Abstract
Autosomal Dominant Polycystic Kidney Disease (ADPKD) is caused by the mutation of polycystins (PC-1 or PC-2), in which cysts start from the collecting duct to extend to all nephron segments with eventual end stage renal failure. The cyst development is attenuated by a vasopressin V2 receptor antagonist tolvaptan which, however, will not affect proximal tubule cysts devoid of V2 receptor. Aquaporin-11 (AQP11) is expressed selectively in the proximal tubule of the kidney and AQP11-null kidneys have a disruptive PC-1 trafficking to the plasma membrane to develop polycystic kidneys. Here, we analyzed AQP11-null kidneys at the beginning of cyst formation by quantitative proteomic analysis using Tandem Mass Tag (TMT). Among ~ 1200 identified proteins, 124 proteins were differently expressed by > 1.5 or < 0.8 fold change. A pancreatic stone inhibitor or a growth factor, lithostathine-1 (Reg1) was most enhanced by 5 folds which was confirmed by western blot, while mitochondria-related proteins were downregulated. The identified proteins will be new target molecules for the treatment of proximal tubular cysts and helpful to explore the functional roles of AQP11 in the kidney.
Keywords: ADPKD, Proximal tubule, AQP11, Proteome, Reg1
Highlights
-
•
Proteomic analysis of the kidney from AQP11-null mice of proximal tubular specific ADPKD.
-
•
Among ~ 1200 identified proteins, 124 proteins were differently expressed by > 1.5 or < 0.8 fold change.
-
•
Mitochondrial proteins were downregulated reflecting a functional mitochondrial damage in cystic epithelia.
-
•
Reg1 protein was most enhanced by 5 folds which was confirmed by western blot.
1. Introduction
Human Autosomal Dominant Polycystic Kidney Disease (ADPKD) is caused by the mutation of polycystin-1 (PC-1) or polycystin-2 (PC-2) [1], [2], [3]. Cysts originate from the collecting duct in PC-1 null mice with defective cAMP signaling [1], which are marginally attenuated by a V2 receptor antagonist, tolvaptan [4]. Since V2 receptor is absent in the proximal tubule, tolvaptan will not affect the growth of proximal tubular cysts. Moreover, the mechanism for cyst development in the proximal tubule may not be same as in the collecting duct. The therapy against proximal tubular cysts will improve the suboptimal efficiency of tolvaptan therapy for ADPKD [4].
Aquaporin-11 (AQP11) is a new member of aquaporin family which is expressed at the membrane of intracellular organelles such as the endoplasmic reticulum (ER) [5], [6], [7], [8]. Currently, the function of AQP11 is not clear even as a water channel due to its unusual location in the cell. However, AQP11-null mice revealed a striking phenotype of intracellular vacuole formation in the proximal tubule at one week old [5], [9] indicating its critical role in the proximal tubule development. Surprisingly, these cells subsequently turn to cystic epithelia to develop polycystic kidney disease (PKD) at three week old and death at one month old [5]. The mechanism for the development of PKD in AQP11-null mice may not be related to its water channel function.
Our recent study on AQP11-null mice revealed a trafficking defect of PC-1 to the plasma membrane due to its abnormal glycosylation at the ER [10] possibly induced by abnormal environment of the ER with defective water and/or solutes transports. Irrespective of its mechanism, AQP11-null mice will be a good model for ADPKD affecting the proximal tubule selectively with intact collecting ducts, which will be useful to examine the proximal tubular cyst formation in ADPKD. As is the case with conditional knock-out mice of PC-1 [11], the effect of AQP11 deletion is also developmentally dependent as cysts were not observed with the disruption of AQP11 at ten days after birth [9]. Furthermore, these cysts may represent a very early stage of the cyst formation as they are in fact not cysts but dilated proximal tubules [9].
To expand our previous microarray studies [12], we employed a proteomic approach in this study to compare the differentially expressed proteins in AQP11-null kidney to identify key molecules for the development of proximal tubule specific cysts and functional role of AQP11 in the kidney.
2. Materials and methods
2.1. Animals
All procedures performed in animals were approved by the Meiji Pharmaceutical University Committee for Ethics of Experimentation and Animal Care (approved number: 2005). Homogenous AQP11-null mice {AQP11(-/-)} were generated as previously reported [5], [12], [13]. In short, AQP11(-/-) mice were produced by mating heterozygous AQP11-null mice {AQP11(+/-)} since AQP11(-/-) mice are fatal before matured enough to be mated. The genotypes for AQP11 gene mutation were determined by PCR as previously reported [5].
2.2. Protein isolation
Kidneys were isolated from three AQP11-null mice and three wild type mice anesthetized by barbiturate. The kidneys were frozen-powdered with liquid nitrogen by Cryo-Press (MICROTEC CO., LTD, Chiba, Japan), which were then homogenized in 20 volumes of 12 mM sodium deoxycholate (SDC), 12 mM sodium lauryl sulfate (SLS), and 50 mM triethylammonium bicarbonate. The homogenate was centrifuged at 19,000×g at 4 °C for 15 min. The supernatant containing the mixture of proteins was collected, and the protein concentration was determined using a RCDC protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA).
2.3. Trypsin digestion
The proteins were digested with trypsin essentially as previously described [14], [15], with following modifications. 20 μL of 200 mM Triethylamonium bicarbonat/12 mM SDC/12 mM SLS and 2 μL of 200 mM tris (2-carboxylethyl) phosphine hydrochloride/ 120 mM TEAB were added and then the mixture was incubating at 50 °C for 30 min. After the addition of 2 μL of 375 mM iodoacetamide, the mixture was incubated in the dark for 30 min, to which 2 μL of 100 ng/μL trypsin was added with further incubation at 37 °C for indicated periods. Acetonitrile (ACN) and 5% trifluoroacetic acid, 50 μL each, were then added to the digest, followed by the centrifugation at 19,000×g for 15 min. The supernatant was subjected to Tandem Mass Tag (TMT)-labeling as following.
2.4. TMT-labeling
Each 6-plex TMT labeling reagent was re-dissolved with anhydrous ACN. A total of 12 μL of TMT solution was added to the eluate and then incubated for 1 h at the room temperature. The reaction was terminated by adding 2 μL of 5% hydroxyamine. Six samples labeled with TMT reagents were combined and desalted for SCX fractionation using ‘stop and go extraction tips’ (Stage tips) [16] filled with Empore™ C18 sealant and Cation Exchange (3 M, MN, USA).
2.5. LC-MS analysis
Sage tip SCX prefractionated samples were injected into a C18 0.075- × 20-mm trap column (Acclaim PepMap 100; Thermo Fisher Scientific) and then eluted into a C18 0.075- × 120-mm analytical column (Nano HPLC Capillary Column; Nikkyo Technos, Tokyo, Japan) configured to an EASY-nLC 1000 HPLC system (Thermo Scientific, San Jose, CA, USA). The flow rate of the mobile phase was 300 nl/min; mobile phase (A) consisted of 0.1% formic acid and mobile phase (B) with 0.1% formic acid/100% acetonitrile. Separated peptides were subjected to Q-ExactiveTM (Thermo Scientific) operated in data-dependent mode to switch automatically between full-scan MS and MS/MS acquisition. The ten most intense full-scan peaks were selected with an isolation window of 2.4 Da.
2.6. Protein identification and quantification
Database searches were performed using the SEQUEST algorithm incorporated in Proteome Discoverer 1.4 (Thermo Fisher Scientific). The search parameters were as follows: enzyme, trypsin; variable modification, oxidation of M residue; static modification, TMT labeling of N-terminal and K residues; peptide ion mass tolerance, 10 ppm; fragment ion mass tolerance, 0.02 Da. The identified peptides were searched against the decoy database with false discovery rate (FDR) set as 0.01 using Percolator scoring validated by posterior error probability. Peptide quantification was also performed using Proteome Discoverer 1.4. Both peptide identification and quantification were performed in an overall workflow in Proteome Discoverer. KEGG pathway and Swiss-Prot keywords of proteins were assigned by using the DAVID Bioinformatics Database.
2.7. Western Blotting
Protein of 20 μg in each lane was separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (e-PAGEL 15%, ATTO CORPORATION, Tokyo, Japan) according to the manufacturer's protocol. After completion of electrophoresis, proteins were transferred onto PVDF membranes and detected with a mouse antibody against lithostathine-1 (Reg1) (1: 2000; R&D System, Minneapolis, MN, USA). Alkaline phosphatase conjugated secondary antibodies (Merck KGaA, Darmstadt, Germany) were diluted 1: 30,000. To calibrate the expression levels of Reg1, β- actin was used as an internal control with a rabbit anti-β-actin monoclonal antibody (Abcam, Cambridge, UK). Antigens on the membrane were detected with ProtoBlot® II AP Systems with Stabilized Substrate (Promega, Fitchburg, WI, USA). Gel images were converted to densitograms using Image J software 1.45I (http://rsb.info.nih.gov/ij/).
3. Results
3.1. Proteomic analysis of the kidney
Proteins from six kidneys (three AQP11-null mice and three wild type mice: SET1) were extracted and digested. Each sample was labeled with 6-plex TMT reagent and then mixed to be fractionated by C18-SCX STAGE-Tip and analyzed by LC-MS/MS. We also analyzed another set (SET2) of each three mouse kidneys by the same protocol. A total of 2044 proteins were identified by combining two LC-MS/MS analyses, 1420 and 1835 protein each, in which the proteins were mostly shared (~ 1200). The selected proteins whose average three reporter ion ratios of AQP11-null mouse were > 1.5 or < 0.80 were 126 (62 down-regulated and 162 up-regulated in AQP-null mouse) (Table 1 and Table 2, respectively)(see supplements Table 1, Table 2 in more details).
Table 1.
Up-regulated proteins in AQP11-null kidneys.
| UniProt accession | Description | Gene name | Average | Set 1 | Set 2 |
|---|---|---|---|---|---|
| P43137 | Lithostathine-1 | Reg1 | 9.69 | 15.33 ± 7.28 | 4.05 ± 2.52 |
| Q91VW3 | SH3 domain-binding glutamic acid-rich-like protein 3 | Sh3bgrl | 2.59 | 3.15 ± 0.62 | 2.04 ± 0.82 |
| Q04447 | Creatine kinase B-type | Ckb | 2.49 | 1.99 ± 0.36 | 2.98 ± 0.94 |
| Q9ET54 | Palladin | Palld | 2.39 | 2.29 ± 0.38 | 2.50 ± 1.00 |
| P20152 | Vimentin | Vim | 2.33 | 2.82 ± 0.35 | 1.84 ± 0.51 |
| Q60847 | Collagen alpha-1(XII) chain | Col12a1 | 2.27 | 2.61 ± 0.15 | 1.93 ± 0.42 |
| P97314 | Cysteine and glycine-rich protein 2 | Csrp2 | 2.25 | 2.97 ± 0.29 | 1.53 ± 0.42 |
| Q8BFW7 | Lipoma-preferred partner homolog | Lpp | 2.22 | 1.19 ± 0.09 | 3.25 ± 0.91 |
| P08905 | Lysozyme C-2 | Lyz2 | 2.22 | 1.85 ± 0.53 | 2.58 ± 0.93 |
| P39061 | Collagen alpha-1(XVIII) chain | Col18a1 | 2.17 | 2.17 ± 0.17 | 2.18 ± 0.49 |
| Q61207 | Prosaposin | Psap | 2.15 | 2.56 ± 0.26 | 1.74 ± 0.35 |
| P68033 | Actin, alpha cardiac muscle 1 | Actc1 | 2.03 | 2.38 ± 0.21 | 1.68 ± 0.11 |
| P11859 | Angiotensinogen | Agt | 2.03 | 2.62 ± 0.87 | 1.43 ± 0.34 |
Table 2.
Down-regulated proteins in AQP11-null kidneys.
| UniProt accession | Description | Gene name | Average | Set 1 | Set 2 |
|---|---|---|---|---|---|
| Q8QZW3 | Protein FAM151A | Fam151a | 0.28 | 0.26 ± 0.11 | 0.30 ± 0.04 |
| O35728 | Cytochrome P450 4A14 | Cyp4a14 | 0.37 | 0.67 ± 0.31 | 0.06 ± 0.12 |
| Q8BH00 | Aldehyde dehydrogenase family 8 member A1 | Aldh8a1 | 0.41 | 0.70 ± 0.11 | 0.12 ± 0.11 |
| Q91×52 | L-xylulose reductase | Dcxr | 0.44 | 0.58 ± 0.25 | 0.30 ± 0.05 |
| Q8K0L3 | Acyl-coenzyme A synthetase ACSM2, mitochondrial | Acsm2 | 0.44 | 0.54 ± 0.15 | 0.34 ± 0.09 |
| Q8CHT0 | Delta-1-pyrroline-5-carboxylate dehydrogenase, mitochondrial | Aldh4a1 | 0.47 | 0.55 ± 0.19 | 0.39 ± 0.09 |
| P13707 | Glycerol-3-phosphate dehydrogenase [NAD(+)], cytoplasmic | Gpd1 | 0.48 | 0.54 ± 0.17 | 0.42 ± 0.09 |
| Q9D964 | Glycine amidinotransferase, mitochondrial | Gatm | 0.48 | 0.62 ± 0.13 | 0.34 ± 0.06 |
| P16460 | Argininosuccinate synthase | Ass1 | 0.48 | 0.60 ± 0.14 | 0.36 ± 0.06 |
| Q8BWT1 | 3-ketoacyl-CoA thiolase, mitochondrial | Acaa2 | 0.49 | 0.57 ± 0.13 | 0.42 ± 0.07 |
| O88338 | Cadherin-16 | Cdh16 | 0.50 | 0.55 ± 0.12 | 0.46 ± 0.07 |
These proteins were functionally classified by Swiss-Prot Keywords. As shown in Table 3, phosphoproteins and acetylation-related proteins were predominant in both groups. Cytoplasm, metal-binding, and Ubl conjugation proteins were up-regulated, while mitochondrial, transporting and nucleotide-binding proteins were down-regulated (Table 3).
Table 3.
Swiss Prot-keywords classification of significantly up-regulated and down-regulated proteins in AQP11-null kidneys.
| SP-keyword | All |
Up-regurate |
Down-regurate |
|||
|---|---|---|---|---|---|---|
| Count | % | Count | % | Count | % | |
| Phosphoprotein | 1278 | 62.6 | 41 | 65.1 | 37 | 60.7 |
| Acetylation | 1084 | 53.1 | 31 | 49.2 | 38 | 62.3 |
| Cytoplasm | 797 | 39.0 | 21 | 33.3 | ||
| Nucleus | 490 | 24.0 | ||||
| Alternative splicing | 455 | 22.3 | ||||
| Metal-binding | 376 | 18.4 | 17 | 27.0 | ||
| Mitochondrion | 335 | 16.4 | 31 | 50.8 | ||
| Transport | 330 | 16.2 | 11 | 18.0 | ||
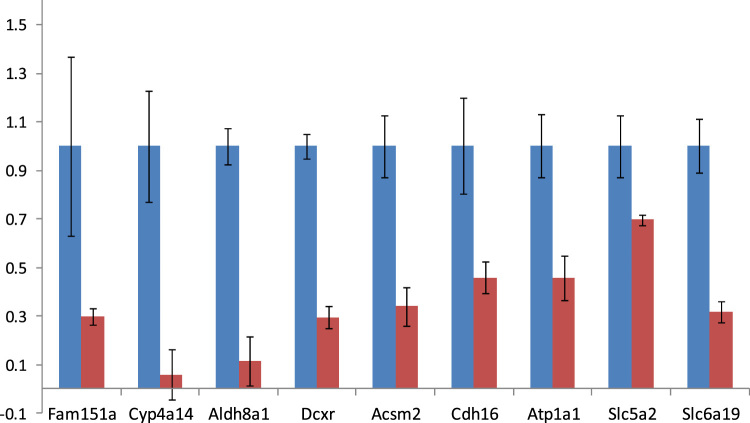
Fig. 1, Fig. 2 illustrated SET2 proteins with altered expressions in AQP11-null and wild type mouse kidneys in Table 1, Table 2. Extracellular matrix proteins such as fibulin-5 (Fbln5), fibronectin (Fn1), and annexin A2 (Anexa2) were enhanced in AQP11-null kidneys, which agrees with the results of previous PKD mouse models [17], [18]. Moreover, vimentin (Vim), prosaposin (Psap) and angiotensinogen (Agt) were also enhanced, which were unique to this study. On the other hand, mitochondrial-function-related proteins including l-xylulose reductase (Dcxr) and sodium/potassium-transporting ATPase subunit alpha-1 (Atp1a1) were depressed in AQP11-null kidneys, suggesting a mitochondrial defect of the cyst epithelium.
Fig. 1.
Up-regulated proteins in AQP11-null kidneys. MS signal ratios were plotted by quantity proteomic analysis by using Tandem Mass Tag. The intensity of knockout (KO) mice (red bar) was expressed as the fold change from the intensity of wild-type (wt) mice (blue bar). Values were means ± SD (n = 3). All were significantly different at p < 0.01. Reg1: Lithostathine-1, Sh3bgrl: SH3 domain-binding glutamic acid-rich-like protein 3, Ckb: Creatine kinase B-type, Palld: Palladin, Vim: Vimentin, Agt: Angiotensinogen, Psap: Prosaposin, Csrp2: Cysteine and glycine-rich protein 2, Serpinf: Pigment epithelium-derived factor.
Fig. 2.
Down-regulated proteins in AQP11-null kidneys. MS signal ratios were plotted by quantity proteomics analysis by using TMT. The intensity of the KO mice (red bar) was expressed as the fold change from the intensity of wild-type (wt) mice (blue bar). Values were means ± SD (n = 3). All were significantly different at p < 0.01. Fam151a: Protein FAM151A, Cyp4a14: Cytochrome P450 4A14, Aldh8a1: Aldehyde dehydrogenase family 8 member A1, Dcxr: L-xylulose reductase, Acsm2: Acyl-coenzyme A synthetase ACSM2, mitochondrial, Cdh16: Cadherin-16, Atp1a1: Sodium/potassium-transporting ATPase subunit alpha-1, Slc5a2: Sodium/glucose cotransporter 2, Slc6a19: Sodium-dependent neutral amino acid transporter.
3.2. Expression of Reg1 transcript in the kidney
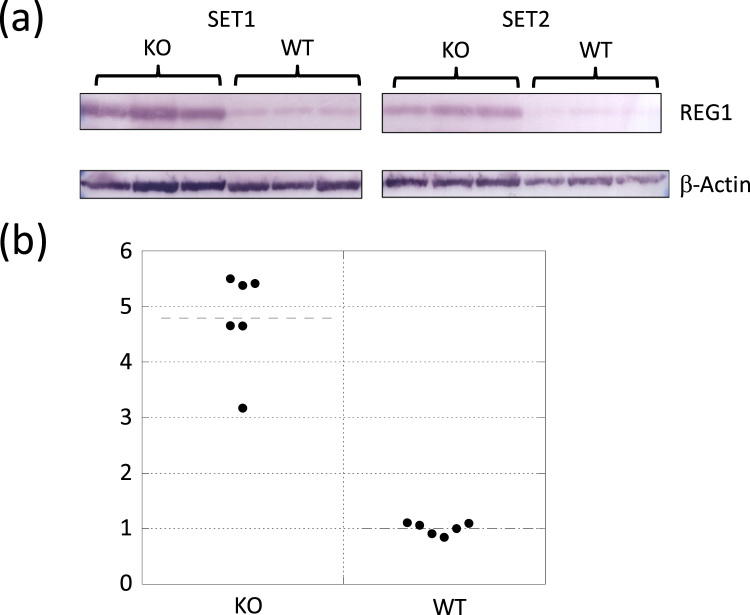
Notably, a pancreatic growth factor, lithostathine-1 (Reg1) was most increased by 8 folds. To further verify the identified proteins, western blotting analysis was performed to determine the increase of Reg1 protein in AQP11 null or wild type mice (Fig. 3). Densitometry analysis on the expression levels of Reg1 protein was shown in Fig. 3(b). Reg1 was 4.8 times highly expressed in AQP11-null mice than in wild type mice. Thus, the increase of Reg1 protein in the KO mouse kidney was confirmed.
Fig. 3.
Protein expression levels from Western blot. (a) Expression levels of Reg1 and β-actin protein in the kidney were compared between AQP11-null (KO) mice and wild-type (WT) mice. (b) The graph with plotted densitometry analysis. The expression level was normalized by that of β actin. The values were expressed as fold changes from the wild-type mice. The two groups were statistically different at p < 0.01.
4. Discussion
This is the first report on proteomic analyses of AQP11-null kidneys, a unique proximal-tubule-specific model for PKD [10]. As the cyst formation in ADPKD and most PKD animal models is initiated from the collecting duct [1], our results will give a clue to understand the mechanism for the cyst formation and the basis to identify key molecules for the development of proximal tubular cysts in ADPKD. The results should also be useful to speculate the function of AQP11, which has not yet been well characterized.
First, the predominant up-regulation of Reg1 in this study is noteworthy. Furthermore, the increase in Reg1 by LC-MS was validated by western blot (Fig. 3). Reg1 was first identified in the pancreas as an anti-stone forming molecule [19], [20]. However, it also has a function of a growth factor for pancreas. As Reg1 is predominantly expressed in the pancreas, it may be trapped in the kidney and the kidney may not produce it. We examined the Reg1 transcript by PCR and indeed found it (supplement Fig. 1). Furthermore, it has been also identified in human kidney and urine which is increased in diabetic patients [21], [22], suggesting that Reg1 may be involved in diabetic kidney hypertrophy as a kidney growth factor. As EGF in the fluid of collecting duct cysts has been shown to stimulate the cyst growth, a similar role of Reg1 in proximal tubule cysts will be anticipated [1]. Reg1 may also be involved in the kidney development albeit with a limited phenotypic report on Reg1-null mice [23]. Further studies are necessary to examine the role of Reg1 in cyst growth and kidney development to document its importance.
Another interesting observation in this study was decreased cadherin and increased fibronectin in AQP11-null kidneys. Both are markers for epithelial-mesenchymal transition (EMT) [24], [25], [26], [27], which has been shown to be associated with kidney cyst formation in ADPKD and pck rats [28], [29], [30]. Moreover, proteins related to wound healing such as fibronectin1 and Ptk7 were also induced in AQP11-null kidneys. In fact, the increase in extracellular matrix proteins such as vimentin has also been reported in jck mouse, a polycystic kidney disease model of the collecting duct [18]. Thus, EMT may also be involved in cyst formation and development in the proximal tubule.
The observed increases of prosaposin (Pap) and angiotensinogen (Agt) have not been reported in previous proteomic analyses of PKD [17], [18]. Prosaposin, a precursor of saposins, is expressed mainly in the brain but it is also highly expressed in the kidney [31]. Saposin is indispensable for lysosomal hydrolysis of sphingolipids in the brain and has been shown to promote neurite outgrowth and nerve regeneration as well as to prevent cell death [32], whose expression has been markedly increased in response to brain injury such as ischemia [33], [34]. Therefore, saposins may also be involved in the survival of the proximal tubule in the absence of intracellular AQP11 and important for the survival of the cyst epithelium in the proximal tubule of PKD.
Angiotensinogen (Agt) may facilitate wound healing by local production of angiotensin II which has proinflammatory and profibrotic functions. In fact, active inflammation and fibrosis have been observed in PKD [35], [36]. Based on these results, angiotensin II receptor blockers (ARB) and angiotensin converting enzyme inhibitors (ACEI) have been tried to inhibit the cyst development in animal models [37] and patients [35], [37], [38]. However, the results were equivocal. Although the receptor for angiotensin II is expressed both in the proximal tubule and in the collecting duct, the effect of these drugs will be more dominant on the proximal tubule as Agt has been shown to be expressed primarily in cysts originated from the proximal tubule and in cyst-derived cells with proximal tubule characteristics [39]. AQP11-null kidneys with selective proximal tubular cysts will be useful to examine the effect of these drugs on cyst progression, which will be our future project.
On the other hand, the decrease of mitochondrial proteins of AQP11-null kidneys in this study as well as in a previous report by microarray analysis [12] is unique to proximal tubular cysts. The decrease of mitochondrial proteins will lead to cellular damages especially in proximal tubular cells as they have many mitochondria [40] as revealed by intracellular vacuole formation in AQP11-null kidneys [5], [9]. In fact, granular swollen epithelial proximal tubular cells have been reported in mitochondrial nephropathy [41]. Therefore, the formation of intracellular vacuoles in AQP11-null kidneys may be caused by mitochondrial defect. Furthermore, mitochondria may also play a role in the development of kidney cysts, especially the proximal tubular cysts.
In conclusion, our proteomic data at the beginning of the cyst formation in AQP11-null kidneys identified several interesting molecules involved in PKD development specifically in the proximal tubule. These will be useful for the development of new therapy against proximal tubular cysts in ADPKD as well as for exploring the role of intracellular AQP11 in the kidney.
Acknowledgments
We thank Ms. S. Nakada for technical assistance and Dr. S. Sasaki for critical reading of the manuscript. T. Saito appreciates the post-doctoral fellowship from Meiji Pharmaceutical University. This work was supported by JSPS KAKENHI Grant nos. 24591243 and 15K09302 to K. Ishibashi.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.11.003.
Contributor Information
Tatsuya Saito, Email: tsaito@my-pharm.ac.jp.
Yasuko Tanaka, Email: ystanaka@my-pharm.ac.jp.
Yoshiyuki Morishita, Email: ymori@jichi.ac.jp.
Kenichi Ishibashi, Email: kishiba@my-pharm.ac.jp.
Appendix A. Transparency document
Supplementary material
References
- 1.Guay-Woodford L.M. Murine models of polycystic kidney disease: molecular and therapeutic insights. Am. J. Physiol. Renal Physiol. 2003;285:F1034–F1049. doi: 10.1152/ajprenal.00195.2003. [DOI] [PubMed] [Google Scholar]
- 2.Song X., Di Giovanni V., He N., Wang K., Ingram A., Rosenblum N.D. Systems biology of autosomal dominant polycystic kidney disease (ADPKD): computational identification of gene expression pathways and integrated regulatory networks. Hum. Mol. Genet. 2009;18:2328–2343. doi: 10.1093/hmg/ddp165. [DOI] [PubMed] [Google Scholar]
- 3.Watnick T., He N., Wang K., Liang Y., Parfrey P., Hefferton D. Mutations of PKD1 in ADPKD2 cysts suggest a pathogenic effect of trans-heterozygous mutations. Nat. Genet. 2000;25:143–144. doi: 10.1038/75981. [DOI] [PubMed] [Google Scholar]
- 4.Gattone V.H., 2nd, Wang X., Harris P.C., Torres V.E. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat. Med. 2003;9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 5.Morishita Y., Matsuzaki T., Hara-chikuma M., Andoo A., Shimono M., Matsuki A. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol. Cell. Biol. 2005;25:7770–7779. doi: 10.1128/MCB.25.17.7770-7779.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yakata K., Tani K., Fujiyoshi Y. Water permeability and characterization of aquaporin-11. J. Struct. Biol. 2011;174:315–320. doi: 10.1016/j.jsb.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Gorelick D.A., Praetorius J., Tsunenari T., Nielsen S., Agre P. Aquaporin-11: a channel protein lacking apparent transport function expressed in brain. BMC Biochem. 2006;7:14. doi: 10.1186/1471-2091-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rojek A., Fuchtbauer E.M., Fuchtbauer A., Jelen S., Malmendal A., Fenton R.A. Liver-specific Aquaporin 11 knockout mice show rapid vacuolization of the rough endoplasmic reticulum in periportal hepatocytes after amino acid feeding. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;304:G501–G515. doi: 10.1152/ajpgi.00208.2012. [DOI] [PubMed] [Google Scholar]
- 9.Rutzler M., Rojek A., Damgaard M.V., Andreasen A., Fenton R.A., Nielsen S. Temporal deletion of Aqp11 in mice is linked to the severity of cyst-like disease. Am. J. Physiol. Renal Physiol. 2017;312:F343–F351. doi: 10.1152/ajprenal.00065.2016. [DOI] [PubMed] [Google Scholar]
- 10.Inoue Y., Sohara E., Kobayashi K., Chiga M., Rai T., Ishibashi K. Aberrant glycosylation and localization of polycystin-1 cause polycystic kidney in an AQP11 knockout model. J. Am. Soc. Nephrol. 2014;25:2789–2799. doi: 10.1681/ASN.2013060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piontek K., Menezes L.F., Garcia-Gonzalez M.A., Huso D.L., Germino G.G. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat. Med. 2007;13:1490–1495. doi: 10.1038/nm1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okada S., Misaka T., Tanaka Y., Matsumoto I., Ishibashi K., Sasaki S. Aquaporin-11 knockout mice and polycystic kidney disease animals share a common mechanism of cyst formation. FASEB J. 2008;22:3672–3684. doi: 10.1096/fj.08-111872. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y., Watari M., Saito T., Morishita Y., Ishibashi K. Enhanced autophagy in polycystic kidneys of AQP11 null mice. Int. J. Mol. Sci. 2016;17:E1993. doi: 10.3390/ijms17121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichimura K., Kawashima Y., Nakamura T., Powell R., Hidoh Y., Terai S. Medaka fish, Oryzias latipes, as a model for human obesity-related glomerulopathy. Biochem. Biophys. Res. Commun. 2013;431:712–717. doi: 10.1016/j.bbrc.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masuda T., Saito N., Tomita M., Ishihama Y. Unbiased quantitation of Escherichia coli membrane proteome using phase transfer surfactants. Mol. Cell. Proteomics. 2009;8:2770–2777. doi: 10.1074/mcp.M900240-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adachi J., Hashiguchi K., Nagano M., Sato M., Sato A., Fukamizu K. Improved proteome and phosphoproteome analysis on a cation exchanger by a combined acid and salt gradient. Anal. Chem. 2016;88:7899–7903. doi: 10.1021/acs.analchem.6b01232. [DOI] [PubMed] [Google Scholar]
- 17.Han C.L., Chien C.W., Chen W.C., Chen Y.R., Wu C.P., Li H. A multiplexed quantitative strategy for membrane proteomics: opportunities for mining therapeutic targets for autosomal dominant polycystic kidney disease. Mol. Cell. Proteomics. 2008;7:1983–1997. doi: 10.1074/mcp.M800068-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Valkova N., Yunis R., Mak S.K., Kang K., Kultz D. Nek8 mutation causes overexpression of galectin-1, sorcin, and vimentin and accumulation of the major urinary protein in renal cysts of jck mice. Mol. Cell. Proteomics. 2005;4:1009–1018. doi: 10.1074/mcp.M500091-MCP200. [DOI] [PubMed] [Google Scholar]
- 19.Jin C.X., Hayakawa T., Ko S.B., Ishiguro H., Kitagawa M. Pancreatic stone protein/regenerating protein family in pancreatic and gastrointestinal diseases. Intern. Med. 2011;50:1507–1516. doi: 10.2169/internalmedicine.50.5362. [DOI] [PubMed] [Google Scholar]
- 20.Unno M., Yonekura H., Nakagawara K., Watanabe T., Miyashita H., Moriizumi S. Structure, chromosomal localization, and expression of mouse reg genes, reg I and reg II. A novel type of reg gene, reg II, exists in the mouse genome. J. Biol. Chem. 1993;268:15974–15982. [PubMed] [Google Scholar]
- 21.Verdier J.M., Dussol B., Casanova P., Daudon M., Dupuy P., Berthezene P. Evidence that human kidney produces a protein similar to lithostathine, the pancreatic inhibitor of CaCO3 crystal growth. Eur. J. Clin. Investig. 1992;22:469–474. doi: 10.1111/j.1365-2362.1992.tb01492.x. [DOI] [PubMed] [Google Scholar]
- 22.Sobajima H., Niwa T., Shikano M., Naruse S., Kitagawa M., Nakae Y. Urinary excretion of pancreatic stone protein in diabetic nephropathy. Intern. Med. 1998;37:500–503. doi: 10.2169/internalmedicine.37.500. [DOI] [PubMed] [Google Scholar]
- 23.Ose T., Kadowaki Y., Fukuhara H., Kazumori H., Ishihara S., Udagawa J. Reg I-knockout mice reveal its role in regulation of cell growth that is required in generation and maintenance of the villous structure of small intestine. Oncogene. 2007;26:349–359. doi: 10.1038/sj.onc.1209799. [DOI] [PubMed] [Google Scholar]
- 24.Hay E.D. An overview of epithelio-mesenchymal transformation. Acta Anat. 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J. Am. Soc. Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 26.Schieren G., Rumberger B., Klein M., Kreutz C., Wilpert J., Geyer M. Gene profiling of polycystic kidneys. Nephrol. Dial. Transplant. 2006;21:1816–1824. doi: 10.1093/ndt/gfl071. [DOI] [PubMed] [Google Scholar]
- 27.Thiery J.P., Acloque H., Huang R.Y., Nieto M.A. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Boca M., D'Amato L., Distefano G., Polishchuk R.S., Germino G.G., Boletta A. Polycystin-1 induces cell migration by regulating phosphatidylinositol 3-kinase-dependent cytoskeletal rearrangements and GSK3beta-dependent cell mechanical adhesion. Mol. Biol. Cell. 2007;18:4050–4061. doi: 10.1091/mbc.E07-02-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Togawa H., Nakanishi K., Mukaiyama H., Hama T., Shima Y., Sako M. Epithelial-to-mesenchymal transition in cyst lining epithelial cells in an orthologous PCK rat model of autosomal-recessive polycystic kidney disease. Am. J. Physiol. Renal Physiol. 2011;300:F511–F520. doi: 10.1152/ajprenal.00038.2010. [DOI] [PubMed] [Google Scholar]
- 30.Venugopal J., McDermott J., Sanchez G., Sharma M., Barbosa L., Reif G.A. Ouabain promotes partial epithelial to mesenchymal transition (EMT) changes in human autosomal dominant polycystic kidney disease (ADPKD) cells. Exp. Cell Res. 2017;355:142–152. doi: 10.1016/j.yexcr.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoneshige A., Suzuki K., Kojima N., Matsuda J. Regional expression of prosaposin in the wild-type and saposin D-deficient mouse brain detected by an anti-mouse prosaposin-specific antibody. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009;85:422–434. doi: 10.2183/pjab.85.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolter T., Sandhoff K. Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu. Rev. Cell Dev. Biol. 2005;21:81–103. doi: 10.1146/annurev.cellbio.21.122303.120013. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien J.S., Carson G.S., Seo H.C., Hiraiwa M., Kishimoto Y. Identification of prosaposin as a neurotrophic factor. Proc. Natl. Acad. Sci. USA. 1994;91:9593–9596. doi: 10.1073/pnas.91.20.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unuma K., Chen J., Saito S., Kobayashi N., Sato K., Saito K. Changes in expression of prosaposin in the rat facial nerve nucleus after facial nerve transection. Neurosci. Res. 2005;52:220–227. doi: 10.1016/j.neures.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Loghman-Adham M., Soto C.E., Inagami T., Cassis L. The intrarenal renin-angiotensin system in autosomal dominant polycystic kidney disease. Am. J. Physiol. Renal Physiol. 2004;287:F775–F788. doi: 10.1152/ajprenal.00370.2003. [DOI] [PubMed] [Google Scholar]
- 36.Ravichandran K., Ozkok A., Wang Q., Mullick A.E., Edelstein C.L. Antisense-mediated angiotensinogen inhibition slows polycystic kidney disease in mice with a targeted mutation in Pkd2. Am. J. Physiol. Renal Physiol. 2015;308:F349–F357. doi: 10.1152/ajprenal.00478.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rysz J., Gluba-Brzozka A., Franczyk B., Banach M., Bartnicki P. Combination drug versus monotherapy for the treatment of autosomal dominant polycystic kidney disease. Expert Opin.Pharmacother. 2016;17:2049–2056. doi: 10.1080/14656566.2016.1232394. [DOI] [PubMed] [Google Scholar]
- 38.Schrier R.W. Renal volume, renin-angiotensin-aldosterone system, hypertension, and left ventricular hypertrophy in patients with autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2009;20:1888–1893. doi: 10.1681/ASN.2008080882. [DOI] [PubMed] [Google Scholar]
- 39.Hatanaka M., Kaimori J.Y., Yamamoto S., Matsui I., Hamano T., Takabatake Y. Azilsartan Improves salt sensitivity by modulating the proximal tubular Na+-H+ exchanger-3 in mice. PLoS One. 2016;11:e0147786. doi: 10.1371/journal.pone.0147786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arrell D.K., Elliott S.T., Kane L.A., Guo Y., Ko Y.H., Pedersen P.L. Proteomic analysis of pharmacological preconditioning: novel protein targets converge to mitochondrial metabolism pathways. Circ. Res. 2006;99:706–714. doi: 10.1161/01.RES.0000243995.74395.f8. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi A., Goto Y., Nagata M., Yamaguchi Y. Granular swollen epithelial cells: a histologic and diagnostic marker for mitochondrial nephropathy. Am. J. Surg. Pathol. 2010;34:262–270. doi: 10.1097/PAS.0b013e3181cb4ed3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material