Abstract
Key points
Autologous cardiac progenitor cell (CPC) therapy is a promising approach for treatment of heart failure (HF). There is an unmet need to identify inherent deficits in aged/diseased human CPCs (hCPCs) derived from HF patients in the attempts to augment their regenerative capacity prior to use in the clinical setting.
Here we report significant functional correlations between phenotypic properties of hCPCs isolated from cardiac biopsies of HF patients, clinical parameters of patients and expression of the P2Y14 purinergic receptor (P2Y14R), a crucial detector for extracellular UDP‐sugars released during injury/stress.
P2Y14R is downregulated in hCPCs derived from HF patients with lower ejection fraction or diagnosed with diabetes.
Augmenting P2Y14R expression levels in aged/diseased hCPCs antagonizes senescence and improves functional responses.
This study introduces purinergic signalling modulation as a potential strategy to rejuvenate and improve phenotypic characteristics of aged/functionally compromised hCPCs prior to transplantation in HF patients.
Abstract
Autologous cardiac progenitor cell therapy is a promising alternative approach to current inefficient therapies for heart failure (HF). However, ex vivo expansion and pharmacological/genetic modification of human cardiac progenitor cells (hCPCs) are necessary interventions to rejuvenate aged/diseased cells and improve their regenerative capacities. This study was designed to assess the potential of improving hCPC functional capacity by targeting the P2Y14 purinergic receptor (P2Y14R), which has been previously reported to induce regenerative and anti‐senescence responses in a variety of experimental models. c‐Kit+ hCPCs were isolated from cardiac biopsies of multiple HF patients undergoing left ventricular assist device implantation surgery. Significant correlations existed between the expression of P2Y14R in hCPCs and clinical parameters of HF patients. P2Y14R was downregulated in hCPCs derived from patients with a relatively lower ejection fraction and patients diagnosed with diabetes. hCPC lines with lower P2Y14R expression did not respond to P2Y14R agonist UDP‐glucose (UDP‐Glu) while hCPCs with higher P2Y14R expression showed enhanced proliferation in response to UDP‐Glu stimulation. Mechanistically, UDP‐Glu stimulation enhanced the activation of canonical growth signalling pathways ERK1/2 and AKT. Restoring P2Y14R expression levels in functionally compromised hCPCs via lentiviral‐mediated overexpression improved proliferation, migration and survival under stress stimuli. Additionally, P2Y14R overexpression reversed senescence‐associated morphology and reduced levels of molecular markers of senescence p16INK4a, p53, p21 and mitochondrial reactive oxygen species. Findings from this study unveil novel biological roles of the UDP‐sugar receptor P2Y14 in hCPCs and suggest purinergic signalling modulation as a promising strategy to improve phenotypic properties of functionally impaired hCPCs.
Keywords: cardiac progenitor cells, extracellular UDP‐sugars, P2Y14R, cellular senescence
Key points
Autologous cardiac progenitor cell (CPC) therapy is a promising approach for treatment of heart failure (HF). There is an unmet need to identify inherent deficits in aged/diseased human CPCs (hCPCs) derived from HF patients in the attempts to augment their regenerative capacity prior to use in the clinical setting.
Here we report significant functional correlations between phenotypic properties of hCPCs isolated from cardiac biopsies of HF patients, clinical parameters of patients and expression of the P2Y14 purinergic receptor (P2Y14R), a crucial detector for extracellular UDP‐sugars released during injury/stress.
P2Y14R is downregulated in hCPCs derived from HF patients with lower ejection fraction or diagnosed with diabetes.
Augmenting P2Y14R expression levels in aged/diseased hCPCs antagonizes senescence and improves functional responses.
This study introduces purinergic signalling modulation as a potential strategy to rejuvenate and improve phenotypic characteristics of aged/functionally compromised hCPCs prior to transplantation in HF patients.
Abbreviations
- CPCs
cardiac progenitor cells
- EF
ejection fraction
- hCPCs
human CPCs
- HF
heart failure
- HSCs
haematopoietic stem cells
- LVAD
left ventricular assist device
- mGFP
monomeric green fluorescent protein
- MOI
multiplicity of infection
- P2Y14R
P2Y14 purinergic receptor
- ROS
reactive oxygen species
Introduction
Heart failure (HF) remains a major cause of death worldwide due to current inefficient therapies. Autologous stem cell therapy holds promise for mediating cardiac repair following injury. c‐Kit+ cardiac‐derived progenitor cells (CPCs) have proven efficacy in improving cardiac function following myocardial infarction in small animal models (Beltrami et al. 2003; Ellison et al. 2013). The Stem Cell Infusion in Patients with Ischemic Cardiomyopathy (SCIPIO) clinical trial using autologous c‐Kit+ CPCs (hCPCs) validated the feasibility and safety of this approach (Bolli et al. 2011; Chugh et al. 2012). However, hCPCs from multiple patients exhibit varying growth rates and senescence features, which possibly accounts for the modest and inconsistent therapeutic outcomes observed in the clinical setting. Hence, there is an unmet need to identify the molecular components implicated in rejuvenating hCPCs and enhancing their regenerative capacity towards an improved therapeutic outcome in HF patients.
Stem cell capacity to detect stress‐induced extracellular signals is critical for initiating regenerative responses following injury. In this regard, it is necessary to understand the molecular mechanism regulating purinergic receptor signalling mediated by extracellular nucleotides released during injury/stress. This study focuses on extracellular UDP‐conjugated sugars and their membrane receptor P2Y14 (P2Y14R).
UDP‐conjugated sugars such as UDP‐glucose (UDP‐Glu) are synthesized in the cytosol then translocated into the lumen of endoplasmic reticulum and Golgi apparatus where they regulate carbohydrate synthesis and mediate glycosylation reactions by serving as donor substrates (Abeijon & Hirschberg, 1992; Berninsone & Hirschberg, 1998). In addition to their established intracellular roles, UDP‐sugars are secreted from the cell where they act as extracellular signalling molecules (Kreda et al. 2007, 2008). UDP‐Glu is usually released at lower rates than other nucleotides such as ATP, but hydrolysed at a much slower rate, which results in higher steady‐state levels of UDP‐Glu in the extracellular compartment (Lazarowski et al. 2003). As an extracellular signalling molecule, UDP‐Glu binds to and activates the ubiquitously expressed Gαi protein‐coupled P2Y14R, also known as GPR105. Since its initial characterization over 15 years ago (Chambers et al. 2000), an increasing body of literature has described diverse physiological responses downstream of P2Y14R in a variety of tissues. P2Y14R regulates cell proliferation (Scrivens & Dickenson, 2005), migration (Jokela et al. 2014) and maturation (Skelton et al. 2003). Moreover, UDP‐Glu triggers chemotaxis of macrophages (Xu et al. 2012), neutrophils (Sesma et al. 2012; Barrett et al. 2013) and a subset of haematopoietic stem cells (HSCs) (Kook et al. 2013) in a P2Y14R‐dependent manner. Furthermore, HSCs isolated from P2Y14R knockout mice show impaired capability of restoring hematopoiesis in irradiated mice compared to HSCs derived from wild‐type mice, indicating an indispensible role of P2Y14R in promoting regenerative responses after injury (Cho et al. 2014).
Impaired regenerative capacity of P2Y14R‐deficient HSCs was coupled with increased susceptibility to senescence in response to various stress stimuli, including chemotherapy, irradiation, environmental stresses and ageing (Cho et al. 2014). Induction of cellular senescence is regulated by an intricate signalling network that involves activation of p16INK4a, p53 and p21 signalling pathways along with generation of high levels of reactive oxygen species (ROS) (Ziegler et al. 2015). Interestingly, P2Y14R deletion in HSCs resulted in upregulation of molecular markers of senescence, including p16INK4a and ROS (Cho et al. 2014). These results highlight a potential role of P2Y14R in antagonizing stem cell senescence. Here, we hypothesize that P2Y14R mediates similar pro‐regenerative and anti‐senescence responses in hCPCs derived from cardiac biopsies of HF patients.
The present study was designed to assess correlations between P2Y14R expression, hCPC phenotypic characteristics and clinical parameters of patients from which hCPCs were derived. These results were extended by testing the effect of P2Y14R activation or overexpression upon improving hCPC functional responses and reversing senescence‐associated phenotypes. Findings from this study are discussed in the context of augmenting hCPC phenotypic properties ex vivo via pharmacological or genetic engineering approaches.
Methods
Ethical approval
Human tissue specimens used in this proposal are derived from heart failure patients undergoing left ventricular assist device (LVAD) implantation surgeries. These tissue samples are routine discards from the surgical procedure. There is no additional risk to the patient as the samples are thrown away if not used and would need to be harvested as part of the procedure. Details relating to patient involvement and consent are being conducted and approved by the Sharp Hospital where the samples are acquired (IRB #120686). The samples provided for use in this proposal are de‐identified and of no potential therapeutic or diagnostic value to the patients. Therefore, these samples are considered non‐human subjects research working with cell lines procured as part of the Sussman lab environment and available for use in relevant studies involving the biology of human cardiac stem cells.
Human cardiac progenitor cell isolation and culture
Human CPCs (hCPCs) (Table 1) were isolated from cardiac biopsies of heart failure patients undergoing LVAD implantation surgeries as previously described (Mohsin et al. 2013). Briefly, tissue was minced and digested in collagenase (150 U mg mL−1) (Worthington Biochemical Corporation; Lakewood, NJ, USA) at 37°C for 2 h. Digested tissue was sorted with c‐Kit‐labelled beads (Miltenyi Biotec; San Diego, CA, USA) according to the manufacturer's protocol. Pelleted c‐Kit+ cells were cultured and expanded in hCPC growth media composed of Ham's F12 (Fisher Scientific; Hampton, NH, USA), 10% embryonic stem cell screened fetal bovine serum (ES FBS), 5 mU mL−1 human erythropoietin (Sigma Aldrich; St. Louis, MO, USA), 10 ng mL−1 basic fibroblast growth factor (Peprotech; Rocky Hill, NJ, USA) and 1% penicillin/streptomycin/glutamine (PSG), and incubated in a humidified atmosphere of 5% CO2 and 95% air at 37°C.
Table 1.
Clinical profile of patients used for stem cell isolation
| Patient ID | Age (years) | Sex | EF (%) | Cardiac index | Diabetes | Hyperchole‐sterolaemia | Smoking | Infarct | Ischaemia | Ace inhibitor | β‐Blocker | Anticoagulant | NYHA score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H10‐004 | 81 | M | 8% | 2.0 | √ | √ | √ | √ | √ | √ | X | √ | IV |
| H12‐047 | 72 | M | 8% | 1.1 | √ | X | X | X | X | X | X | √ | IV |
| H10‐001 | 68 | M | 11% | 1.6 | √ | X | X | X | X | √ | X | √ | IV |
| H12‐053 | 59 | M | 15% | 1.7 | √ | √ | X | √ | √ | √ | √ | √ | IV |
| H10‐014 | 73 | M | 17% | 1.6 | X | √ | √ | − | √ | X | X | √ | IV |
| H11‐020 | 68 | F | 20% | 1.7 | X | X | X | X | X | X | X | √ | IV |
| H13‐061 | 65 | M | 20% | 1.6 | X | X | X | √ | X | X | √ | √ | |
| H13‐070 | 42 | M | 20% | 1.5 | X | X | X | X | X | X | √ | √ | IV |
| H13‐065 | 54 | M | — | — | — | — | — | — | — | — | — | — | — |
EF: ejection fraction; NYHA: New York Heart Association; patient information: (√) positive; (X) negative; (—) unavailable.
Real‐time reverse transcriptase polymerase chain reaction
Total RNA isolation from cultured hCPCs and cDNA synthesis were performed using Quick‐RNA MiniPrep (Zymo Research; Irvine, CA, USA) and iScript cDNA Synthesis Kit (Bio‐Rad; Hercules, CA, USA), respectively, according to manufacturers’ protocols. iQ SYBER Green (Bio‐Rad) was used to prepare samples for quantitative real‐time polymerase chain reaction (qRT‐PCR). Primer sequences are listed in Table 2.
Table 2.
List of qRT‐PCR primers
Cell proliferation assay
hCPC proliferation was assessed using CyQuant fluorescent nucleic acid‐based dye (Life Technologies; Carlsbad, CA, USA) according to manufacturer's protocol. CyQuant dye labels live cells where the fluorescence intensity directly correlates with cell number. In agonist stimulation experiments, hCPCs were seeded in serum‐starved medium (Ham's F12 medium supplemented with 2.5% ES FBS) in a 96‐well plate (500 cells well−1) and then treated with CyQuant reagent for 1 h; fluorescence intensity was measured using a plate reader to record baseline (day 0) readings. Cells were treated with or without UDP‐glucose (100 μm) (Sigma Aldrich) and after 24 h CyQuant dye was added and the day 1 reading recorded. In overexpression experiments, hCPC proliferation was assessed 5 days after culture in growth medium using haemocytometer counting.
Cell migration assay
hCPC single‐cell suspensions were cultured in a 96‐well plate coated with growth factor reduced (GFR) Matrigel (BD Biosciences; San Jose, CA, USA) (1600 cells well−1) in serum‐free Ham's F12 medium and incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air. After 2 h, the cell culture plate was mounted on a DMI6000 Leica time‐lapse live imaging microscope equipped with a digital camera, an automatic shutter, a motorized x–y stage and an OKO stage top incubator to maintain 37°C, 5% CO2 and 95% air throughout the duration of the experiment. Bright field images of the selected fields were collected with a 5× objective every 30 min for 2 h. Cell migration was assessed by measuring the distance that cells travelled from origin using Leica LAX software. Cell velocity was calculated by dividing distance travelled from origin over time.
Cell death assay
hCPCs were cultured in a 6‐well plate (30,000 cells well−1) in growth medium overnight. The next day, cells were subjected to either serum‐free medium for 48 h or H2O2 (300 μm) (Sigma Aldrich) in serum‐free medium for 24 h. After the indicated times, cells were resuspended in 700 μl Annexin V binding buffer (BD Pharmingen; Franklin Lakes, NJ, USA). Cells were stained with Annexin V (BD Pharmingen) for 10 min to detect apoptotic cells. Cells were pelleted and resuspended in 300 μl Annexin V binding buffer to minimize non‐specific binding. Then cells were stained with propidium iodide (PI) (Invitrogen; Carlsbad, CA, USA) for 1 min to detect necrotic cells. The percentage of apoptotic/necrotic cells was measured using flow cytometric analysis.
Morphometric analysis
Bright field images of cultured hCPCs were obtained using a Leica DMIL inverted tissue culture phase contrast microscope (10× magnification). Cell morphology was assessed by measuring Length‐to‐Width ratio (L/W = Feret/min Feret), Area and Roundness using ImageJ software.
MitoSOX staining
To detect levels of mitochondrial ROS, hCPCs were labelled with MitoSOX Red (ThermoFisher; Waltham, MA, USA) according to manufacturer's protocol. Briefly, hCPCs were cultured in a six‐well plate (30 cells well−1) overnight then loaded with 5 μm MitoSOX reagent and incubated at 37°C, 5% CO2 and 95% air for 10 min. Cells were washed with PBS, detached using cell stripper/TrypLE (1:1), pelleted and resuspended in fresh growth medium. MitoSOX mean fluorescence intensity (MFI) was assessed using flow cytometry.
Protein isolation, SDS‐PAGE and immunoblot analysis
hCPCs cultured in a six‐well plate were collected in 50 μL of sample buffer, sonicated and boiled. Protein lysates were run on 4–12% NuPage Novex Bis Tris gel (Invitrogen), transferred onto a polyvinylidene fluoride (PVDF) membrane and blocked in Odyssey blocking buffer for 1 h at room temperature. Blocked membranes were incubated with primary antibodies at 4°C overnight, then washed several times in Tris‐buffered saline Tween‐20 solution (TBST) and incubated with secondary antibodies (1:5000) for 1 h at room temperature. Fluorescence signal was detected using an Odyssey imaging scanner and quantified using ImageJ software (Amersham Biosciences; Little Chalfont, UK). Antibodies used are listed in Table 3.
Table 3.
List of antibodies
| Antibody | Vendor | Catalogue no. | Immunoblotting dilution |
|---|---|---|---|
| pERK1/2Thr202/Tyr204 | CST (Danvers, MA, USA) | 9101 | 1:500 |
| ERK1/2 | CST | 9107 | 1:500 |
| pAKTSer473 | CST | 9271 | 1:100 |
| AKT1/2 | Santa Cruz Biotechnology (Dallas, TX, USA) | sc‐1619 | 1:500 |
| GPR105 (P2Y14R) | Invitrogen | PA5‐34087 | 1:500 |
| p16INK4A | R&D Systems (Minneapolis, MN, USA) | AF5779 | 1 μg mL−1 |
| p53 | Santa Cruz Biotechnology | FL‐393 | 1:500 |
| p21 | R&D Systems | AF1047 | 0.5 μg mL−1 |
| GAPDH | SICGEN (Cantanhede, Portugal) | AB0067‐200 | 1:2000 |
CST, Cell Signaling Technology; GAPDH: glyceraldehyde‐3‐phosphate dehydrogenase (loading control).
Lentiviral‐mediated transduction of human cardiac progenitor cells
hCPCs were seeded in a six‐well plate (30,000 cells well−1) overnight then transduced with lentiviral particles encoding monomeric green fluorescent protein (multiplicity of infection (MOI) 2; hCPC‐mGFP) or P2Y14R fused to mGFP (MOI 0.2; hCPC‐Y14) (lentiviral plasmids were purchased from Origene (Rockville, MD, USA), P2Y14R‐mGFP plasmid SKU: RC224391L2). P2Y14R overexpression was performed in three representative hCPC lines: H10‐001, H12‐053 and H13‐065.
Statistical analysis
Quantified data are presented as the means ± SEM of data from at least three experiments. Statistical analysis was performed using a two‐tailed Student's t test or Pearson correlation analysis, as indicated, where P < 0.05 represents a significant difference (GraphPad Prism software version 5.0; La Jolla, CA, USA).
Results
Differential expression of P2Y14R in hCPCs isolated from multiple heart failure patients
Correlations between P2Y14R expression in hCPCs and clinical parameters of patients were assessed. P2Y14R expression in hCPCs directly correlated with ejection fraction (EF) of patients from which hCPCs were derived where lower EF corresponded to lower P2Y14R expression, i.e. higher normalized cycle number (R 2 = −0.8833, P = 0.0036) (Fig. 1 A). Diabetes, a common comorbidity in HF patients, reduces number and functional capacity of CPC population (Leonardini & Avogaro, 2013). Interestingly, P2Y14R expression was significantly downregulated in hCPCs isolated from cardiac biopsies of diabetic HF patients compared to non‐diabetic control group (normalized cycle number in diabetic group 25.94 ± 1.26 and non‐diabetic group 21.81 ± 0.73, P = 0.0291) (Fig. 1 B). The impact of P2Y14R activation with its agonist UDP‐glucose (UDP‐Glu) was assessed in multiple hCPC lines. hCPCs with lower P2Y14R expression derived from diabetic HF patients with lower EF did not show increased proliferation in response to UDP‐Glu stimulation (H10‐001: 1.086 ± 0.065 fold change, P = 0.2230; H12‐053: 0.997 ± 0.068 fold change, P = 0.2173) (Fig. 1 C). In contrast, proliferation of hCPC lines with higher P2Y14R expression isolated from non‐diabetic HF patients with relatively higher EF was enhanced following UDP‐Glu treatment (Fig. 1 C) (H13‐061: 1.498 ± 0.133 fold change, P = 0.0333; H13‐070: 1.803 ± 0.057 fold change, P = 0.0008). Collectively, these findings show significant correlations between clinical parameters of HF patients and expression/functional response of P2Y14R in hCPCs.
Figure 1. Differential expression of P2Y14 receptor in human CPCs isolated from multiple heart failure patients.
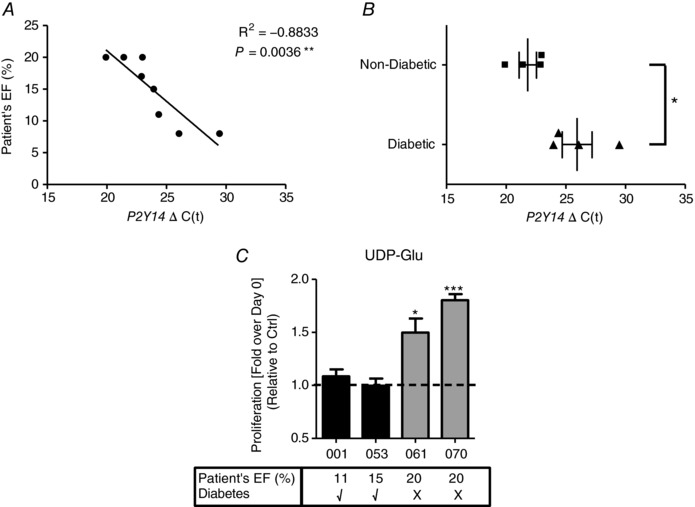
A, expression of P2Y14 receptor mRNA in hCPCs isolated from cardiac biopsies of heart failure (HF) patients by qRT‐PCR analysis plotted against patients’ ejection fraction (EF) showing a significant correlation (Pearson R 2 = −0.8833) where low EF corresponds to low P2Y14 receptor expression (high cycle number [ΔC(t)]). B, P2Y14 mRNA expression is also significantly downregulated in hCPCs isolated from HF patients diagnosed with diabetes mellitus. Cycle numbers were normalized to 18S and data are represented relative to hCPCs from non‐diabetic patients. * P < 0.05 indicates significant difference as measured by unpaired Student's t test. C, hCPCs derived from multiple HF patients showed differential response to P2Y14R agonist UDP‐glucose (UDP‐Glu). Each bar represents a hCPC line with corresponding clinical information in the table below. While hCPCs isolated from diabetic HF patients with low EF did not respond to UDP‐Glu, hCPCs from non‐diabetic patients with relatively higher EF exhibited enhanced proliferation following UDP‐Glu treatment for 24 h. Cell proliferation was assessed using CyQuant fluorescent nucleic acid‐based dye that labels live cells where the fluorescence intensity directly correlates with the proliferation rate. * P < 0.05 and *** P < 0.001 indicate significant difference from non‐treated control as measured by paired Student's t test.
Activation of ERK1/2 and AKT signalling by the P2Y14R agonist UDP‐glucose
Mechanistically, hCPC stimulation with UDP‐Glu prompted activation of canonical proliferation signalling pathways ERK1/2 and AKT as indicated by increased phosphorylation at ERK1/2Thr202/Tyr204 (5 min: 1.70 ± 0.15 fold change; 10 min: 1.61 ± 0.14 fold change; 15 min: 1.22 ± 0.23 fold change, P = 0.0051) (Fig. 2 A–C) and AKTSer473 (5 min: 1.40 ± 0.15 fold change; 10 min: 1.35 ± 0.11 fold change, P = 0.0017) (Fig. 2 D–F).
Figure 2. Activation of ERK1/2 and AKT signalling by the P2Y14R agonist UDP‐glucose.
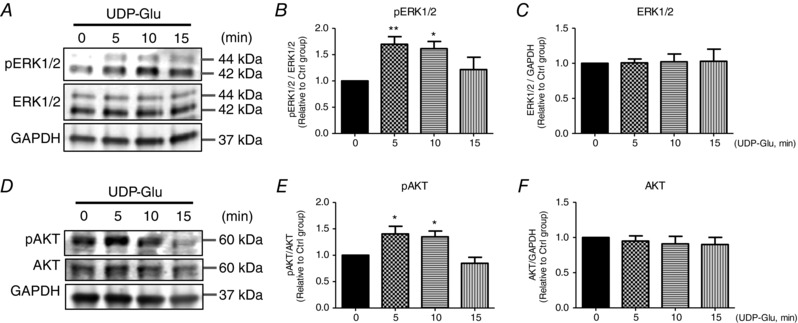
hCPC immunoblotting analysis (A and D) and corresponding quantification (B and C, E and F) showing enhanced phosphorylation of ERK1/2 and AKT (indicating activation) in response to UDP‐Glu (100 μm) treatment. pERK1/2Thr202/Tyr204 and pAKTSer473 were normalized to total ERK1/2 (n = 5) and total AKT levels (n = 6), respectively. Total ERK1/2 (n = 8) and total AKT levels (n = 8) were normalized to GAPDH (glyceraldehyde‐3‐phosphate dehydrogenase; loading control). Data are represented relative to 0 min (no UDP‐Glu treatment). * P < 0.05 and ** P < 0.01 indicate significant difference from 0 min as measured by one‐way ANOVA followed by Dunnett's post hoc test.
Improving hCPC proliferation and migration by P2Y14R overexpression
To assess whether augmenting P2Y14R expression improves hCPC phenotypic properties, cells were transduced with lentiviral particles encoding P2Y14R fused to mGFP (hCPC‐Y14) or mGFP alone (hCPC‐mGFP) as a control. Transduction efficiency was 98.47 ± 0.3828% for hCPC‐mGFP and 94.47 ± 4.007% for hCPC‐Y14 as assessed by flow cytometric analysis for percentage of GFP+ cells (Fig. 3 A and B). Expression of the P2Y14R‐mGFP fused construct was confirmed by immunoblotting (Fig. 3 C).
Figure 3. Improving hCPC proliferation and migration by P2Y14R overexpression.
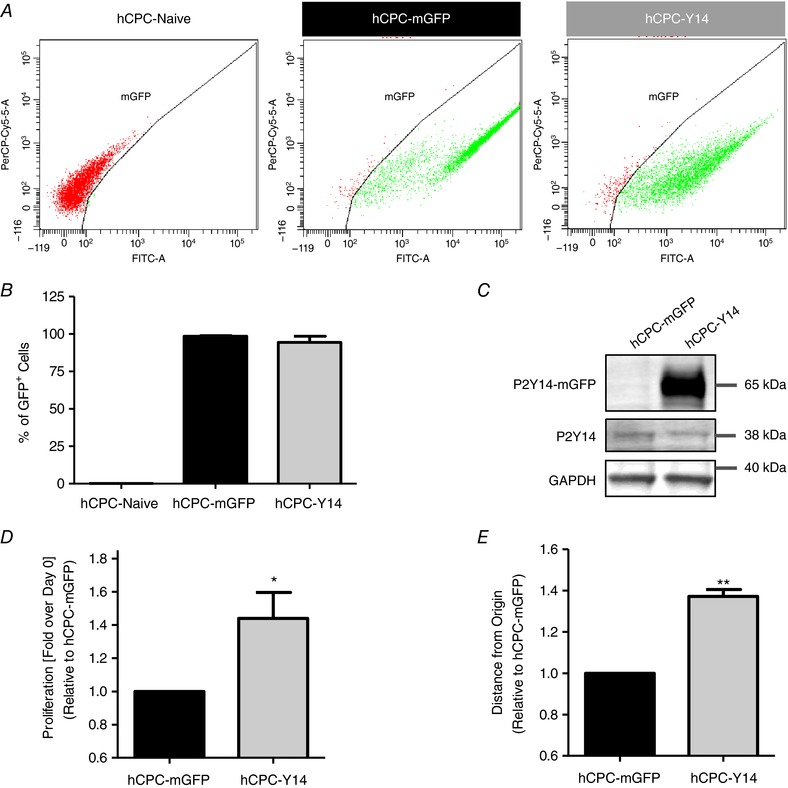
Transduction efficiency of hCPCs with lentiviral particles encoding for P2Y14R and mGFP (hCPC‐Y14) or mGFP alone (hCPC‐mGFP) as a control by flow cytometry analysis (A) with corresponding quantification of percentage of GFP+ cells (98% for hCPC‐mGFP and 94% for hCPC‐Y14; B). C, a representative immunoblot showing a 38 kDa band corresponding to endogenous P2Y14R in hCPCs overexpressing P2Y14R and mGFP (hCPC‐Y14) and control hCPCs expressing mGFP alone (hCPC‐mGFP) in addition to a shifted 65 kDa P2Y14R band in hCPC‐Y14 validating overexpression of the P2Y14R‐mGFP fused construct at the protein level. D, proliferation analysis over the course of 5 days demonstrating that hCPC‐Y14 cells exhibit enhanced proliferation. Cell proliferation was assessed as described in Fig. 1. E, migration analysis showing that P2Y14R overexpression significantly enhances hCPC migration on GFR Matrigel as indicated by increased distance that cells travelled from origin. Cell migration was monitored for 2 h using live‐cell imaging. * P < 0.05 and ** P < 0.01 indicate significant difference from hCPC‐mGFP as measured by paired Student's t test.
P2Y14R stimulates cell proliferation and migration in a variety of experimental models (Sesma et al. 2012; Barrett et al. 2013; Kook et al. 2013; Jokela et al. 2014). Similarly, P2Y14R overexpression significantly improved hCPC proliferation (1.441 ± 0.1563 fold change, P = 0.0372) (Fig. 3 D) and enhanced cell migration as shown by increased distance travelled from the origin on GFR Matrigel (1.372 ± 0.03291 fold change, P = 0.0077) (Fig. 3 E). These results confirm the role of P2Y14R in stimulating hCPC proliferative and migratory responses.
Enhancing hCPC resistance to cell death by P2Y14R overexpression
P2Y14R expression is essential for HSC resistance to various stress stimuli (Cho et al. 2014). To test whether P2Y14R plays similar roles in hCPCs, hCPC‐Y14 and control hCPC‐mGFP were subjected to nutrient deprivation by culturing in serum‐free medium for 48 h. The percentage of cells undergoing apoptosis or necrosis was assessed using Annexin V or propidium iodide (PI), respectively (Fig. 4 A). hCPC‐Y14 showed significantly higher resistance to cell death as indicated by an increase in the percentage of Annexin V−/PI− healthy population (1.12 ± 0.0056 fold change, P = 0.0022) and a decrease in apoptotic cells (0.803 ± 0.0277 fold change, P = 0.0191) with no significant effect on percentage of necrotic cells (Fig. 4 B).
Figure 4. Enhancing hCPC survival under serum deprivation or oxidative stress by P2Y14R overexpression.
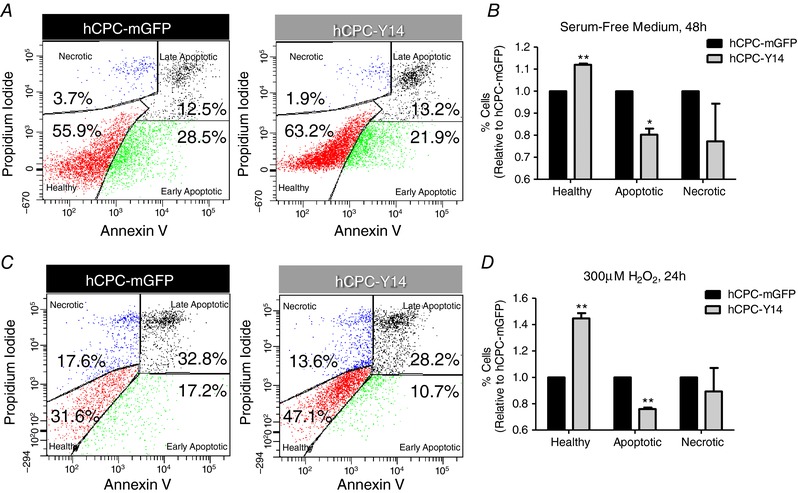
Cell death analysis in hCPCs subjected to serum‐free medium for 48 h (A and B) or H2O2 (300 μm) in serum‐free medium for 24 h (C and D) showing that hCPC‐Y14 exhibit a higher percentage of live cells, lower levels of apoptotic cells and no significant effect on cell necrosis. Cell apoptosis and necrosis were assessed by Annexin V and propidium iodide (PI) staining, respectively, followed by flow cytometric analysis. * P < 0.05 and ** P < 0.01 indicate significant difference from hCPC‐mGFP control group as measured by paired Student's t test.
Additionally, hCPC‐Y14 and hCPC‐mGFP cell death was assessed following H2O2‐induced oxidative stress (300 μm) in serum‐free medium for 24 h (Fig. 4 C). Similarly, hCPC‐Y14 demonstrated enhanced survival (1.447 ± 0.0395 fold change, P = 0.0077) and reduced apoptosis (0.76 ± 0.0101 fold change, P = 0.0018) with no significant effect on cell necrosis (Fig. 4 D). These findings suggest a pro‐survival role mediated by P2Y14R under stress conditions.
Reversing hCPC senescence‐associated phenotypes by P2Y14R overexpression
P2Y14R deletion enhances stress‐induced senescence in HSCs (Cho et al. 2014). To demonstrate whether P2Y14R mediates anti‐senescence responses in hCPCs, the impact of P2Y14R overexpression upon cell morphology and expression of senescence markers was assessed. hCPC‐Y14 exhibited a ‘spindly’ morphology characteristic of a more proliferative and less senescent hCPC population (Fig. 5 A). This was indicated by increased cell length/width ratio (from 3.848 ± 0.0884 to 5.325 ± 0.5607 arbitrary units, P = 0.0405) (Fig. 5 B), decreased area (from 5868 ± 465.4 to 4873 ± 280.4 arbitrary units, P = 0.1411) (Fig. 5 C) and roundness (from 0.2967 ± 0.0022 to 0.2534 ± 0.0097 arbitrary units, P = 0.0048) (Fig. 5 D).
Figure 5. Reversing hCPC senescence‐associated morphology by P2Y14R overexpression.
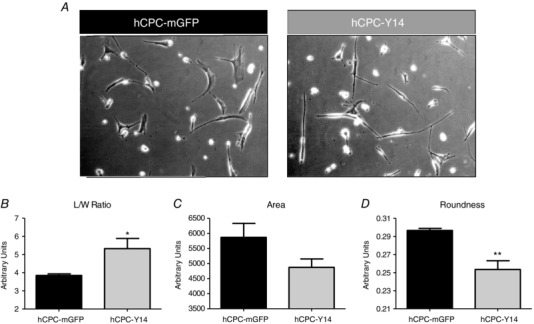
A, representative bright field images showing that hCPC‐Y14 cells restore a ‘spindly’ morphology characteristic of a more proliferative and less senescent hCPC population. Morphometric analysis demonstrating increased length/width ratio (B), reduced surface area (C) and roundness (D) in hCPC‐Y14 cells compared to hCPC‐mGFP control. Cell morphology was analysed using ImageJ software. * P < 0.05 and ** P < 0.01 indicate significant difference from hCPC‐mGFP control as assessed by unpaired Student's t test.
P2Y14R‐induced changes in morphology were associated with downregulation of canonical molecular markers of senescence p16INK4a (0.2917 ± 0.0475 fold change, P = 0.0045) (Fig. 6 A and D), p53 (0.798 ± 0.0245 fold change, P = 0.0144) (Fig. 6 B and E) and p21 (0.5590 ± 0.0842 fold change, P = 0.0012) (Fig. 6 C and F). Moreover, P2Y14R overexpression reduced levels of mitochondrial ROS as shown by diminished MitoSOX mean fluorescence intensity (MFI) in hCPC‐Y14 compared to hCPC‐mGFP (from 485.5 ± 33.89 to 329.3 ± 11.23 arbitrary units, P = 0.0119) (Fig. 6 G). These findings implicate an anti‐senescence role for P2Y14R in hCPCs.
Figure 6. Reducing molecular markers of senescence in hCPCs by P2Y14R overexpression.
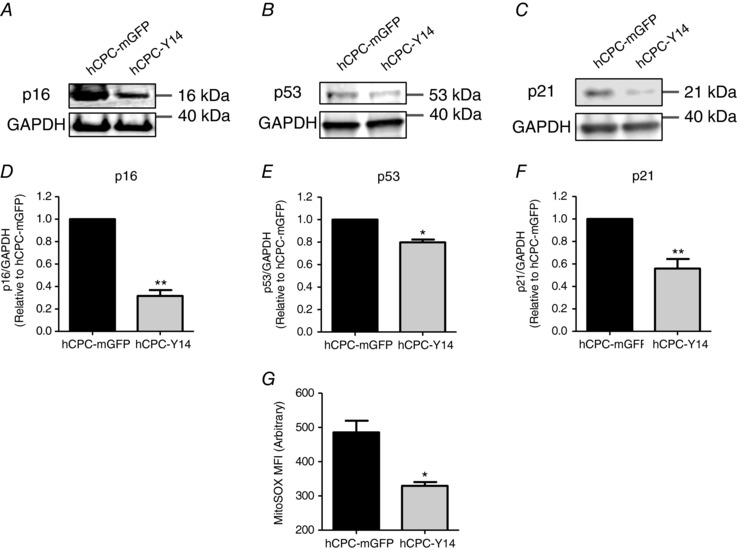
Representative immunoblots (A, B and C) and corresponding quantification (D, E and F) showing downregulation of canonical markers of senescence p16INK4A, p53 and p21, respectively. p16INK4A, p21 and p53 protein levels were normalized to GAPDH (loading control). * P < 0.05 and ** P < 0.01 indicate significant difference from hCPC‐mGFP as measured by paired Student's t test. G, hCPC‐Y14 cells exhibit reduced MitoSOX mean fluorescence intensity indicative of lesser levels of mitochondrial ROS. * P < 0.05 indicates significant difference from hCPC‐mGFP as measured by unpaired Student's t test.
Discussion
Human CPCs (hCPCs) isolated from heart failure (HF) patients with genetic predispositions, concomitant underlying chronic diseases such as diabetes, obesity and hypertension, and/or habitual stressors such as alcoholism and smoking exhibit severely compromised regenerative potential. Therefore, there is an unequivocal need to reveal the molecular mechanisms involved in rejuvenating these cells and enhancing their phenotypic characteristics towards a better reparative potential of the damaged myocardium (Mohsin et al. 2012, 2013; Kulandavelu et al. 2016). This study introduces P2Y14R as an important regulator of hCPC proliferative, migratory and survival responses. Moreover, findings from the present study reveal an anti‐senescence role mediated by P2Y14R in hCPCs through retaining cellular morphology and molecular signature that typify a more youthful stem cell population (Fig. 7).
Figure 7. P2Y14R‐mediated responses in hCPCs.
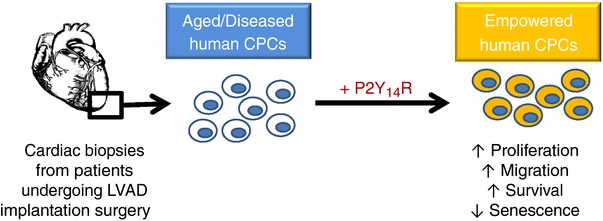
A schematic diagram showing the physiological responses mediated by P2Y14R in hCPCs derived from HF patients undergoing LVAD implantation surgery. Engineering aged/diseased hCPCs with P2Y14R antagonizes cellular senescence and improves functional responses indicated by increased proliferation, migration and survival under stress stimuli.
The regenerative capacity of CPCs progressively declines with age and age‐related chronic diseases (Capogrossi, 2004; Dimmeler & Leri, 2008; Cesselli et al. 2011) rendering limited therapeutic potential of stem cells isolated from HF patients (Bolli et al. 2011; Chugh et al. 2012). Rejuvenation of hCPCs with advanced biological age is crucial for augmenting functional activity and regenerative properties. Findings from this study demonstrate that P2Y14R overexpression reverses senescence‐associated phenotypes in aged hCPCs derived from HF patients. P2Y14R‐overexpressing hCPCs exhibit a ‘spindly’ morphology and reduced levels of p16INK4a, p53, p21 and mitochondrial ROS characteristic of a less senescent stem cell population. These results are consistent with the anti‐senescence roles of P2Y14R previously demonstrated in HSCs where P2Y14R deletion resulted in enhanced susceptibility to stress‐induced senescence and elevated expression levels of the senescence markers p16INK4a, hypophosphorylated retinoblastoma protein (Rb) and ROS (Cho et al. 2014).
Myocardial regeneration mediated by resident or adoptively transferred progenitor cells is severely limited due to poor survival rates in the unfavourable environment of the necrotic tissue (Hofmann et al. 2005; Rota et al. 2007). Antagonizing senescence in aged hCPCs has proven efficacy in improving survival capability and functional capacity both in vitro and in vivo (Mohsin et al. 2012, 2013). Similarly, P2Y14R‐mediated anti‐senescence responses were associated with improved hCPC survival under various stress stimuli including serum deprivation and H2O2‐induced oxidative stress.
Additionally, P2Y14R overexpression significantly improved hCPC proliferation by 1.44‐fold as assessed by haemocytometer counting after 5 days in culture. These data extend previous findings showing enhancement of hCPC proliferative capacity using different interventional approaches. Engineering cells with Pim1 kinase improves hCPC proliferation by 1.2‐ to 1.4‐fold as assessed by CyQuant assay after 3 days in culture (Mohsin et al. 2013). A more profound effect on hCPC proliferation was achieved by targeting Pim1 to specific intracellular compartments. Overexpressing nuclear‐targeted Pim1 increases hCPC proliferation by 2.21‐fold whereas targeting Pim1 to mitochondria enhances proliferative response by 2.78‐fold as determined by CyQuant assay after 3 days in culture (Samse et al. 2015). In addition to genetic engineering, pharmacological approaches have been pursued to enhance hCPC proliferative capacity. ATP preconditioning improves atrial‐derived c‐Kit+ hCPC proliferation by ∼2‐fold as measured by BrdUrd incorporation (Ferreira‐Martins et al. 2009). Modulating oxygen levels is an alternative approach that significantly impacts proliferative response in hCPCs. Transferring human endomyocardial biopsy‐derived c‐Kit+ cardiac stem cells from atmospheric oxygen (21%) to physiological oxygen (5%) improved cell proliferation by 1.4‐fold by day 3 and 1.5‐fold by day 4 in culture as assessed by a TC10 automated cell counter (Bellio et al. 2016). Collectively, multiple molecular interventions improve CPC growth to varying levels; however, the differences observed could be partially impacted by varying origins and culture procedures of isolated c‐Kit+ cells, techniques used to measure cell proliferation and/or time points at which cell proliferation was assessed.
In addition to improving hCPC survival and proliferation, P2Y14R overexpression enhanced migratory responses on GFR Matrigel, indicating an overall improvement in cellular properties critical for the regeneration process. These findings are in agreement with prior literature showing a central role of P2Y14R in mediating proliferation of keratinocytes (Jokela et al. 2014), migration of neutrophils (Sesma et al. 2012; Barrett et al. 2013) and haematopoietic stem cells (HSCs) (Kook et al. 2013) in addition to maintaining the regenerative capacity of HSCs following injury (Cho et al. 2014).
Purinergic receptors are activated by extracellular nucleotides released following injury/stress to induce cellular responses required for the repair process (Burnstock, 1997; Erb et al. 2006). Lack of purinergic receptor expression could lead to impaired regenerative responses to injury. Striking correlations existed between P2Y14R expression in hCPCs and clinical parameters of HF patients from which hCPCs were derived. hCPCs from patients with relatively lower ejection fraction (EF) exhibited lower P2Y14R expression. In addition, P2Y14R was significantly downregulated in hCPCs isolated from HF patients diagnosed with diabetes, previously linked to a functionally defective CPC population due to enhanced inflammation, hyperglycaemia, hyperlipidaemia and oxidative stress in diabetic patients (Leonardini & Avogaro, 2013). Altogether, these findings point to P2Y14R as a biomarker of hCPC functional capacity that can be used to enrich for a more youthful subset of hCPC population.
Future studies will be dedicated to assessing the inclusion of additional steps to further augment the functional capacity of hCPCs engineered with P2Y14R (hCPC‐Y14). For instance, hCPC‐Y14 can be subjected to hypoxia preconditioning since unpublished data from our group and recent reports show that modulating O2 concentration significantly impacts the regenerative potential of CPCs (Bellio et al. 2016). It is also likely that purinergic receptors act in a cooperative manner. Studies to evaluate other potential purinergic receptor candidates to improve the regenerative capacity of hCPCs are currently underway. Co‐expression of more than one purinergic receptor could induce synergistic effects towards a better therapeutic outcome. In vitro results will be extended to an in vivo model of myocardial infarction to test the reparative potential of modified hCPCs for injured myocardium.
Overall, identifying intrinsic deficits in hCPCs is an unequivocally essential step towards improving therapeutic efficacy. Defective hCPCs derived from HF patients with relatively lower ejection fraction and/or diagnosed with diabetes show reduced P2Y14R expression levels and impaired functional responses. P2Y14R overexpression significantly augmented hCPC function and antagonized cellular senescence. These findings set the stage to assess P2Y14R overexpression as a potential interventional approach to augment the therapeutic outcome of stem cells derived from injured myocardium for treatment of HF through autologous stem cell therapy.
Additional information
Protection of human subjects
Source
Human tissue specimens used in this proposal are derived from heart failure patients undergoing left ventricular assist device (LVAD) implantation surgeries. These tissue samples are routine discards from the surgical procedure. There is no additional risk to the patient as the samples are thrown away if not used and would need to be harvested as part of the procedure. Details relating to patient involvement and consent are being conducted and approved by the Sharp Hospital where the samples are acquired (IRB no. 120686). The samples provided for use in this proposal are de‐identified and of no potential therapeutic or diagnostic value to the patients. Therefore, these samples are considered non‐human subjects research working with cell lines procured as part of the Sussman lab environment and available for use in relevant studies involving the biology of human cardiac stem cells.
Justification of use
Cells derived from human tissue are more clinically relevant than those obtained from experimental animal models. The scope of the proposed experiments has not changed.
Competing interests
M.A.S. is co‐founder and chief scientific officer of CardioCreate, Inc. The other authors have no competing interests to declare.
Author contributions
F.G.K. and M.A.S. designed the experiments. F.G.K., W.K. and A.K. performed the experiments. F.G.K. analysed data. W.P.D. contributed to data acquisition by providing cardiac explants derived from heart failure patients. M.M.M. participated in isolating hCPCs from tissue specimens of heart failure patients. K.I., B.N., R.A. and M.C. contributed to lentiviral plasmid validation, plasmid expansion and lentiviral particle generation. F.G.K. and M.A.S. wrote the article. All authors read and approved the final article and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed. Experiments have been performed in Dr. Mark Sussman's laboratory at San Diego State University.
Funding
This work is supported by National Institutes of Health (F32HL131299 Ruth L. Kirschstein Postdoctoral Individual National Research Service Award (NRSA) to F.G.K.; 1R37HL091102, 1R01HL105759, 5R01HL067245, 1R01HL113656, 1R01HL117163, 1R01HL113647 and 5R01HL105759 to M.A.S.) and Foundation Leducq.
Translational perspective
The regenerative capacity of stem cells is largely dependent on their ability to communicate with the extracellular environment and react with the proper responses. In this regard, transmembrane purinergic receptors represent a major detector for extracellular nucleotides released during injury/stress and serve as an intracellular platform harbouring numerous signalling pathways that play critical roles in regulating inflammatory and regenerative responses required for the healing process following injury. Findings from this study demonstrate significant correlations between P2Y14 purinergic receptor (P2Y14R) expression in human cardiac progenitor cells (hCPCs) isolated from cardiac biopsies of heart failure patients and their clinical profiles. P2Y14R expression was downregulated in hCPCs derived from heart failure patients with low ejection fraction or diagnosed with diabetes. Augmenting P2Y14R expression levels in aged/diseased hCPCs reverses cellular senescence and boosts survival, proliferative and migratory responses. Restoring the youthful phenotype of hCPCs enhances engraftment and regenerative capacity. Therefore, engineering hCPCs with P2Y14R could improve reparative potential upon adoptive transfer into infarcted myocardium with the long‐term goal of translational application in autologous stem cell therapy. P2Y14R‐engineered hCPCs could display enhanced capability to detect extracellular UDP‐sugars released during injury/stress and improve therapeutic efficacy of hCPCs. Indeed, this concept is not restricted to hCPCs and can be extended in other types of stem cells used for autologous or allogeneic cell therapy for treatment of HF.
Acknowledgements
We sincerely acknowledge Dr Walter Dembitsky from Sharp Memorial Hospital for providing the Sussman lab with the human heart biopsy samples used for our cell isolations.
Edited by: Michael Hogan & Troy Hornberger
References
- Abeijon C & Hirschberg CB (1992). Topography of glycosylation reactions in the endoplasmic reticulum. Trends Biochem Sci 17, 32–36. [DOI] [PubMed] [Google Scholar]
- Barrett MO, Sesma JI, Ball CB, Jayasekara PS, Jacobson KA, Lazarowski ER & Harden TK (2013). A selective high‐affinity antagonist of the P2Y14 receptor inhibits UDP‐glucose‐stimulated chemotaxis of human neutrophils. Mol Pharmacol 84, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellio MA, Rodrigues CO, Landin AM, Hatzistergos KE, Kuznetsov J, Florea V, Valasaki K, Khan A, Hare JM & Schulman IH (2016). Physiological and hypoxic oxygen concentration differentially regulates human c‐Kit+ cardiac stem cell proliferation and migration. Am J Physiol Heart Circ Physiol 311, H1509–H1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E & Urbanek K (2003). Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776. [DOI] [PubMed] [Google Scholar]
- Berninsone P & Hirschberg CB (1998). Nucleotide sugars, nucleotide sulfate, and ATP transporters of the endoplasmic reticulum and Golgi apparatus. Ann N Y Acad Sci 842, 91–99. [DOI] [PubMed] [Google Scholar]
- Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J & Anversa P (2011). Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378, 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Burnstock G (1997). The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology 36, 1127–1139. [DOI] [PubMed] [Google Scholar]
- Capogrossi MC (2004). Cardiac stem cells fail with aging: A new mechanism for the age‐dependent decline in cardiac function. Circ Res 94, 411–413. [DOI] [PubMed] [Google Scholar]
- Cesselli D, Beltrami AP, D'Aurizio F, Marcon P, Bergamin N, Toffoletto B, Pandolfi M, Puppato E, Marino L & Signore S (2011). Effects of age and heart failure on human cardiac stem cell function. Am J Pathol 179, 349–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, Foley JJ, Zhu Y, McLaughlin MM, Murdock P & McMillan L (2000). A G protein‐coupled receptor for UDP‐glucose. J Biol Chem 275, 10767–10771. [DOI] [PubMed] [Google Scholar]
- Cho J, Yusuf R, Kook S, Attar E, Lee D, Park B, Cheng T, Scadden DT & Lee BC (2014). Purinergic P2Y14 receptor modulates stress‐induced hematopoietic stem/progenitor cell senescence. J Clin Invest 124, 3159–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P & Bolli R (2012). Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation 126, S54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S & Leri A (2008). Aging and disease as modifiers of efficacy of cell therapy. Circ Res 102, 1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, Henning BJ, Stirparo GG, Papait R, Scarfo M, Agosti V, Viglietto G, Condorelli G, Indolfi C, Ottolenghi S, Torella D & Nadal‐Ginard B (2013). Adult c‐kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 154, 827–842. [DOI] [PubMed] [Google Scholar]
- Erb L, Liao Z, Seye CI & Weisman GA (2006). P2 receptors: intracellular signaling. Pflugers Arch 452, 552–562. [DOI] [PubMed] [Google Scholar]
- Ferreira‐Martins J, Rondon‐Clavo C, Tugal D, Korn JA, Rizzi R, Padin‐Iruegas ME, Ottolenghi S, De Angelis A, Urbanek K, Ide‐Iwata N, D'Amario D, Hosoda T, Leri A, Kajstura J, Anversa P & Rota M (2009). Spontaneous calcium oscillations regulate human cardiac progenitor cell growth. Circ Res 105, 764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH & Drexler H (2005). Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation 111, 2198–2202. [DOI] [PubMed] [Google Scholar]
- Jokela TA, Karna R, Makkonen KM, Laitinen JT, Tammi RH & Tammi MI (2014). Extracellular UDP‐glucose activates P2Y14 receptor and induces signal transducer and activator of transcription 3 (STAT3) Tyr705 phosphorylation and binding to hyaluronan synthase 2 (HAS2) promoter, stimulating hyaluronan synthesis of keratinocytes. J Biol Chem 289, 18569–18581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook S, Cho J, Lee SB & Lee B‐C (2013). The nucleotide sugar UDP‐glucose mobilizes long‐term repopulating primitive hematopoietic cells. J Clin Invest 123, 3420–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreda SM, Okada SF, van Heusden CA, O'Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC & Lazarowski ER (2007). Coordinated release of nucleotides and mucin from human airway epithelial Calu‐3 cells. J Physiol 584, 245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreda SM, Seminario‐Vidal L, van Heusden C & Lazarowski ER (2008). Thrombin‐promoted release of UDP‐glucose from human astrocytoma cells. Br J Pharmacol 153, 1528–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulandavelu S, Karantalis V, Fritsch J, Hatzistergos KE, Loescher VY, McCall F, Wang B, Bagno L, Golpanian S & Wolf A (2016). Pim1 kinase overexpression enhances ckit+ cardiac stem cell cardiac repair following myocardial infarction in swine. J Am Coll Cardiol 68, 2454–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Shea DA, Boucher RC & Harden TK (2003). Release of cellular UDP‐glucose as a potential extracellular signaling molecule. Mol Pharmacol 63, 1190–1197. [DOI] [PubMed] [Google Scholar]
- Leonardini A & Avogaro A (2013). Abnormalities of the cardiac stem and progenitor cell compartment in experimental and human diabetes. Arch Physiol Biochem 119, 179–187. [DOI] [PubMed] [Google Scholar]
- Mohsin S, Khan M, Nguyen J, Alkatib M, Siddiqi S, Hariharan N, Wallach K, Monsanto M, Gude N, Dembitsky W & Sussman MA (2013). Rejuvenation of human cardiac progenitor cells with Pim‐1 kinase. Circ Res 113, 1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsin S, Khan M, Toko H, Bailey B, Cottage CT, Wallach K, Nag D, Lee A, Siddiqi S, Lan F, Fischer KM, Gude N, Quijada P, Avitabile D, Truffa S, Collins B, Dembitsky W, Wu JC & Sussman MA (2012). Human cardiac progenitor cells engineered with Pim‐I kinase enhance myocardial repair. J Am Coll Cardiol 60, 1278–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quijada P, Hariharan N, Cubillo JD, Bala KM, Emathinger JM, Wang BJ, Ormachea L, Bers DM, Sussman MA & Poizat C (2015). Nuclear calcium/calmodulin‐dependent protein kinase II signaling enhances cardiac progenitor cell survival and cardiac lineage commitment. J Biol Chem 290, 25411–25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin‐Iruegas ME, Gonzalez A, Rizzi R, Small N, Muraski J, Alvarez R, Chen X, Urbanek K, Bolli R, Houser SR, Leri A, Sussman MA & Anversa P (2007). Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci USA 104, 17783–17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samse K, Emathinger J, Hariharan N, Quijada P, Ilves K, Völkers M, Ormachea L, De La Torre A, Orogo AM & Alvarez R (2015). Functional effect of Pim1 depends upon intracellular localization in human cardiac progenitor cells. J Biol Chem 290, 13935–13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrivens M & Dickenson JM (2005). Functional expression of the P2Y14 receptor in murine T‐lymphocytes. Br J Pharmacol 146, 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrivens M & Dickenson JM (2006). Functional expression of the P2Y14 receptor in human neutrophils. Eur J Pharmacol 543, 166–173. [DOI] [PubMed] [Google Scholar]
- Sesma JI, Kreda SM, Steinckwich‐Besancon N, Dang H, Garcia‐Mata R, Harden TK & Lazarowski ER (2012). The UDP‐sugar‐sensing P2Y14 receptor promotes Rho‐mediated signaling and chemotaxis in human neutrophils. Am J Physiol Cell Physiol 303, C490–C498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton L, Cooper M, Murphy M & Platt A (2003). Human immature monocyte‐derived dendritic cells express the G protein‐coupled receptor GPR105 (KIAA0001, P2Y14) and increase intracellular calcium in response to its agonist, uridine diphosphoglucose. J Immunol 171, 1941–1949. [DOI] [PubMed] [Google Scholar]
- Xu J, Morinaga H, Oh D, Li P, Chen A, Talukdar S, Lazarowski E, Olefsky JM & Kim JJ (2012). GPR105 ablation prevents inflammation and improves insulin sensitivity in mice with diet‐induced obesity. J Immunol 189, 1992–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DV, Wiley CD & Velarde MC (2015). Mitochondrial effectors of cellular senescence: beyond the free radical theory of aging. Aging Cell 14, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


