Abstract
Key points
Obesity during pregnancy and childbirth is associated with labour dystocia leading to instrumental or operative delivery, but the underlying pathophysiological mechanisms remain unclear and insufficient uterine contractility has been suggested.
This study examined whether reduced myometrial mitochondrial capacity or quantity could contribute as a pathophysiological mechanism to labour dystocia.
Data did not support reduced myometrial mitochondrial capacity or quantity in the myometrium at term in obese women, but a reduced myocyte density with increased triglyceride content was demonstrated, which could lead to poorer uterine contractility.
These results add to the understanding of systemic effects of obesity, placing also the myometrium at term as an affected non‐adipose tissue.
Abstract
Obesity is known to increase the risk of labour dystocia and insufficient energy supply, due to reduced mitochondrial capacity or quantity, could be a possible mechanism leading to reduced efficiency of uterine contractility during labour. In the present study of 36 women having an elective Caesarean section at term, obesity did not change mitochondrial phenotype in the myometrial myocyte obtained from uterine biopsies taken at delivery. Respiration rates in isolated mitochondria were unaffected by obesity. No indication of reduced content, investigated by quantification of the complexes of the respiratory chain, or altered regulation, examined by myometrial mRNA levels of genes related to mitochondrial biogenesis and inflammation, was detected. Yet we found increased myometrial triglyceride content in the obese group (2.39 ± 0.26 vs. 1.56 ± 0.20 mm, P = 0.024), while protein content and citrate synthase activity per gram wet weight myometrium were significantly lower in the obese (109.2 ± 7.2 vs. 139.4 ± 5.6 mg g−1, P = 0.002, and 24.8 ± 1.0 vs. 29.6 ± 1.4 U g−1 wet wt, P = 0.008, respectively). These differences were substantiated by our histological findings where staining for nuclei, cytoplasm, glycogen and collagen supported the idea of a smaller muscle content in the myometrium in obese women. In conclusion no indication of myometrial mitochondrial dysfunction in the isolated state was found, but the observed increase of lipid content might play a role in the pathophysiological mechanisms behind labour dystocia in obese women.
Keywords: mitochondria, pregnancy, obesity, myometrium, intramuscular fat
Key points
Obesity during pregnancy and childbirth is associated with labour dystocia leading to instrumental or operative delivery, but the underlying pathophysiological mechanisms remain unclear and insufficient uterine contractility has been suggested.
This study examined whether reduced myometrial mitochondrial capacity or quantity could contribute as a pathophysiological mechanism to labour dystocia.
Data did not support reduced myometrial mitochondrial capacity or quantity in the myometrium at term in obese women, but a reduced myocyte density with increased triglyceride content was demonstrated, which could lead to poorer uterine contractility.
These results add to the understanding of systemic effects of obesity, placing also the myometrium at term as an affected non‐adipose tissue.
Introduction
In 2014 the World Health Organisation estimated that the worldwide prevalence of obesity (body mass index (BMI) ≥ 30 kg m−2) among adult women had reached 15%, with a parallel increase in number of obese childbearing women (Mendis et al. 2014). Obesity during pregnancy and childbirth is associated with several serious complications (Castro & Avina, 2002; Mantakas & Farrell, 2010; Chung et al. 2012), including labour dystocia (Zhang et al. 2007a; Cedergren, 2009; Walsh et al. 2011). Obese women are reported to have more than twice as high a risk of having a Caesarean section due to labour dystocia than normal‐weight women (Walsh et al. 2011) and in the USA up to half of acute Caesarean sections in nulliparous women have dystocia as an indication (Zhang et al. 2010). The evidence of an increased risk of inefficient uterine contractility during labour in obese women is well‐established (Zhang et al. 2007a; Walsh et al. 2011; Magann et al. 2013; Highley et al. 2016) and spontaneous contractions in myometrial strips from obese women have been shown to decline with increasing body weight (Zhang et al. 2007a), yet these findings are controversial (Chiossi et al. 2010; Higgins et al. 2010; Crankshaw et al. 2017). The underlying pathophysiological mechanisms are still poorly understood and proposed suggestions are primarily centred around obesity altering hormonal and lipid levels, ultimately leading to changes in the myocyte function or composition, hence reducing uterine contractility (Lowe & Corwin, 2011; Carlson et al. 2015).
Inhibitory effects of adipokines on contractions in human myometrium strips have indeed been reported (Moynihan et al. 2006; Hehir et al. 2008; Hehir & Morrison, 2012; Mumtaz et al. 2015). However, the physiological importance remains unclear, as not all adipokines increase with obesity and levels of adipokines during labour have yet to be investigated (Alsaif et al. 2015). Another hypothesis is that raised plasma cholesterol disrupts the caveolae of the myocytes of the myometrium – this could affect the function of ion channels essential for hyperpolarization of the myocyte membrane during myometrial contraction, but also alter the properties or the number of oestrogen and oxytocin receptors (Lowe & Corwin, 2011). Elevated plasma cholesterol levels do seem to inhibit contractions in human and rat myometrial strips ex vivo (Smith et al. 2005; Zhang et al. 2007b), but changes in the oxytocin receptor system or gap junctions between the myocytes associated to obesity have not been substantiated (Garabedian et al. 2013; Grotegut et al. 2013). Dysregulation of the expression of potassium channels important for onset of labour has also been demonstrated in obese women (Parkington et al. 2014). Finally obesity does not seem to have an impact on the proportion of smooth muscle cell and extracellular matrix in the human myometrium at term either (Sweeney et al. 2013, 2014).
A more general hypothesis of obesity effects is the lipid overflow hypothesis (Mittendorfer, 2011), which suggests that when the individual limits of physical expandability of the adipose tissue are surpassed, other tissues, e.g. skeletal or cardiac muscle, are exposed to excess free fatty acid (FFA) levels, leading to intramyocellular fat storage and mitochondrial stress due to incomplete fatty acid oxidation (Consitt et al. 2009; Tumova et al. 2016; Schrauwen‐Hinderling et al. 2016; Di Meo et al. 2017). In skeletal muscle it still remains unsettled whether the observed lower ATP synthesis capacity in individuals with obesity (Bakkman et al. 2010; Vijgen et al. 2013) is due to a lower concentration of mitochondria, dysfunction or a combination of the two (Consitt et al. 2009; Holloway et al. 2009; Tumova et al. 2016; Jorgensen et al. 2017). Adverse effects of obesity in skeletal muscle on the mitochondrial electron transport chain (Ritov et al. 2010) and on expression of genes of the oxidative metabolism and mitochondrial biogenesis, such as the peroxisome proliferator‐activated receptor γ (PPAR‐γ) and PPAR co‐activator 1 α (PGC‐1α) (Maples et al. 2015), have been reported. In our previous study conducted in a rat model of obesity in pregnancy, mitochondrial oxidative capacity and morphology in the myometrium appeared unaffected (Gam et al. 2015), but such knowledge is not available for the human myometrium at term. In the present study we therefore aimed to investigate whether pre‐pregnancy obesity alters lipid metabolism and mitochondrial phenotype in the human myometrium at term.
Methods
Ethical approval
The study was approved by the regional ethical committee of Copenhagen, Denmark (Protocol no. H‐1‐2012‐070). The research was carried out in accordance with the standards set by the World Medical Association's Declaration of Helsinki and oral and written informed consent to participate in the study was obtained from all women.
Anthropometric and clinical data
Thirty‐six pregnant adult women with a singleton pregnancy attending antenatal care at Rigshospitalet, Denmark and having a term elective Caesarean section were included in a random sequence during the period October 2013 to December 2015. Nineteen were normal weight (BMI 18.5–24.9 kg m−2) and seventeen obese (BMI ≥ 30 kg m−2) (WHO Expert Committee on Physical Status, 1995). The pre‐pregnancy BMI was calculated using self‐reported height and weight from the antenatal file. The exclusion criteria were a known substance addiction including smoking, known comorbidities including diabetes or psychiatric illnesses or the use of medicine known to affect muscular contractions. Age, gestational age, parity and indication for Caesarean section were obtained from the medical records. An hour prior to surgery, blood samples, blood pressure, height and weight were obtained.
Blood sampling and myometrial biopsies
Venous blood samples were drawn into EDTA vials from vena cubitalis after at least 6 h fasting and blood sample analyses were done immediately. Myometrial biopsies (length 4 cm × depth 0.5 cm × height 1 cm, approximately 2–4 g) were isolated from the upper part of the uterine incision (transverse isthmic incision). All women were, 2–3 min before harvest of the myometrial biopsy, given an intramyometrial injection of oxytocin (Syntocinon® 10 IU) after delivery of child and placenta according to the Danish national guidelines for prevention of postpartum haemorrhage. The main part of the biopsies was immediately soaked in ice‐cold KCl medium for preparation of isolated mitochondria and subsequent mitochondrial respiratory measurement. The remaining parts were either frozen in liquid nitrogen (approximately 200–250 mg of myometrium) and placed in a −80°C freezer for subsequent biochemical analyses, or immersion‐fixed in 2% paraformaldehyde and 0.1% glutaraldehyde (approximately 8 mm3) for subsequent histology analyses, or immersion‐fixed in 2% v/v glutaraldehyde in 0.05 m sodium phosphate buffer (pH 7.2) (approximately 8 mm3) for subsequent ultrastructural analysis.
Mitochondrial respiratory measurements
Incubation media
Three different media were used in the respiratory experiments: (1) KCl medium containing 100 mm KCl, 5 mm MgCl2, 50 mm Tris, 1 mm EDTA, pH 7.4 at 0°C; (2) ATP medium consisting of KCl medium with 1 mm ATP and 0.2% BSA; (3) MSTPi medium containing 225 mm mannitol, 75 mm sucrose, 20 mm Tris base, 0.5 mm EDTA and 10 mm KH2PO4, pH 7.0 at 25°C. All reagents were purchased from Sigma‐Aldrich (St Louis, MO, USA).
Preparation of isolated mitochondria
Mitochondria from the myometrium were isolated using a modified protocol (Wikstrom et al. 1975; Fritzen et al. 2007). Approximately 3 g myometrium was washed briefly in ice‐cold KCl medium, subsequently minced rapidly with scissors in 20 mL ATP medium containing 100 U of proteinase, and left in this medium for 10 min with occasional stirring. The resulting tissue fragments were rinsed in ATP medium and homogenized in a Teflon Potter Elvehjem homogenizer for 6 min at 0°C (200 rpm, 0.405 mm vessel–pestle clearance) followed with a Büchner funnel filtration. The mitochondrial preparation was obtained after three consecutive centrifugations at 4°C. The first centrifugation was done at 400 g for 5 min and the supernatant collected. In the second centrifugation this supernatant was spun at 5400 g for 10 min and the pellet from this centrifugation was resuspended in approximately 8 mL of KCl medium and spun at 6700 g for 10 min in the third centrifugation. The supernatant was decanted and the resulting pellet was weighed and resuspended (1:1) in MSTPi medium.
Mitochondrial oxygen consumption measurements
Five Oroboros Oxygraph‐2k instruments (Innsbruck, Austria) were used to operate 10 oxygraph chambers in parallel at 25°C. Data acquisition and analyses were performed using DatLab software (Oroboros). Ten microlitres of mitochondrial suspension was added to each chamber containing 2 mL of MSTPi medium. Stirrer speed was 700 rpm in all measurements. Various respiratory parameters were measured by sequential substrate addition. Chamber concentrations after substrate addition were as follows: malate (2 mm), pyruvate (0.5 mm), palmitoyl carnitine (10 μm), ADP (2 mm), succinate (5 mm), exogenous cytochrome c (0.04 μm), rotenone (50 nm). Each respiration period after addition of new substrate was recorded for 5–10 min ensuring that steady state was reached. Between experiments, the chambers were washed thoroughly with first ethanol and then water. State 4 respiration was defined as oxygen consumption with pyruvate or palmitoyl carnitine in the absence of ADP or any metabolic poisons or inhibitors. State 3 respiration was defined as ADP‐stimulated respiration. respiration was reached by addition of succinate and complex II respiration alone was determined by addition of rotenone, a complex I inhibitor. Mitochondrial integrity was verified by observing that addition of cytochrome c did not cause a significant increase in respiration. The ATP formation to oxygen consumption ratio (P/O2) was obtained by measuring the amount of oxygen needed to consume 0.4 μmol of ADP. The respiratory control ratio (RCR) was calculated as the ratio between states 3 and 4. Oxygen consumption rate measurements were normalized to protein content of the mitochondrial suspensions and expressed as nmol oxygen per min per mg mitochondrial protein.
Biochemical analyses
Plasma triglycerides, cholesterol, high‐density lipoprotein (HDL), low‐density lipoprotein (LDL) and glucose were measured by enzymatic assay (respective reagents: TRIGL, CHOL2, HDLC3, LDLC3, GLUC3; Roche Diagnostics, Rotkreuz, Switzerland), and plasma insulin was measured by a sandwich electrochemiluminescense immunoassay (reagent: insulin; Roche Diagnostics, Rotkreuz, Switzerland) on a Cobas 8000 analyser (Roche Diagnostics, Rotkreuz, Switzerland) shortly after venous blood sampling. Plasma FFA levels were subsequently measured by using the chemical kit NEFA C ACS‐ACOD Method (code no. 99‐75406‐01, Wako Chemicals GmbH, Neuss, Germany). Insulin sensitivity was estimated by the homeostatic model assessment of insulin resistance (HOMA‐IR) and calculated with the formula [fasting insulin (mU L−1) × fasting glucose (mm)/22.5] (Matthews et al. 1985). Protein concentrations in the mitochondrial suspensions or myometrial tissue samples were determined by a modified Lowry method (Lowry et al. 1951) with BSA as standard. Citrate synthase (CS) activity in the mitochondrial suspensions or myometrial tissue samples was assayed according to Shepherd & Garland (1969). Triglyceride content in the myometrial samples was measured by hydrolysis coupled to enzymatic determination of glycerol (Kates, 1986) and absorbance changes at 340 nm were followed by spectrophotometry (Wieland, 1984).
RNA purification and quantitative real‐time PCR
RNA was extracted from the myometrial tissue using Qiazol (Qiagen, Valencia, CA, USA) and RNA was cleaned using RNeasy® Mini Kit (Qiagen) according to the manufacturer's instruction. Total RNA was mixed (at a concentration above 0.15 μg μL−1 for a total of 1.5 μg RNA in 20 μL volume) with reverse transcriptase, random hexamer primers and nucleotides and cDNA synthesis performed using the High Capacity cDNA Reverse Transcription Kit with RNase inhibitor (Applied Biosystems, Carlsbad, CA, USA) as described by de Melo et al. (2011). Amplification mixtures were amplified using a SYBR Green mastermix (Applied Biosystems) according to standard conditions ((95°C, 10 min) × 1, (95°C, 15 s; 60°C, 1 min; 95°C, 15 s; 60°C, 15 s; 95°C, 15 s) × 50 cycles in a total volume of 10 μL with a melting curve from 60 to 100°C) in 384‐well plates in duplicates on an ABI VIIA7 real‐time PCR system (Applied Biosystems). Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) mRNA levels were used for normalization between samples. The primers were designed according to the National Centre for Biotechnology Information ‘primer design tool’ online using Homo sapiens and adhered to the Minimum Information for Publication of Quantitative (MIQE) Real‐Time PCR Experiments guidelines. Primer sequences are described in Table 1. As there was no significant difference in glyceraldehyde‐3‐phpshpate dehydrogenase (GAPDH) mRNA levels between groups (P = 0.69) and expression level was very similar, GAPDH was selected for normalisation between samples.
Table 1.
Primer sequences used for quantitative real‐time PCR in this study
| Gene | Accession no. | Forward primer pair | Reverse primer pair |
|---|---|---|---|
| GAPDH | NM_002046.5 | AGGGCTGCTTTTAACTCTGGT | CCTGGAAGATGGTGATGGGAT |
| CD68 | NM_001040059.1 | CACCTGCTTCTCTCATTCCC | TTGTACTCCACCGCCATGTA |
| PGC‐1α | NM_013261.3 | GAGAAGCGAGAGTCTGAGAGG | GTTCTGTCCGTGTTGTGTCAG |
| PGC‐1β | NM_133263.3 | AAGAAGCACCCAGAGCGAA | ATGGTCTCCAAAGGAACAGGA |
| PPAR‐γ | NM_138712.3 | TTAGATGACAGCGACTTGGCA | TGGGCTTCACATTCAGCAAAC |
| TFAM | NM_003201.2 | GAGGCAGGAGTTTCGTTTTCA | ATATCACAGAACACCGTGGCT |
| CPT1A | NM_001876.3 | ATTTTGCTGTCGGTCTTGGAC | ACCAGTCGCTCACGTAATTTG |
Western blot
Myometrial tissue was mixed with a modified RIPA lysis buffer with protease and phosphatase inhibitors (an aqueous solution at pH 7.4 of: 50 mm Tris–HCl, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 0.25% deoxycholate, 1% Triton X‐100, 1 μg mL−1 pepstatin A, 1 mm Na3VO4, 1 mm NaF, Phosphatase inhibitor 1 and 2 (Sigma‐Aldrich) and a complete protease inhibitor cocktail (Roche, Basel, Switzerland). Tissue samples were mechanically homogenized using a Tissuelyzer (Qiagen) for 1 min at 30 Hz and 15 min incubation on ice, repeated 3 times. Samples were rotated end over for 1 h at 4°C before being centrifuged for 30 min at 20,000 g at 4°C and the supernatant transferred to a new tube. Protein concentration was determined using the Bio‐Rad DC kit (Bio‐Rad, Hercules, CA, USA) with BSA used as standard. All determinations were done in triplicate. Protein lysates were heated at 50°C for 2 min in NuPAGE LDS Sample Buffer Invitrogen (Thermo Fisher Scientific, Carlsbad, CA, USA) and proteins, 25 μg per lane, were separated by SDS‐PAGE electrophoresis (4% to 12% NuPAGE Bis‐Tris precast gels) and transferred to polyvinylidene diflouride membranes (0.20 A constant for 1 h). The membranes were stained with Ponceau‐S staining for transfer quality control and blocked at room temperature for 1 h in 5% skim milk in Tris‐buffered saline containing 0.1% Tween‐20 (TBS‐T) and incubated with primary antibody (diluted in 5% BSA or skim milk in TBS‐T) overnight at 4°C. Membranes were washed and incubated with an appropriate secondary antibody for 1 h at room temperature and visualized using ECL or Supersignal West Femto (Thermo Scientific, Pierce Technology, Omaha, NE, USA) employing a G:BOX imager (Syngene, Cambridge, UK). Densitometry analysis was performed using the ImageJ software (open source: http://rsbweb.nih.gov). The β‐actin protein levels were used for normalization between samples. The total OXPHOS antibody cocktail (cat. no ab‐110413) was obtained from Abcam (Cambridge, UK) and β‐actin (ACTB, cat. no. Sc‐47778) was obtained from Santa Cruz Biotechnology (Dallas, TX, USA).
Light microscopy
Paraffin embedding and histological staining
Myometrial biopsies of approximately 8 mm3 were immersion‐fixed in 2% paraformaldehyde and 0.1% glutaraldehyde and embedded in paraffin. Using a microtome the embedded myometrium samples were cut into 5 μm sections and mounted on glass microscope slides. The sections were dewaxed through graded concentrations of alcohol and xylene. For nucleus and cytoplasm visualization, sections were stained with haematoxylin–eosin (HE). Sections were incubated for 4 min in Mayers Hemalun, followed by a 5 min wash in running tap water followed by 0.1% eosin in Walpole acetate buffer pH 4.6 for 1 min and a short wash in running tap water. Finally sections were dehydrated in ascending ethanol to 99% and mounted with Pertex. For glycogen visualization, sections were stained with periodic acid–Schiff (PAS). Sections were incubated for 5 min in periodic acid, followed by a 5 min wash in water, then 40 min in Schiff's reagent followed by a 10 min wash in water. Sections were then counterstained in haematoxylin for 5 min and washed in water for 10 min. Sections were finally dehydrated in ascending ethanol to 99% and mounted with Pertex. For collagen visualization, sections were stained with Sirius Red. They were postfixed for 18 h in Bouin solution, followed by a 20 min wash in water. Sections were then incubated in Weigert's solution for 10 min, followed by a 5 min wash in water. Finally they were incubated in Sirius Red solution for 15 min, dehydrated in ascending ethanol to 99% and mounted with Pertex. For immunohistochemical staining sections were primarily boiled for 15 min in triethylene glycol buffer (pH 9) for antigen retrieval. They were then pre‐incubated in 10 min in 2% bovine serum albumin followed by 18 h incubation at 4°C with the primary antibody anti‐cytochrome c oxidase subunit IV (COX IV) (ab16056, Abcam), diluted 1:300. The immunoreaction was amplified by incubation for 40 min with biotinylated secondary antibody immunoglobulins goat anti‐rabbit (BA‐1000, Vector Laboratories, Burlingame, CA, USA) 1:200. Endogenous peroxidase was then blocked with 3% hydrogen peroxide. Sections were then incubated for 30 min with preformed avidin and biotinylated horseradish peroxidase macromolecular complex (Elite ABC, code no. PK‐6100, Vector Laboratories). Finally the reaction was developed by use of 3,3‐diaminobenzidine (KEM‐EN‐TEC Diagnostics, Taastrup, Denmark, cat. no. 4170) for 15 min, followed by 2 min incubation in 0.5% copper sulphate (Merck, Darmstadt, Germany), diluted in Tris buffer containing 0.05% Tween. Counterstaining was performed with Mayer's hemalun.
Image acquisition and processing
Images for quantification were recorded using a Zeiss Axioplan 2 plus microscope (Jena, Germany) fitted with a Photometrics CoolSNAP camera (Tucson, AZ, USA) and analysis was performed using Image‐Pro Plus 7.0 software. Images for quantification were recorded at ×20 magnification and each image represented 1,760,000 μm2 of tissue. Different representative areas were chosen and the area of stained structures (HE, PAS, Sirius Red or COX IV) was measured by selecting a coloured region of interest. The average total area measured per subject for each staining in μm2 was 1,505,000 for HE, 1,425,000 for PAS, 1,373,000 for Sirius Red and 1,059,000 for COX IV. Automatically, areas the with same colour were measured. Sections with insufficient muscle tissue or poor staining results were excluded. Means ± SEM were calculated for samples in each group. Representative images of the HE, PAS, Sirius Red and COX IV staining are shown in Fig. 1 A–H.
Figure 1. Representative sections of the myometrium from women at term stained with HE (A and B), PAS (C and D), Sirius Red (E and F) and COX IV (G and H).
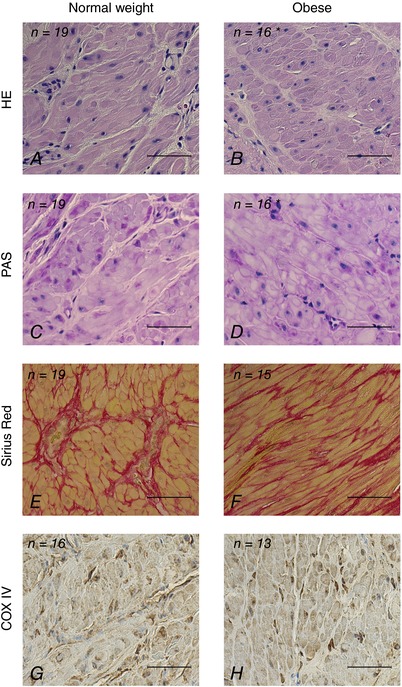
Percentage of stained area for normal weight (BMI >18 and <25 kg m−2) vs. obese women (BMI ≥30 kg m−2) was respectively 2.17 ± 0.12 vs. 1.79 ± 0.13% (P = 0.024) for nucleus stain (HE), 4.80 ± 0.23 vs. 4.12 ± 0.32% (P = 0.049) for glycogen stain (PAS), 36.35 ± 1.20 vs. 33.08 ± 1.36% (P = 0.076) for collagen stain (Sirius Red) and 2.60 ± 0.19 vs. 2.64 ± 0.22% (P = 0.927) for mitochondrial stain (anti COX IV antibody). Results are given as means ± SEM. Data were log‐transformed prior to analysis and signficant difference between groups was evaluated by Student's t test; *significant difference between groups. Scale bars: 50 μm.
Electron microscopy
Seven randomly chosen specimen blocks from each group were chosen for descriptive examination. Blocks were rinsed three times in 0.15 m sodium cacodylate buffer (pH 7.2) and subsequently postfixed in 1% w/v OsO4 in 0.12 m sodium cacodylate buffer (pH 7.2) for 2 h. The specimens were dehydrated in graded series of ethanol, transferred to propylene oxide and embedded in Epon according to standard procedures. Sections, approximately 80 nm thick, were cut with a UC7 microtome (Leica, Vienna, Austria) and collected on copper grids with Formvar supporting membranes, stained with uranyl acetate and lead citrate, and subsequently examined with a Philips CM 100 transmission electron microscope (TEM) (Philips, Eindhoven, The Netherlands), operated at an accelerating voltage of 80 kV. Digital images were recorded with an OSIS Veleta digital slow scan 2 × 2 k CCD camera and the ITEM software package (Olympus Soft Imaging Corp, Münster, Germany).
Statistics
Statistical analysis was performed using SAS 9.4 (SAS Institute, Cary, NC, USA). Normal distributions were checked with histograms and the Kolmogorov–Smirnov test. Data were log‐transformed prior to analysis when appropriate to obtain a normal distribution. A mixed model ANOVA was used for the analysis of the effect of obesity and substrate on oxidative capacity data in the isolated mitochondria. There were no significant interactions between effects. On all other data Student's unpaired two‐tailed t test was used for analysis of the effect of obesity. Results are given as means ± SEM unless otherwise stated. Statistical significance was set at the 95% level (P < 0.05).
Results
Characteristics of the groups of the study
Clinical and anthropometric characteristics of the 36 participating women showed that age, height, gestational age, blood pressure and obstetric history did not differ between the two BMI groups (Table 2). Weight gain during pregnancy was significantly lower in the obese group compared to the normal weight group (P = 0.006). Fasting venous insulin levels were on average almost twice as high in women with obesity (128 ± 13 vs. 68 ± 10 pmol L−1, P = 0.002) – accordingly the HOMA‐IR was also twice as high (3.92 ± 0.38 vs. 2.03 ± 0.43 mm.mU L−1, P < 0.001) – while fasting venous FFA levels were lower in women with obesity (0.28 ± 0.04 vs. 0.47 ± 0.04 mmol L−1, P = 0.043) (Table 3). Fasting venous plasma glucose, cholesterol, HDL, LDL and triglyceride levels did not differ between the groups.
Table 2.
Clinical and anthropometric characteristics of the normal weight group (18.5 < BMI < 25 kg m−2) and obese group (BMI ≥ 30 kg m−2)
| Normal wt | Obese | ||
|---|---|---|---|
| (n = 19) | (n = 17) | P | |
| Age (years) | 35 (24–44) | 35 (26–41) | 0.926 |
| Height (m) | 1.65 (1.58–1.74) | 1.68 (1.54–1.77) | 0.200 |
| Weight prior to pregnancy (kg) | 59 (50–65) | 93 (79–133)* | <0.001 |
| Weight at delivery (kg) | 75 (62–93) | 101 (78–132)* | <0.001 |
| Weight gain during pregnancy (kg) | 18 (9–30) | 10 (−10 to 24)* | 0.006 |
| BMI prior to pregnancy (kg m−2) | 21 (18–24) | 33 (30–45)* | <0.001 |
| BMI at delivery (kg m−2) | 27 (24–35) | 36 (30–45)* | <0.001 |
| Systolic blood pressure (mmHg) | 125 (108–150) | 130 (105–150) | 0.219 |
| Diastolic blood pressure (mmHg) | 74 (60–104) | 78 (59–94) | 0.754 |
| Parity | 1 (0–2) | 1 (0–3) | 0.254 |
| Number of previous Caesarean sections | 0 (0–2) | 1 (0–3) | 0.155 |
| Gestational age at Caesarean section (days) | 272 (264–280) | 269 (265–275) | 0.079 |
| Indication for elective Caesarean section | |||
| Maternal request | 12 | 10 | |
| Two or more previous Caesarean sections | 2 | 2 | |
| Breech presentation | 2 | 3 | |
| Other medical reason† | 3 | 2 | |
| Clinical history of dystocia during labor | 1 | 2 |
Clinical and anthropometric characteristics from women having a term elective Caesarean section grouped by BMI. Results are given as median and range for each group. Significance of difference between groups was evaluated by Student's t test; *significant difference between groups. †Abdominal cerclage, lichen sclerosus, anal fistula.
Table 3.
Plasma parameters of the normal weight group (18.5 < BMI < 25 kg m−2) and obese group (BMI ≥ 30 kg m−2)
| Normal wt | Obese | ||
|---|---|---|---|
| (n = 19) | (n = 17) | P | |
| Glucose (mm) | 4.4 ± 0.2 | 4.5 ± 0.1 | 0.420 |
| Insulin (pmol L−1)† | 68 ± 10 | 128 ± 13* | 0.002 |
| HOMA‐IR (mm.mU L−1)† | 2.03 ± 0.43 | 3.92 ± 0.38* | <0.001 |
| Cholesterol (mm) | 6.6 ± 0.3 | 6.8 ± 0.3 | 0.617 |
| HDL (mm) | 1.96 ± 0.10 | 1.86 ± 0.11 | 0.573 |
| LDL (mm) | 3.9 ± 0.3 | 4.2 ± 0.3 | 0.378 |
| Triglycerides (mm) | 2.71 ± 0.29 | 2.61 ± 0.19 | 0.867 |
| FFA (mm) | 0.47 ± 0.04 | 0.28 ± 0.04* | 0.043 |
Plasma parameters from the women having a term elective Caesarean section grouped by BMI. Fasting venous plasma samples were taken approximately 1 h prior to delivery. HOMA‐IR, homeostatic model assessment of insulin resistance; HDL, high density lipoprotein; LDL, low density lipoprotein; FFA, free fatty acid. Results are given as mean ± SEM. Data was logtransformed prior to analysis and significance of difference between groups was evaluated by Student's t test; *significant difference between groups. † n = 18 and n = 16, respectively in the normal‐weight and obese group, due to missing analysis of insulin.
Characteristics of the myometrium
Biochemical analyses of the myometrium revealed that triglyceride content was significantly higher in the women with obesity (2.39 ± 0.26 vs. 1.56 ± 0.20 mm, P = 0.024) (Table 4). Protein content and CS activity in g wet weight myometrium was significantly lower in the group with obesity (109.2 ± 7.2 vs. 139.4 ± 5.6 mg g−1 wet wt, P = 0.002, and 24.8 ± 1.0 vs. 29.6 ± 1.4 U g−1 wet wt, P = 0.008, respectively), but CS activity‐to‐protein content did not differ between the groups. None of the complexes of the respiratory chain examined by Western blot differed. Myometrial mRNA levels of genes related to mitochondrial biogenesis (PGC‐1α, PGC‐1β, transcriptional mitochondrial factor A (TFAM), carnitine palmitoyl transferase 1A (CPT1A)) and inflammation (PPAR‐γ and CD68), examined by quantitative real‐time PCR, did not differ between the groups (Table 5).
Table 4.
Biochemical analyses of the myometrium at term of the normal weight group (18.5 < BMI < 25 kg m−2) and obese group (BMI ≥ 30 kg m−2)
| Normal wt | Obese | ||
|---|---|---|---|
| (n = 19) | (n = 17) | P | |
| Triglycerides (mm) | 1.56 ± 0.20 | 2.39 ± 0.26* | 0.024 |
| Protein (mg g−1 wet wt) | 139.4 ± 5.6 | 109.2 ± 7.2* | 0.002 |
| CS activity (U g−1 wet wt) | 29.6 ± 1.4 | 24.8 ± 1.0* | 0.008 |
| CS activity (U mg−1 protein) | 0.22 ± 0.01 | 0.24 ± 0.02 | 0.363 |
| Complex I (protein, AU) | 2.06 ± 0.38 | 2.33 ± 0.65 | 0.825 |
| Complex II (protein, AU) | 1.55 ± 0.21 | 2.25 ± 0.39 | 0.589 |
| Complex III (protein, AU) | 1.09 ± 0.12 | 1.06 ± 0.18 | 0.659 |
| Complex IV (protein, AU) | 1.90 ± 0.26 | 2.36 ± 0.45 | 0.425 |
| Complex V (protein, AU) | 0.99 ± 0.07 | 1.04 ± 0.10 | 0.653 |
Triglycerides, protein by wet weight, CS activity expressed by wet weight or protein and complex I, II, III, IV and V of the respiratory chain in the myometrium in women at term grouped by BMI. CS, citrate synthase. Results are given as mean ± SEM. Data were log‐transformed prior to analysis and significance of difference between groups was evaluated by Student's t test; *significant difference between groups.
Table 5.
mRNA levels of genes related to mitochondrial biogenesis and inflammation in the myometrium at term of the normal weight group (18.5 < BMI < 25 kg m−2) and obese group (BMI ≥ 30 kg m−2)
| Normal wt | Obese | ||
|---|---|---|---|
| (n = 19) | (n = 17) | P | |
| CD68 (mRNA, AU) | 1.23 ± 0.12 | 1.02 ± 0.34 | 0.127 |
| PGC‐1α (mRNA, AU) | 1.14 ± 0.26 | 1.80 ± 0.16 | 0.120 |
| PGC‐1β (mRNA, AU) | 1.07 ± 0.09 | 1.10 ± 0.09 | 0.827 |
| PPAR‐γ (mRNA, AU) | 1.30 ± 0.19 | 1.27 ± 0.12 | 0.839 |
| TFAM (mRNA, AU) | 1.31 ± 0.07 | 1.30 ± 0.75 | 0.513 |
| CPT1A (mRNA, AU) | 1.09 ± 0.07 | 1.17 ± 0.71 | 0.997 |
RNA levels of genes related to mitochondrial biogenesis and inflammation measured by quantitative real‐time PCR in the myometrium at term normalized to GAPDH mRNA levels grouped by BMI. CD68, cluster of differentiation 68; PGC‐1α, peroxisome proliferator‐activated receptor γ coactivator 1 α; PCG‐1β, peroxisome proliferator‐activated receptor γ coactivator 1 β; PPAR‐γ, peroxisome proliferator‐activated receptor γ; TFAM, mitochondrial transcriptional factor A; CPT1A carnitine palmitoyltransferase 1A. Results are given as mean ± SEM. Data were log‐transformed prior to analysis and significance of difference between groups was evaluated by Student's t test; no significant difference between groups was found.
Oxidative capacity in the myometrium
The oxygen consumption was measured in mitochondria isolated from the myometrium as described in Methods. In state 4 and 3 respiration, as well as state 3 with activation of complex II with succinate and inhibition of complex I with rotenone, oxygen consumption in the isolated myometrial mitochondria did not differ significantly between the two groups, regardless of whether the oxygen consumption substrate was a carbohydrate (pyruvate) or a fatty acid (palmitoyl carnitine) (Table 6). Still, the respiratory control ratio (RCR) was on average 20% lower in the obese group compared with control regardless of the substrate supplied (P = 0.004). The effect of adding succinate or rotenone did not differ between groups. However, the effect of ADP and rotenone was significantly reduced when palmitoyl carnitine was the substrate. The phosphorylation efficiency, expressed by the ATP formation‐to‐oxygen consumption ratio (P/O2), did not differ between the groups (Table 6). The CS activity in the isolated mitochondrial suspension did not differ between the groups (0.19 ± 0.03 vs. 0.19 ± 0.02 U mg−1 protein, P = 0.538).
Table 6.
Oxidative capacity (nmol O2 min−1 mg protein−1) in isolated mitochondria from the myometrium at term of the normal weight group (18.5 < BMI < 25 kg m−2) and obese group (BMI ≥ 30 kg m−2)
| Fixed effect | ||||||||
|---|---|---|---|---|---|---|---|---|
| Normal wt | Obese | BMI | PY or PC | Effect interaction | ||||
| Line | Respiration state | Substrates | (n = 19) | (n = 17) | P | P | P | |
| 1 | State 4 respiration | M + PY | 4.2 ± 0.4 | 5.8 ± 1.0 | 0.328 | 0.837 | 0.560 | |
| M + PC | 4.7 ± 0.5 | 5.5 ± 0.9 | ||||||
| 2 | State 3 respiration | M + PY + D | 12.4 ± 1.2 | 13.9 ± 2.4 | 0.521 | 0.129 | 0.632 | |
| M + PC + D | 11.2 ± 1.4 | 10.8 ± 2.0 | ||||||
| 3 |
|
M + PY + D + S | 20.5 ± 1.9 | 21.6 ± 3.8 | 0.374 | 0.145 | 0.768 | |
| M + PC + D + S | 17.9 ± 2.0 | 17.1 ± 2.9 | ||||||
| 4 | Cytochrome c control | M + PY + D + S + C | 21.3 ± 2.0 | 22.9 ± 3.9 | 0.470 | 0.264 | 0.756 | |
| M + PC + D + S + C | 19.5 ± 2.2 | 18.9 ± 3.2 | ||||||
| 5 | Complex II respiration | M + PY + D + S + C + R | 16.2 ± 1.5 | 17.0 ± 3.0 | 0.422 | 0.994 | 0.894 | |
| M + PC + D + S + C + R | 16.6 ± 1.7 | 15.8 ± 2.2 | ||||||
| 6 | ADP effect | For PY: line 2 − line 1 | 8.2 ± 0.9 † | 8.1 ± 1.7† | 0.067 | 0.013 | 0.521 | |
| For PC: line 2 − line 1 | 6.5 ± 1.0 | 5.3 ± 1.4 | ||||||
| 7 | Succinate effect | For PY: line 3 − line 2 | 8.1 ± 0.9 | 7.7 ± 1.5 | 0.252 | 0.149 | 0.962 | |
| For PC: line 3 − line 2 | 6.7 ± 0.8 | 6.2 ± 1.0 | ||||||
| 8 | Cytochrome c effect | For PY: line 4 − line 3 | 0.9 ± 0.2 | 1.2 ± 0.3 | 0.590 | 0.163 | 0.310 | |
| For PC: line 4 − line 3 | 1.6 ± 0.4 | 1.4 ± 0.3 | ||||||
| 9 | Rotenone effect | For PY: line 4 − line 5 | 6.0 ± 0.9† | 5.8 ± 1.3† | 0.142 | 0.008 | 0.097 | |
| For PC: line 4 − line 5 | 2.9 ± 1.0 | 2.0 ± 1.3 | ||||||
| 10 | RCR | For PY: line 2/line 1 | 3.1 ± 0.2† | 2.5 ± 0.2† , ‡ | 0.004 | 0.002 | 0.855 | |
| For PC: line 2/line 1 | 2.5 ± 0.2 | 2.0 ± 0.2‡ | ||||||
| 11 | P/O2 | M + PY + D | 4.7 ± 0.4 | 4.1 ± 0.3 | 0.526 | — | — | |
Oxygen consumption measured in isolated mitochondria from the myometrium of women at term grouped by BMI and by PY or PC. C, cytochrome c; D, adenosine diphosphate; M, malate; PC, palmitoyl carnitine; P/O2, phosphate to oxygen ratio; PY, pyruvate; R, rotenone; RCR, respiratory control ratio; S, succinate. Results are given as mean ± SEM. Data were log‐transformed prior to analysis and significance of difference between groups was evaluated by a mixed model ANOVA. Significant difference is indicated: †with respect to significant difference between substrate, ‡with respect to significant difference between BMI groups.
Light and electron microscopy
Representative sections stained for visualization of nucleus and cytoplasm (haematoxylin–eosin, HE), glycogen (periodic acid–Schiff, PAS), collagen (Sirius Red) and mitochondria (COX IV) from the myometrium were evaluated (Fig. 1). The stained areas for nuclei (HE) and glycogen (PAS) in the obese group compared to the normal weight group were significantly smaller (respectively 1.79 ± 0.13 vs. 2.17 ± 0.12%, P = 0.024, and 4.12 ± 0.32 vs. 4.80 ± 0.23%, P = 0.049) and it was noted that the collagen stained area (Sirius Red) tended to be smaller (33.08 ± 1.36 vs. 36.35 ± 1.20%, P = 0.076). No difference was detected between groups in the COX IV stained area. TEM images from the myometrium of seven women from each group disclosed no difference in morphology and localization of mitochondria in myocytes between the groups. The mitochondria in the myocytes appeared mainly to have perinuclear and subsarcolemmal localization (Fig. 2), often seemingly arranged as ‘pearls on a string’ (Fig. 2 A and 2 B). Perinuclear mitochondria were associated with rough endoplasmic reticulum, Golgi apparatus and clusters of glycogen (Fig. 2 C and 2 D), whereas subsarcolemmal mitochondria were seen in proximity to invaginations (caveolae) of the plasma membrane (Fig. 2 E).
Figure 2. Localization of the mitochondria in the myometrial myocyte.
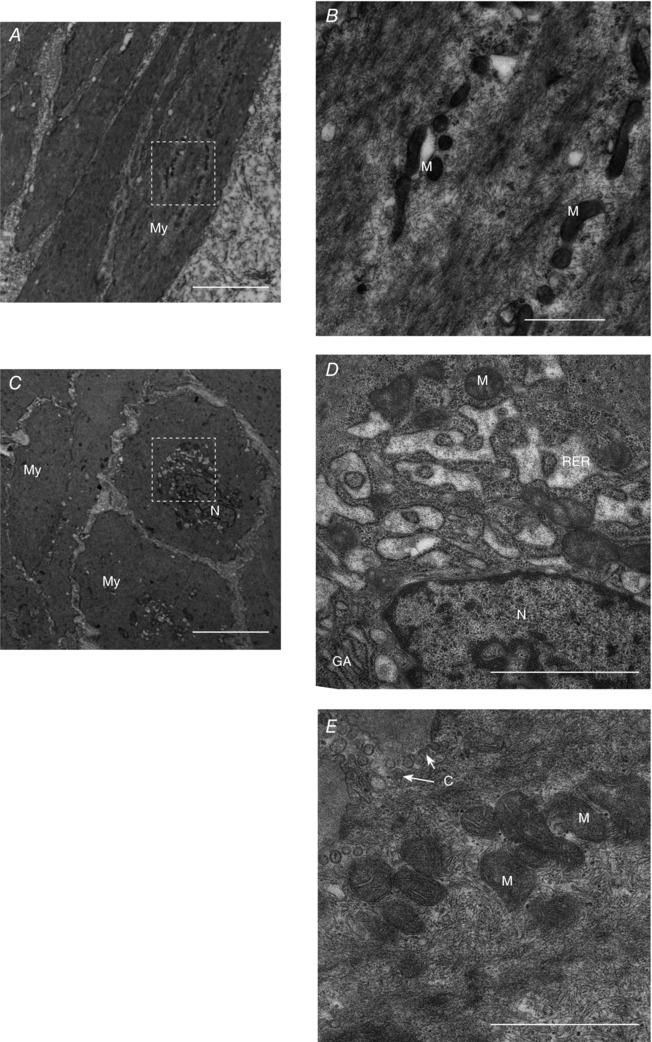
A and C, longitudinal (A) and cross‐sectional (C) TEM micrographs of myocytes from the myometrium at term with perinuclear and subsarcolemmal mitochondria. B and D, perinuclear mitochondria are arranged as ‘pearls on a string’ (B) or in relation to other organelles (D). E, subsarcolemmal mitochondria in association with caveolae. C, caveolae; GA, Golgi apparatus; M, mitochondria; My, myocyte; N, nucleus; RER, rough endoplasmic reticulum. Scale bars: 5 μm (A and C), 1 μm (B, D and E).
Discussion
A reduction of myometrial mitochondrial capacity or quantity, or both, mediated by obesity could decrease the efficiency of uterine contractility during labour (Lowe & Corwin, 2011). The present study shows, however, that obesity during pregnancy does not change mitochondrial content, morphology, localization or function in the myocyte in the myometrium. Yet, we find strong indications of reduced muscle content with increased triglyceride levels in the myometrium at term. This latter observation may enable further understanding of the biological mechanisms behind labour dystocia in obesity.
Mitochondrial content in the myometrial myocyte seems unaffected by obesity as CS activity and amounts of mitochondrial complexes, as expressed per total myometrial protein, did not differ between normal weight and obese pregnant women. Markers of mitochondrial biogenesis, PGC‐1α, PGC‐1β, CPT1A or TFAM, were also unaffected, rendering no indication of altered regulation of mitochondria. Although TEM micrographs of the myocytes did not provide any indication of alteration in mitochondrial localization or morphology, the finding of a distinct perinuclear or subsarcolemmal localization in proximity to caveolae, as previously described in rats (Gam et al. 2015), might be of importance for understanding the contraction–relaxation cycle in the myometrial myocyte, as energy‐dependent caveolae endocytosis seems essential for normal contractile function (Wray, 2007). Additionally altered organization of the mitochondria could be of importance for contractile function of the myocyte and interestingly the linear relationship or ‘pearls on a string’ formation noted in some of the images gives the distinct impression that the mitochondria are organized in a structured entity, as seen in other muscle tissue (Vincent et al. 2016). Further exploration of this would require other methods (e.g. serial block face scanning and subsequent 3D reconstruction) than those presented in the present study, but could provide new insight on how the myometrial myocyte contracts.
Mitochondrial function also seemed unaffected, with the exception of a significantly decreased RCR in obese women. Mitochondrial RCR depends on numerous factors and is a complex entity – a change in almost any part of the oxidative phosphorylation will change RCR (Brand & Nicholls, 2011) and RCR is usually taken as an indicator of the ‘quality’ of the mitochondrial preparation. The ‘quality’ can also be assessed by the increase in oxygen consumption seen by addition of exogenous cytochrome c. However the cytochrome c effect on oxygen consumption did not differ between groups and the effect did not correlate to RCR either (analysis not shown) – suggesting that different qualities of the isolated mitochondria are reflected by the two parameters. It is also of note that the P/O2 did not differ between groups. It is tempting to suggest that the difference in RCR is somehow related to the finding of increased triglyceride levels in the myometrium, although at this stage it remains a speculation.
However, the triglyceride levels were 1.5 times higher in the myometrium of obese women and, accordingly, both protein and CS per wet weight content were decreased. The decrease in protein content in the obese women can to some extent be explained by the increase in triglyceride content: the difference between groups in protein and triglyceride content is 1.51 mg g−1 and 0.83 mm, respectively, and taking the molar weight of triglyceride to 850 g mol−1, approximately half of the difference in protein content can be attributed to the increased triglyceride content (0.83 mm/850 g mol−1 = 0.71 mg g−1). These differences are substantiated by our histological findings. Light microscopy sections stained for nuclei, cytoplasm, glycogen and collagen supported the idea of a smaller density of myocytes in the myometrium in obese women, while no difference was seen between groups for mitochondrial staining. This might be due to exclusion of more COX IV stained sections, because of poor staining results. Our histological findings are in contrast with those of Sweeney et al. (2013), where no influence of BMI on smooth muscle cell or extracellular matrix in the myometrium was revealed. Their study had a similar study population and size, but their approach to the analysis of the stained sections differed from the present study by both sample handling and quantification method. Especially the macroscopic dissection of myometrial strips with elimination of presumptive non‐muscular tissue could lead to unrepresentative sampling. The same group also did a study on TEM images of myometrial myocytes to identify cell and nuclear volume in the myometrium at term in obese women where no effects were identified (Sweeney et al. 2014). Although a large number of TEM images from each patient was examined, the area actually examined in each case was smaller than what we examined by light microscopy and combined with a different quantification method, this might account for the diverging results. No information on the myometrial composition in regards to triglyceride, protein or citrate synthase content has been reported in any of these previous studies (Sweeney et al. 2013, 2014). To our knowledge, there are no studies reporting such measurement in the myometrium at term in obese women and studies modelling obesity during pregnancy in rats convey rather conflicting findings (Elmes et al. 2011; Gam et al. 2015). As samples from the myometrium in the present study were isolated during Caesarean section, our sampling was not contaminated by, for example, adipose tissue, which is supported by the histology presented.
Elevated plasma FFA levels or increased dietary fat content are known to increase intramyocellular lipid content in skeletal muscle tissue, suggesting that skeletal muscle stores fat simply if the availability of fatty acids is high (Schrauwen‐Hinderling et al. 2006). A similar mechanism may be in play in the myometrium, but at this stage we have not identified the location of the triglycerides in the myometrium. The issue of lipotoxicity presumably only arises when the fatty acid oxidation capacity in the skeletal muscle is surpassed (Sorensen, 2012) and it is unknown if this occurs in the myometrium. Animal studies clearly indicate that in early pregnancy myocytes first proliferate, whilst in late pregnancy a substantial hypertrophy of the myocytes occurs (Shynlova et al. 2009) – and this could affect the duration of exposure to FFA oxidation in the myometrial myocytes. Indeed obesity did not seem to have any influence on the expression of gene markers of inflammation, respectively CD68 and PPAR‐γ, which differs from the findings in skeletal muscle in obese subjects (Consitt et al. 2009; Schrauwen et al. 2010; Sorensen, 2012). Is it credible that these obese women are exposed to higher FFA levels? We do not have non‐fasting plasma parameters to compare with the fasting values and a study by Buijs et al. (2003) strongly indicated that the lipolytic response to fasting in abdominally obese women is reduced. This mechanism could explain the lower FFA levels found in the pregnant obese women – thus not excluding higher amounts of FFA levels in the non‐fasting state leading to intramyocellular lipid storage in the myometrium. Fasting plasma cholesterol triglycerides, HDL, and LDL were not different between groups, although increased non‐fasting levels in the late pregnancy in obese women have been reported (Merzouk et al. 2000; Dube et al. 2012) and non‐fasting plasma lipid levels would have been interesting.
We assume glucose homeostasis was normal in both groups (women with a BMI above 27 kg m−2 or familiar disposition to diabetes are routinely proposed an oral glucose tolerance test in the second trimester in Denmark) and fasting blood glucose was also equal in both groups. Although fasting seems to decrease insulin levels equally in both lean and obese women (Buijs et al. 2003), the obese women in our study still had twice as high a HOMA‐IR as the normal‐weight women. Bearing in mind that insulin resistance is a normal physiological response in pregnancy (Herrera & Ortega‐Senovilla, 2010; Catalano & Shankar, 2017), this significantly higher level of plasma insulin, combined with the increased insulin resistance in obese women, would be expected to decrease lipolysis in the adipose tissue, stimulate hepatic FFA uptake and possibly stimulate fat storage in the myometrium, by a mechanism similar to the one seen in skeletal muscle (Herrera & Ortega‐Senovilla, 2010). These effects in synergy with fasting could thus explain the lower FFA levels in the obese women, as well as the elevated triglyceride content in the myometrium.
Weight gain during pregnancy differed greatly between groups and, for some of the obese women, pregnancy was accompanied by a weight loss or very small weight gain. This phenomenon is well known (Nohr et al. 2008) and might even reduce perinatal risks associated with obesity (Bogaerts et al. 2015), although fetal growth might also be impaired by no or very low maternal weight gain in obese women (Catalano et al. 2014). The groups in the present study are too small and too diverse in their weight gain to meaningfully stratify groups according to weight gain and pre‐pregnancy BMI, but it is difficult not to speculate on how FFA levels would be affected by lesser weight gain or no weight gain, and how this ultimately could affect the myometrium. Weight loss during pregnancy in obese women could increase general FFA levels, but at the same time weight loss is often accompanied by a change in food consumption – this, combined with the physiological effects of pregnancy, makes it difficult to predict exactly what the availability of FFA would be.
In this study we present new insight on the myometrium at term in normal weight and obese women. Mitochondria in the term myometrium in healthy normal‐weight and obese women are described in detail in terms of amount, function, morphology and localization and it seems clear that obesity per se does not influence mitochondrial phenotype in the myometrium. The increased triglyceride in the myometrium in obese women seems linked to a smaller density of myocytes, which in itself might decrease contractility force. In turn this could contribute to the pathophysiological mechanisms of labour dystocia. However, to verify this hypothesis, more detailed studies of triglyceride localization and of the lipid metabolism in the myometrium, in combination with myometrial contractility studies, as well as studies in labouring women, are needed. Nonetheless, the presented results add to the understanding of systemic effects of obesity, placing also the myometrium at term as an affected non‐adipose tissue.
Additional information
Competing interests
The authors declare that they have no competing interest with the contents of this article.
Author contributions
C.M.B.F.G., B.Q., P.D., O.H.M. and L.H.L. planned and designed the study. C.M.B.F.G., P.D., E.R.M. and L.E. were responsible for subject inclusion. C.M.B.F.G. acquired the blood and tissue samples. C.M.B.F.G. conducted the mitochondrial respiratory experiments. C.M.B.F.G., K.Q. and S.S.P. conducted the histological analyses. C.M.B.F.G., O.H.M. and L.H.L. conducted the biochemical analyses, including gene and protein expression analyses. C.M.B.F.G., L.E. and O.H.M. did the statistical analyses. All authors contributed in writing the manuscript, approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed. The coordination of the correspondence between authors was organized by C.M.B.F.G.
Funding
The study is part of C.M.B.F.G.’s PhD project funded by the Faculty of Health and Medical Sciences, University of Copenhagen, and by the PhD program of Diabetes and Metabolism, Faculty of Health Sciences, University of Southern Denmark.
Acknowledgements
We thank Mr Ib Therkelsen, Panum NMR Centre, and Mrs Bettina Starup Mentz, Section for Cellular and Metabolic Research, for expert technical assistance during the conductance of the experiments. We also thank Ms Zhila Nikrozi, Core Facility for Integrated Microscopy, and Mrs Heidi Marie Paulsen, Endocrinology Research Section, for expert technical assistance in section preparation for electron microscopy and light microscopy, respectively.
References
- Alsaif S, Mumtaz S & Wray S (2015). A short review of adipokines, smooth muscle and uterine contractility. Life Sci 125, 2–8. [DOI] [PubMed] [Google Scholar]
- Bakkman L, Fernstrom M, Loogna P, Rooyackers O, Brandt L & Lagerros YT (2010). Reduced respiratory capacity in muscle mitochondria of obese subjects. Obes Facts 3, 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaerts A, Ameye L, Martens E & Devlieger R (2015). Weight loss in obese pregnant women and risk for adverse perinatal outcomes. Obstet Gynecol 125, 566–575. [DOI] [PubMed] [Google Scholar]
- Brand MD & Nicholls DG (2011). Assessing mitochondrial dysfunction in cells. Biochem J 435, 297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs MM, Burggraaf J, Wijbrandts C, de Kam ML, Frolich M, Cohen AF, Romijn JA, Sauerwein HP, Meinders AE & Pijl H (2003). Blunted lipolytic response to fasting in abdominally obese women: evidence for involvement of hyposomatotropism. Am J Clin Nutr 77, 544–550. [DOI] [PubMed] [Google Scholar]
- Carlson NS, Hernandez TL & Hurt KJ (2015). Parturition dysfunction in obesity: time to target the pathobiology. Reprod Biol Endocrinol 13, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro LC & Avina RL (2002). Maternal obesity and pregnancy outcomes. Curr Opin Obstet Gynecol 14, 601–606. [DOI] [PubMed] [Google Scholar]
- Catalano PM, Mele L, Landon MB, Ramin SM, Reddy UM, Casey B, Wapner RJ, Varner MW, Rouse DJ, Thorp JM Jr, Saade G, Sorokin Y, Peaceman AM & Tolosa JE (2014). Inadequate weight gain in overweight and obese pregnant women: what is the effect on fetal growth? Am J Obstet Gynecol 211, 137.e1–137.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano PM & Shankar K (2017). Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ 356, j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedergren MI (2009). Non‐elective caesarean delivery due to ineffective uterine contractility or due to obstructed labour in relation to maternal body mass index. Eur J Obstet Gynecol Reprod Biol 145, 163–166. [DOI] [PubMed] [Google Scholar]
- Chiossi G, Costantine MM, Betancourt A, Hankins GD, Longo M, Saade GR & Bytautiene E (2010). Effect of maternal body mass index on in vitro response to tocolytics in term myometrium. Am J Obstet Gynecol 203, 261.e1–261.e5. [DOI] [PubMed] [Google Scholar]
- Chung JH, Melsop KA, Gilbert WM, Caughey AB, Walker CK & Main EK (2012). Increasing pre‐pregnancy body mass index is predictive of a progressive escalation in adverse pregnancy outcomes. J Matern Fetal Neonatal Med 25, 1635–1639. [DOI] [PubMed] [Google Scholar]
- Consitt LA, Bell JA & Houmard JA (2009). Intramuscular lipid metabolism, insulin action, and obesity. IUBMB Life 61, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crankshaw DJ, O'Brien YM, Crosby DA & Morrison JJ (2017). Maternal body mass index and spontaneous contractility of human myometrium in pregnancy. J Perinatol 37, 492–497. [DOI] [PubMed] [Google Scholar]
- de Melo JF, Aloulou N, Duval JL, Vigneron P, Bourgoin L, Leandro CG, de Castro CM & Nagel MD (2011). Effect of a neonatal low‐protein diet on the morphology of myotubes in culture and the expression of key proteins that regulate myogenesis in young and adult rats. Eur J Nutr 50, 243–250. [DOI] [PubMed] [Google Scholar]
- Di Meo S, Iossa S & Venditti P (2017). Skeletal muscle insulin resistance: role of mitochondria and other ROS sources. J Endocrinol 233, R15–R42. [DOI] [PubMed] [Google Scholar]
- Dube E, Gravel A, Martin C, Desparois G, Moussa I, Ethier‐Chiasson M, Forest JC, Giguere Y, Masse A & Lafond J (2012). Modulation of fatty acid transport and metabolism by maternal obesity in the human full‐term placenta. Biol Reprod 87, 14, 1–11. [DOI] [PubMed] [Google Scholar]
- Elmes MJ, Tan DS, Cheng Z, Wathes DC & McMullen S (2011). The effects of a high‐fat, high‐cholesterol diet on markers of uterine contractility during parturition in the rat. Reproduction 141, 283–290. [DOI] [PubMed] [Google Scholar]
- Fritzen AJ, Grunnet N & Quistorff B (2007). Flux control analysis of mitochondrial oxidative phosphorylation in rat skeletal muscle: pyruvate and palmitoyl‐carnitine as substrates give different control patterns. Eur J Appl Physiol 101, 679–689. [DOI] [PubMed] [Google Scholar]
- Gam CM, Mortensen OH, Qvortrup K, Damm P & Quistorff B (2015). Effect of high‐fat diet on rat myometrium during pregnancy—isolated myometrial mitochondria are not affected. Pflugers Arch 467, 1539–1549. [DOI] [PubMed] [Google Scholar]
- Garabedian MJ, Hansen WF, McCord LA, Manning MA, O'Brien JM & Curry TE, Jr. (2013). Up‐regulation of oxytocin receptor expression at term is related to maternal body mass index. Am J Perinatol 30, 491–497. [DOI] [PubMed] [Google Scholar]
- Grotegut CA, Gunatilake RP, Feng L, Heine RP & Murtha AP (2013). The influence of maternal body mass index on myometrial oxytocin receptor expression in pregnancy. Reprod Sci 20, 1471–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehir MP, Glavey SV & Morrison JJ (2008). Uterorelaxant effect of ghrelin on human myometrial contractility. Am J Obstet Gynecol 198, 323.e1–323.e5. [DOI] [PubMed] [Google Scholar]
- Hehir MP & Morrison JJ (2012). The adipokine apelin and human uterine contractility. Am J Obstet Gynecol 206, 359.e1–359.e5. [DOI] [PubMed] [Google Scholar]
- Herrera E & Ortega‐Senovilla H (2010). Disturbances in lipid metabolism in diabetic pregnancy – Are these the cause of the problem? Best Pract Res Clin Endocrinol Metab 24, 515–525. [DOI] [PubMed] [Google Scholar]
- Higgins CA, Martin W, Anderson L, Blanks AM, Norman JE, McConnachie A & Nelson SM (2010). Maternal obesity and its relationship with spontaneous and oxytocin‐induced contractility of human myometrium in vitro. Reprod Sci 17, 177–185. [DOI] [PubMed] [Google Scholar]
- Highley LL, Previs RA, Dotters‐Katz SK, Brancazio LR & Grotegut CA (2016). Cesarean delivery among women with prolonged labor induction. J Perinat Med 44, 759–766. [DOI] [PubMed] [Google Scholar]
- Holloway GP, Bonen A & Spriet LL (2009). Regulation of skeletal muscle mitochondrial fatty acid metabolism in lean and obese individuals. Am J Clin Nutr 89, 455S–462S. [DOI] [PubMed] [Google Scholar]
- Jorgensen W, Rud KA, Mortensen OH, Frandsen L, Grunnet N & Quistorff B (2017). Your mitochondria are what you eat: a high‐fat or a high‐sucrose diet eliminates metabolic flexibility in isolated mitochondria from rat skeletal muscle. Physiol Rep 5, e13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates M (1986). Techniques in Lipidology. Elsevier, New York. [Google Scholar]
- Lowe NK & Corwin EJ (2011). Proposed biological linkages between obesity, stress, and inefficient uterine contractility during labor in humans. Med Hypotheses 76, 755–760. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL & Randall RJ (1951). Protein measurement with the Folin phenol reagent. J Biol Chem 193, 265–275. [PubMed] [Google Scholar]
- Magann EF, Doherty DA, Sandlin AT, Chauhan SP & Morrison JC (2013). The effects of an increasing gradient of maternal obesity on pregnancy outcomes. Aust N Z J Obstet Gynaecol 53, 250–257. [DOI] [PubMed] [Google Scholar]
- Mantakas A & Farrell T (2010). The influence of increasing BMI in nulliparous women on pregnancy outcome. Eur J Obstet Gynecol Reprod Biol 153, 43–46. [DOI] [PubMed] [Google Scholar]
- Maples JM, Brault JJ, Shewchuk BM, Witczak CA, Zou K, Rowland N, Hubal MJ, Weber TM & Houmard JA (2015). Lipid exposure elicits differential responses in gene expression and DNA methylation in primary human skeletal muscle cells from severely obese women. Physiol Genomics 47, 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF & Turner RC (1985). Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419. [DOI] [PubMed] [Google Scholar]
- Mendis S, Armstrong T, Bettcher D, Branca F, Lauer J, Mace C, Poznyak V, Leanne R, Da Costa E Silva V & Stevens G (2014). Global Status Report on Noncommunicable Diseases 2014. WHO Press, Geneva. [Google Scholar]
- Merzouk H, Meghelli‐Bouchenak M, Loukidi B, Prost J & Belleville J (2000). Impaired serum lipids and lipoproteins in fetal macrosomia related to maternal obesity. Biol Neonate 77, 17–24. [DOI] [PubMed] [Google Scholar]
- Mittendorfer B (2011). Origins of metabolic complications in obesity: adipose tissue and free fatty acid trafficking. Curr Opin Clin Nutr Metab Care 14, 535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynihan AT, Hehir MP, Glavey SV, Smith TJ & Morrison JJ (2006). Inhibitory effect of leptin on human uterine contractility in vitro. Am J Obstet Gynecol 195, 504–509. [DOI] [PubMed] [Google Scholar]
- Mumtaz S, AlSaif S, Wray S & Noble K (2015). Inhibitory effect of visfatin and leptin on human and rat myometrial contractility. Life Sci 125, 57–62. [DOI] [PubMed] [Google Scholar]
- Nohr EA, Vaeth M, Baker JL, Sorensen T, Olsen J & Rasmussen KM (2008). Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr 87, 1750–1759. [DOI] [PubMed] [Google Scholar]
- Parkington HC, Stevenson J, Tonta MA, Paul J, Butler T, Maiti K, Chan EC, Sheehan PM, Brennecke SP, Coleman HA & Smith R (2014). Diminished hERG K+ channel activity facilitates strong human labour contractions but is dysregulated in obese women. Nat Commun 5, 4108. [DOI] [PubMed] [Google Scholar]
- Ritov VB, Menshikova EV, Azuma K, Wood R, Toledo FG, Goodpaster BH, Ruderman NB & Kelley DE (2010). Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. Am J Physiol Endocrinol Metab 298, E49–E58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauwen‐Hinderling VB, Hesselink MK, Schrauwen P & Kooi ME (2006). Intramyocellular lipid content in human skeletal muscle. Obesity (Silver Spring) 14, 357–367. [DOI] [PubMed] [Google Scholar]
- Schrauwen‐Hinderling VB, Kooi ME & Schrauwen P (2016). Mitochondrial function and diabetes: consequences for skeletal and cardiac muscle metabolism. Antioxid Redox Signal 24, 39–51. [DOI] [PubMed] [Google Scholar]
- Schrauwen P, Schrauwen‐Hinderling V, Hoeks J & Hesselink MK (2010). Mitochondrial dysfunction and lipotoxicity. Biochim Biophys Acta 1801, 266–271. [DOI] [PubMed] [Google Scholar]
- Shepherd D & Garland PB (1969). The kinetic properties of citrate synthase from rat liver mitochondria. Biochem J 114, 597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shynlova O, Tsui P, Jaffer S & Lye SJ (2009). Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol 144(Suppl 1), S2–S10. [DOI] [PubMed] [Google Scholar]
- Smith RD, Babiychuk EB, Noble K, Draeger A & Wray S (2005). Increased cholesterol decreases uterine activity: functional effects of cholesterol alteration in pregnant rat myometrium. Am J Physiol Cell Physiol 288, C982–C988. [DOI] [PubMed] [Google Scholar]
- Sorensen TI (2012). Is obesity a healthy active response to an expected future lack of energy rather than a passive storage of surplus energy? Obes Facts 5, 431–435. [DOI] [PubMed] [Google Scholar]
- Sweeney EM, Crankshaw DJ, O'Brien Y, Dockery P & Morrison JJ (2013). Stereology of human myometrium in pregnancy: influence of maternal body mass index and age. Am J Obstet Gynecol 208, 324.e1–324.e6. [DOI] [PubMed] [Google Scholar]
- Sweeney EM, Dockery P, Crankshaw DJ, O'Brien YM, Walsh JM & Morrison JJ (2014). Human uterine lower segment myometrial cell and nuclear volume at term: influence of maternal age. J Anat 225, 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumova J, Andel M & Trnka J (2016). Excess of free fatty acids as a cause of metabolic dysfunction in skeletal muscle. Physiol Res 65, 193–207. [DOI] [PubMed] [Google Scholar]
- Vijgen GH, Bouvy ND, Hoeks J, Wijers S, Schrauwen P & van Marken Lichtenbelt WD (2013). Impaired skeletal muscle mitochondrial function in morbidly obese patients is normalized one year after bariatric surgery. Surg Obes Relat Dis 9, 936–941. [DOI] [PubMed] [Google Scholar]
- Vincent AE, Ng YS, White K, Davey T, Mannella C, Falkous G, Feeney C, Schaefer AM, McFarland R, Gorman GS, Taylor RW, Turnbull DM & Picard M (2016). The spectrum of mitochondrial ultrastructural defects in mitochondrial myopathy. Sci Rep 6, https://doi.org/10.1038/srep30610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J, Foley M & O'Herlihy C (2011). Dystocia correlates with body mass index in both spontaneous and induced nulliparous labors. J Matern Fetal Neonatal Med 24, 817–821. [DOI] [PubMed] [Google Scholar]
- WHO Expert Committee on Physical Status (1995). Physical Status: The Use and Interpretation of Anthropometry Technical Report Series No. 854. WHO, Geneva. [PubMed] [Google Scholar]
- Wieland O (1984). Glycerol In Methods of Enzymatic Analysis, 3rd edn, ed. Bergmeyer HU, vol. VI, pp. 504–510. Verlag Chemie, Weinheim. [Google Scholar]
- Wikstrom M, Ahonen P & Luukkainen T (1975). The role of mitochondria in uterine contractions. FEBS Lett 56, 120–123. [DOI] [PubMed] [Google Scholar]
- Wray S (2007). Insights into the uterus. Exp Physiol 92, 621–631. [DOI] [PubMed] [Google Scholar]
- Zhang J, Bricker L, Wray S & Quenby S (2007a). Poor uterine contractility in obese women. BJOG 114, 343–348. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kendrick A, Quenby S & Wray S (2007b). Contractility and calcium signaling of human myometrium are profoundly affected by cholesterol manipulation: implications for labor? Reprod Sci 14, 456–466. [DOI] [PubMed] [Google Scholar]
- Zhang J, Landy HJ, Branch DW, Burkman R, Haberman S, Gregory KD, Hatjis CG, Ramirez MM, Bailit JL, Gonzalez‐Quintero VH, Hibbard JU, Hoffman MK, Kominiarek M, Learman LA, Van Veldhuisen P, Troendle J & Reddy UM (2010). Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol 116, 1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]


