Abstract
Objective
Repetitive subconcussive head impacts (RSHI) may lead to structural, functional, and metabolic alterations of the brain. While differences between males and females have already been suggested following a concussion, whether there are sex differences following exposure to RSHI remains unknown. The aim of this study was to identify and to characterize sex differences following exposure to RSHI.
Methods
Twenty-five collegiate ice hockey players (14 males and 11 females, 20.6 ± 2.0 years), all part of the Hockey Concussion Education Project (HCEP), underwent diffusion-weighted magnetic resonance imaging (dMRI) before and after the Canadian Interuniversity Sports (CIS) ice hockey season 2011–2012 and did not experience a concussion during the season. Whole-brain tract-based spatial statistics (TBSS) were used to compare pre- and postseason imaging in both sexes for fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). Pre- and postseason neurocognitive performance were assessed by the Immediate Post-Concussion Assessment and Cognitive Test (ImPACT).
Results
Significant differences between the sexes were primarily located within the superior longitudinal fasciculus (SLF), the internal capsule (IC), and the corona radiata (CR) of the right hemisphere (RH). In significant voxel clusters (p < 0.05), decreases in FA (absolute difference pre- vs. postseason: 0.0268) and increases in MD (0.0002), AD (0.00008), and RD (0.00005) were observed in females whereas males showed no significant changes. There was no significant correlation between the change in diffusion scalar measures over the course of the season and neurocognitive performance as evidenced from postseason ImPACT scores.
Conclusions
The results of this study suggest sex differences in structural alterations following exposure to RSHI. Future studies need to investigate further the underlying mechanisms and association with exposure and clinical outcomes.
Abbreviations: AD, axial diffusivity; CIS, Canadian Interuniversity Sports; CR, corona radiata; dMRI, diffusion magnetic resonance imaging; EC, external capsule; FA, fractional anisotropy; HCEP, Hockey Concussion Education Project; IC, internal capsule; ImPACT, Immediate Post-Concussion Assessment and Cognitive Test; LH, left hemisphere; MD, mean diffusivity; MRI, magnetic resonance imaging; NCAA, National Collegiate Athletic Association; rs, Spearman's rank correlation coefficient; RD, radial diffusivity; RH, right hemisphere; RSHI, repetitive subconcussive head impacts; SD, standard deviation; SLF, superior longitudinal fasciculus; TBI, traumatic brain injury; TBSS, tract-based spatial statistics; WM, white matter
Keywords: Diffusion tensor imaging, Ice hockey, Repetitive subconcussive head impacts, Sex difference, Traumatic brain injury, White matter
Highlights
-
•
This study explores sex differences following repetitive subconcussive head impacts.
-
•
Decreases in FA were observed in females over the course of one ice hockey season.
-
•
Females also showed increases in MD, AD, and RD over the course of the season.
-
•
In contrast, males showed no significant changes in these diffusion measures.
-
•
The results suggest sex differences in structural alterations following subconcussion.
1. Introduction
Concussion is a common injury in contact sports, with an incidence ranging between 1.6 and 3.1 per 1000 athlete exposures (Agel et al., 2007a, Agel et al., 2007b, Flik et al., 2005). Women are at higher risk than men for sustaining a sports-related concussion and they represent a large proportion of the athletic community in organized sports (Abrahams et al., 2014, Black et al., 2017, Covassin et al., 2003, Gessel et al., 2007). In fact, female participation in National Collegiate Athletic Association (NCAA) sanctioned sports is currently at an all-time high, where an estimated 43% (~ 210,000) of all collegiate student-athletes are women (Irick, 2015). However, despite the high number of female athletes, females remain an understudied population, as only a small number of studies have focused on female athletes. Moreover, evidence from these studies suggests that females have worse outcomes following concussion compared with males (Baker et al., 2016, Broshek et al., 2005, Colvin et al., 2009, Covassin et al., 2013, Covassin et al., 2012, Covassin et al., 2007, Majerske et al., 2008, Miller et al., 2016, Zuckerman et al., 2014). Specifically, women reported more post-concussive symptoms with greater symptom severity (Zuckerman et al., 2014), performed worse on neurocognitive tests (Broshek et al., 2005, Colvin et al., 2009, Covassin et al., 2013, Covassin et al., 2012, Covassin et al., 2007, Majerske et al., 2008), and demonstrated longer periods of recovery compared to males (Baker et al., 2016, Miller et al., 2016, Zuckerman et al., 2014).
Following a concussion, brain alterations have been detected using advanced neuroimaging techniques (for review see Shenton et al., 2012). One of these advanced techniques is diffusion magnetic resonance imaging (dMRI), which has been repeatedly used to detect and to characterize white matter (WM) alterations related to brain injury (Koerte et al., 2015, Shenton et al., 2012). However, to date, there is only one study using dMRI that has investigated sex differences in structural brain alterations following a concussion (Fakhran et al., 2014). This study included 47 male and 22 female individuals after a confirmed concussion (Fakhran et al., 2014). In this study, findings indicated that male concussed individuals demonstrated decreased fractional anisotropy (FA) in the uncinate fasciculus compared to concussed females or controls (Fakhran et al., 2014).
Even more common than concussions are subconcussive head impacts in contact sports. Evidence here suggests that repetitive subconcussive head impacts (RSHI) may also result in structural, functional, and metabolic alterations of the brain (for review see Koerte et al., 2015). Of note, dMRI has shown sensitivity to detect even subtle WM alterations related to RSHI (Koerte et al., 2015). Furthermore, dMRI parameters have predicted impairments in executive function, attention, memory, speed of processing, and learning following traumatic brain injury (TBI) (Caeyenberghs et al., 2011a, Caeyenberghs et al., 2011b, Caeyenberghs et al., 2014). Detection of sex-specific WM changes related to RSHI could facilitate an individualized clinical management at an early stage of potential brain injury. However, to date, there are no studies investigating sex differences in brain alterations following exposure to RSHI. Thus, the aim of this study is to evaluate potential sex differences in the brain's WM following exposure to RSHI in a sample of collegiate ice hockey players using dMRI.
2. Materials and methods
2.1. Participants and procedures
All study participants were part of the Hockey Concussion Education Project (HCEP), which was conducted during the Canadian Interuniversity Sports (CIS) ice hockey seasons of 2009–2010 and 2011–2012. The present study analyzed participants of the 2011–2012 HCEP, which used clinical examination, neurocognitive assessment, and pre- and postseason magnetic resonance imaging (MRI) as well as sequential testing and imaging at three time points after any concussion among ice hockey players (Echlin, 2012). Data from the HCEP have already been analyzed with respect to other specific research questions (Chamard et al., 2012, Echlin, 2010, Echlin, 2012, Echlin et al., 2014, Echlin et al., 2010a, Echlin et al., 2012, Echlin et al., 2010b, Echlin et al., 2010c, Helmer et al., 2014, Koerte et al., 2012b, Pasternak et al., 2014, Sasaki et al., 2014). The study was approved by ethics committees at each CIS university, and was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants prior to the investigations.
For the 2011–2012 HCEP, exclusion criteria were general MRI exclusion criteria (e.g., metallic or electronic implants), structural MRI abnormalities, previous eye surgery, severe cognitive impairment, and/or a history of any psychiatric or neurological diseases. The team physician conducted the pre- and postseason clinical examinations. Moreover, concussions during the season were diagnosed and reported by an independent designated specialist physician who attended the games. In this context, concussion was defined with respect to the Zürich consensus statement, which met the criteria by a later consensus conference (McCrory et al., 2009, McCrory et al., 2013). For the present study, only HCEP participants that (1) did not experience a concussion during the course of the 2011–2012 CIS ice hockey season, and (2) completed pre- and postseason dMRI were included in the analyses.
In total, 45 ice hockey players (25 males and 20 females) were enrolled in the 2011–2012 HCEP. Among this cohort, 5 males and 6 females sustained a concussion during the season and were therefore excluded from the analysis. An additional 9 participants were excluded due to the following reasons: missing pre- or postseason dMRI (4 males), poor scan quality in either pre- or postseason dMRI sequences (2 males and 1 female), incidental finding of a large arachnoidal cyst (1 female), or age more than eight standard deviations (SDs) above the cohort's mean age (1 female). Thus, 25 participants (14 males and 11 females) were included in the analyses (Table 1).
Table 1.
Participant-related characteristics.
| Males | Females | p-Value | ||
|---|---|---|---|---|
| Number of players | 14 | 11 | – | |
| Age (in years) (mean ± SD) |
21.7 ± 1.3 | 19.2 ± 1.8 | 0.0005 | |
| Handedness (right/left/ambidextrous) |
10/3/1 | 10/1/0 | 0.6040 | |
| ImPACT score (preseason testing) (mean ± SD) |
Verbal memory | 90.9 ± 4.5 | 91.0 ± 8.6 | 0.3615 |
| Visual memory | 83.7 ± 8.6 | 85.4 ± 10.0 | 0.5358 | |
| Visual motor speed | 44.1 ± 4.2 | 42.7 ± 3.7 | 0.3712 | |
| Reaction time | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.0862 | |
| ImPACT score (postseason testing) (mean ± SD) |
Verbal memory | 89.4 ± 7.7 | 94.7 ± 4.1 | 0.0608 |
| Visual memory | 81.8 ± 11.9 | 79.2 ± 9.9 | 0.4623 | |
| Visual motor speed | 47.4 ± 5.3 | 42.9 ± 5.4 | 0.0344 | |
| Reaction time | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.4613 | |
This table gives an overview of participant-related characteristics, including the number of male and female participants, age, handedness, and pre- and postseason scores according to the four composite scores (verbal memory, visual memory, visual motor speed, and reaction time) derived from the results of the Immediate Post-Concussion Assessment and Cognitive Test (ImPACT). One female participant did not undergo neurocognitive assessment by the ImPACT.
2.2. Cognitive testing
Neurocognitive function was assessed using the Immediate Post-Concussion Assessment and Cognitive Test (ImPACT) before the beginning of the season and at the end of the season (ImPACT Applications Inc., San Diego, CA, USA; https://www.impacttest.com). The ImPACT is a computer-based assessment composed of a concussion symptom inventory as well as 6 modules for assessment of neurocognitive function. Although it is primarily applied in subjects with reported concussion, it can also be used to evaluate neurocognitive function in general. Based on the results obtained from the 6 neurocognitive test modules, 4 composite scores were generated (verbal memory, visual memory, visual motor speed, and reaction time). ImPACT composite scores have already been used in previous investigations among the 2011–2012 HCEP participants (Echlin et al., 2012, Sasaki et al., 2014). The ImPACT results were independently evaluated by a neuropsychologist.
2.3. Acquisition of dMRI
All imaging was performed on a 3T MRI scanner with an eight-channel head coil array (Achieva, Philips Medical Systems). A sequence with two averages and 60 non-colinear diffusion directions (TR/TE: 7015 ms/60 ms, b: 0 and 0.7 ms/mm2, 70 slices) was acquired using a 2.2 mm isotropic voxel size and a 100 × 100 matrix reconstructed into a 112 × 112 matrix with a resolution of 2 × 2 × 2.2 mm3.
Between preseason and postseason imaging, a scanner update took place (gradient coil change). A hardware update could potentially affect diffusion measures. However, since the present study compares the change in dMRI over the course of one season for each individual (postseason minus preseason) and the update would have affected all included data sets in the same way, this should not have confounded our longitudinal results.
2.4. Analysis of dMRI
2.4.1. Data processing
First, quality checks were performed by visually inspecting diffusion-weighted data sets using 3D Slicer (http://www.slicer.org; version 4.5.0-1, Surgical Planning Laboratory, Brigham and Women's Hospital, Boston, MA, USA) (Fedorov et al., 2012). To remove misalignments, an affine registration with the baseline volume was conducted for the data sets of each participant, and eddy current corrections were carried out using the MCFLIRT and eddy tools of the FMRIB Software Library (FSL, version 5.0.9; The Oxford Centre for Functional MRI of the Brain, Oxford, UK). Then, automated OTSU masks covering the entire brain were generated for each participant, excluding non-brain areas and background noise (3D Slicer, version 4.5.0-1). The resulting brain masks were again visually assessed for quality, and were manually edited where necessary (e.g., incorrect overlap of the mask with brain volume, missing voxels within the brain volume). A diffusion tensor was estimated for each voxel using a multivariate linear fitting algorithm, and three pairs of eigenvalues and eigenvectors were obtained. Diffusion scalar measures, which included FA, mean diffusivity (MD, also known as trace), axial diffusivity (AD), and radial diffusivity (RD), were then calculated for each voxel based on these values, as described previously (Koerte et al., 2012b, Sasaki et al., 2014).
2.4.2. White matter analysis
For analysis of WM diffusion properties, tract-based spatial statistics (TBSS) were carried out (Smith et al., 2006). All analysis protocols and detailed descriptions of the TBSS approach, which is part of FSL, are freely available (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS) (Jenkinson et al., 2012).
TBSS was conducted separately for FA, MD, AD, and RD, whereas the WM skeleton was generated based on FA maps. The individual maps were aligned and registered to the FMRIB58_FA template, which is in the same space as the MNI152 standard space image (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FMRIB58_FA). The mean FA map was projected to the FMRIB58_skeleton to create a mean FA skeleton. The FA threshold was set at > 0.3 to exclude peripheral tracts where there was considerable inter-subject variability or partial volume effects (Koerte et al., 2012b, Sasaki et al., 2014). MD, AD, and RD maps were registered to the FMRIB58_FA template by applying the nonlinear transformation obtained from the FA registration.
The voxels that formed the skeletons were extracted for each individual scan using the fslsplit command. This step was a prerequisite for subsequent subtraction of the participant-specific data sets obtained during pre- and postseason scanning using the fslmaths command. To depict the change in diffusion scalar measures over the course of the ice hockey season, the preseason data sets were subtracted from the postseason data sets, which generated skeletonized delta maps for each participant for FA, MD, AD, and RD, respectively. The delta maps were then merged across participants into a single file using the fslmerge command.
2.5. Statistical analyses
To identify voxel clusters with statistically significant group differences between females and males in the change in diffusion scalar measurements over the course of the play season, unpaired t-tests were performed applying the randomise command (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/GLM), adjusted for age and handedness. The random permutation number was set at 5000, and a p-value of < 0.05 was considered statistically significant, following threshold-free cluster enhancement and correction for multiple comparisons. The resulting statistical maps for FA, MD, AD, and RD were visualized in FSLView (version 3.2.0). Then, using the FSL cluster tool, we extracted the size of the statistically significant voxel clusters for FA, MD, AD, and RD, respectively. For improved illustration, the statistically significant voxel clusters were enlarged using the tbss_fill command.
Then, the statistical map for each of the diffusion scalar measures, thresholded at p < 0.05, was transformed into a binary map using fslmaths. These binary maps distinguished between statistically significant and non-significant voxels. Then, average diffusion scalar measures were extracted from the statistically significant voxel clusters for each participant and visualized by scatter plots using GraphPad Prism (version 7.0; GraphPad Software Inc., La Jolla, CA, USA). The spatial location of the significant voxel clusters was determined in relation to WM anatomy using the atlasquery command in combination with the ICBM-DTI-81 white-matter labels atlas (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases) (Mori et al., 2008).
Additionally, means ± SD were calculated for the participants' four composite scores derived from the results of the ImPACT evaluations. Mann-Whitney and Fisher exact tests were performed to assess differences between male and female participants. The individuals' change in FA, MD, AD, and RD values derived from the statistically significant voxel clusters as identified using TBBS were correlated with the postseason ImPACT composite scores using Spearman's rank correlation coefficient (rs). To adjust for multiple comparisons, we controlled the false discovery rate using the Benjamini & Hochberg procedure (Benjamini and Hochberg, 1995). GraphPad Prism (version 7.0) was used for these statistical tests, with the significance level set at p < 0.05.
3. Results
3.1. Participant characteristics
Table 1 shows participant-related characteristics and pre- and postseason scores of the four composite scores derived from the ImPACT assessments. There was a statistically significant difference in age between female and male participants (21.7 ± 1.3 vs. 19.2 ± 1.8 years, p = 0.0005; Table 1).
3.2. White matter diffusion
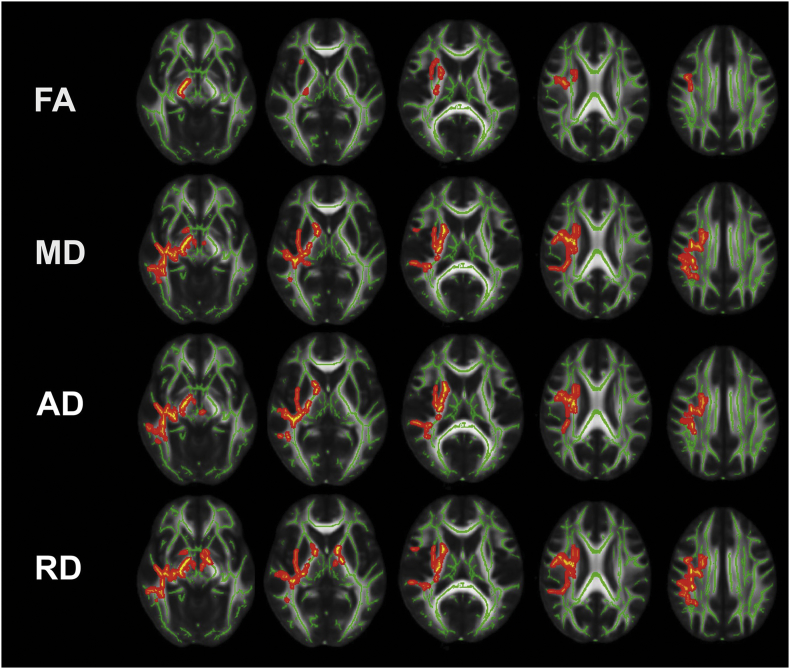
Voxel clusters with statistically significant differences between male and female participants in change over time (postseason minus preseason) are shown for FA, MD, AD, and RD in Fig. 1.
Fig. 1.
Results of the tract-based spatial statistics (TBSS) analysis I.
This figure illustrates the results of the TBSS analysis (axial view). Voxel clusters with statistically significant differences (p < 0.05) in change over time (postseason minus preseason data sets) between male and female participants are highlighted in red to yellow. The TBSS analysis was carried out for fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). Voxels of the statistically significant clusters are thickened into local tracts on a standardized FA skeleton (FMRIB58_FA-skeleton; green) and a standardized diffusion-weighted image (FMRIB58_FA). The left side in each image corresponds to the right hemisphere (RH). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The statistically significant FA cluster primarily includes the superior longitudinal fasciculus (SLF), internal capsule (IC), and corona radiata (CR) of the right hemisphere (RH; Fig. 1). There was no statistically significant FA cluster detected in the left hemisphere (LH; Fig. 1). In the statistically significant cluster, FA values did not change significantly in male participants over the course of one season (pre- vs. postseason: 0.6202 ± 0.0121 vs. 0.6270 ± 0.0131, p > 0.05), whereas a decrease in FA in female participants was observed (pre- vs. postseason: 0.6247 ± 0.0147 vs. 0.5978 ± 0.0184, p < 0.05; Fig. 1, Fig. 2). The statistically significant FA cluster had a size of 1494 voxels.
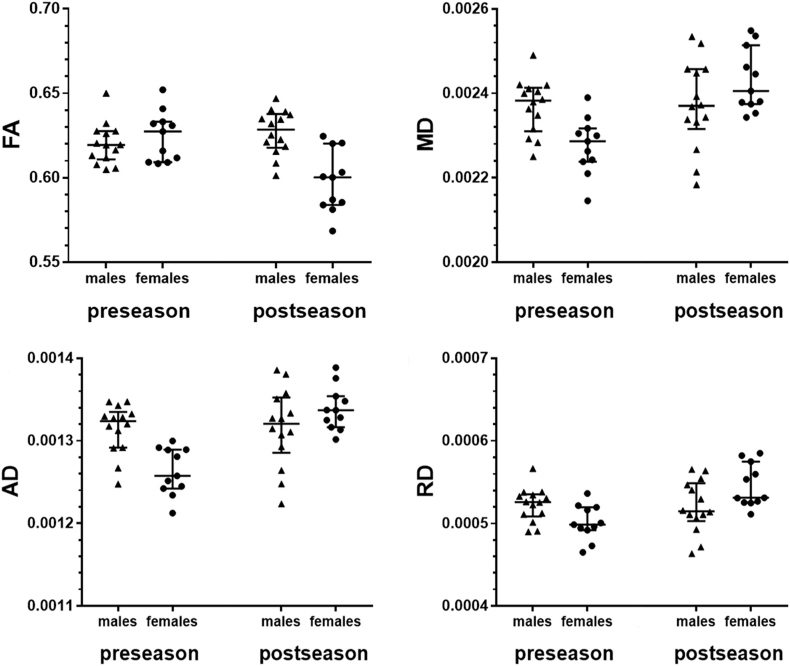
Fig. 2.
Results of the tract-based spatial statistics (TBSS) analysis II.
This figure depicts scatter plots of average values in the voxel clusters with statistically significant group differences (p < 0.05; Fig. 1) for fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). The values are shown for males vs. females and pre- vs. postseason data, respectively. Circles or triangles represent individual values, whereas horizontal bars represent the median and interquartile range. There was a statistically significant difference between pre- and postseason FA, MD, AD, and RD in female participants (p < 0.05). In contrast, no statistically significant changes were observed in males over the course of one season with respect to FA, MD, AD, and RD (p > 0.05).
The statistically significant MD cluster mainly includes the SLF, IC, CR, and the external capsule (EC) of the RH, whereas the LH again showed no statistically significant cluster (Fig. 1). In the significant voxel cluster, MD did not change significantly in male participants (pre- vs. postseason: 0.002369 ± 0.00007 vs. 0.002373 ± 0.00011, p > 0.05), whereas female participants demonstrated an increase in MD (pre- vs. postseason: 0.002276 ± 0.00007 vs. 0.002431 ± 0.00008, p < 0.05; Fig. 1, Fig. 2). The statistically significant MD cluster was composed of 7481 voxels.
Regarding both AD and RD, values increased in female participants over the course of one season (AD: pre- vs. postseason: 0.001263 ± 0.00003 vs. 0.001339 ± 0.00003, p < 0.05; RD: pre- vs. postseason: 0.000501 ± 0.00002 vs. 0.000546 ± 0.00003, p < 0.05), whereas they did not in male participants (AD: pre- vs. postseason: 0.001315 ± 0.00003 vs. 0.001316 ± 0.00005, p > 0.05; RD: pre- vs. postseason: 0.000523 ± 0.00002 vs. 0.000520 ± 0.00003, p > 0.05; Fig. 1, Fig. 2). Again, statistically significant clusters primarily involved the SLF, IC, CR, and EC of the RH (Fig. 1). The statistically significant AD cluster had a size of 6110 voxels, and the statistically significant RD cluster included 7355 voxels.
3.3. Correlation of diffusion scalar measures with ImPACT scores
Table 1 shows the results of pre- and postseason ImPACT assessments regarding the four composite scores (verbal memory, visual memory, visual motor speed, and reaction time). There were no statistically significant differences between female and male participants except for visual motor speed at postseason assessment, where male athletes demonstrated significantly improved function in visual motor speed compared to females (p = 0.0344; Table 1).
Furthermore, there were no statistically significant correlations of postseason ImPACT composite scores with individuals' change in FA, MD, AD, or RD over the season of play derived from significant voxel clusters.
4. Discussion
This study revealed sex-specific differences of change in diffusion measures over the course of one ice hockey season (Fig. 1, Fig. 2). Statistically significant voxel clusters were observed in several brain regions, including the SLF, IC, CR, and EC (Fig. 1). More specifically, in these voxel clusters female athletes demonstrated a decrease in FA and an increase in MD, AD, and RD whereas, in contrast, diffusion measures did not change significantly over the course of the season in male athletes (Fig. 2).
Changes in WM diffusivity over time can be observed during aging but have also been associated with a variety of psychiatric or neurological diseases such as mild TBI (Assaf and Pasternak, 2008, Westlye et al., 2010). Evidence suggests that RSHI may also lead to detectable WM alterations (Koerte et al., 2012a, Koerte et al., 2012b, Lipton et al., 2013, McAllister et al., 2014). In this context, decreased FA and increased AD and RD have been shown to be associated with heading the ball in soccer (Koerte et al., 2012a, Lipton et al., 2013), whereas increased MD has been reported in contact-sports athletes compared to non-contact sports athletes after one season (McAllister et al., 2014). However, although these studies included male and female athletes in their study cohorts, sex-specific differences in WM diffusivity were not reported. To the best of our knowledge, we here demonstrate for the first time widespread statistically significant differences between female and male athletes following RSHI for changes in diffusion measures.
Sex differences in the change of WM diffusivity were predominantly located within the RH. The underlying mechanisms may potentially include differences in vulnerability, developmental characteristics, or differences in exposure to head impacts. Future studies will need to elucidate reasons for asymmetric changes due to RSHI and the underlying mechanisms for sex-specific differences in the change of WM diffusivity following RSHI. There are two main components that may play a role regarding sex-specific WM diffusivity changes over time. First, sex differences following exposure to RSHI could be associated with differences in RSHI incidences and intensities. Studies have reported that female athletes are at greater risk for concussions when compared to males (Covassin et al., 2003, Forward et al., 2014, Marar et al., 2012), which has been associated with smaller neck girth and weaker neck muscles compared to males (Tierney et al., 2005). This increased risk for brain trauma may also be the case when exposed to RSHI and could explain why differences in change in diffusion measures occurred over the course of one ice hockey season between male and female participants (Fig. 1, Fig. 2). Second, sex differences in the change of WM diffusivity following RSHI could be due to physiological or hormonal differences between males and females, as suggested by investigations among patients suffering from TBI (Djebaili et al., 2005, Emerson et al., 1993, Kupina et al., 2003, Roof and Hall, 2000). Both estrogen and progesterone, which exist in different concentrations in males and females, may have neuroprotective effects after TBI, with previous data suggesting that females may profit from a higher neuroprotective effect (Djebaili et al., 2005, Kupina et al., 2003, Roof and Hall, 2000). However, an opposite situation has also been observed in a study where estrogen was administered to rats prior to inducing a TBI, leading to the observation that estrogen exacerbated injury in female rats but not in males (Emerson et al., 1993). Furthermore, greater rates of basal glucose metabolism and cerebral blood flow in females have been suggested as contributing to differences between the sexes in response to concussion (Andreason et al., 1994, Esposito et al., 1996). In females increased demands for glucose and increased blood flow may lead to an exacerbation of the neuro-metabolic cascade after injury (Broshek et al., 2005). However, it is important to note that most of the previous study results have been restricted to moderate to severe TBI rather than to RSHI, or they have been conducted in animal models, thus leaving open the question of whether such results are directly translatable to human RSHI.
In concussion, sex differences in neurocognitive and clinical outcome have been shown, with the number of symptoms and symptom severity being higher among concussed females (Zuckerman et al., 2014). Furthermore, worse verbal, visual, and motor speed deficits have been reported in females (Covassin et al., 2012, Covassin et al., 2007, Majerske et al., 2008), and symptom duration was prolonged when compared to males (Baker et al., 2016, Miller et al., 2016, Zuckerman et al., 2014). The present study used the ImPACT assessment to test pre- and postseason neurocognitive performance. Although no statistically significant differences were found between female and male athletes at preseason evaluation, at postseason assessment, male athletes demonstrated significantly improved function in visual motor speed compared to their female counterparts (Table 1). However, there was no statistically significant correlation of change in diffusion scalar measures over the course of the season of play and postseason ImPACT composite scores. In this context, it is important to note that the ImPACT assessment, which has been designed for the detection of concussion-related symptoms, may not be sufficiently sensitive for the detection of subtle neurocognitive alterations following RSHI. It is therefore not surprising that we did not find significant correlations with postseason ImPACT scores in our study that focused on RSHI. More sensitive methods to assess the effects of RSHI are currently being developed (Echemendia et al., 2016, Koerte et al., 2017, Zhang et al., 2013). However, it could also be the case that major cognitive changes due to RSHI occur later and, thus, may not have been detectable by postseason ImPACT assessments. Thus, further studies are needed to explore the relationship between sex-specific changes in WM diffusivity and potential subtle neurocognitive changes, using more sensitive neurocognitive measures. Furthermore, additional complementary techniques such as electrophysiological measurements, analyses of functional connectivity, and evaluation of cerebral blood flow may help to investigate further and to enhance our understanding of the underlying mechanisms following RSHI, and particularly to explore WM diffusivity differences between males and females related to RSHI. Regarding concussion, different modalities have already been applied to study sex differences. In contrast, approaches using different techniques or even multi-modal setups in RSHI are just emerging (Covassin and Elbin, 2011, Koerte et al., 2015, Resch et al., 2017).
There are limitations to this study that need to be taken into account when interpreting the data. First, without a control group, the difference between pre- and postseason dMRI cannot be attributed to RSHI only and other factors such as training might play a role. However, the changes found confirm the existing literature on WM alterations following exposure to RSHI. Second, results from this study may not be generalizable to other sports and thus need to be followed-up by further studies in larger cohorts and including other sports. Third, head impact forces and frequencies were not measured in our present study. Future studies should include quantitative assessments of head impact exposure to understand better the underlying mechanisms of sex-specific differences in alterations in WM diffusivity, and we need to determine whether or not the observed sex differences can distinctly be attributed to RSHI exposure. Fourth, there was a statistically significant difference in age between female and male participants (Table 1). Although the analyses performed in this study were adjusted for age, we cannot categorically rule out any potential effect of age on WM diffusivity changes following RSHI. Fifth, group-wise analysis using TBSS may not be sensitive to the spatial location of changes in diffusion properties in heterogeneous conditions such as exposure to RSHI. However, results of this study provide an overview of several regions involved that should be investigated further regarding subject-specific changes. Finally, despite these limitations, we think that the present study demonstrates for the first time sex differences in WM alterations following exposure to RSHI, which, importantly, may pave the way for future research on sex-specific alterations.
5. Conclusions
Previous research has shown that exposure to RSHI during a single varsity ice hockey season can result in significant alterations in WM diffusivity. The results of this study further suggest sex differences in WM diffusivity following exposure to RSHI. The underlying mechanisms remain to be elucidated but may include an increased vulnerability of the female brain to RSHI. Future studies are also needed to investigate the association between neurocognitive and clinical outcome with brain alterations in more detail.
Acknowledgments
Acknowledgments
The authors acknowledge the players and staffs of two CIS varsity ice hockey teams for their participation in the HCEP, and the participating physicians, observers, and volunteers for their contributions to the HCEP. The authors would also like to acknowledge the contributions of the University of British Columbia MRI Center and all of the associated researchers and employees, especially Ms. Trudy Harris and Ms. Linda Chandler.
Disclosure
Funding for this work was provided to the HCEP and Dr. Echlin by the Ontario Trillium Foundation, The Dave Irwin Foundation for Brain Injury, the Ontario Neurotrauma Foundation, Air Canada, The Ontario Ministry of Health and Long-Term Care, The Ontario Ministry of Tourism Culture and Sport and The Ontario Ministry of Education. The authors of this study were supported by the NIH (U01 NS 093334: IKK, MES; R01 R01NS100952: IKK; R01 HD 090641: SB), the Veterans Affairs (VA Merit Award I01 RX00928: MES), the Department of Defense Congressionally Directed Medical Research Programs (W81XWH-08-2-0159: MES), the German Academic Exchange Service PROMOS award (VS), the LMU Munich's Institutional Strategy LMU excellent within the framework of the German Excellence Initiative, and by the Canadian Institutes of Health Research Frederick Banting and Charles Best Doctoral Award (CL).
Footnotes
Previous presentation of data:
Portions of this work were presented in poster form at the 12th World Congress on Brain Injury, New Orleans, LA, USA, March 29–April 1, 2017.
Contributor Information
Nico Sollmann, Email: Nico.Sollmann@tum.de.
Paul S. Echlin, Email: Psechlin@gmail.com.
Vivian Schultz, Email: VivianSchultz@gmx.de.
Petra V. Viher, Email: Petra.Viher@puk.unibe.ch.
Amanda E. Lyall, Email: Alyall@bwh.harvard.edu.
Yorghos Tripodis, Email: Yorghos@bu.edu.
David Kaufmann, Email: David.Kaufmann@charite.de.
Elisabeth Hartl, Email: Elisabeth.Hartl@med.uni-muenchen.de.
Philipp Kinzel, Email: Pkinzel@bwh.harvard.edu.
Lorie A. Forwell, Email: Lforwell@uwo.ca.
Andrew M. Johnson, Email: Ajohnson@uwo.ca.
Elaine N. Skopelja, Email: Eskopelj@iu.edu.
Christian Lepage, Email: Clepage@uottawa.ca.
Sylvain Bouix, Email: Sylvain@bwh.harvard.edu.
Ofer Pasternak, Email: Ofer@bwh.harvard.edu.
Alexander P. Lin, Email: Aplin@bwh.harvard.edu.
Martha E. Shenton, Email: Shenton@bwh.harvard.edu.
Inga K. Koerte, Email: Ikoerte@bwh.harvard.edu.
References
- Abrahams S., Fie S.M., Patricios J., Posthumus M., September A.V. Risk factors for sports concussion: an evidence-based systematic review. Br. J. Sports Med. 2014;48:91–97. doi: 10.1136/bjsports-2013-092734. [DOI] [PubMed] [Google Scholar]
- Agel J., Dick R., Nelson B., Marshall S.W., Dompier T.P. Descriptive epidemiology of collegiate women's ice hockey injuries: National Collegiate Athletic Association Injury Surveillance System, 2000–2001 through 2003–2004. J. Athl. Train. 2007;42:249–254. [PMC free article] [PubMed] [Google Scholar]
- Agel J., Dompier T.P., Dick R., Marshall S.W. Descriptive epidemiology of collegiate men's ice hockey injuries: National Collegiate Athletic Association Injury Surveillance System, 1988–1989 through 2003–2004. J. Athl. Train. 2007;42:241–248. [PMC free article] [PubMed] [Google Scholar]
- Andreason P.J., Zametkin A.J., Guo A.C., Baldwin P., Cohen R.M. Gender-related differences in regional cerebral glucose metabolism in normal volunteers. Psychiatry Res. 1994;51:175–183. doi: 10.1016/0165-1781(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Assaf Y., Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J. Mol. Neurosci. 2008;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- Baker J.G., Leddy J.J., Darling S.R., Shucard J., Makdissi M., Willer B.S. Gender differences in recovery from sports-related concussion in adolescents. Clin. Pediatr. 2016;55:771–775. doi: 10.1177/0009922815606417. (Phila) [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. [Google Scholar]
- Black A.M., Sergio L.E., Macpherson A.K. The epidemiology of concussions: number and nature of concussions and time to recovery among female and male Canadian varsity athletes 2008 to 2011. Clin. J. Sport Med. 2017;27(1):52–56. doi: 10.1097/JSM.0000000000000308. [DOI] [PubMed] [Google Scholar]
- Broshek D.K., Kaushik T., Freeman J.R., Erlanger D., Webbe F., Barth J.T. Sex differences in outcome following sports-related concussion. J. Neurosurg. 2005;102:856–863. doi: 10.3171/jns.2005.102.5.0856. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K., Leemans A., Coxon J., Leunissen I., Drijkoningen D., Geurts M., Gooijers J., Michiels K., Sunaert S., Swinnen S.P. Bimanual coordination and corpus callosum microstructure in young adults with traumatic brain injury: a diffusion tensor imaging study. J. Neurotrauma. 2011;28:897–913. doi: 10.1089/neu.2010.1721. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K., Leemans A., Geurts M., Linden C.V., Smits-Engelsman B.C., Sunaert S., Swinnen S.P. Correlations between white matter integrity and motor function in traumatic brain injury patients. Neurorehabil. Neural Repair. 2011;25:492–502. doi: 10.1177/1545968310394870. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K., Leemans A., Leunissen I., Gooijers J., Michiels K., Sunaert S., Swinnen S.P. Altered structural networks and executive deficits in traumatic brain injury patients. Brain Struct. Funct. 2014;219:193–209. doi: 10.1007/s00429-012-0494-2. [DOI] [PubMed] [Google Scholar]
- Chamard E., Theoret H., Skopelja E.N., Forwell L.A., Johnson A.M., Echlin P.S. A prospective study of physician-observed concussion during a varsity university hockey season: metabolic changes in ice hockey players. Part 4 of 4. Neurosurg. Focus. 2012;33(E4):1–7. doi: 10.3171/2012.10.FOCUS12305. [DOI] [PubMed] [Google Scholar]
- Colvin A.C., Mullen J., Lovell M.R., West R.V., Collins M.W., Groh M. The role of concussion history and gender in recovery from soccer-related concussion. Am. J. Sports Med. 2009;37:1699–1704. doi: 10.1177/0363546509332497. [DOI] [PubMed] [Google Scholar]
- Covassin T., Elbin R.J. The female athlete: the role of gender in the assessment and management of sport-related concussion. Clin. Sports Med. 2011;30:125–131. doi: 10.1016/j.csm.2010.08.001. (x) [DOI] [PubMed] [Google Scholar]
- Covassin T., Swanik C.B., Sachs M.L. Sex differences and the incidence of concussions among collegiate athletes. J. Athl. Train. 2003;38:238–244. [PMC free article] [PubMed] [Google Scholar]
- Covassin T., Schatz P., Swanik C.B. Sex differences in neuropsychological function and post-concussion symptoms of concussed collegiate athletes. Neurosurgery. 2007;61:345–350. doi: 10.1227/01.NEU.0000279972.95060.CB. (discussion 350-341) [DOI] [PubMed] [Google Scholar]
- Covassin T., Elbin R.J., Harris W., Parker T., Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am. J. Sports Med. 2012;40:1303–1312. doi: 10.1177/0363546512444554. [DOI] [PubMed] [Google Scholar]
- Covassin T., Elbin R.J., Bleecker A., Lipchik A., Kontos A.P. Are there differences in neurocognitive function and symptoms between male and female soccer players after concussions? Am. J. Sports Med. 2013;41:2890–2895. doi: 10.1177/0363546513509962. [DOI] [PubMed] [Google Scholar]
- Djebaili M., Guo Q., Pettus E.H., Hoffman S.W., Stein D.G. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J. Neurotrauma. 2005;22:106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- Echemendia R.J., Bruce J.M., Meeuwisse W., Comper P., Aubry M., Hutchison M. Long-term reliability of ImPACT in professional ice hockey. Clin. Neuropsychol. 2016;30:328–337. doi: 10.1080/13854046.2016.1158320. [DOI] [PubMed] [Google Scholar]
- Echlin P.S. Concussion education, identification,and treatment within a prospective study of physician-observed junior ice hockey concussions: social context of this scientific intervention. Neurosurg. Focus. 2010;29 doi: 10.3171/2010.10.FOCUS10222. [DOI] [PubMed] [Google Scholar]
- Echlin P.S. A prospective study of physician-observed concussion during a varsity university ice hockey season. Part 1 of 4. Neurosurg. Focus. 2012;33(E1):1–7. doi: 10.3171/2012.9.FOCUS12287. [DOI] [PubMed] [Google Scholar]
- Echlin P.S., Johnson A.M., Riverin S., Tator C.H., Cantu R.C., Cusimano M.D., Taunton J.E., Upshur R.E., Hall C.R., Forwell L.A., Skopelja E.N. A prospective study of concussion education in 2 junior ice hockey teams: implications for sports concussion education. Neurosurg. Focus. 2010;29 doi: 10.3171/2010.9.FOCUS10187. [DOI] [PubMed] [Google Scholar]
- Echlin P.S., Tator C.H., Cusimano M.D., Cantu R.C., Taunton J.E., Upshur R.E., Czarnota M., Hall C.R., Johnson A.M., Forwell L.A., Driediger M., Skopelja E.N. Return to play after an initial or recurrent concussion in a prospective study of physician-observed junior ice hockey concussions: implications for return to play after a concussion. Neurosurg. Focus. 2010;29 doi: 10.3171/2010.9.FOCUS10210. [DOI] [PubMed] [Google Scholar]
- Echlin P.S., Tator C.H., Cusimano M.D., Cantu R.C., Taunton J.E., Upshur R.E., Hall C.R., Johnson A.M., Forwell L.A., Skopelja E.N. A prospective study of physician-observed concussions during junior ice hockey: implications for incidence rates. Neurosurg. Focus. 2010;29 doi: 10.3171/2010.9.FOCUS10186. [DOI] [PubMed] [Google Scholar]
- Echlin P.S., Skopelja E.N., Worsley R., Dadachanji S.B., Lloyd-Smith D.R., Taunton J.A., Forwell L.A., Johnson A.M. A prospective study of physician-observed concussion during a varsity university ice hockey season: incidence and neuropsychological changes. Part 2 of 4. Neurosurg. Focus. 2012;33(E2):1–11. doi: 10.3171/2012.10.FOCUS12286. [DOI] [PubMed] [Google Scholar]
- Echlin P.S., Johnson A.M., Holmes J.D., Tichenoff A., Gray S., Gatavackas H., Walsh J., Middlebro T., Blignaut A., MacIntyre M., Anderson C., Fredman E., Mayinger M., Skopelja E.N., Sasaki T., Bouix S., Pasternak O., Helmer K.G., Koerte I.K., Shenton M.E., Forwell L.A. The sport concussion education project. A brief report on an educational initiative: from concept to curriculum. J. Neurosurg. 2014;121:1331–1336. doi: 10.3171/2014.8.JNS132804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson C.S., Headrick J.P., Vink R. Estrogen improves biochemical and neurologic outcome following traumatic brain injury in male rats, but not in females. Brain Res. 1993;608:95–100. doi: 10.1016/0006-8993(93)90778-l. [DOI] [PubMed] [Google Scholar]
- Esposito G., Van Horn J.D., Weinberger D.R., Berman K.F. Gender differences in cerebral blood flow as a function of cognitive state with PET. J. Nucl. Med. 1996;37:559–564. [PubMed] [Google Scholar]
- Fakhran S., Yaeger K., Collins M., Alhilali L. Sex differences in white matter abnormalities after mild traumatic brain injury: localization and correlation with outcome. Radiology. 2014;272:815–823. doi: 10.1148/radiol.14132512. [DOI] [PubMed] [Google Scholar]
- Fedorov A., Beichel R., Kalpathy-Cramer J., Finet J., Fillion-Robin J.C., Pujol S., Bauer C., Jennings D., Fennessy F., Sonka M., Buatti J., Aylward S., Miller J.V., Pieper S., Kikinis R. 3D slicer as an image computing platform for the quantitative imaging network. Magn. Reson. Imaging. 2012;30:1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flik K., Lyman S., Marx R.G. American collegiate men's ice hockey: an analysis of injuries. Am. J. Sports Med. 2005;33:183–187. doi: 10.1177/0363546504267349. [DOI] [PubMed] [Google Scholar]
- Forward K.E., Seabrook J.A., Lynch T., Lim R., Poonai N., Sangha G.S. A comparison of the epidemiology of ice hockey injuries between male and female youth in Canada. Paediatr. Child Health. 2014;19:418–422. doi: 10.1093/pch/19.8.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessel L.M., Fields S.K., Collins C.L., Dick R.W., Comstock R.D. Concussions among United States high school and collegiate athletes. J. Athl. Train. 2007;42:495–503. [PMC free article] [PubMed] [Google Scholar]
- Helmer K.G., Pasternak O., Fredman E., Preciado R.I., Koerte I.K., Sasaki T., Mayinger M., Johnson A.M., Holmes J.D., Forwell L.A., Skopelja E.N., Shenton M.E., Echlin P.S. Hockey concussion education project, part 1. Susceptibility-weighted imaging study in male and female ice hockey players over a single season. J. Neurosurg. 2014;120:864–872. doi: 10.3171/2013.12.JNS132093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irick E. National Collegiate Athletic Association; 2015. Student-athlete participation: 1981–82 – 2014–15; NCAA sports sponsorship and participation rates report. http://www.ncaa.org/sites/default/files/Participation%20Rates%20Final.pdf
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. Fsl. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Koerte I.K., Ertl-Wagner B., Reiser M., Zafonte R., Shenton M.E. White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA. 2012;308:1859–1861. doi: 10.1001/jama.2012.13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte I.K., Kaufmann D., Hartl E., Bouix S., Pasternak O., Kubicki M., Rauscher A., Li D.K., Dadachanji S.B., Taunton J.A., Forwell L.A., Johnson A.M., Echlin P.S., Shenton M.E. A prospective study of physician-observed concussion during a varsity university hockey season: white matter integrity in ice hockey players. Part 3 of 4. Neurosurg. Focus. 2012;33(E3):1–7. doi: 10.3171/2012.10.FOCUS12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte I.K., Lin A.P., Willems A., Muehlmann M., Hufschmidt J., Coleman M.J., Green I., Liao H., Tate D.F., Wilde E.A., Pasternak O., Bouix S., Rathi Y., Bigler E.D., Stern R.A., Shenton M.E. A review of neuroimaging findings in repetitive brain trauma. Brain Pathol. 2015;25:318–349. doi: 10.1111/bpa.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte I.K., Nichols E., Tripodis Y., Schultz V., Lehner S., Igbinoba R., Chuang A.Z., Mayinger M., Klier E.M., Muehlmann M., Kaufmann D., Lepage C., Heinen F., Schulte-Korne G., Zafonte R., Shenton M.E., Sereno A.B. Impaired cognitive performance in youth athletes exposed to repetitive head impacts. J. Neurotrauma. 2017;34:2389–2395. doi: 10.1089/neu.2016.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupina N.C., Detloff M.R., Bobrowski W.F., Snyder B.J., Hall E.D. Cytoskeletal protein degradation and neurodegeneration evolves differently in males and females following experimental head injury. Exp. Neurol. 2003;180:55–73. doi: 10.1016/s0014-4886(02)00048-1. [DOI] [PubMed] [Google Scholar]
- Lipton M.L., Kim N., Zimmerman M.E., Kim M., Stewart W.F., Branch C.A., Lipton R.B. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268(3):850–857. doi: 10.1148/radiol.13130545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerske C.W., Mihalik J.P., Ren D., Collins M.W., Reddy C.C., Lovell M.R., Wagner A.K. Concussion in sports: postconcussive activity levels, symptoms, and neurocognitive performance. J. Athl. Train. 2008;43:265–274. doi: 10.4085/1062-6050-43.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marar M., McIlvain N.M., Fields S.K., Comstock R.D. Epidemiology of concussions among United States high school athletes in 20 sports. Am. J. Sports Med. 2012;40:747–755. doi: 10.1177/0363546511435626. [DOI] [PubMed] [Google Scholar]
- McAllister T.W., Ford J.C., Flashman L.A., Maerlender A., Greenwald R.M., Beckwith J.G., Bolander R.P., Tosteson T.D., Turco J.H., Raman R., Jain S. Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology. 2014;82:63–69. doi: 10.1212/01.wnl.0000438220.16190.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory P., Meeuwisse W., Johnston K., Dvorak J., Aubry M., Molloy M., Cantu R. Consensus statement on concussion in sport: the 3rd international conference on concussion in sport held in Zurich, November 2008. Br. J. Sports Med. 2009;43(Suppl. 1):i76–90. doi: 10.1136/bjsm.2009.058248. [DOI] [PubMed] [Google Scholar]
- McCrory P., Meeuwisse W.H., Aubry M., Cantu B., Dvorak J., Echemendia R.J., Engebretsen L., Johnston K., Kutcher J.S., Raftery M., Sills A., Benson B.W., Davis G.A., Ellenbogen R.G., Guskiewicz K., Herring S.A., Iverson G.L., Jordan B.D., Kissick J., McCrea M., McIntosh A.S., Maddocks D., Makdissi M., Purcell L., Putukian M., Schneider K., Tator C.H., Turner M. Consensus statement on concussion in sport: the 4th international conference on concussion in sport held in Zurich, November 2012. Br. J. Sports Med. 2013;47:250–258. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- Miller J.H., Gill C., Kuhn E.N., Rocque B.G., Menendez J.Y., O'Neill J.A., Agee B.S., Brown S.T., Crowther M., Davis R.D., Ferguson D., Johnston J.M. Predictors of delayed recovery following pediatric sports-related concussion: a case-control study. J. Neurosurg. Pediatr. 2016;17:491–496. doi: 10.3171/2015.8.PEDS14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S., Oishi K., Jiang H., Jiang L., Li X., Akhter K., Hua K., Faria A.V., Mahmood A., Woods R., Toga A.W., Pike G.B., Neto P.R., Evans A., Zhang J., Huang H., Miller M.I., van Zijl P., Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O., Koerte I.K., Bouix S., Fredman E., Sasaki T., Mayinger M., Helmer K.G., Johnson A.M., Holmes J.D., Forwell L.A., Skopelja E.N., Shenton M.E., Echlin P.S. Hockey concussion education project, part 2. Microstructural white matter alterations in acutely concussed ice hockey players: a longitudinal free-water MRI study. J. Neurosurg. 2014;120:873–881. doi: 10.3171/2013.12.JNS132090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch J.E., Rach A., Walton S., Broshek D.K. Sport concussion and the female athlete. Clin. Sports Med. 2017;36:717–739. doi: 10.1016/j.csm.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Roof R.L., Hall E.D. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J. Neurotrauma. 2000;17:367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Pasternak O., Mayinger M., Muehlmann M., Savadjiev P., Bouix S., Kubicki M., Fredman E., Dahlben B., Helmer K.G., Johnson A.M., Holmes J.D., Forwell L.A., Skopelja E.N., Shenton M.E., Echlin P.S., Koerte I.K. Hockey concussion education project, part 3. White matter microstructure in ice hockey players with a history of concussion: a diffusion tensor imaging study. J. Neurosurg. 2014;120:882–890. doi: 10.3171/2013.12.JNS132092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton M.E., Hamoda H.M., Schneiderman J.S., Bouix S., Pasternak O., Rathi Y., Vu M.A., Purohit M.P., Helmer K., Koerte I., Lin A.P., Westin C.F., Kikinis R., Kubicki M., Stern R.A., Zafonte R. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6:137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Tierney R.T., Sitler M.R., Swanik C.B., Swanik K.A., Higgins M., Torg J. Gender differences in head-neck segment dynamic stabilization during head acceleration. Med. Sci. Sports Exerc. 2005;37:272–279. doi: 10.1249/01.mss.0000152734.47516.aa. [DOI] [PubMed] [Google Scholar]
- Westlye L.T., Walhovd K.B., Dale A.M., Bjornerud A., Due-Tonnessen P., Engvig A., Grydeland H., Tamnes C.K., Ostby Y., Fjell A.M. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb. Cortex. 2010;20:2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Zhang M.R., Red S.D., Lin A.H., Patel S.S., Sereno A.B. Evidence of cognitive dysfunction after soccer playing with ball heading using a novel tablet-based approach. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S.L., Apple R.P., Odom M.J., Lee Y.M., Solomon G.S., Sills A.K. Effect of sex on symptoms and return to baseline in sport-related concussion. J. Neurosurg. Pediatr. 2014;13:72–81. doi: 10.3171/2013.9.PEDS13257. [DOI] [PubMed] [Google Scholar]