Abstract
Background
IgG concentrations in cerebrospinal fluid generally range from 20 to 45 mg/L. In multiple sclerosis immune reactions lead to intrathecal synthesis of specific IgGs that can be detected in biological fluid samples both quantitatively and qualitatively by isoelectric focusing of supplementary oligoclonal IgG bands.
Method
A simple tool, using the MATLAB application, to facilitate and improve isoelectric focusing profile analysis is presented and evaluated in terms of its sensitivity, repeatability and reproducibility. A comparison between human readers and semi-automatic method has also been performed.
Results
Results from the semi-automatic method were found to be equivalent or superior to generally employed laboratory methods. Repeatability analysis for semi-automatic processing yielded coefficients of variation (CVs) in the 3–7% range, and using a sample with an estimated IgG concentration of 200 mg/L, four bands were still visible after dilution to 5 mg/L, corresponding to band concentrations of 1.1–1.6 mg/L. Discordances between visual inspection and automatic analysis only appear at threshold levels for interpretation (the gray zone).
Conclusion
The semi-automatic method has acceptable performance for routine implementation.
Keywords: Electrophoretogram, Oligoclonal bands, Isoelectric focusing, Repeatability, Sensitivity
1. Introduction
The incidence of Multiple Sclerosis (MS) in France is one per 1000, making it the leading cause of acquired non-traumatic severe disability in young patients. MS involves inflammatory lesions of white matter in the central nervous system (CNS) that spread over time. Positive evidence of MS can be obtained with Magnetic Resonance Imaging (MRI) demonstrating the occurrence of 3 out of 4 Barkhof brain and spinal cord criteria [1]. The combination of two suggestive MRI lesions associated with a positive cerebrospinal fluid (CSF) analysis also indicates disease [1]. Detection of oligoclonal bands (OCBs) in CSF by isoelectric focusing (IEF) is a common diagnostic tool [2].
Chromogenic staining of immunoblots facilitates detection. The higher the concentration of the IgG bands, the greater the intensity of the colors and the more readable the profile, making interpretation easier. Analyzing a profile involves counting the number of visible IgG bands. A profile with at least two IgG bands in CSF which are not present in a serum sample taken at the same time is said to be oligoclonal.
Chromogen interaction with the immunoblot support determines the baseline or background noise. Other forms of noise (white and dark spots [salt and pepper], blots, stain irregularities) may appear on the profile, increasing the chance of misinterpretation for the operator who must distinguish peaks as band intensity versus background and noise.
Patients with MS exhibit a wide range of IgG electrophoretic profiles in CSF samples, and the skill in interpretation consists of distinguishing false-negatives and positives in samples to correctly classify the profile as oligoclonal or not in the presence of background noise. Counting bands is not an easy task and the operator frequently needs help. New technology based on computerized analysis can provide an important tool to supplement human reading. Automated analysis can also determine other properties such as surface area, band location and the intensity of the band. This information may be useful for further studies and patient follow-up.
In the past decade, methods for filtering, segmenting, and detecting gel bands, as well as rectifying geometries, have been described [3], [4]. Major applications and interfaces to fine tune DNA band analysis have been developed [5], [6] based on Gaussian mixture models [7] and wavelet transformation [8]
However, semi-automatic techniques require manual setting of sensitivity thresholds. Problems with grayscale intensity also occur and end-users must often adjust parameters for individual bands.
Other methods automatically filter and smooth grayscale intensities, which can cause true bands to disappear while false bands remain. Blob areas may compute as rectangular markers, decreasing reproducibility. Performance assessment of these kinds of tools and associated interfaces is, to our knowledge, absent from the literature. Use of a DNA Gel Analyzer interface for CSF profiles does not give satisfactory results (Fig. 1). Methods designed to process DNA electrophoresis need to be adapted to be relevant to IgG isoelectric focusing. OCB analysis does not require fine band tuning, but contrast enhancement and elimination of false readings and artefacts.
Fig. 1.
Analysis of a manual ROI CSF profile by GelAnalyser software.
Existing software can, however be adapted to IgG isoelectric focusing. This article briefly describes a tool based on Gaussian adaptation (GA) that we have developed [9] to enhance peak detection. These tools were then assessed (accreditation process) according to good laboratory practice (GLP), in terms of reproducibility [10].
2. Materials and methods
2.1. Semi-automatic image profile processing
IgG isoelectric focusing was performed according to the instructions of the agarose gel and immunoblot membrane (10 cm × 8 cm) supplier (Helena Biosciences Europe, Gateshead, UK). The “IgG IEF Chromogen” contains 3-amino-9-ethyl-carbazole powder that we dissolve in 50 mL of methanol. The “IgG IEF Primary Antibody” contains sheep anti-human IgG antiserum and the “IgG IEF Secondary Antibody” contains anti-sheep IgG- peroxidase conjugate.
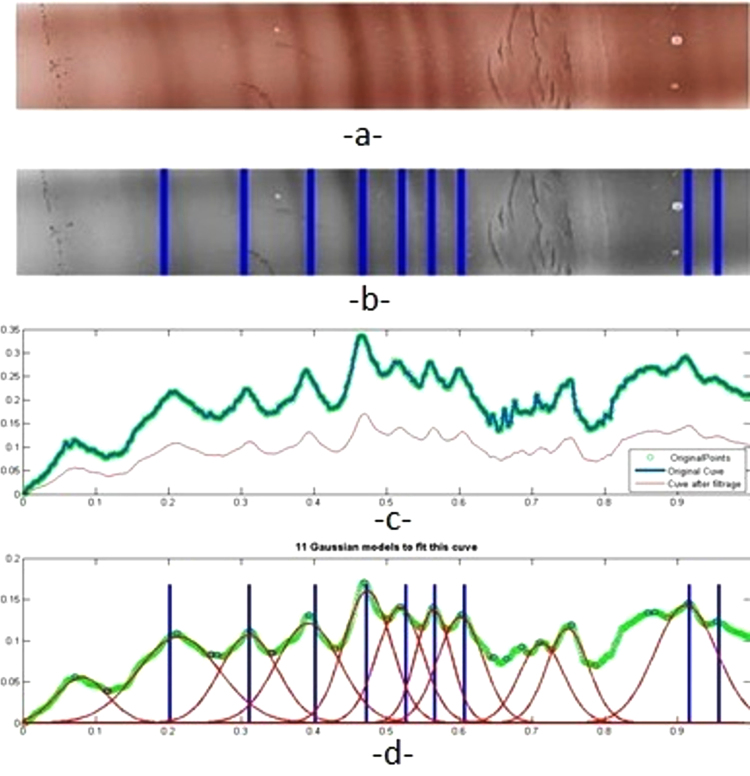
A scanned membrane (Epson V750 PRO scanner [Epson, Levallois-Perret, France], 48 bit color, 3 color values scaled from 0 to 1, 600 dpi) contains ten profiles of different patients. From the 10 profiles you can select one that defines the Region Of Interest (ROI) that forms the basis for our experiments (Fig. 2A). The ROI corresponds to the migration zone of IgG on this type of support.
Fig. 2.
Illustration of the difficulty of automatic interpretation of a 2D image.
Misinterpretations described previously are due to image artefacts like salt and pepper noise (Fig. 2B), blur artefacts and false lanes (Fig. 2A, B), horizontal lane smile deformations, and baseline variations.
Automatic peak detection processing is performed in 3 stages. The first stage involves selection of a rectangular ROI as a 2D image (Fig. 2A,B). On this ROI, a grayscale conversion is performed (Fig. 2C) as well as a 2D image correction by artefact detection and straight-line realignment (Fig. 2D). Finally, the 2D signal is converted to a 1D signal (Fig. 3, Fig. 4). The second stage involves peak detection on the 1D image (Fig. 3, Fig. 4) and the third stage is the location of peaks on the 2D image (Fig. 2E) which can be regarded as a synthesis of the whole process. Each of these steps must be performed to improve peak detection, and we have developed tools for each step which are described in our previous work [9].
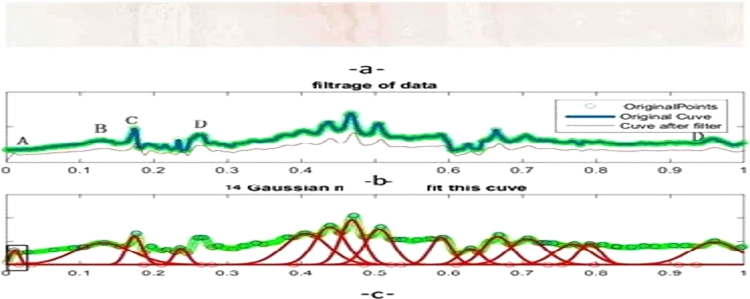
Fig. 3.
Peak detection. (a) a dilute electrophoresis.(b) shows a calculated profile (blue) with a median fitness line (x) computing each column of vertical pixels. (c) red curve is the post filter median curve. Small peaks and troughs have been removed. Thus, if evolution starts at point A with a relatively small variance (the red bell curve), then evolution will take place along the red curve. The process may also yield peaks at B and C, as long as adjacent valleys to the right of these points remain. The red curve before point A is not computed because of noise. From point A, 14 Gaussian models are found. A noise zone occurs within zone D, so D is eliminated. The left peak near to D may be a fail peak (an artefact??), which would need to be evaluated by an expert. The number of Gaussian components is obtained by a simple peak detection algorithm.
Fig. 4.
a) Original scanned color image, b) gray scale image, c) filtered 1D signal, d GA representation.
Because the existing approaches cannot achieve accurate automatic computation, in the present work, we propose an electrophoresis analysis method based on Gaussian Adaptation (GA) [11], [12], [13]. The principle is that we can find a functional Gaussian decomposition, that is to say a set of Gaussian functions whose shape will be adapted at each peak found in the 1D smoothed and filtered signal coming from the electrophoresis profile.
As the GA method is an iterative process, the initial value of the Gaussian characteristics (mean and standard deviation, location) and the stop value (precision of approximation of the shape reconstruction) must be set.
In our case, first and second statistical moment can be calculated provided that the distribution of parameters and image structure is known [Fig. 3].
To set the initial number and characteristics of the Gaussian functions, we compute the number of peaks with a classical function of peak detection available on MATLAB, ‘findPeaks’ (MATLAB, MathWorks, Meudon, France), with the parameter’ MeanPeakdistance’ equal to 25 points. The number of peaks is increased by 20%, because we assume that the error of the Matlab function is not more than 20% for a smoothed and filtered signal. The algorithm is started by using the characteristics (location and width, that is to say, mean and standard deviation) of the first detected peak with the MATLAB function. The final number of Gaussian components is obtained when the detection of two peaks shows no difference with two consecutive parameter settings, e.g. peak width. Post-processing treatment allows us to remove undesirable peaks (Fig. 4-d).
The whole process is illustrated in Fig. 4. The scanned ROI electrophoresis (Fig. 4-a), gray scale of ROI (Fig. 4-b), raw signal (Fig. 4-c, green), the rolling ball baseline and the filtered signal baseline (Fig. 4-c, red) are determined and the number of peaks is counted by the GA algorithm (Fig. 4-d) and can be compared to the original 2D image (Fig. 4a–b).
2.2. Evaluation and comparison of methods
The performance of the semi-automated tool was evaluated by visual assessment and by determining the repeatability (within one gel), detection sensitivity, reproducibility (across different gels) and the comparison between human readers and the semi-automatic method.
The electrophoretic profiles were read with and without magnification by an expert biologist and a laboratory technician. Both were blinded to the other's interpretation and to the clinical context. The sensitivity and specificity of visual assessment were determined.
2.2.1. Sensitivity analysis
According to the manufacturer, a discrete band can be detected when the CSF sample contains at least 0.28 mg/L IgG. To verify this, we started with a pure CSF sample containing 50.87 mg/L IgG according to the nephelometric assay and made 50% serial dilutions across 8 gel lanes, together with one internal quality control. For this sample 10 bands were visible before dilution.
For the computer-assisted analysis, sensitivity was tested by counting the number of peaks and their amplitude and by computing the surface area of the bands using successive dilutions of a sample with an initial IgG concentration of 200 mg/L. Mean and standard deviations were determined together with the CV for all variables.
2.2.2. Repeatability
For isoelectric focusing, we defined repeatability as the requirement for a sample to present the same number of bands of equivalent appearance when tested in the 9 wells of a single gel (with internal quality control in the 10th well). Repeatability was tested on two samples:
-
–
a patient CSF sample containing 50.87 mg/L IgG
-
–
a control sample (Sebia, Lisses, France) containing 201 mg/L IgG
Each sample was applied 9 times on the same gel. The gel profiles were read by two expert biologists and the software was used to count peaks from the same IgG-positive CSF sample used for the repeatability analysis. To assess the repeatability of these tools, we assessed the coefficients of variation (CVs) of the five peaks from five applications displaying the largest surface areas.
2.2.3. Reproducibility or intermediary reliability
The test was performed on successive batches, usually once or twice à day, using daily internal quality control samples. The internal quality control was tested on 30 different gels. The profiles were analyzed by two expert readers and by the semi-automatic method.
2.2.4. Visual inspection vs semi-automated inspection
31 electrophoretic profiles were compared by the two modalities. All profiles compared were from patients suspected of neurodegenerative disease, and none of the patients had detectable oligoclonal bands in the serum. We assessed the concordance between experts and compared the performance of the expert readers and the semi-automatic method.
3. Results
3.1. Manual processing
3.1.1. Repeatability
Repeatability was analyzed on a CSF sample and on a serum sample. On the CSF sample, which is the more difficult to read, we counted 8–10 bands (mean = 9 bands, CV = 9%). On the serum sample with a higher protein content, readers counted 9 times 9 bands and one time 10 bands. There was no difference in the visual reading between readers for each sample analyzed.
3.1.2. Reproducibility
On the control sample, over the 30 daily readings, readers could read either 10 or 11 bands, (CV = 4.72%) and there was no difference in the visual reading between readers for each sample analyzed.
The closeness of the repeatability and intermediary reliability results with CVs less than 10% demonstrated the robustness of the method.
3.1.3. Sensitivity analysis
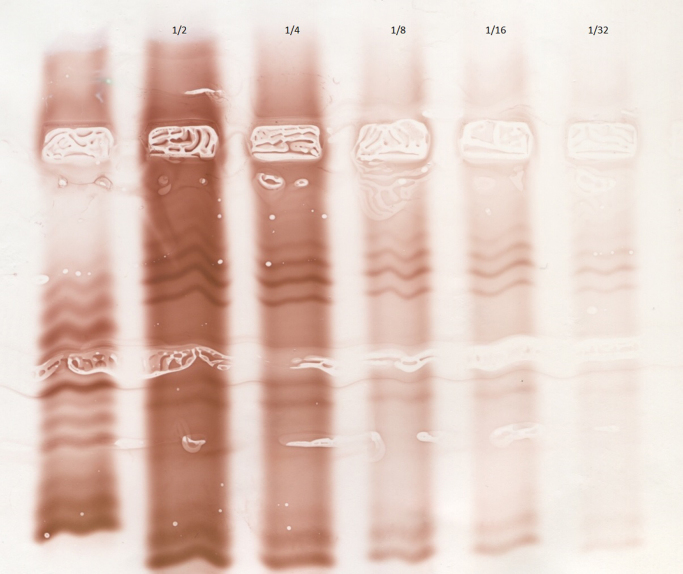
In the final 1/32 dilution, corresponding to 3.15 mg/L IgG, three (or sometimes four) bands were still visible (Fig. 5). The visibility of the bands can be further improved by increasing the time of chromogen contact (20 min instead of 10), but with the risk of increasing artefact images.
Fig. 5.
Sensitivity analysis. Three bands (defining the oligoclonal profile) are still visible on the 1/32 dilution (3.5 mg/L IgG).
3.2. Semi-automatic processing
3.2.1. Repeatability
A serum sample was used for analysis and five or more bands were visible. Repeatability was determined for the five most readily visible bands among the 10 identified in the profile. Semi-automatic processing yielded CVs in the 3–7% range, demonstrating the method's precision (Table 1)
Table 1.
Surface area of the five bands used for the repeatability test of five applications.
| Band | Surface area (mm2) |
CV (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Application 1 | Application 2 | Application 3 | Application 4 | Application 5 | Mean | SD | ||
| 1 | 5.41 | 5.43 | 5.74 | 5.78 | 5.64 | 5.60 | 0.17 | 3.03 |
| 2 | 6.30 | 5.53 | 5.75 | 5.88 | 5.79 | 5.85 | 0.28 | 4.79 |
| 3 | 6.38 | 5.48 | 5.84 | 6.01 | 6.02 | 5.95 | 0.33 | 5.55 |
| 4 | 6.52 | 5.68 | 5.90 | 6.30 | 5.54 | 5.99 | 0.41 | 6.84 |
| 5 | 5.60 | 5.92 | 4.95 | 5.52 | 5.12 | 5.42 | 0.39 | 7.1 |
3.2.2. Sensitivity analysis
Using a sample with an estimated IgG concentration of 200 mg/L IgG, four bands were still visible after dilution to 5 mg/L, corresponding to a relative concentration in the bands of 1.1–1.6 mg/L. Dilution proportionality remained valid up to 1/20 dilution but was no longer valid at 1/40.
Like the visual analysis, four bands were also detected by semi-automatic analysis, and this method can also quantify the peak surface area for each band (Table 2). Since the background noise concentration is not taken into account in our calculations, the concentrations shown in Table 2 are partially overestimated, suggesting that the analytical sensitivity is probably better than the values stated above.
Table 2.
Location and relative concentration (expressed as mg/L) of oligoclonal bands. Relative concentration was determined from the initial concentration (band surface area/total band surface area) for each dilution.
| IgG concentration (mg/L) | 200 |
100 |
50 |
20 |
10 |
5 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bands | Location (pixels) | Relative concentration | Bands | Relative concentration | Bands | Relative concentration | Bands | Relative concentration | Bands | Relative concentration | Bands | Relative concentration |
| 1 | 46 | 17,52 | 1 | 9,01 | 1 | 3,08 | 1 | 1,52 | 1 | 1,1 | 1 | 1,1 |
| 2 | 79 | 19,12 | 2 | 10,49 | 2 | 5,69 | 2 | 2,37 | 2 | 1,89 | 2 | 1,54 |
| 3 | 112 | 20,88 | 3 | 12,14 | 3 | 10,48 | 3 | 3,99 | 3 | 2,63 | 3 | 1,10 |
| 4 | 147 | 22,06 | 4 | 10,98 | 4 | 7,91 | 4 | 3,29 | 4 | 1,87 | ||
| 5 | 333 | 21,66 | 5 | 10,7 | 5 | 4,78 | 5 | 2,31 | 5 | 1,52 | 5 | 1,24 |
| 6 | 364 | 16,28 | 6 | 7,49 | ||||||||
| 7 | 404 | 15,68 | 7 | 7,49 | 7 | 1,35 | 7 | 1,04 | ||||
| 8 | 424 | 15,02 | 8 | 7,41 | ||||||||
| 9 | 569 | 21,38 | 9 | 10,46 | 9 | 5,54 | 9 | 1,85 | 9 | 0,97 | ||
| 10 | 611 | 26,36 | 10 | 13,79 | 10 | 11,13 | 10 | 3,58 | ||||
| 11 | 647 | 3,92 | ||||||||||
3.3. Method comparison
For the 31 electrophoretic profiles from patients, 16 of them were deemed to be oligoclonal (3 bands or more) and 15 were deemed non-oligoclonal. The concordance between the readers approaches 100%: both readers classified the same samples as oligoclonal or non-oligoclonal, with a maximum difference of 1 band (5 samples). None of the corresponding serum samples exhibited an oligoclonal profile.
The comparison between visual reading and automatic reading was: 24/31 profiles were concordant, 4 visually oligoclonal profiles were said to be said non-oligoclonal by the automatic inspection and 3 visually non-oligoclonal profiles were said to be oligoclonal by the automatic method.
For the 3 visually non oligoclonal profiles, in two cases, the experts counted 2 bands vs 3 bands for the automatic method. In one case the experts give 0 bands vs 3 for the automated method.
For the four visually oligoclonal profiles, the experts counted 3 or 4 bands vs 2 bands for the automated method.
These 7 cases are the gray area of interpretation. Some experts consider 2 or more bands in the CSF to be a positive oligoclonal pattern. If this criterion is adopted, six of the seven discordant cases become concordant.
4. Discussion
We used a serum sample to determine the reproducibility of the procedure. No target CVs or repeatability and reproducibility data are currently available in the literature for comparison [14].
The software exhibited satisfactory repeatability, yielding CVs below 10% (mean 5.46%) in all cases, illustrating the robustness of the method.
The sensitivity analysis was also satisfactory since at the concentration limit for band visibility (5 mg/L) 4 of the 11 bands seen at a concentration of 200 mg/L remained visible, a sufficient number to determine the oligoclonal profile. This level of sensitivity could be used to develop the isoelectric focusing technique to other biological fluids such as tears where the IgG concentration is in the 20–30 mg/L range. Concentration proportionality was valid up to the 1/20 dilution but not at 1/40, although the oligoclonal profile remained identifiable. Improvement in the tool's discriminatory power should resolve this problem.
Discordances appear at the threshold level of interpretation (3 bands to qualify a profile as oligoclonal). For simple situations (outside the gray zone) the comparison is very satisfactory. In the gray zone an algorithm to improve the levels of contrast in automatic reading should be used [15]
It was also noteworthy that the number of peaks detected by the software declined proportionally with protein content, suggesting that the supplementary peaks detected by the software were not necessarily artefacts but perhaps bands that were undetectable visually. The challenge will thus be to determine the minimal peak surface area that can confirm the presence of a true OCB and rule out an artefact. For this, the system will have to be “trained”. Currently, the algorithm is so precise that it detects even very small peaks, even peaks undetectable visually. This needs to be balanced against the risk of detecting undesirable peaks.
The good performance of the tools in terms of repeatability and sensitivity suggests a promising application for the software. Automatic recognition of artefacts will contribute to the quality of the results. Generally, the automated method detects more peaks than visual analysis. This larger number of peaks can be explained in part by identification of artifacts as peaks and in part by the threshold set (theoretically, based on the recognition of the image by the human eye) for distinguishing between a band and background noise. The definition of this threshold can be further improved by including other factors such as the lighting angle and the overall profile that an expert uses for the visual analysis.
Routine use of a computer-assisted analysis technique for semi-automatic processing of CSF gel profiles may require changes in laboratory practices to allow co-existence of visual and automatic analysis according to revalidation under ISO standards. To our knowledge, there is no routine assisted-reading application for isoelectric focusing of CSF and serum in MS, in contrast to electrophoresis of blood proteins.
The main obstacle for identifying OCBs in CSF is the requirement for a lumbar puncture to obtain a sample. Lumbar puncture is an invasive technique that can be painful for the patient and is generally not performed during an outpatient visit. As tears are a more accessible and less complex body fluid and sampling is much less invasive, our team has started research on analysis for oligoclonal profiles in tears [16], [17], [18]. Furthermore, the concentrations of IgG studied during the dilution tests in this work correspond to those found in the analysis of the tear profiles. To evaluate the true analytical sensitivity of our method it would be necessary to subtract the background noise and estimate the protein content of this background noise. However, the results of the dilution study indicating the possibility of identifying bands with concentrations close to 1.5 mg/L, suggest that the technique of isoelectric focusing technique with semi-automated reading could be applicable to tears if very specific image processing tools are developed to deal with the very minimal contrast seen in tear sample profiles. This might avoid some of the lumbar punctures needed for the diagnosis of MS, in that it might be possible to limit lumbar punctures to patients who have had a negative result on their tear sample.
Acknowledgements
We thank Christophe Herlin for his technical support. This work was supported by the French “Programme Hospitalier de Recherche Clinique” (No NCT02043964)
Contributor Information
G. Forzy, Email: forzy.gerard@ghicl.net.
L. Peyrodie, Email: laurent.peyrodie@yncrea.fr.
References
- 1.Polman C.H., Reingold S.C., Banwell B., Clanet M., Cohen J.A., Filippi M. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freedman M.S., Thompson E.J., Deisenhammer F., Giovannoni G., Grimsley G., Keir G. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch. Neurol. 2005;62(6):865–870. doi: 10.1001/archneur.62.6.865. [DOI] [PubMed] [Google Scholar]
- 3.Kaabouch N., Schultz Richard R. A 2-D gel electrophoresis DNA image analysis algorithm with automatic thresholding. Int. Soc. Opt. Eng. Proc. (SPIE) 2007;6508 (65081H-1 to 65081H-12) [Google Scholar]
- 4.Adiga P.S., Bhornra A., Turri M.G., Nicod A., Datta S.R., Jeavons P. Automatic analysis of agarose gel images. Bioinformatics. 2001;17:1084–1090. doi: 10.1093/bioinformatics/17.11.1084. [DOI] [PubMed] [Google Scholar]
- 5.Bajla I., Hollander L., Fluch S., Burg K., Kollar M. An alternative method for electrophoretic gel image analysis in the GelMaster software. Comput. Methods Prog. Biomed. 2005;77:209–231. doi: 10.1016/j.cmpb.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 6.I. Lazar, GelAnalyzer: Freeware 1D gel electrophoresis image analysis software, 2010. Available online: 〈http://www.gelanalyzer.com〉 (Accessed 27 October 2017).
- 7.Polanski A., Marczyk M., Pietrowska M., Widlak P., Polanska J. Signal partitioning algorithm for highly efficient gaussian mixture modeling in mass spectrometry. PLoS One. 2015;10(7):1–19. doi: 10.1371/journal.pone.0134256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreira B., Sousa A., Mendonça A., Campilho A. Automatic lane segmentation in TLC images using the continuous wavelet transform. Comput. Math. Methods Med. 2013;2013:1–19. doi: 10.1155/2013/218415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boudet S., Peyrodie L., Wang Z., Forzy G. Semi-automated image analysis of gel electrophoresis of cerebrospinal fluid for oligoclonal band detection. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2016;8:744–747. doi: 10.1109/EMBC.2016.7590809. [DOI] [PubMed] [Google Scholar]
- 10.Espiño M., Abraira V., Arroyo R., Bau L., Cámara C., Campos-Ruiz L. Assessment of the reproducibility of oligoclonal IgM band detection for its application in daily clinical practice. Clin. Chim. Acta. 2015;438:67–69. doi: 10.1016/j.cca.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Kjellström G., Taxén L. Stochastic Optimization in System Design. IEEE Trans. Circ. Syst. CAS. 1981;28(7):702–715. [Google Scholar]
- 12.Kjellström G. Evolution as a statistical optimization algorithm. Evolut. Theory. 1996;11:105–117. [Google Scholar]
- 13.Kjellström G. On the efficiency of gaussian adaptation. J. Optim. Theory Appl. 1991;71:589–597. [Google Scholar]
- 14.Vassault A., Grafmeyer D., de Graeve J., Cohen R., Beaudonnet A., Bienvenu J. Quality specifications and allowable standards for validation of methods used in clinical biochemistry. Ann. Biol. Clin. 1999;57:685. [PubMed] [Google Scholar]
- 15.Otsu N., Threshold A. Selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979;9:62–66. [Google Scholar]
- 16.Devos D., Forzy G., de Seze J., Caillez S., Louchart P., Gallois P., Hautecoeur P. Silver stained isoelectrophoresis of tears and cerebrospinal fluid in multiple sclerosis. J. Neurol. 2001;248(8):672–675. doi: 10.1007/pl00007833. [DOI] [PubMed] [Google Scholar]
- 17.Calais G., Forzy G., Crinquette C., Mackowiak A., de Seze J., Blanc F. Tears analysis in clinically isolated syndrome as new multiple sclerosis criterion. Mult. Scler. 2010;16(1):87–92. doi: 10.1177/1352458509352195. [DOI] [PubMed] [Google Scholar]
- 18.Lebrun C., Forzy G., Collongues N., Cohen M., de Seze J., Hautecoeur P. Tear analysis as a tool to detect oligoclonal bands in radiologically isolated syndrome. Rev. Neurol. 2015;171(4):390–393. doi: 10.1016/j.neurol.2014.11.007. [DOI] [PubMed] [Google Scholar]