The ADT+ and ADT− groups did not differ on ability to have an erection or ability to climax at baseline (p values ≥ .10), but the ADT+ group was less able to have an erection or climax than the ADT− group at six months (p values ≤ .04) and 12 months (p values ≤ .01). The ADT+ group was less able to have an erection or climax than the CA− group at all assessments (p values < .01). See Table 2 for effect sizes. Examinations of within-group change over time indicated that while the ADT+ group worsened over time on levels of sexual desire, ability to have an erection, and ability to climax (p values < .01) the CA− group did not change over time on these outcomes (p values ≥ .06). The ADT− group did not change over time on levels of sexual desire (p = .62) but improved over time on ability to have an erection and ability to climax (p values < .01).
Figure 2.
Estimated means for sexual function items.
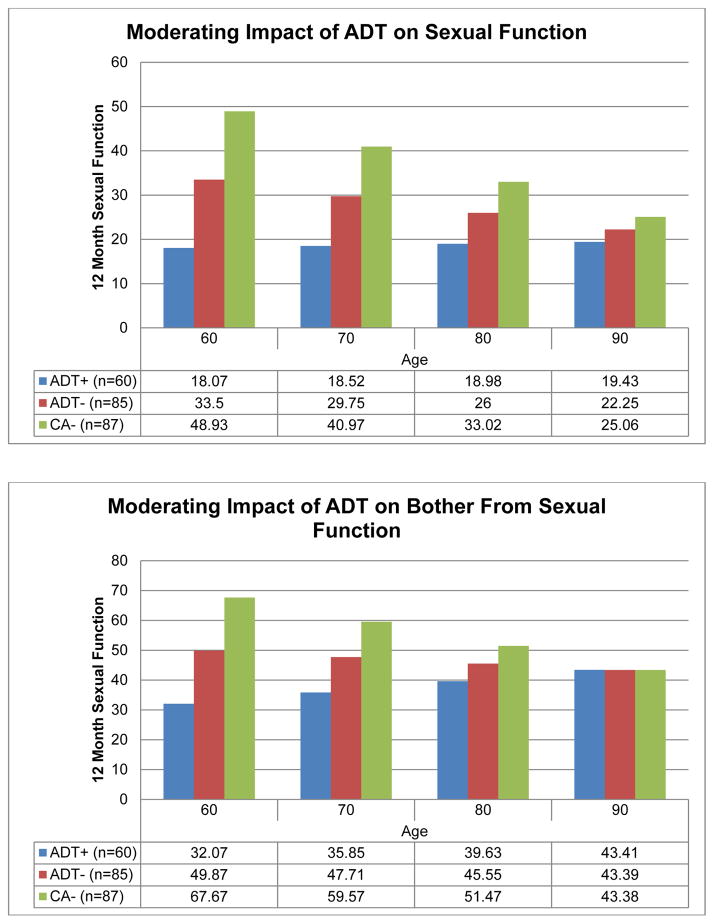
Moderators of Change in Sexual Function and Bother
Exploratory analyses examined potential baseline moderators of the effect of ADT on sexual function and bother from sexual function. Age moderated the effect of ADT on both sexual function and bother (p values ≤ .03; see Figure 3). After controlling for baseline sexual function, younger ADT+ group participants reported significantly worse sexual function at 12 months than similarly aged men who did not receive ADT; however, this trend was not observed among older participants. Specifically, analyses indicated that ADT recipients 83 years of age or older when starting ADT did not report significantly worse sexual function than men who did not receive ADT. Similarly, ADT recipients aged 78 or older when starting ADT did not report significantly worse sexual bother than men who did not receive ADT. Body mass index at baseline, baseline medical comorbidities, education and marital status were not associated with changes in sexual function (p values = .26) or bother (p values ≥ .21).
Figure 3.
Adjusted means for sexual function items.
Discussion
The current study sought to describe the trajectory and relative magnitude of changes in sexual function and sexual bother in men with prostate cancer during a one-year period after the initiation of ADT. Results confirmed the hypothesis that prostate cancer patients receiving ADT would report worsening sexual function and increasing bother over time compared to prostate cancer patients who underwent RP only and were not receiving ADT and men with no history of cancer. Effect sizes for the differences in sexual function were large to very large while the differences in sexual bother ranged from small to very large. This pattern was also evident on single-item measures of sexual desire, ability to have an erection and ability to reach climax with effect sizes ranging from medium to very large. These findings build upon results from a study of 1,269 men enrolled in the CaPSURE™ trial.[12] Among the 64 men who received ADT in that trial, sexual function worsened immediately after treatment with little or no recovery at year one after the initial decline. The increase in sexual bother in these men largely paralleled the course of sexual function, but to a lesser degree, and the investigators suggested this was evidence that patients seem to adjust to the changes. In the current study, bother increased significantly over time; at baseline and six months, there was no difference in sexual bother between men on ADT and men who underwent RP and were not on ADT. By 12 months, however, men on ADT reported significantly more bother than men who underwent RP only and non-cancer controls. We suggest this is evidence that men on ADT do not adjust to the changes and that sexual bother represents an important and relatively neglected target for intervention. However, definitive evidence for this can only come from studies with longer-term follow up.[13] Certainly, the conflicting findings and conclusions to date highlight the need for additional research in this area.
Our findings also confirmed the hypothesis that prostate cancer patients treated with RP only who were not receiving ADT would report improvement over time in sexual function. Both erectile function and ability to climax improved over time. Consistent with previous studies demonstrating that sexual desire is relatively preserved after RP,[23] level of desire did not change over the course of the study but remained similar to that of men with no history of cancer and significantly higher than that reported by men on ADT. With respect to sexual bother, as hypothesized, RP patients’ bother decreased over time in a pattern that mirrored the improvement over time in sexual function. This pattern of results is consistent with the results of the CaPSURE™ trial referenced previously.[12] The men who underwent RP (n = 757) in that trial demonstrated the greatest decline in sexual function in the more immediate post-treatment period with recovery after year one. Results for sexual bother paralleled that of sexual function although recovery in bother was greater within the first year than the next. Similarly, in the Prostate Cancer Outcomes Study[25] 1,213 men who underwent RP reported the highest rates of erectile dysfunction and sexual bother at six months following RP. Both sexual function and bother demonstrated relative improvements at one year with the reduction in the proportion of men experiencing moderate to great bother mirroring the reduction in the proportion of men reporting difficulty maintaining an erection. Notably, many of the studies in this area to date have lacked adequate comparison groups. Our study and its findings build upon previous results via its carefully controlled design and its inclusion of multiple comparison groups, including men with no history of cancer and men currently receiving ADT.
Results of the exploratory analyses indicated that age moderated the effect of ADT on both sexual function and bother. Younger age predicted relatively poorer sexual function and greater bother at 12 months in men on ADT compared to men not on ADT. This was not the case in older men. Older men on ADT did not report significantly worse sexual function or more bother than older men who were not on ADT. These findings are consistent with a large retrospective review of CaPSURE registry data investigating the effect of age on prostate cancer outcomes which found that treatments have an effect on outcomes depending on age and whether the treatment is local (as in RP or radiation) or nonlocal (as in ADT or surveillance).[39] In that study, men < 60 years of age were more likely to report greater declines and better recovery in sexual function as well as worse sexual bother over time compared to men > 70 years of age. Analyses of the effect of treatment showed that rates of sexual dysfunction were treatment-dependent for men < 60 years but similar across treatments for men > 70 years of age while sexual bother worsened after local treatment both for men < 60 years and men > 70 years. Similarly, a recent cross-sectional study found that while the majority of men on ADT for metastatic prostate cancer experienced a decline in sexual function, there was variability in the men’s degree of bother, with younger men reporting higher levels of sexual bother.[9] These recent findings as well as our own underscore the clinical relevance of bother and the need for interventions that target sexual bother, whether or not such interventions include medical therapies aimed at improving sexual function. When sexual function is not expected to improve, as is the case for most men on ADT, sexual bother is likely to be a more meaningful indicator of a man’s psychological adjustment to the effects of ADT.
Limitations
Limitations of the study should be noted. First, patients in the current study were followed for only 12 months following the initiation of ADT. Other studies of sexual function and recovery in prostate cancer have followed patients over periods as long as 24 months while still others have assessed patients at time points as far out as five or even 10 years from diagnosis. These studies demonstrate the persistent effects of ADT and the previously noted time-dependent patterns of recovery in men who undergo RP. To the best of our knowledge, however, no longitudinal prospective studies have examined the relative effect on both function and bother of continuing ADT in men with prostate cancer compared to controls, including age- and education-matched men with no history of cancer. Second, our focus on treatment-based differences may be overly simplistic as it does not allow for the possibility of patient subgroups based on distinct trajectories of function and/or bother separate from treatment. The current study lacked sufficient statistical power to conduct analyses such as growth curve modeling that would have allowed for the identification of such subgroups. Third, we did not assess participants’ use of medical and sexual aids or the extent of penile rehabilitation. These variables might have more fully explained the observed relationships, although this seems unlikely in the context of ADT. Fourth, the present study examined only clinical and demographic variables as potential predictors of change over time in men on ADT. There is evidence that constructs such as masculinity and relationship intimacy play a role in how men respond to prostate cancer[40] and to administration of ADT.[5] [5, 29] Future studies should examine a broader range of potential predictors, including personal and interpersonal factors in both the patient and partner, as well as quantify the importance of these factors,[3] in samples sufficiently large to identify whether there may be distinct trajectories of function and bother in patients on ADT.
Clinical Implications
Sexual function is a widely assessed and important patient-reported outcome in men being treated for prostate cancer. This study highlights the significance of assessing sexual bother in addition to function. Unlike men treated with RP, most men on ADT will never fully recover their baseline sexual function. Thus, interventions designed to manage expectations for post-treatment outcomes, especially the expectations of younger men, should be considered. Intervention strategies that target sexual bother over function in men on ADT are likely to be more effective at mitigating the sexual effects associated with this treatment. Helping couples build and maintain satisfying relationship intimacy (whether by modifying and/or redefining their sexual practices or not) is likely to more positively affect men’s psychological well-being than medical or sexual aids targeting sexual dysfunction.
Supplementary Material
Acknowledgments
This work was supported by the following grants from the National Cancer Institute: R01 CA132803 (PI: Jacobsen) and R25 CA090314 (PI: Jacobsen). None of the authors have any financial disclosures.
Footnotes
Among participants in the ADT+ group, 55 completed the baseline assessment on or before the day of their first ADT injection; five were assessed within 21 days after their first ADT injection (M = 12 days, SD = 6.58). Significant findings comparing groups on change over time in all outcomes measured remained significant regardless of whether these five participants were included in the analyses.
References
- 1.American Cancer Society Facts and Figures. Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- 2.Grossmann M, Cheung AS, Zajac JD. Androgens and prostate cancer; pathogenesis and deprivation therapy. Best Pract Res Clin Endocrinol Metab. 2013;27:603–616. doi: 10.1016/j.beem.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Fode M, Sonksen J. Sexual funtion in elderly men receiving androgen deprivation therapy (ADT) Sex Med Rev. 2014;2:36–46. doi: 10.1002/smrj.17. [DOI] [PubMed] [Google Scholar]
- 4.Cetin K, Li S, Blaes AH, Stryker S, Liede A, Arneson TJ. Prevalence of patients with nonmetastatic prostate cancer on androgen deprivation therapy in the United States. Urol. 2013;81:1184–1189. doi: 10.1016/j.urology.2013.02.050. [DOI] [PubMed] [Google Scholar]
- 5.Donovan KA, Walker LM, Wassersug RJ, Thompson LM, Robinson JW. Psychological effects of androgen-deprivation therapy on men with prostate cancer and their partners. Cancer. 2015;121:4286–4299. doi: 10.1002/cncr.29672. [DOI] [PubMed] [Google Scholar]
- 6.Grossmann M, Zajac JD. Management of side effects of androgen deprivation therapy. Endocrinol Metab Clin North Am. 2011;40:655–671. x. doi: 10.1016/j.ecl.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Stensvold A, Dahl AA, Brennhovd B, et al. Bother problems in prostate cancer patients after curative treatment. Urol Oncol. 2013;31:1067–1078. doi: 10.1016/j.urolonc.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 9.Benedict C, Traeger L, Dahn JR, et al. Sexual bother in men with advanced prostate cancer undergoing androgen deprivation therapy. J Sex Med. 2014;11:2571–2580. doi: 10.1111/jsm.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brassell SA, Elsamanoudi SI, Cullen J, Williams ME, McLeod DG. Health-related quality of life for men with prostate cancer - an evaluation of outcomes 12–24 months after treatment. Urol Oncol. 2013;31:1504–1510. doi: 10.1016/j.urolonc.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Lubeck DP, Grossfeld GD, Carroll PR. The effect of androgen deprivation therapy on health-related quality of life in men with prostate cancer. Urol. 2001;58(Supp 2A):94–100. doi: 10.1016/s0090-4295(01)01250-x. [DOI] [PubMed] [Google Scholar]
- 12.Huang GJ, Sadetsky N, Penson DF. Health related quality of life for men treated for localized prostate cancer wtih long-term quality followup. J Urol. 2010;183:2206–2212. doi: 10.1016/j.juro.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sevilla C, Maliski SL, Kwan L, Connor SE, Litwin MS. Long-term quality of life in disadvantaged men with prostate cancer on androgen-deprivation therapy. Prostate Cancer Prostatic Dis. 2012;15:237–243. doi: 10.1038/pcan.2011.71. [DOI] [PubMed] [Google Scholar]
- 14.Tal R, Alphs HH, PK, Nelson CJ, Mulhall JP. Erectile function recovery rate after radical prostatectomy: a meta-analysis. J Sex Med. 2009;6:2538–2546. doi: 10.1111/j.1743-6109.2009.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Resnick MJ, Loyama KT, Fan K, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368:436–4445. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sivarajan G, Prabhu V, Taksler GB, Laze J, Lepor H. Ten-year outcomes of sexual function after radical prostatectomy: results of a prospective longitudinal study. Eur Urol. 2014;65:58–65. doi: 10.1016/j.eururo.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Lee JK, Assel M, Thong AE, et al. Unexpected long-term improvements in urinary and erectile function in a large cohort of men with self-reported outcomes following radical prostatectomy. Eur Urol. 2015;68:899–905. doi: 10.1016/j.eururo.2015.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabbani F, Schiff J, Piecuch M, et al. Time course of recovery of erectile function after radical retropubic prostatectomy: does anyone recover after 2 years? J Sex Med. 2010;7:3984–3990. doi: 10.1111/j.1743-6109.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- 19.Matthew AG, Alibhai SMH, Davidson T, et al. Helath-related quality of life outcomes following radical prostatectomy: long-term outcomes. Qual Life Res. 2014;23:2809–2317. doi: 10.1007/s11136-014-0664-1. [DOI] [PubMed] [Google Scholar]
- 20.Nelson CJ, Scardino PT, Eastham JA, Mulhall JP. Back to baseline: erectle function recovery after radical prostatectomy from the patients’ perspective. J Sex Med. 2013;10:1643. doi: 10.1111/jsm.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levinson AW, Lavery HJ, Ward NT, Su L, Pavlovich CP. Is a return to baseline sexual function possible? An analysis of sexual function outcomes following laparoscopic radical prostatectomy. World J Urol. 2011:29. doi: 10.1007/s00345-010-0616-5. [DOI] [PubMed] [Google Scholar]
- 22.Frey AU, Sonksen J, Fode M. Neglected side effects after radical prostatectomy: a systematic review. J Sex Med. 2014;11:374–385. doi: 10.1111/jsm.12403. [DOI] [PubMed] [Google Scholar]
- 23.Le JD, Cooperberg MR, Sadetsky N, et al. Changes in specific domains of sexual function and sexual bother after radical prostatectomy. BJU Int. 2010;106:1022–1029. doi: 10.1111/j.1464-410X.2010.09231.x. [DOI] [PubMed] [Google Scholar]
- 24.Steinsvik EA, Axcrona K, Dahl AA, Eri LM, Stensvold A, Fossa SD. Can sexual bother after radical prostatectomy be predicted preoperatively? Findings from a prospective national study of the relation between sexual function, activity and bother. BJU Int. 2012;109:1366–1374. doi: 10.1111/j.1464-410X.2011.10598.x. [DOI] [PubMed] [Google Scholar]
- 25.Penson DF, McLerran D, Feng Z, et al. 5-year urinary and sxual outcomes after radical prostatectomy: results from the Prostate Cancer Outcomes study. J Urol. 2005;173:1701–1705. doi: 10.1097/01.ju.0000154637.38262.3a. [DOI] [PubMed] [Google Scholar]
- 26.Kimura M, Banez LL, Polascik TJ, et al. Sexual bother and function after radical prostatectomy: predictors of sexual bother recovery in men despite persistent post-operative sexual dysfunction. Andrology. 2013;1:256–261. doi: 10.1111/j.2047-2927.2012.00036.x. [DOI] [PubMed] [Google Scholar]
- 27.Nelson CJ, Deveci S, Stasi J, Scardino PT, Mulhall JP. Sexual bother following radical prostatectomy. J Sex Med. 2010;7:129–135. doi: 10.1111/j.1743-6109.2009.01546.x. [DOI] [PubMed] [Google Scholar]
- 28.Litwin MS. Measuring health related quality of life in men with prostate cancer. J Urol. 1994;152:1882–1887. doi: 10.1016/s0022-5347(17)32407-2. [DOI] [PubMed] [Google Scholar]
- 29.Lee M, Jim HS, Fishman M, et al. Depressive symptomatology in men receiving androgen deprivation therapy for prostate cancer: a controlled comparison. Psychoncology. 2014;24:472–477. doi: 10.1002/pon.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urol. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 32.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 33.Clay CA, Perera S, Wagner JM, Miller ME, Nelson JB, Greenspan SL. Physical function in men with prostate cancer on androgen deprivation therapy. Phys Ther. 2007;87:1325–1333. doi: 10.2522/ptj.20060302. [DOI] [PubMed] [Google Scholar]
- 34.Newell A, Rosenbloom PS. Mechanisms of skill acquisition and the law of practice. Cognitive skills and their acquisition. Hillsdale, NJ: Lawrence Earlbaum Associates; 1981. pp. 1–55. [Google Scholar]
- 35.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 36.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 37.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press; 2008. [Google Scholar]
- 38.Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav Res Methods. 2009;41:924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- 39.Hampson LA, Cowan JE, Zhao S, Carroll PR, Cooperberg MR. Impact of age on quality-of-life outcomes after treatment for localized prostate cancer. Eur Urol. 2015;68:480–486. doi: 10.1016/j.eururo.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Zaider T, Manne S, Nelson C, Mulhall J, Kissane D. Loss of masculine identity, marital affection, and sexual bother in men with localized prostate cancer. J Sex Med. 2012;9:2724–2732. doi: 10.1111/j.1743-6109.2012.02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.