Highlights
-
•
A novel purely synthetic topical haemostatic agent is proposed for use in endonasal surgery.
-
•
The mechanism is based on the self-assembling tendency of four repeating peptide sequences.
-
•
The haemostatic agent was used in 60 patients undergoing endonasal powered turbinoplasty.
-
•
Routine post-operative followup at 4 weeks has shown no re-bleeding or adhesion formation in all 60 patients.
-
•
Pending further research, the agent shows promise in endonasal surgery as both a haemostatic and anti-adhesive adjunct.
Abbreviations: RADA, short peptide made of 4 amino acids (Arginine-Alanine-Aspartic acid-Alanine) repeated 4 times; FEES, Functional Endoscopic Endonasal Surgery
Keywords: PuraStat, Haemostasis, Endonasal surgery, Endoscopic nasal surgery, Endoscopic turbinoplasty
Abstract
Introduction
Recently, a novel purely synthetic topical haemostatic agent (PuraStat®) has been proposed in surgery based on the self-assembling tendency of some repeating peptide sequences. This transparent, ready to use hydrogel appears suitable for use in FEES with low rates of post-operative re-bleeding and adhesion formation.
A first series of 60 patients experiencing endonasal powered turbinoplasty across various hospitals in Sydney using PuraStat® was observed for postoperative re-bleeding and adhesion formation.
Discussion
In all 60 patients, no post-operative re-bleeding was observed, while healing went well in absence of adhesion formation. Effective haemostasis with PuraStat® is well documented in other surgical fields, but its use in FEES and adhesion prevention is relatively novel.
Conclusion
Synthetic self-assembling peptides appear to be indicated in this area. Further studies are needed to confirm their potential for adhesion prevention.
1. Introduction
There has been a great evolution in nasal surgery over the past few decades. Contemporary nasal surgery involves endoscopic surgery targeted at the nasal sinuses and the turbinates. Due to the highly vascular nature of nasal tissue, post-operative haemostasis has always been a concern. The use of tamponade through the insertion of nasal packing is still commonly used, but this is at the cost of the patient's comfort. Excessive pressure caused by tamponade can also be detrimental to the outcomes of delicately performed sinus surgery such as the preservation of the mucociliary function. In addition, delayed healing and crust formation can lead to the formation of endonasal adhesions, themselves a source of chronic nasal infection. Therefore, the ideal setting would be to leave an operated nose completely unviolated by the use packing in favour of an alternative haemostatic agent offering satisfactory healing and no adhesions. PuraStat® is a novel topical haemostatic agent based on nanotechnologies looking like a transparent hydrogel suitable for endoscopic use and that could achieve these various goals. We have been using this product in 60 cases of endonasal surgery since August 2016 with great satisfaction and would share our learning here.
2. Methods
2.1. Registration and ethics
This study has been registered in the Research Registry as per the Declaration of Helsinki. The research registry number is researchregistry3085. Ethical approval was not needed as this case series involved the use of a haemostatic agent licensed for use in exudative haemorrhage, with haemostatic agents routinely used in all patients undergoing endonasal surgery, and no changes being made to the operative procedure or post-operative followup. This case series has been reported in line with the PROCESS criteria [1].
2.2. Study design
This study was a prospective consecutive multi centre case series conducted from August 2016 to October 2016.
2.3. Setting
Patients were managed across two public and one private hospitals in Sydney. Post-operative followup was conducted in one hospital clinic and the two private clinics of the operating surgeon.
2.4. Participants
We have been using PuraStat® mainly in endoscopic powered turbinoplasty in patients with severe allergic rhinitis and intractable nasal obstruction resistant to medical treatment. The inclusion criterion for this study was all patients undergoing endoscopic powered turbinoplasty using PuraStat® as a haemostatic adjunct. The exclusion criterion for this study was any concurrent use of alternative haemostatic adjuncts, such as another haemostatic agent or the use of nasal packing. All patients, a total of 60, undergoing endoscopic powered turbinoplasty meeting our inclusion/exclusion criteria between August and October were included. Patients were followed up 4 weeks postoperatively through an in-person clinic appointment for endoscopic evaluation.
2.5. Pre-intervention considerations
All patients in this study were deemed fit for surgery by standard pre-operative anaesthetics review. No pre-intervention considerations specific to the use of PuraStat® was required for this study.
2.6. Materials deployed
PuraStat® (3D-Matrix, Tokyo, Japan) is made of a fully-synthetic peptide solution that forms a colorless peptide hydrogel at neutral pH (Fig. 1). The peptide named RADA16 consists of four naturally occurring amino acids repeated four times to create a 16 amino acid poly-peptide chain. Contact between the product and liquid such as blood causes the acidic peptide solution to be neutralized or become alkaline and, as a result, the peptide molecule, which has a β structure, quickly forms fibres in the aqueous solution, yielding a peptide hydrogel. The hydrogel quickly coats the point of bleeding and, by physically closing the superficial part of the broken blood vessel, causes coagulation in the deeper part of the vascular wall resulting in haemostasis ([2], [3], [4]).
Fig. 1.
PuraStat® peptide hydrogel.
In addition, the nano-structure of the 3D-nanofiber structure appears similar to the natural extracellular matrix, which results in adequate adherence of the cells and tissue [3]. Any product remaining after surgery is absorbed over time although some residue may remain for longer than 30 days ([4]).
PuraStat® is indicated for haemostasis in exudative haemorrhage (oozing) from blood vessels and parenchyma of solid organs encountered during surgery when haemostasis by ligation or standard means is insufficient or impractical. It comes in the form of a pre-filled sterile syringe filled with a clear, 2.5% (range 1.8–3.0%) aqueous peptide solution. It doesn’t require any preparation and is stored at refrigerator temperature (from 2 °C to 8 °C). The nozzle has to be connected to the syringe and ideally the first drop should be discarded. PuraStat® is made available in three fill volume variants (1, 3 and 5 mL). The product has no swelling capacity [5].
2.7. Peri-intervention considerations
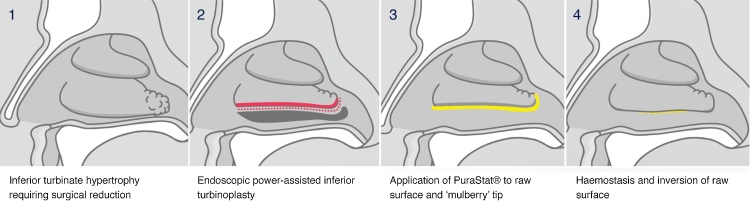
We perform an endoscopic powered turbinoplasty to provide volumetric reduction of the inferior turbinate through removal of the inferior turbinate bone and resection of the lateral lamella of turbinate tissue. The posterior end of the inferior turbinate, which is commonly described as a “mulberry” due to its swollen and variegated appearance, is also reduced. The procedure is quite commonly performed as a day surgery procedure, therefore it is imperative to achieve appropriate haemostasis to discharge the patients on the same day of surgery. However, the inferior turbinate is a significantly vascular structure, particularly in its posterior end and the turbinoplasty involves the creation of a large raw surface which can ooze significantly. Therefore, to avoid nasal packing, we have used PuraStat® to reduce bleeding (Fig. 2).
Fig. 2.
Application of PuraStat® in endoscopic inferior turbinoplasty.
After sub-mucosal tissue resection, any type of severe bleeding should be treated (we regularly use bipolar cautery), and mainly at the end of the resection on the oozing resected area. Application technique is key to effective use of PuraStat®. The applicator tip must be positioned directly at the bleeding point with close supposition to the tissue and a thin and even layer of product has to be applied. A slow application better allows the β-sheets of peptides to self-assemble in contact with blood, form the 3D-matrix and cap the bleeding point. One mL covers a 1 cm2 area hence a 5 mL syringe is used in a bilateral turbinoplasty (2.5 mL per side). One should wait for a minute to check for sufficient haemostasis. In the case of ongoing bleeding, additional PuraStat® can be applied but underneath the initial layer to access the fresh blood, continuing to apply slow and even pressure on the plunger while withdrawing the syringe. In endonasal application, a longer applicator nozzle is required to adequately cover the posterior end of the inferior turbinate. However, the fluidity and transparent nature of the product in such vertical application helps to easily cover the resected area. After application, it is important to not disturb the product (by suction or any kind of compression) as peptides will not re-assemble.
2.8. Proceduralist
All 60 patients were operated on by a Sydney based otolaryngologist sub specialized in rhinology with over 10 years of experience performing endoscopic powered turbinoplasties.
2.8.1. Quality control
All endoscopic powered turbinoplasties were performed in keeping with standard pre-operative and operative procedure. To ensure maximal efficacy of PuraStat®, application of the product was performed strictly using the recommended application technique.
2.8.2. Post-intervention considerations
Our patients were requested to perform daily nasal irrigation starting the day after surgery as per standard post-operative procedure. Visual inspection using a nasal endoscope was performed in routine post-operative followup at 4 weeks.
3. Results
In a 3 month period, we have been using this product in 60 cases of endoscopic powered turbinoplasty meeting our inclusion/exclusion criteria. The use of PuraStat® in endonasal surgery is a novel practice unreported on in the present literature. Application technique has a low difficulty learning curve with effective application usually achieved on the first use. All patients reported compliance with daily nasal irrigation. No re-bleeding was noted in routine post-operative followup at 4 weeks and visual inspection using a nasal endoscope showed normal healing in absence of any adhesion formation in all the patients. No patients were lost to followup (Fig. 3).
Fig. 3.
Video displaying use of PuraStat® in ENT surgery.
4. Discussion
PuraStat® is made of chemically engineered amino acids thus the synthetic origin material can eliminate the potential risk of infections caused by existing hemostatic agents containing blood derivatives.
In cardiovascular surgery, a first uncontrolled prospective study was performed in 2012 in which the product was applied to 33 vascular anastamoses (coronary or peripheral) in 25 heparinised patients. The product under the code name TDM-621 was applied to hemorrhagic bleeding before and after protamine administration. Haemostatic efficacy was 87.9% (29 out of 33 cases) and no postoperative bleeding was observed as well as no severe side effects in relation to the product [6].
In digestive surgery, a clinical controlled study was performed in 2014 in a group of 20 patients undergoing rectal cancer resection. PuraMatrix®, an equivalent product, was applied to 10 patients and paired with 10 conventionally operated patients without a haemostatic adjunct as the control. No abnormal effects were observed in the 10 patients treated 2–3 months postoperatively, and they displayed significantly reduced post-operative drainage to the control group [7]. Additionally in digestive endoscopy, an initial clinical study performed in 2014 involving 12 patients undergoing endoscopic surgery for gastric tumours found haemostasis achieved with TDM-621 to be remarkably effective in 11 patients, effective in 1 patient and globally considered convenient for operability with nil adverse effects noted [8].
Multiple subsequent studies in digestive endoscopy since 2016 have further identified PuraStat® to be effective as an operative haemostatic adjunct in accelerating the healing phase and significantly reducing the rates of delayed bleeding even amongst high-risk bleeding lesions [9], [10], [5], [11].
However, while it’s haemostatic and accelerated healing effects are well documented elsewhere, it’s use in the specialty of endonasal surgery and it’s effects on adhesion formation have yet to be extensively investigated. While many current haemostatic products are used in endonasal surgery with varying rates of efficacy, none have undergone a clinical study demonstrating a zero rate of adhesion formation. Clinical controlled studies will be required to further investigate whether this product has reproducibly low rates of adhesion formation as presently suggested.
5. Conclusion
Multiple clinical studies have been published to date identifying the efficacy of PuraStat® as a haemostatic agent in cardiovascular, digestive and digestive endoscopic surgery. This is the first case series reporting on the haemostatic and anti-adhesive properties of PuraStat® in the setting of endonasal surgery. Our initial results lead us to consider PuraStat® as well suited for use in endonasal surgery but additional clinical controlled studies in humans or animals comparing adhesions rates with alternative haemostatic products and the control are required for further evaluation of the anti-adhesive properties.
Conflicts of interest
None.
Dr Arjuna Ananda is a speaker in an unpaid expert testimony for PuraStat®.
3D-Matrix, the manufacturer of PuraStat®, will be funding an animal study in late 2017/early 2018 to further investigate the effects of PuraStat®, to be conducted by the same three authors as this case series. None of the three authors are employees of 3D-Matrix, and none of the three authors will be paid for this case series or the subsequent animal study.
Funding
None.
Ethical approval
All patients involved in this case series had consented to the use of PuraStat®, which is a licensed haemostatic product in Australia. Monitoring post-operative bleeding and adhesion formation rates is standard procedure for all patients undergoing endonasal surgery. The institutions at which the operations were performed, Royal Prince Alfred Hospital, Strathfield Private Hospital and Campbelltown Private Hospital did not require specific ethical approval for this case series as the operative procedure did not differ from standard operative practice and PuraStat® was already approved for use as a haemostatic product by the surgical department in all three institutions.
Consent
All patients in this case series consented to the use of haemostatic products, such as PuraStat® which is licensed for use in exudative haemorrhage when haemostasis by ligation or standard means is insufficient or impractical, as part of their consent for endonasal surgery. Consent was given by all patients or guardians/next of kin for their deidentified intra-operative and post-operative outcomes to be included in this case series. No images of patients or volunteers were taken for the purposes of this case series.
Author contribution
Dr Michael Fook-Ho Lee: Writing the paper, case series concept and design, diagram illustrator, operative assistant.
Dr Zhiyuan Ma: Secondary author, operative assistant.
Dr Arjuna Ananda: Otolaryngologist primarily responsible for operating and post-operative followup on all patients in this case series.
Dr Maurice Bagot d’Arc: Consultant for advice on case series preparation and application technique for PuraStat.
Guarantor
Dr Michael Fook-Ho Lee, Medical Officer at Royal North Shore Hospital, Sydney, NSW, Australia.
Dr Zhiyuan Ma, Medical Officer at Royal Prince Alfred Hospital, Sydney, NSW, Australia.
Dr Arjuna Ananda, Head of the Ear, Nose and Throat Department at Royal Prince Alfred Hospital, Sydney, NSW, Australia.
Acknowledgment
Dr Maurice Bagot d’Arc is acknowledged for 3D-Matrix consultant for advice and lectures. Present address: BluePharm Consulting, 17 rue Davioud, Paris, 75016, France.
Contributor Information
M.F. Lee, Email: 94michaellee@gmail.com.
Z. Ma, Email: zhi1992@gmail.com.
A. Ananda, Email: info@drananda.com.au.
References
- 1.Agha R.A., Fowler A.J., Rammohan S., Barai I., Orgill D.P., the PROCESS Group The process statement: preferred reporting of case series in surgery. Int. J. Surg. 2016;36(Pt A):319–323. doi: 10.1016/j.ijsu.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 2.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotechnol. 2003;21(10):1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 3.Zhao X., Pan F., Lu J.R. Recent development of peptide self-assembly. Prog. Nat. Sci. 2008;18:653–660. [Google Scholar]
- 4.Uraoka T., Ochiai Y., Fujimoto A., Goto O., Kawahara Y., Kobayashi N., Kanai T., Matsuda S., Kitagawa Y., Yahagi N. A novel fully synthetic and self-assembled peptide solution for endoscopic submucosal dissection-induced ulcer in the stomach. Gastrointest. Endosc. 2016;83(June (6)):1259–1264. doi: 10.1016/j.gie.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Masuhara H., Fujii T., Watanabe Y., Koyama N., Tokuhiro K. Novel infectious agent-free hemostatic material (TDM-621) in cardiovascular surgery. Ann. Thorac. Cardiovasc. Surg. 2012;18(5):444–451. doi: 10.5761/atcs.oa.12.01977. [DOI] [PubMed] [Google Scholar]
- 6.Kondo Y., Nagasaka T., Kobayashi S., Kobayashi N., Fujiwara T. Management of peritoneal effusion by sealing with a self-assembling nanofiber polypeptide following pelvic surgery. Hepatogastroenterology. 2014;61(March-April (130)):349–353. [PubMed] [Google Scholar]
- 7.Yoshida M., Goto N., Kawaguchi M., Koyama H., Kuroda J., Kitahora T., Iwasaki H., Suzuki S., Kataoka M., Takashi F., Kitajima M. Initial clinical trial of a novel hemostat, TDM-621, in the endoscopic treatments of the gastric tumors. J. Gastroenterol. Hepatol. 2014;29(Suppl. 4):77–79. doi: 10.1111/jgh.12798. [DOI] [PubMed] [Google Scholar]
- 8.Pioche M., Camus M., Rivory J., Leblanc S., Lienhart I., Barret M., Chaussade S., Saurin J.C., Prat F., Ponchon T. A self-assembling matrix-forming gel can be easily and safely applied to prevent delayed bleeding after endoscopic resections. Endosc. Int. Open. 2016;4(April (4)):E415–9. doi: 10.1055/s-0042-102879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subramanian S., Chedgy F.J.Q., Kandiah K., Thayalasekaran S., Longcroft-Wheaton G., Bhandari P. The use of a novel extracellular scaffold matrix for haemostasis during endoscopic resection in patients at high risk of bleeding: a little goes a long way. United Eur. Gastroenterol. J. 2016;2(Suppl. 1) [Google Scholar]
- 10.Tsiamouulos Z.P., Rajaratnam R., Sibbons P.D., Saunders B.P. Use of a novel synthetic, topical gel to enhance healing post endoscopic mucosal resection: a randomised, blinded preclinical study. Gastrointest. Endosc. 2017;85(May (5)):AB512. [Google Scholar]
- 11.PuraStat® . PuraStat®; Tokyo, Japan: 2016. Instructions For Use. 3D Matrix Medical Technology. [Google Scholar]