Abstract
Key points
Maternal training during gestation enhances offspring body composition and energy substrates handling in early adulthood.
Offspring nutrition also plays a role as some beneficial effects of maternal training during gestation disappear after consumption of a high‐fat diet.
Abstract
Maternal exercise during gestation has been reported to modify offspring metabolism and health. Whether these effects are exacerbated when offspring are receiving a high‐fat diet remains unclear. Our purpose was to evaluate the effect of maternal exercise before and during gestation on the offspring fed a high‐fat/high‐sucrose diet (HF) by assessing its body composition, pancreatic function and energy substrates handling by two major glucose‐utilizing tissues: liver and muscle. Fifteen‐week‐old nulliparous female Wistar rats exercised 4 weeks before as well as during gestation at a constant submaximal intensity (TR) or remained sedentary (CT). At weaning, pups from each group were fed either a standard diet (TRCD or CTCD) or a high‐fat/high‐sucrose diet (TRHF or CTHF) for 10 weeks. Offspring from TR dams gained less weight compared to those from CT dams. Selected fat depots were larger with the HF diet compared to control diet (CD) but significantly smaller in TRHF compared to CTHF. Surprisingly, the insulin secretion index was higher in islets from HF offspring compared to CD. TR offspring showed a higher muscle insulin sensitivity estimated by the ratio of phosphorylated protein kinase B to total protein kinase B compared with CT offspring (+48%, P < 0.05). With CD, permeabilized isolated muscle fibres from TR rats displayed a lower apparent affinity constant (K m) for pyruvate and palmitoyl coenzyme A as substrates compared to the CT group (−46% and −58%, respectively, P < 0.05). These results suggest that maternal exercise has positive effects on young adult offspring body composition and on muscle carbohydrate and lipid metabolism depending on the nutritional status.
Keywords: gestation, exercise, offspring, insulin sensitivity, pancreas, metabolism
Key points
Maternal training during gestation enhances offspring body composition and energy substrates handling in early adulthood.
Offspring nutrition also plays a role as some beneficial effects of maternal training during gestation disappear after consumption of a high‐fat diet.
Introduction
Initially based on epidemiological studies from Barker and colleagues (Barker et al. 1989), the developmental origins of health and disease (DOHaD) concept suggests that environmental conditions during the gestational and perinatal periods may increase the susceptibility to many metabolic diseases in adulthood. Thus, many observational and experimental studies have established a link between a poor maternal nutritional environment and a low birth weight with a higher susceptibility to develop several diseases in adulthood such as cardiovascular diseases (Barker et al. 1989; Osmond et al. 1993; Frankel et al. 1996), hypertension (Barker et al. 1992; Leon et al. 1996), insulin resistance and hyperinsulinaemia (Barker et al. 1993; Phillips et al. 1994), pancreatic islets dysfunction (Dahri et al. 1991; Garofano et al. 1997), Type 2 diabetes mellitus (T2DM) (Hales et al. 1991; Lithell et al. 1996) or metabolic syndrome (Barker et al. 1993). In the same way, maternal obesity increases offspring susceptibility to obesity, cardiometabolic diseases and metabolic syndrome (Barker, 1998; Ghosh et al. 2001; Taylor et al. 2005; Hanson & Gluckman, 2014). Regular physical activity is known to help with weight loss and to decrease susceptibility to T2DM by increasing insulin sensitivity and non‐insulin‐stimulated glucose uptake (Wojtaszewski et al. 2000; Richter et al. 2004). Moderate aerobic exercise during healthy pregnancy has no detrimental effects on the mother's health (Clapp, 1991; Wolfe et al. 1994; Platt et al. 2013) and has been reported to positively impact the cardiovascular system and to reduce the incidence of gestational diabetes (Ruchat et al. 2012; Cordero et al. 2015; Mottola & Artal, 2016). The effect of maternal exercise training on offspring has been less studied and appears to be more controversial. It has positive and/or negative effects on offspring birth weight, body composition, and cardiovascular and cognitive functions, depending on the exercise parameters (Clapp et al. 2002; Hopkins et al. 2010; Dayi et al. 2012; Newcomer et al. 2012; Raipuria et al. 2015). Moreover, there have been few studies on the metabolic consequences of maternal exercise training on the offspring whatever its age. In rodents, voluntary maternal training using running wheels improves offspring glucose tolerance, insulin sensitivity and body composition (Carter et al. 2012, 2013; Stanford et al. 2015; Vega et al. 2015). In addition, compulsory treadmill training before and during gestation is associated with a better pancreatic islet insulin secretory capacity in offspring at weaning (Quiclet et al. 2016). These positive effects of maternal exercise have also been reported with high‐fat diet consumption in offspring. Indeed, voluntary maternal exercise may protect the offspring from obesity and liver steatosis, with a better insulin sensitivity and glucose tolerance when fed a high‐fat diet (Wasinski et al. 2015; Sheldon et al. 2016). Our hypothesis is that such apparent better glucose handling ability in offspring could be due to either a better functioning of the pancreas or changes in the metabolism of the main tissues utilizing glucose, i.e. liver and muscle, in response to chronic exercise done by the mother before and during gestation. Therefore, we investigated how a chronic submaximal endurance treadmill exercise done before gestation and during all the gestational periods would act on the young adult offspring pancreatic function, as well as on its liver and muscle metabolism, to determine their contribution in the overall glucose handling.
Methods
Ethical approval
All experimental procedures were carried out in accordance with European Directive 2010/63/UE and complied with the principles and standards for reporting animal experiments in The Journal of Physiology (Grundy, 2015). They were reviewed by the local Institutional Ethics Committee for Animal Care and Use (LBFA‐U1055‐KC‐01) and authorized by the French Ministry of Research. Rats were anaesthetized by an i.p. injection of sodium pentobarbital (5 mg (100 g)−1 body weight) and killed by decapitation.
Animals
Fifteen‐week‐old nulliparous female Wistar rats (Charles River Laboratories, Saint Germain‐Nuelles, France) were housed three per cage with access to food (A03, SAFE Diets, Augy, France) and water ad libitum. The animal facility was thermoneutral (22 ± 2°C) and on a 12 h:12 h light/dark cycle. After a 1‐week acclimatization period, female rats were assigned to either a sedentary (Control, CT, n = 10) or trained (Trained, TR, n = 9) group. Body weight and food consumption were measured once a week during breeding and pregnancy. Trained females were exercised using a motorized treadmill (Bioseb, Vitrolles, France) 5 days per week during the 4 weeks before gestation and during the first 18 days of gestation. Sedentary female rats were kept in their home cage. During the first 3 weeks of training, the speed and the duration of the exercise session were gradually increased to reach a speed of 25 m min−1 corresponding to 55% of their maximal aerobic speed for 60 min (Quiclet et al. 2016). After 4 weeks of controlled exercise, male Wistar rats were housed for 1 week with females from each group for mating purpose. The males did not exercise at any stage of the study. Vaginal smears were performed every day until spermatozoa were found as a sign of the first day of gestation. Forty‐eight hours after birth, litters were culled to eight pups. To ensure the maximum number of males per litter, pups were cross‐fostered between litters from the same group and age. Only male pups were considered in the study to avoid the additional confounding influence of hormonal variations. Mothers from the TR group did not exercise during nursing. Pups were weighed on postnatal days 7, 14 and 21 (D7, D14, D21). At weaning, pups from each group were separated in two groups. Half of the animals were fed a high‐fat/high‐sucrose diet (HF) (36% fat, 16.6% sucrose, ref. 260HF (5.5 kcal g−1), SAFE Diets) and the other half a standard control diet (CD) (5.1% fat, 4.4% total sugars, ref A03 (3.4 kcal g−1), SAFE Diets) during 10 weeks ad libitum without any controlled physical activity. Mothers were killed after nursing and offspring at 3 months of age, as described above. Selected skeletal muscles, fat depots and organs were dissected out and weighed in order to estimate changes in body composition and/or collected and stored at −80°C for further measurements.
Intraperitoneal glucose tolerance test
An intraperitoneal glucose tolerance test (ipGTT) was performed at 12 weeks of age after 16 h of fasting. Glucose was injected intraperitoneally at 1 g kg−1 body weight. Blood glucose was measured in blood from tail veins before injection (time 0 min) and 5, 10, 15, 20, 25, 30, 35, 40, 45, 60, 90 and 120 min after glucose injection, using an Accu‐Chek glucometer (Roche Diabetes Care, Meylan, France). Area under the curve was related to time 0 min. Blood samples were collected at time 0 min to determine plasma insulin using commercial radioimmunoassay (RIA) kits (Merck Millipore Corporation, Darmstadt, Germany).
Intraperitoneal insulin tolerance test
At 13 weeks of age, an intraperitoneal insulin tolerance test (ipITT) was performed on pups from each group. Rats were fasted for 6 h. Insulin was intraperitoneally injected at 1 mIU g−1 body weight. Blood glucose was measured in blood from tail vein before injection (time 0 min) and 20, 40, 60, 90 and 120 min after insulin injection using an automated blood glucose analyser (Sensostar, DiaSys Diagnostic Systems, Holzheim, Germany). Area over the curve was related to time 0 min. Blood samples were collected at time 0 min to determine plasma insulin by RIA kit (Merck Millipore Corporation).
Insulin load test and analysis of insulin signalling in tissues
After a 6 h fast, 13‐week‐old rats were intraperitoneally injected either with physiological saline (NaCl 0.9% w/v) (CTCD−, CTHF−, TRCD−, TRHF−) or with insulin (10 mIU g−1 body weight) (CTCD+, CTHF+, TRCD+, TRHF+). Rats were killed 15 min after injection, and gastrocnemius muscle and liver were rapidly removed and frozen. Protein kinase B (PKB) phosphorylation level, as an indicator of insulin pathway activation, was determined by western blotting (Vial et al. 2015). Tissues were lysed in PBS buffer containing 1% NP‐40, 0.5% sodium deoxycholate, 0.1% SDS supplemented with 5 mm EDTA, 1 mm Na3VO4, 20 mm NaF, 1 mm DTT and protease inhibitors cocktail (Sigma P2714, Saint Quentin Fallavier, France). Proteins were separated by SDS–10% PAGE and then transferred onto polyvinylidene difluoride (PVDF) membranes. Membranes were incubated overnight with primary antibodies (anti‐total Akt/PKB (Cell Signaling Technology, Leiden, The Netherlands, cat. no. 9272, RRID:AB_329827) and anti‐phospho‐Akt/PKBSer473 (Cell Signaling Technology, cat. no. 9271, RRID:AB_329825) antibodies). The antibody–protein duo was marked with a horseradish peroxidase‐conjugated secondary antibody (AbD Serotec, Hercules, CA, USA, cat. no. 172‐1019, RRID:AB_11125143) and revealed using the Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific, Waltham, MA, USA).
Pancreas insulin content
Pancreas total insulin content was determined in pancreas from rats injected with NaCl during the insulin load test. Pancreata were removed just after the animals were killed and stored at −80°C. They were then homogenized in 6 mL of 0.18 m HCl in 70% ethanol and incubated overnight on a rotating wheel (20 r.p.m.) at 4°C. The day after, homogenates were sonicated twice for 10 s and then incubated for 2 days on a rotating wheel (20 r.p.m., 4°C). Then, homogenates were centrifuged (150 g, 5 min, 4°C) and supernatants collected and centrifuged (3200 g, 20 min, 4°C). The resulting supernatants were collected and stored at −80°C until the insulin content assay with RIA kit (Merck Millipore Corporation).
Islets isolation and glucose‐stimulated insulin secretion test
Pancreatic islets isolation was performed on the rats used for ipGTT and ipITT after a 1 week recovery period. Rats were anaesthetized as described above. A laparotomy was performed and the pancreas was exposed as much as possible. The pancreatic duct was clamped with a haemostat at its duodenal insertion ensuring no injury to the pancreatic tissue. The bile duct at the proximal end was isolated. It was cut with fine scissors at one‐third of the way across and a 26 G catheter was inserted and fixed in the bile duct. A collagenase solution (10 mL of 1 mg mL−1 collagenase from Clostridium histolyticum type XI (Sigma‐Aldrich, Saint‐Quentin Fallavier, France) in Hanks’ balanced salt solution (HBSS)) was slowly injected. The pancreas was removed carefully and put in a tube containing 7.5 mL HBSS at 4°C and then transferred to a water‐bath pre‐set at 37°C for 11 min. After incubation, the tube was vigorously shaken by hand for 15 s and 25 mL of HBSS, 5% fetal bovine serum (FBS) was added. The tube was centrifuged (250 g, 2 min, 4°C). The supernatant was poured off and the pellet filtered through a wire mesh. After the addition of 25 mL of HBSS, 5% FBS, the tube was centrifuged (250 g, 2 min, 4°C) and the supernatant poured off. The washing procedure was repeated two more times. After the last centrifugation, 5 mL of the supernatant was kept with the pellet. A density gradient was then prepared by pouring 10 mL of Histopaque 1.119 (Sigma‐Aldrich) in a 50 mL tube. This phase was overlaid with 10 mL of Histopaque 1.077. Islets were then resuspended in 7.5 mL Histopaque 1.119 + 3.5 mL Histopaque 1.077. The suspension was layered on the Histopaque 1.077 phase followed by 10 mL of HBSS on top. The gradient was centrifuged (1750 g, 20 min, 20°C) with slow acceleration and no braking. The islets were then collected from each of the interfaces and washed 3 times in 25 mL of HBSS, 5% FBS (once at 350 g, 5 min, 4°C and 2 times at 250 g, 2 min, 4°C). The islets were then cultured overnight at 37°C in RPMI, 10% FBS, 1% sodium pyruvate, 1% antibiotic/antimycotic solution. The day after, the islets were incubated with glucose to determine their insulin release capacity. After 1 h preincubation in 2.8 mm “low” glucose medium at 37°C, islets were incubated for 1 h at 37°C in 2.8 mm “low” glucose medium and supernatants were collected. Islet insulin secretion was then stimulated by incubation with 16.7 mm “high” glucose medium for 1 h at 37°C and supernatants were collected. Residual total insulin in the islets was extracted in 0.18 m HCl, 70% ethanol and collected. Samples were stored at −80°C until insulin assay by RIA kit (Merck Millipore Corporation). Islet insulin secretion index was calculated by dividing the insulin concentration in the medium after “high” glucose incubation by the insulin concentration in the medium after “low” glucose incubation.
In situ study of mitochondrial respiration
The mitochondrial respiration was studied in situ in saponin‐skinned fibres from plantaris muscle after a 16 h fast as previously described (Kuznetsov et al. 2008). Briefly, fibres were separated under binocular microscope in solution A (2.77 mm CaK2EGTA, 7.23 mm K2EGTA (100 nm free Ca2+), 6.56 mm MgCl2 (1 mm free Mg2+), 20 mm taurine, 0.5 mm DTT, 50 mm potassium methane sulfonate (160 mm ionic strength), 5.7 mm Na2ATP, 15 mm creatine phosphate, 20 mm imidazole, pH 7.1) at +4°C and permeabilized in solution A added with 50 μg mL−1 of saponin for 30 min. Permeabilized fibres were then rinsed for 15 min in solution B (2.77 mm CaK2EGTA, 7.23 mm K2EGTA (100 nm free Ca2+), 6.56 mm MgCl2 (1 mm free Mg2+), 20 mm taurine, 0.5 mm DTT, 50 mm potassium methane sulfonate (160 mm ionic strength), 3 mm phosphate, 2 mm ADP, and 2 mg mL−1 fatty acid‐free bovine serum albumin (BSA), 20 mm imidazole, pH 7.1) to wash out adenine nucleotides and creatine phosphate. For each muscle, 3–8 mg of skinned fibres were incubated in 1.5 mL of solution B at +22°C under continuous stirring and their oxygen consumption was measured using a Clark‐type electrode (Hansatech Oxygraph Instruments, Pentney, King's Lynn, UK) . After measurements, fibres were removed, dried and weighed. Respiration rates were expressed as micromoles of oxygen per minute per gram of dry weight.
Mitochondrial metabolism was studied using a protocol adapted from Ponsot et al. (2005). Stepwise additions of substrates: pyruvate (10–1500 μm), palmitoyl‐d,l‐carnitine (PC; 10–400 μm), or palmitoyl coenzyme A (PCoA; 10–400 μm) were done until the maximal respiration rate was reached in the presence of 2 mm ADP, 4 mm malate and 2 mm carnitine (added at the beginning of PCoA protocol). Under these conditions, the available substrate concentration is the only limiting factor of the mitochondrial respiration. Apparent affinity constant values (K m) for pyruvate, PCoA and PC were calculated using a non‐linear mono‐exponential fitting based on the Michaelis–Menten model equation. As an internal control for compromised integrity of mitochondrial preparation, mitochondrial membrane permeability was randomly assessed with the addition of cytochrome c at the end of protocols (Kuznetsov et al. 2008).
Western blotting
Total content and/or phosphorylation levels of proteins were determined by western blotting using anti‐phosphoenolpyruvate carboxykinase (Santa Cruz Biotechnology, Santa Cruz, CA, USA), cat. no. sc‐32879, RRID:AB_2160168), anti‐fatty acid synthase (Cell Signaling Technology, cat. no. 3180S, RRID:AB_2100796) and anti‐glycogen synthase kinase‐3 (GSK3, Cell Signaling Technology, cat. no. 9315, RRID:AB_490890, pGSK3, Cell Signaling Technology, cat. no. 9323, RRID:AB_2115201) antibodies. Liver samples were homogenized in a buffer containing 50 mm Tris, 50 mm NaCl, 1 mm EDTA, 0.5% NP‐40, 0.1% SDS, 5% glycerol, 1 mm Na3VO4 supplemented with a protease inhibitor cocktail (Complete EDTA free, Sigma Aldrich). Proteins were subjected to SDS–10% PAGE and separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes that were incubated overnight with primary antibodies. The antibody–protein duo was marked with a horseradish peroxidase‐conjugated secondary antibody (Millipore, cat. no. AQ132P, RRID:AB_92785 or AbD Serotec, cat. no. 172‐1019, RRID:AB_11125143) and revealed with enhanced chemiluminescence as described above.
Hepatic glycogen concentration
A quantity of 100 mg of liver was hydrolysed in KOH for measurements of glycogen by a method derived from Keppler and Decker (Keppler & Decker, 1974). Glucose obtained from hydrolysis was determined with a Glucose Assay Kit (Sigma Diagnostics, Isle d'Abeau, France).
Enzymatic assays
Liver and gastrocnemius muscle samples were homogenized at 4°C in PBS supplemented with protease and phosphatase inhibitor cocktails. Citrate synthase (CS, EC:2.3.3.1) activity was assessed according to Srere (Srere & Brooks, 1969) on homogenates at 30°C. Results were expressed as μmol min−1 (g protein)−1. Activity of the 3‐hydroxyacyl‐coenzyme A (CoA) dehydrogenase (HAD, EC 1.1.1.35) was assessed by measuring the decrease in absorbance at 340 nm resulting from the oxidation of nicotinamide adenine dinucleotide (NADH) and the reduction of S‐acetoacetyl‐CoA. The measurement was performed in 60 μm EDTA, 200 μm NADH, H+, 40 mm imidazole, pH 7. After the initial measurement (non‐specific activity), 50 μm S‐acetoacetyl‐CoA was added to determine the specific activity expressed in μmol min−1 (g protein)−1. Enolase (EC 4.2.1.11) activity was determined by incubating 1 mg protein of homogenate in 2 mm 2‐phosphoglycerate (2‐PGA), 50 mm imidazole, 4 mm magnesium acetate, 40 mm KCl, 0.2% BSA, 2 mm ADP, 3 U mL−1 pyruvate kinase, 5 U mL−1 lactate dehydrogenase and 1 mm β‐NADH at 37°C. Total activity was determined by measuring optical density at 340 nm for 10 min. Non‐specific activity was assessed in the same conditions but without 2‐PGA. Specific activity was then calculated by subtracting non‐specific activity from total activity and results were expressed as μmol min−1 (g protein)−1.
Statistical analysis
Data were expressed as means ± SEM. Data were analysed for specific effect of the diet or the maternal training, using Student's t tests or two‐way ANOVAs with the Holm–Sidak post hoc test. Kruskall–Wallis tests were applied when values were not normally distributed. P < 0.05 was considered significant.
Results
Exercise training before and during gestation does not alter maternal body composition and food consumption
Body weight of female Wistar rats was monitored for both groups during breeding, mating and gestation (Table 1). There was no difference between the two groups in maternal body weight at the beginning of the study (196 ± 7 g vs. 199 ± 9 g for CT and TR groups, respectively), after the first 4 weeks of training (250 ± 8 g vs. 250 ± 6 g for CT and TR groups, respectively) and after lactation (262 ± 9 g vs. 255 ± 6 g for CT and TR groups, respectively). There was no significant difference in relative tissue weights between the groups. Food intake was similar in the two groups throughout the entire protocol including the lactating period (Table 1).
Table 1.
Maternal outcomes
| CT | TR | P values | |
|---|---|---|---|
| Body weight (g) | |||
| Before the study | 196 ± 7 | 199 ± 9 | n.s. |
| Before gestation | 250 ± 8 | 250 ± 6 | n.s. |
| During lactation | 262 ± 9 | 255 ± 6 | n.s. |
| Food intake (g day−1) | |||
| At the beginning of the study | 18.8 ± 0.4 | 17.9 ± 0.6 | n.s. |
| During gestation | 21.1 ± 1.1 | 20.3 ± 0.7 | n.s. |
| During lactation | 70.3 ± 1.5 | 73.0 ± 1.4 | n.s. |
| Organ weight (g kg−1 body weight) | |||
| Pancreas | 4.45 ± 0.62 | 4.80 ± 0.63 | n.s. |
| Liver | 25.27 ± 0.37 | 27.11 ± 1.16 | n.s. |
| Kidney | 3.76 ± 0.10 | 3.56 ± 0.12 | n.s. |
| Fat | 35.02 ± 2.91 | 30.85 ± 2.00 | n.s. |
| Muscles | 7.52 ± 0.25 | 7.33 ± 0.25 | n.s. |
Data are means ± SEM (n = 10 for CT and n = 8 for TR). Fat mass was calculated as the sum of retroperitoneal, urogenital and mesenteric fat depots. Muscle mass was calculated as the sum of gastrocnemius, plantaris and soleus muscles. n.s., not significant.
Exercise training before and during gestation has no effect on the litter size and sex ratio but decreases offspring body weight
At birth, the number of pups and the sex ratio per litter were not significantly different between CT and TR groups (Table 2). However, the number of male pups per litter tended to be higher in the TR group compared to the CT group (P = 0.057). Offspring body weight was measured at postnatal days 7, 14 and 21. Pup body weights were estimated by averaging pup body weights for each litter. Pups from TR mothers showed a lower body weight at postnatal days 7 and 21, and a tendency to be lower at day 14 (P = 0.087) when compared with pups from the CT group (Table 2).
Table 2.
Litter outcomes
| CT | TR | P values | |
|---|---|---|---|
| Litter size | 11.4 ± 0.9 | 13.1 ± 0.6 | n.s. |
| Number of males | 5.1 ± 0.5 | 6.8 ± 0.7 | n.s. |
| Number of females | 6.3 ± 0.7 | 6.3 ± 0.5 | n.s. |
| D7 body weight (g) | 16.4 ± 0.5 | 13.9 ± 0.5* | P = 0.003 |
| D14 body weight (g) | 33.5 ± 0.9 | 31.0 ± 1.0 | n.s. |
| D21 body weight (g) | 53.5 ± 1.3 | 49.0 ± 1.5* | P = 0.036 |
Data are means ± SEM (n = 10 for CT and n = 9 for TR). * P < 0.05 vs. CT. D7, D14, D21: postnatal day 7, 14, 21. n.s., not significant.
Offspring body weight and fat mass are increased by HF diet and decreased by maternal training
Offspring caloric intake was higher during the first 8 weeks of diet, except during the third week of diet, in HF group when compared with CD group (Fig. 1). Additionally, HF fed rats gained more weight during the 10 weeks of HF diet exposure compared with CD rats (Fig. 1) and were heavier at 3 months of age (Table 3). Body weight gain was significantly smaller in offspring from TR dams compared with those from CT dams from the 9th week to the end of the diet (−5 to −9%, P < 0.05), whatever the diet (CD or HF) (Fig. 1). Neither maternal exercise nor HF diet modified kidney, muscle or pancreas weight. HF diet fed rats showed higher liver and fat masses (Table 3). However, under HF diet, offspring fat mass was lower in TR rats when compared with CT rats (Table 3).
Figure 1. Maternal exercise reduces offspring body weight gain whatever the diet.

Rat offspring caloric intake was measured during the 7 weeks following weaning (A). Body weight was monitored for 10 weeks after weaning (B). Data are means ± SEM; n = 4–9 in A and n = 25–32 in B. * P < 0.05 vs. CTCD, $ P < 0.05 vs. CTHF, § P < 0.05 CTHF and TRHF vs. control diet groups (CTCD + TRCD).
Table 3.
Offspring outcomes at 3 months of age
| CTCD | CTHF | TRCD | TRHF | |
|---|---|---|---|---|
| Body weight (g) | 392 ± 11 | 467 ± 21* | 371 ± 7* | 437 ± 6†, ‡ |
| Organ weight (g) | ||||
| Liver | 13.23 ± 0.46 | 14.78 ± 0.37* | 12.93 ± 0.35 | 15.06 ± 0.34† |
| Kidney | 1.20 ± 0.03 | 1.24 ± 0.04 | 1.19 ± 0.02 | 1.22 ± 0.02 |
| Fat | 13.79 ± 0.68 | 37.71 ± 2.18* | 13.85 ± 0.76 | 30.03 ± 1.41†, ‡ |
| Muscles | 2.28 ± 0.05 | 2.15 ± 0.05 | 2.18 ± 0.05 | 2.15 ± 0.03 |
| Pancreas | 1.42 ± 0.05 | 1.24 ± 0.09 | 1.36 ± 0.06 | 1.29 ± 0.05 |
| Liver glycogen concentration (μmol (g tissue)−1) | 263.9 ± 13.1 | 181.7 ± 12.7* | 281.3 ± 21.7 | 193.2 ± 12.5† |
Data are means ± SEM (n = 24, n = 6–8 for liver glycogen concentration) and were analysed for diet or training effect against their respective control groups with: * P < 0.05 vs. CTCD, † P < 0.05 vs. TRCD, ‡ P < 0.05 vs. CTHF. Fat mass was calculated as the sum of retroperitoneal, epididymal and mesenteric fats depots. Muscle mass was the sum of gastrocnemius, plantaris and soleus muscles.
HF diet reduces glucose tolerance and increases insulinaemia independently of maternal training
The 16 h‐fasting glycaemia was higher in TRHF rats compared with TRCD and CTCD rats (Fig. 2 A). Basal insulinaemia was higher in animals fed the HF diet compared with rats fed the CD (+307%, P < 0.05) (Table 4). During the ipGTT, glycaemia of CTHF and TRHF rats was higher than that of CTCD and TRCD rats (Fig. 2 A). Area under the curve, used as a measure of overall glucose disposal, was higher in CTHF and TRHF rats compared with TRCD rats (inset Fig. 2 A).
Figure 2. Ten weeks of high‐fat/high‐sucrose diet alter 3‐month‐old offspring glucose tolerance with no change in overall insulin sensitivity.

Rat offspring underwent an ipGTT at 3 months of age (A) to assess whole body glucose tolerance. Following a 16 h fast, glucose was intraperitoneally injected at 1 g kg−1 body weight. Blood glucose levels were monitored before injection (0) and 5, 10, 15, 20, 25, 30, 35, 40, 45, 60, 90 and 120 min after glucose injection. Inset graph shows the areas under the curve of ipGTT related to the value at T = 0 min. Rat offspring also underwent an ipITT at 3 months of age (B) to assess whole body insulin sensitivity. Following a 6 h fast, insulin was intraperitoneally injected at 1 mIU g−1 body weight. Blood glucose levels were monitored before injection (0) and 10, 20, 30, 40, 50, 60, 90 and 120 min after insulin injection. Inset graph shows the areas over the curve of ipITT related to the value at T = 0 min. Data are means ± SEM; n = 8–12. * P < 0.05 vs. CTCD, # P < 0.05 vs. TRCD.
Table 4.
Offspring glycaemia, insulinaemia and pancreatic insulin content
| CTCD | CTHF | TRCD | TRHF | |
|---|---|---|---|---|
| Glycaemia (mg dL−1) | 120.8 ± 4.3 | 135.6 ± 2.4* | 120.9 ± 4.1 | 141.4 ± 4.4† |
| Insulinaemia (ng mL−1) | 0.46 ± 0.09 | 1.13 ± 0.20* | 0.29 ± 0.08 | 1.23 ± 0.16† |
| Pancreatic insulin content (ng mg−1 fresh weight) | 199.3 ± 38.2 | 197.8 ± 37.0 | 167.8 ± 28.6 | 147.1 ± 11.8 |
Data are means ± SEM (n = 5–12) and were analysed for diet or training effect against their respective control groups with: * P < 0.05 vs. CTCD, † P < 0.05 vs. TRCD.
Maternal training and 10 weeks of HF diet consumption do not modify offspring insulin tolerance
After a 6 h fast, CTHF and TRHF animals showed a higher glycaemia compared with CTCD and TRCD animals (+15%, P < 0.05) (Table 4). After insulin injection, blood glucose levels were not different between the four groups during the whole test (Fig. 2 B). Area over the curve during ipITT was not affected by maternal exercise or HF diet (inset Fig. 2 B).
Maternal training and HF diet increase muscle insulin pathway activation
The insulin signalling pathway was studied by measuring the ratio of the level of the phosphorylated form of PKB (pPKB) over its total content (PKB) both in liver (Fig. 3 A) and gastrocnemius muscle (Fig. 3 B) after either a NaCl or an insulin load. In liver, insulin pathway activation was not affected by maternal training nor by 10 weeks of HF diet since the pPKB/PKB ratios were similar between the four groups whether in basal (after NaCl injection) or in stimulated conditions (after insulin load injection) (Fig. 3 A and C). In gastrocnemius muscle, HF diet had an overall effect on pPKB/PKB ratio with an increase in basal conditions (+96%, P < 0.001). In insulin‐stimulated conditions, HF diet and maternal training had an overall effect on the increase of the pPKB/PKB ratio (+71% and +48%, P < 0.001 and P < 0.05, respectively) (Fig. 3 B and D).
Figure 3. Muscle insulin pathway activation is increased by maternal exercise and HF diet.
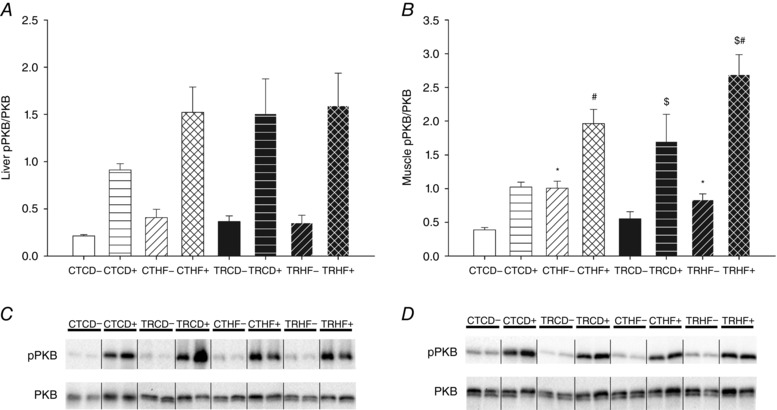
Rat offpring underwent an insulin load test at 3 months of age to assess liver (left) and muscle (right) insulin pathway activation as an indicator of insulin sensitivity. Insulin bolus was intraperitoneally injected at 10 mIU g−1 body weight to half of the rats in each group (CTCD+, CTHF+, TRCD+ and TRHF+) and the others were injected with NaCl (CTCD−, CTHF−, TRCD− and TRHF−). Liver (A) and gastrocnemius muscle (B) samples were collected 15 min after injection in order to determine pPKB/PKB ratio (Ser473) by western blotting. Representative western blots showing phosphorylated and total (pPKB and PKB, respectively) PKB content in liver and gastrocnemius muscle (C and D, respectively). Quantification of the signals was expressed in arbitrary units. Data are means ± SEM; n = 8. * P < 0.05 vs. CD−; $ P < 0.05 vs. CT+; # P < 0.05 vs. CD+.
Maternal training and HF diet have no effect on pancreatic insulin content
Pancreatic insulin content was not affected by maternal exercise nor HF diet (Table 4).
HF diet increases islet insulin secretion index
Insulin secretion from isolated islets was not significantly different between the four groups, whether in low glucose or in high glucose conditions (data not shown). The islets’ insulin secretion index was higher in rats fed a HF diet (+38% vs. CD, P < 0.05) whatever the maternal training condition (TR or CT) (Fig. 4).
Figure 4. HF diet increases islet insulin secretion index in 3‐month‐old offspring.
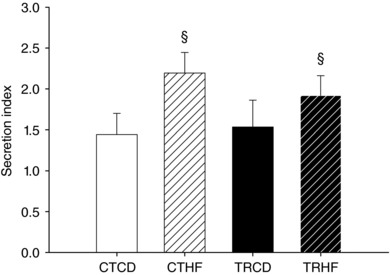
Offspring pancreatic islets were isolated at 3 months of age in order to assess their insulin secretory capacity. The day after isolation, islets were incubated in a low glucose concentration (2.8 mm) medium and then in a high glucose concentration (16.7 mm) medium. Supernatants were collected and islets’ insulin secretion determined by RIA. Insulin secretion index was calculated by dividing insulin concentration in the medium after high glucose incubation by that after low glucose incubation. Data are means ± SEM; n = 11–12. § P < 0.05 vs. control diet groups (CTCD + TRCD).
Maternal training increases offspring muscle mitochondrial affinity for pyruvate and PCoA under CD
Apparent affinity constants for substrates (K m) are reported in Table 5. For pyruvate, the K m was significantly lower in TRCD rats compared with CTCD rats (−46%, P < 0.05). Moreover, maternal training had an overall effect on the reduction of K m for pyruvate. For PCoA substrate, the K m was also significantly lower in the TRCD group compared with CTCD (−58%, P < 0.05) and TRHF (−71%, P < 0.05) groups, and showed a high tendency to be lower compared with the CTHF group (−25%, P = 0.059). For PC, the K m was similar in the four groups. Maximal oxygen consumption (V max) in the presence of pyruvate and PC was similar in the four groups (Table 5). V max with PCoA substrate was higher in HF animals compared with CD animals and higher in CTHF rats vs. CTCD rats (+35%, P < 0.05) (Table 5).
Table 5.
Plantaris muscle mitochondrial maximal respiration and affinity for pyruvate, PCoA and PC
| CTCD | CTHF | TRCD | TRHF | |
|---|---|---|---|---|
| V max pyruvate (μmol O2 min−1 g−1 dry weight) | 8.27 ± 0.64 | 7.74 ± 0.46 | 7.38 ± 0.38 | 6.78 ± 0.47 |
| V max PCoA (μmol O2 min−1 g−1 dry weight) | 2.29 ± 0.15 | 3.08 ± 0.14*, § | 2.44 ± 0.24 | 2.75 ± 0.21§ |
| V max PC (μmol O2 min−1 g−1 dry weight) | 2.34 ± 0.24 | 3.11 ± 0.41 | 2.28 ± 0.28 | 2.50 ± 0.47 |
| K m pyruvate (μm) | 136 ± 13 | 117 ± 10 | 93 ± 6*, ¥ | 114 ± 10¥ |
| K m PCoA (μm) | 202 ± 29 | 160 ± 31 | 128 ± 51* | 219 ± 44† |
| K m PC (μm) | 159 ± 15 | 183 ± 24 | 223 ± 58 | 147 ± 41 |
Data are means ± SEM (n = 7–15) and were analysed for diet or training effect against their respective control groups with: * P < 0.05 vs. CTCD, † P < 0.05 vs. TRCD, ¥ P < 0.05 vs. control groups (CTCD + CTHF). § P < 0.05 global effect of the diet: high‐fat diet groups (CTHF + TRHF) vs. control diet groups (CTCD + TRCD).
Expression and activity of enzymes of carbohydrate and lipid metabolism and glycogen content in liver
Liver CS activity was significantly lower in offspring from TR dams compared with those from CT dams (−43%, P < 0.05) (Table 6). Liver HAD and enolase activities were lower in rats fed the HF diet (−24% and −16%, respectively, P < 0.05) whatever the maternal training condition (TR or CT) (Table 6). Additionally, enolase activity was lower in TRHF rats compared with CTHF rats (Table 6). Liver phosphoenolpyruvate carboxykinase (PEPCK) expression was not affected by HF diet nor maternal exercise (Table 6). Both maternal training and HF diet had an overall lowering effect on the hepatic fatty acid synthase (FAS) activity (Table 6). Total GSK3 expression was reduced in rats fed a HF diet compared with those fed a CD and tended to be lower in offspring from TR dams compared with those from CT dams (data not shown). However, the pGSK3/GSK3 ratio (Table 6) and pGSK3 level (data not shown) were similar between the four groups. Liver glycogen content was significantly lower in offspring fed a HF diet compared with those fed a CD (−31%, P < 0.05) (Table 3).
Table 6.
Enzymes activities and expression in liver and gastrocnemius muscle
| CTCD | TRCD | CTHF | TRHF | |
|---|---|---|---|---|
| Liver | ||||
| PEPCK (arbitrary units) | 22.4 ± 3.1 | 16.8 ± 2.0 | 18.2 ± 2.1 | 15.9 ± 1.7 |
| FAS (arbitrary units) | 15.7 ± 2.6 | 9.9 ± 2.4¥ | 11.2 ± 1.8§ | 4.9 ± 0.9¥, § |
| pGSK3/GSK3 | 0.44 ± 0.07 | 0.37 ± 0.05 | 0.44 ± 0.10 | 0.55 ± 0.11 |
| CS (μmol min−1 (g protein)−1) | 12.6 ± 1.6 | 7.1 ± 2.0¥ | 15.2 ± 3.5 | 9.1 ± 1.7¥ |
| HAD (μmol min−1 (g protein)−1) | 651.5 ± 65.2 | 717.3 ± 38.4 | 583.3 ± 44.0§ | 460.7 ± 44.0§ |
| Enolase (μmol min−1 (g protein)−1) | 91.1 ± 4.4 | 92.7 ± 5.6 | 85.5 ± 4.3§ | 67.5 ± 4.2†, ‡, § |
| Muscle | ||||
| CS (μmol min−1 (g protein)−1) | 63.8 ± 13.9 | 47.8 ± 9.7 | 51.6 ± 4.0 | 45.9 ± 9.0 |
| HAD (μmol min−1 (g protein)−1) | 83.5 ± 14.0 | 143.9 ± 57.8 | 98.8 ± 7.6 | 112.9 ± 13.8 |
| Enolase (μmol min−1 (mg protein)−1) | 1.6 ± 0.1 | 1.3 ± 0.2 | 1.6 ± 0.2 | 0.9 ± 0.1 |
Data are means ± SEM (n = 6–8) and were analysed for diet or training effect against their respective control groups with: † P < 0.05 vs. TRCD; ‡ P < 0.05 vs. CTHF, ¥ P < 0.05 vs. control groups (CTCD + CTHF), § P < 0.05 vs. control diet groups (CTCD + TRCD). PEPCK, phosphoenolpyruvate carboxykinase.
Discussion
In this study, we show that submaximal maternal endurance training before and during gestation is associated with: (i) a reduction in 3‐month‐old offspring body weight gain whatever the diet, (ii) a lower fat mass when offspring is fed a high‐fat/high‐sucrose diet, (iii) a change in carbohydrate and lipid metabolism indicators in offspring muscle and liver, depending on its nutritional status but (iv) without any changes in pancreas insulin content or islets’ insulin secretion.
The aim of this work was to study the effects of a maternal submaximal training during gestation on body composition, pancreatic function and energy substrate handling of the offspring fed an unbalanced diet. For that, we trained female rats before and during gestation in the same way as in previous work from our group (Quiclet et al. 2016) and studied 3‐month‐old male offspring fed a control diet (CD) or a high‐fat/high‐sucrose diet (HF) from weaning and during 10 weeks. As we culled litters to eight pups, offspring body weight reached a similar level at 28 days of age. We then measured offspring body weight from weaning until 3 months of age. The lower body weight gain in offspring from TR dams, whatever the diet (CD or HF), is consistent with a study conducted on mice swimming during gestation showing a decreased body weight in offspring from trained dams until 2 months of age (Wasinski et al. 2015). However, these early effects of maternal exercise on offspring body weight up to 3 months of age would be masked as offspring become older (>7 months), probably due to catch‐up growth (Carter et al. 2013; Quiclet et al. 2016). In our study, HF diet is associated with an increase in measured fat mass and liver mass. Increase in liver mass is mainly attributable to the overall increase in body weight since the relative liver weight as a function of body weight is not different between groups (data not shown). The fat mass increase under a HF diet is, however, only partly explained by the increase in body weight since fat mass as a function of body weight is also higher in HF groups (data not shown). The lower fat mass that we observed in HF offspring from TR dams compared with those from CT dams is consistent with others who have previously reported a reduced fat mass gain in offspring from trained mothers (Wasinski et al. 2015; Sheldon et al. 2016). The underlying mechanism of that reduction in weight and fat mass gain in the offspring from TR dams is yet to be characterized. Although food intake was similar in our study, the lower body weight and fat mass gain in the offspring from TR dams could be explained by the result of Wasinski et al. who showed that HF diet‐fed adult offspring used more calories during daytime compared with offspring from sedentary mothers (Wasinski et al. 2015). This was associated with an increase in adiponectin and a decrease in leptin expression in skeletal muscle and a reduction of Dnmt31 expression in white adipose tissue. Maternal exercise could also affect offspring basal and glucose metabolisms via altered gene expression and hormone levels in skeletal muscle and white adipose tissue suggesting that offspring from TR dams could be protected against obesity and insulin resistance.
However, in our study, maternal exercise has no effect on offspring glucose tolerance and insulin sensitivity at 3 months of age. This contrasts with data from other studies in which treadmill maternal training was associated with a decrease in glucose tolerance at 7 months of age (Quiclet et al. 2016), whereas voluntary maternal exercise was associated with an increase in glucose tolerance and with a reduction in glycaemia and insulinaemia (Carter et al. 2012, 2013; Stanford et al. 2015; Vega et al. 2015). These discrepancies could be due to the fact that, in these studies, offspring glucose homeostasis was checked at an older age compared with ours (8–16 months vs. 3 months in our work). Another reason could be that maternal exercise was performed throughout gestation and during the first 2 weeks of lactation in the studies of Carter et al. (2012, 2013) while in our case, mothers did not exercise during lactation. This reinforced the importance of the maternal/offspring environment during the lactation period for the future offspring health, calling for further studies on the lactation period. However, we observed an increase in fasting insulinaemia and glycaemia in HF animals and a reduction in their glucose tolerance. These results have also been reported in other studies (Nakajima et al. 2015). These early signs of T2DM observed under HF diet are not associated with a change in overall insulin sensitivity during ipITT. The reduction in glucose tolerance in HF rats could result from an exhaustion of pancreatic β cells that could not secrete enough insulin in response to an increase in blood glucose (Triplitt et al. 2000). In order to confirm this hypothesis, we isolated pancreatic islets in order to measure their insulin secretory capacity by calculating the insulin secretion index. Surprisingly, we did not find any difference in islets’ insulin secretion between the four groups, whether after low glucose or high glucose incubation. Others have shown that a high‐fat diet was able to increase circulating levels of insulin and pancreatic insulin content through an elevated insulin translation (Kanno et al. 2015). Our results suggest that such regulation was not involved here since pancreatic insulin content was not significantly different between groups. However, the insulin secretion index of HF animals is increased compared with the islets of CD animals while it has been reported unchanged after a high‐fat/high‐sucrose diet in previous studies (Sauter et al. 2008; Tang et al. 2014). This higher insulin secretory capacity of the islets could be due to a stimulatory effect on the accumulated lipids in the pancreas due to HF diet exposure, as suggested by others (for review, see Nolan & Prentki, 2008).
We also studied the liver in offspring as one of the main organs involved in glucose handling. Surprisingly, we observed a reduction in liver CS activity suggesting a decrease in mitochondrial density in offspring from TR dams. Indeed, maternal exercise is generally associated with an increase in markers of mitochondrial biogenesis such as PGC1‐α and TFAM in offspring liver and adipose tissue (Raipuria et al. 2015; Sheldon et al. 2016). Maternal training and HF diet are both associated with a reduction in liver FAS expression as in previous works showing that physical exercise is associated with a reduction in liver FAS mRNA expression in animals fed a high‐fat diet (Cho et al. 2014; Wu et al. 2015). In our study, animals were exposed to the HF diet from weaning. Such early exposure of the organism to high amounts of circulating substrates may induce adaptive mechanisms in some developing tissues including a reduction of the amount of enzymes involved in lipogenesis such as FAS. This hypothesis could be confirmed by a study conducted on 6‐week‐old Wistar rats fed a high‐fat diet from this early age to 16 weeks of age that showed a significant reduction in liver FAS mRNA expression in high‐fat fed animals compared with standard‐diet fed animals (Brockman et al. 2014). That lower FAS expression could limit lipogenesis, suggesting that maternal training would affect offspring lipid metabolism. This positive effect of maternal exercise is consistent with the work of Sheldon et al. (2016) where maternal exercise protected adult offspring from HF diet‐induced hepatic steatosis and was associated with increased hepatic triacylglycerol secretion/exportthus preventing hepatic steatosis (Sheldon et al. 2016). To our knowledge, our study is the first to explore the effects of maternal chronic exercise on the expression and activity of some enzymes involved in lipid metabolism in the offspring. The reduction in liver HAD activity in HF rats suggests that this diet would reduce the capacity to use lipids in liver in a similar manner to other studies (Vial et al. 2011). We also observed a reduction in enolase activity in liver of HF animals suggesting that HF diet could limit hepatic glycolysis, as already shown in a study on rats consuming a high‐fat diet (An et al. 2013). Moreover, hepatic glycogen concentration was significantly reduced in offspring fed a HF diet. The reduction in liver glycogen concentration in HF rats cannot be solely explained by the increase in liver weight of HF rats attributable to lipid accumulation, compared to that of CD rats. Indeed the increase in liver weight is 11–16% while the decrease in liver glycogen concentration was by about 30%. This suggests that the absolute liver glycogen content is lower under HF diet, whatever the training status.
Besides the liver, we explored the offspring skeletal muscle as another important tissue in the regulation of glucose handling. The increase in pPKB/PKB ratio in the muscle of HF rats under basal conditions (NaCl injection) is probably the consequence of the increase of insulinaemia in these animals. Under insulin‐stimulated conditions, pPKB/PKB ratios were significantly increased by HF diet and maternal training in the gastrocnemius muscle. These results suggest that maternal exercise and early high‐fat/high‐sucrose diet exposure may enhance insulin sensitivity in muscle but not in liver where pPKB/PKB ratios were similar in the four groups under basal and insulin‐stimulated conditions. This positive effect of maternal training on offspring insulin sensitivity has been previously described in the literature (Carter et al. 2012, 2013; Wasinski et al. 2015). However, the increase in pPKB/PKB ratio in the muscle of HF animals is surprising. Indeed, high‐fat diet consumption is generally associated with a reduction in PKB phosphorylation compared with the standard diet (Nascimento et al. 2006; Cao et al. 2012) while sucrose consumption would have no impact on muscle PKB phosphorylation (Ruzzin et al. 2005). This surprising association between HF diet ingestion and the increase in muscle pPKB/PKB ratio might be due to the fact that, in our study, the rats consume this HF diet from weaning. That could modify the maturation of some tissues (Torres‐Rovira et al. 2013; Sheen et al. 2016) such as skeletal muscle, liver, adipose tissue or pancreas. Skeletal muscle early exposure to high blood insulin concentrationsdue to HF diet consumption could set up compensatory mechanisms as an increase in insulin pathway activation in response to a stimulation. The development of this protective mechanism against insulin resistance could explain why we observed no differences between CD and HF animals during ipITT. These changes in skeletal muscle insulin sensitivity in the offspring were associated with metabolic changes. Plantaris muscle mitochondrial affinity for PCoA and pyruvate was increased, as judged by the lower respective K m values, in offspring from TR dams under CD. Such modifications in substrate affinity are less described than those for ADP (Zoll et al. 2003) but according to Ponsot et al. (2005), change in pyruvate affinity could indicate that the pyruvate carrier, the pyruvate dehydrogenase (PDH) enzyme and/or Krebs cycle could be intrinsic limiting factors for substrate delivery to mitochondrial respiration in the skeletal muscles. This suggests that maternal exercise could induce changes in mitochondrial properties and function in offspring muscle leading to a better access of PCoA and pyruvate to the mitochondrial respiratory chain. Moreover, the larger decrease in K m for PCoA than for pyruvate would signify, according to the Michaelis–Menten model, that there is a higher mitochondrial affinity for lipids. This would signify that even a small change in substrate concentration would have a large impact on the mitochondrial respiration thus helping their use even at low concentrations. We can then speculate that there would be less storage of lipids, thus contributing to the reduced fat mass gain in offspring from TR dams. In animal models challenging the metabolism, changes in kinetic properties of the mitochondria such as affinity for substrate (as reflected by the K m value) have been reported to be associated with alterations in plasma free fatty acids, body weight and fat mass (Malgoyre et al. 2017). Although we did not measure any plasma nor muscle lipid content, the reported changes in the mitochondrial affinity for PCoA and pyruvate could be therefore linked to such changes. Moreover, as we observed no changes in muscle affinity for PC, maternal training may have a specific effect on carnitine palmitoyltransferase‐1 (CPT1), necessary to the PCoA but not to the PC to enter the mitochondria. However, the effect of maternal exercise on mitochondrial affinity for carbohydrates and lipids disappeared if offspring were fed a HF diet. Such a reduction of muscle mitochondrial respiration after high‐fat diet consumption has already been shown for carbohydrates as substrates but not for lipids(Skovbro et al. 2011).
Conclusion
This study shows that compulsory maternal exercise during gestation (i) has a positive impact on young adult offspring skeletal muscle energy substrate handling at the mitochondrial level, (ii) alters offspring body composition, and (iii) limits fat mass increase when exposed to an early high‐fat high‐sucrose diet. Most studies on the consequences of maternal exercise during pregnancy have focused on the mother, the fetus or the newborn. However, its long‐term effects on the offspring are still under debate. These new findings should be taken into account to amend the existing exercise recommendations for pregnant women in order to improve the future health of the offspring or to prevent the onset of metabolic disease in the offspring, especially in the context of an unhealthy or unbalanced diet.
Additional information
Competing interests
None declared.
Author contributions
C.Q. contributed to experimental design, did the experiments, collected data, contributed to the discussion and wrote the manuscript. H.D. contributed to experimental design, discussion, collected data and reviewed the manuscript. P.B. and H.S. collected data and contributed to the discussion. F.S. contributed to experimental design and collected data. G.V. collected data, contributed to the discussion and reviewed the manuscript. E.F. contributed to the design of the study, the discussion and reviewed the manuscript. C.B. and K.C. co‐supervised the entire study, contributed to experimental design, researched data, contributed to the discussion and reviewed the manuscript. C.Q. is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed. This work was carried out in the LBFA and in the LIBM.
Funding
This work was supported by INSERM and SFR Sport Exercice Motricité (SEM). C.Q. was funded by a 3 year fellowship from the French Ministry of Research.
Acknowledgements
The authors thank Cindy Tellier (LBFA, INSERM U1055) for animal care and Anne‐Sophie Gauchez (UMR‐S INSERM U1039) for insulin assays.
C. Batandier and K. Couturier co‐supervised the entire study.
Edited by: Bettina Mittendorfer & Kim E. Barrett
References
- An Y, Xu W, Li H, Lei H, Zhang L, Hao F, Duan Y, Yan X, Zhao Y, Wu J, Wang Y & Tang H (2013). High‐fat diet induces dynamic metabolic alterations in multiple biological matrices of rats. J Proteome Res 12, 3755–3768. [DOI] [PubMed] [Google Scholar]
- Barker DJ (1998). In utero programming of chronic disease. Clin Sci (Lond) 95, 115–128. [PubMed] [Google Scholar]
- Barker DJ, Godfrey KM, Osmond C & Bull A (1992). The relation of fetal length, ponderal index and head circumference to blood pressure and the risk of hypertension in adult life. Paediatr Perinat Epidemiol 6, 35–44. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K & Clark PM (1993). Type 2 (non‐insulin‐dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36, 62–67. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Golding J, Kuh D & Wadsworth ME (1989). Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 298, 564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman DA, Chen X & Gallaher DD (2014). High‐viscosity dietary fibers reduce adiposity and decrease hepatic steatosis in rats fed a high‐fat diet. J Nutr 144, 1415–1422. [DOI] [PubMed] [Google Scholar]
- Cao S, Li B, Yi X, Chang B, Zhu B, Lian Z, Zhang Z, Zhao G, Liu H & Zhang H (2012). Effects of exercise on AMPK signaling and downstream components to PI3K in rat with type 2 diabetes. PLoS One 7, e51709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LG, Lewis KN, Wilkerson DC, Tobia CM, Ngo Tenlep SY, Shridas P, Garcia‐Cazarin ML, Wolff G, Andrade FH, Charnigo RJ, Esser KA, Egan JM, de Cabo R & Pearson KJ (2012). Perinatal exercise improves glucose homeostasis in adult offspring. Am J Physiol Endocrinol Metab 303, E1061–E1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LG, Qi NR, De Cabo R & Pearson KJ (2013). Maternal exercise improves insulin sensitivity in mature rat offspring. Med Sci Sports Exerc 45, 832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Lee I, Kim D, Koh Y, Kong J, Lee S & Kang H (2014). Effect of aerobic exercise training on non‐alcoholic fatty liver disease induced by a high fat diet in C57BL/6 mice. J Exerc Nutrition Biochem 18, 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp JF 3rd (1991). Exercise and fetal health. J Dev Physiol 15, 9–14. [PubMed] [Google Scholar]
- Clapp JF 3rd, Kim H, Burciu B, Schmidt S, Petry K & Lopez B (2002). Continuing regular exercise during pregnancy: effect of exercise volume on fetoplacental growth. Am J Obstet Gynecol 186, 142–147. [DOI] [PubMed] [Google Scholar]
- Cordero Y, Mottola MF, Vargas J, Blanco M & Barakat R (2015). Exercise is associated with a reduction in gestational diabetes mellitus. Med Sci Sports Exerc 47, 1328–1333. [DOI] [PubMed] [Google Scholar]
- Dahri S, Snoeck A, Reusens‐Billen B, Remacle C & Hoet JJ (1991). Islet function in offspring of mothers on low‐protein diet during gestation. Diabetes 40 (Suppl. 2), 115–120. [DOI] [PubMed] [Google Scholar]
- Dayi A, Agilkaya S, Ozbal S, Cetin F, Aksu I, Gencoglu C, Cingoz S, Pekcetin C, Tugyan K, Kayatekin BM & Uysal N (2012). Maternal aerobic exercise during pregnancy can increase spatial learning by affecting leptin expression on offspring's early and late period in life depending on gender. ScientificWorldJournal 2012, 429803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel S, Elwood P, Sweetnam P, Yarnell J & Smith GD (1996). Birthweight, body‐mass index in middle age, and incident coronary heart disease. Lancet 348, 1478–1480. [DOI] [PubMed] [Google Scholar]
- Garofano A, Czernichow P & Breant B (1997). In utero undernutrition impairs rat beta‐cell development. Diabetologia 40, 1231–1234. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Bitsanis D, Ghebremeskel K, Crawford MA & Poston L (2001). Abnormal aortic fatty acid composition and small artery function in offspring of rats fed a high fat diet in pregnancy. J Physiol 533, 815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C & Winter PD (1991). Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303, 1019–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MA & Gluckman PD (2014). Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev 94, 1027–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins SA, Baldi JC, Cutfield WS, McCowan L & Hofman PL (2010). Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity. J Clin Endocrinol Metab 95, 2080–2088. [DOI] [PubMed] [Google Scholar]
- Kanno A, Asahara S, Masuda K, Matsuda T, Kimura‐Koyanagi M, Seino S, Ogawa W & Kido Y (2015). Compensatory hyperinsulinemia in high‐fat diet‐induced obese mice is associated with enhanced insulin translation in islets. Biochem Biophys Res Commun 458, 681–686. [DOI] [PubMed] [Google Scholar]
- Keppler D & Decker K (1974). Glycogen determination with amyloglucosidase In Methods of Enzymatic Analysis, ed. Hans‐Ulrich Bergmeyer, pp. 1127–1131. Academic Press, New York. [Google Scholar]
- Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R & Kunz WS (2008). Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc 3, 965–976. [DOI] [PubMed] [Google Scholar]
- Leon DA, Koupilova I, Lithell HO, Berglund L, Mohsen R, Vagero D, Lithell UB & McKeigue PM (1996). Failure to realise growth potential in utero and adult obesity in relation to blood pressure in 50 year old Swedish men. BMJ 312, 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithell HO, McKeigue PM, Berglund L, Mohsen R, Lithell UB & Leon DA (1996). Relation of size at birth to non‐insulin dependent diabetes and insulin concentrations in men aged 50–60 years. BMJ 312, 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgoyre A, Chabert C, Tonini J, Koulmann N, Bigard X & Sanchez H (2017). Alterations to mitochondrial fatty‐acid use in skeletal muscle after chronic exposure to hypoxia depend on metabolic phenotype. J Appl Physiol (1985) 122, 666–674. [DOI] [PubMed] [Google Scholar]
- Mottola MF & Artal R (2016). Fetal and maternal metabolic responses to exercise during pregnancy. Early Hum Dev 94, 33–41. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Hira T & Hara H (2015). Postprandial glucagon‐like peptide‐1 secretion is increased during the progression of glucose intolerance and obesity in high‐fat/high‐sucrose diet‐fed rats. Br J Nutr 113, 1477–1488. [DOI] [PubMed] [Google Scholar]
- Nascimento EB, Fodor M, van der Zon GC, Jazet IM, Meinders AE, Voshol PJ, Vlasblom R, Baan B, Eckel J, Maassen JA, Diamant M & Ouwens DM (2006). Insulin‐mediated phosphorylation of the proline‐rich Akt substrate PRAS40 is impaired in insulin target tissues of high‐fat diet‐fed rats. Diabetes 55, 3221–3228. [DOI] [PubMed] [Google Scholar]
- Newcomer SC, Taheripour P, Bahls M, Sheldon RD, Foust KB, Bidwell CA & Cabot R (2012). Impact of porcine maternal aerobic exercise training during pregnancy on endothelial cell function of offspring at birth. J Dev Orig Health Dis 3, 4–9. [DOI] [PubMed] [Google Scholar]
- Nolan CJ & Prentki M (2008). The islet beta‐cell: fuel responsive and vulnerable. Trends Endocrinol Metab 19, 285–291. [DOI] [PubMed] [Google Scholar]
- Osmond C, Barker DJ, Winter PD, Fall CH & Simmonds SJ (1993). Early growth and death from cardiovascular disease in women. BMJ 307, 1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DI, Hirst S, Clark PM, Hales CN & Osmond C (1994). Fetal growth and insulin secretion in adult life. Diabetologia 37, 592–596. [DOI] [PubMed] [Google Scholar]
- Platt KM, Charnigo RJ, Kincer JF, Dickens BJ & Pearson KJ (2013). Controlled exercise is a safe pregnancy intervention in mice. J Am Assoc Lab Anim Sci 52, 524–530. [PMC free article] [PubMed] [Google Scholar]
- Ponsot E, Zoll J, N'Guessan B, Ribera F, Lampert E, Richard R, Veksler V, Ventura‐Clapier R & Mettauer B (2005). Mitochondrial tissue specificity of substrates utilization in rat cardiac and skeletal muscles. J Cell Physiol 203, 479–486. [DOI] [PubMed] [Google Scholar]
- Quiclet C, Siti F, Dubouchaud H, Vial G, Berthon P, Fontaine E, Batandier C & Couturier K (2016). Short‐term and long‐term effects of submaximal maternal exercise on offspring glucose homeostasis and pancreatic function. Am J Physiol Endocrinol Metab 311, E508–E518. [DOI] [PubMed] [Google Scholar]
- Raipuria M, Bahari H & Morris MJ (2015). Effects of maternal diet and exercise during pregnancy on glucose metabolism in skeletal muscle and fat of weanling rats. PLoS One 10, e0120980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter EA, Nielsen JN, Jorgensen SB, Frosig C, Birk JB & Wojtaszewski JF (2004). Exercise signalling to glucose transport in skeletal muscle. Proc Nutr Soc 63, 211–216. [DOI] [PubMed] [Google Scholar]
- Ruchat SM, Davenport MH, Giroux I, Hillier M, Batada A, Sopper MM, McManus R, Hammond JA & Mottola MF (2012). Effect of exercise intensity and duration on capillary glucose responses in pregnant women at low and high risk for gestational diabetes. Diabetes Metab Res Rev 28, 669–678. [DOI] [PubMed] [Google Scholar]
- Ruzzin J, Lai YC & Jensen J (2005). Consumption of carbohydrate solutions enhances energy intake without increased body weight and impaired insulin action in rat skeletal muscles. Diabetes Metab 31, 178–188. [DOI] [PubMed] [Google Scholar]
- Sauter NS, Schulthess FT, Galasso R, Castellani LW & Maedler K (2008). The antiinflammatory cytokine interleukin‐1 receptor antagonist protects from high‐fat diet‐induced hyperglycemia. Endocrinology 149, 2208–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen JM, Hsieh CS, Tain YL, Li SW, Yu HR, Chen CC, Tiao MM, Chen YC & Huang LT (2016). Programming effects of prenatal glucocorticoid exposure with a postnatal high‐fat diet in diabetes mellitus. Int J Mol Sci 17, 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon RD, Nicole Blaize A, Fletcher JA, Pearson KJ, Donkin SS, Newcomer SC & Rector RS (2016). Gestational exercise protects adult male offspring from high‐fat diet‐induced hepatic steatosis. J Hepatol 64, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovbro M, Boushel R, Hansen CN, Helge JW & Dela F (2011). High‐fat feeding inhibits exercise‐induced increase in mitochondrial respiratory flux in skeletal muscle. J Appl Physiol (1985) 110, 1607–1614. [DOI] [PubMed] [Google Scholar]
- Srere PA & Brooks GC (1969). The circular dichroism of glucagon solutions. Arch Biochem Biophys 129, 708–710. [DOI] [PubMed] [Google Scholar]
- Stanford KI, Lee MY, Getchell KM, So K, Hirshman MF & Goodyear LJ (2015). Exercise before and during pregnancy prevents the deleterious effects of maternal high‐fat feeding on metabolic health of male offspring. Diabetes 64, 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N, Matsuzaka T, Suzuki M, Nakano Y, Zao H, Yokoo T, Suzuki‐Kemuriyama N, Kuba M, Okajima Y, Takeuchi Y, Kobayashi K, Iwasaki H, Yatoh S, Takahashi A, Suzuki H, Sone H, Shimada M, Nakagawa Y, Yahagi N, Yamada N & Shimano H (2014). Ablation of Elovl6 protects pancreatic islets from high‐fat diet‐induced impairment of insulin secretion. Biochem Biophys Res Commun 450, 318–323. [DOI] [PubMed] [Google Scholar]
- Taylor PD, McConnell J, Khan IY, Holemans K, Lawrence KM, Asare‐Anane H, Persaud SJ, Jones PM, Petrie L, Hanson MA & Poston L (2005). Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat‐rich diet in pregnancy. Am J Physiol Regul Integr Comp Physiol 288, R134–R139. [DOI] [PubMed] [Google Scholar]
- Torres‐Rovira L, Gonzalez‐Anover P, Astiz S, Caro A, Lopez‐Bote C, Ovilo C, Pallares P, Perez‐Solana ML, Sanchez‐Sanchez R & Gonzalez‐Bulnes A (2013). Effect of an obesogenic diet during the juvenile period on growth pattern, fatness and metabolic, cardiovascular and reproductive features of swine with obesity/leptin resistance. Endocr Metab Immune Disord Drug Targets 13, 143–151. [DOI] [PubMed] [Google Scholar]
- Triplitt C, Solis‐Herrera C, Reasner C, DeFronzo RA & Cersosimo E (2000). Classification of diabetes mellitus In Endotext, ed. De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F. & Vinik A. MDText.com, Inc., South Dartmouth, MA, USA. [Google Scholar]
- Vega CC, Reyes‐Castro LA, Bautista CJ, Larrea F, Nathanielsz PW & Zambrano E (2015). Exercise in obese female rats has beneficial effects on maternal and male and female offspring metabolism. Int J Obes (Lond) 39, 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial G, Chauvin MA, Bendridi N, Durand A, Meugnier E, Madec AM, Bernoud‐Hubac N, Pais de Barros JP, Fontaine E, Acquaviva C, Hallakou‐Bozec S, Bolze S, Vidal H & Rieusset J (2015). Imeglimin normalizes glucose tolerance and insulin sensitivity and improves mitochondrial function in liver of a high‐fat, high‐sucrose diet mice model. Diabetes 64, 2254–2264. [DOI] [PubMed] [Google Scholar]
- Vial G, Dubouchaud H, Couturier K, Cottet‐Rousselle C, Taleux N, Athias A, Galinier A, Casteilla L & Leverve XM (2011). Effects of a high‐fat diet on energy metabolism and ROS production in rat liver. J Hepatol 54, 348–356. [DOI] [PubMed] [Google Scholar]
- Wasinski F, Bacurau RF, Estrela GR, Klempin F, Arakaki AM, Batista RO, Mafra FF, do Nascimento LF, Hiyane MI, Velloso LA, Camara NO & Araujo RC (2015). Exercise during pregnancy protects adult mouse offspring from diet‐induced obesity. Nutr Metab (Lond) 12, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtaszewski JFP, Hansen BF, Gade J, Kiens B, Markuns JF, Goodyear LJ & Richter EA (2000). Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes 49, 325–331. [DOI] [PubMed] [Google Scholar]
- Wolfe LA, Brenner IK & Mottola MF (1994). Maternal exercise, fetal well‐being and pregnancy outcome. Exerc Sport Sci Rev 22, 145–194. [PubMed] [Google Scholar]
- Wu H, Jin M, Han D, Zhou M, Mei X, Guan Y & Liu C (2015). Protective effects of aerobic swimming training on high‐fat diet induced nonalcoholic fatty liver disease: regulation of lipid metabolism via PANDER‐AKT pathway. Biochem Biophys Res Commun 458, 862–868. [DOI] [PubMed] [Google Scholar]
- Zoll J, Koulmann N, Bahi L, Ventura‐Clapier R & Bigard AX (2003). Quantitative and qualitative adaptation of skeletal muscle mitochondria to increased physical activity. J Cell Physiol 194, 186–193. [DOI] [PubMed] [Google Scholar]


