Abstract
The challenges of seasonal environments are thought to contribute to brain evolution, but in which way is debated. According to the Cognitive Buffer Hypothesis (CBH) brain size should increase with seasonality, as the cognitive benefits of a larger brain should help overcoming periods of food scarcity via, for instance, increased behavioral flexibility. However, in line with the Expensive Brain Framework (EBF) brain size should decrease with seasonality because a smaller brain confers energetic benefits in periods of food scarcity. Empirical evidence is inconclusive and mostly limited to homoeothermic animals. Here we used phylogenetic comparative analyses to test the impact of seasonality on brain evolution across 30 species of anurans (frogs) experiencing a wide range of temperature and precipitation. Our results support the EBF because relative brain size and the size of the optic tectum were negatively correlated with variability in temperature. In contrast, we found no association between the variability in precipitation and the length of the dry season with either brain size or the sizes of other major brain regions. We suggest that seasonality-induced food scarcity resulting from higher variability in temperature constrains brain size evolution in anurans. Less seasonal environments may therefore facilitate the evolution of larger brains in poikilothermic animals.
Introduction
Brain size varies dramatically between species1 and the recent decades have uncovered a variety of factors that drive this evolution2–10. Consequently, several adaptive hypotheses have been proposed to explain the evolution of brain size in vertebrates. Most of these hypotheses assume that due to its cognitive benefits a larger brain is selected for11–14. For instance, the Cognitive Buffer Hypothesis (CBH)15,16 states that a relatively larger brain allows for increased behavioral flexibility, which facilitates the behavioral buffering of unpredictable changes in the environment17. Central to the CBH is that larger-brained species should perform better in more seasonal habitats where cognitive demands are stronger because the available food sources are more difficult to locate in space or time18,19. A recent study across birds found support for the CBH in a seasonality context, as brain size and environmental variation showed a positive association20. Additional support comes from neotropical parrots in which climatic variability and brain size are positively correlated21. Also, non-migrating birds generally have larger brains than migrating ones22–24, which is interpreted as a cognitive buffer effect in the residential species; better cognitive performance facilitates performance in a changing/harsh environment, while smaller-brained species evade those challenges via migration. Large brains and seasonality therefore seem to go hand in hand.
However, the brain is among the most energetically costly organs in the vertebrate body25,26, environmental factors can therefore impose energetic constraints on its evolution7,27,28. Evolution of a larger brain is hence only possible if the supply of energy is increased, if energy is saved by decreasing allocation to another organ (‘trade-off’)29–33, or by a combination of the two27,34. This energetic perspective on brain evolution is generally termed the expensive brain framework (EBF)7. If the EBF is applied to the challenges of seasonality, animals experiencing periodic energy shortages, which are typical for seasonal habitats, may reduce their brain size to endure those periods. Brain size may therefore be negatively correlated with the intensity of seasonality, especially with the duration of periods of low food availability, if they cannot be fully compensated for by increased foraging effort27,28,35. This may be analogous to the situations on small islands, where the limited resources are suspected to drive the pattern of relatively smaller brains of mammal species inhabiting those islands36–39. The strongest evidence in favor of the EBF stems from strepsirrhine primates in which seasonality was negatively correlated with relative brain size35. In the same group of animals, however also the CBH found support7. It is therefore unclear whether there is a general relationship between seasonality and brain size evolution across vertebrates. This is especially so as this relationship remains largely unexplored in the poikilothermic vertebrates (fishes, amphibians, reptilians), which comprise >75% of all vertebrate species40. The brains of such poikilothermic species should be especially prone to coevolve with environmental/seasonal factors as their scope for metabolic activity completely depends on ambient temperature41. Here we therefore provide the first comparative test of the relationship between seasonality and brain evolution in poikilothermic vertebrates, the anurans.
Environmental seasonality (often characterized by variability in mean temperature and/or precipitation, and the length of the dry season, if applicable) often determines food availability; in frogs mostly via influencing the abundance of insects42,43. Insect mortality is often determined by the degree of environmental seasonality across species43–48, more seasonality therefore results in less food availability. If behavioral flexibility or other cognitive assets help to overcome such periods of food shortage we would expect a positive association between brain size and seasonality (CBH). However, if such periods of food shortage were met by saving energy on overly large brains we would expect a negative association between brain size and seasonality (EBF). We test these opposing predictions in 30 species of Chinese frogs using phylogenetically comparative methods (PGLS; see ‘Methods’) and relate aspects of brain anatomy to three measures of seasonality (Coefficient of variation in precipitation, length of the dry season ‘P2T’, coefficient of variation in temperature; see ‘Material and Methods’ for details).
The CBH and the EBF predict relationships between a species’ whole brain size and the seasonality of its habitat; for brain regions such hypothesis do not exist. However, in birds, the associative areas of the brain (e.g. mesopallium) are known to be involved in learning and hence are the brain regions expected to increase with seasonality20. A rich literature on neuro-ecology further shows that brain architecture often reflects the cognitive challenges of a species’ habitat, quality of diet, or predation risk across a number of taxa9,49–55. For example, forebrain size is positively correlated with habitat complexity across fishes50,51, and the size of the optic tectum is largest in fishes that are piscivorous3,50. Similar patterns are found in some prey species, as predation pressure selects for larger olfactory bulbs and optic tectum in anurans8 and larger forebrain and optic tectum size in guppies (Poecilia reticulata)56. In the present study we test the relationship between seasonality and the size of the five main brain regions (olfactory nerves, olfactory bulbs, telencephalon, optic tectum and cerebellum). If we extrapolate the CBH to those regions we predict that seasonality may be positively associated with the size of brain regions that govern behavioral flexibility such as the telencephalon57. It is less clear which regions would be expected to change in size with seasonality if energetic limitations play a more prominent role in anuran brain evolution as the EBF postulates.
Results
Body size, as characterized by snout-vent length (SVL), showed no significant relationship with seasonal variation in temperature (P = 0.089), suggesting that in our sample of frog species body size and seasonality show no tight relationship. In the separate models in which we tested the relationships of relative brain size and the degree of environmental seasonality we found a negative correlation between relative brain size and coefficient of variation (CV) in mean temperature when controlling for the effects of phylogenetic relationships, SVL and body mass (Fig. 1; λ = 0.1260.670,<0.001, β = −0.259, t = −1.907, P = 0.048). In contrast, relative brain size was neither correlated with CV in precipitation (Appendix file: Figure S1; λ = 0.2340.272,<0.001, β = −0.109, t = −0.988, P = 0.332) nor the length of the dry season (P2T; λ = 0.3970.070,<0.001, β = 0.015, t = 1.919, P = 0.066).
Figure 1.
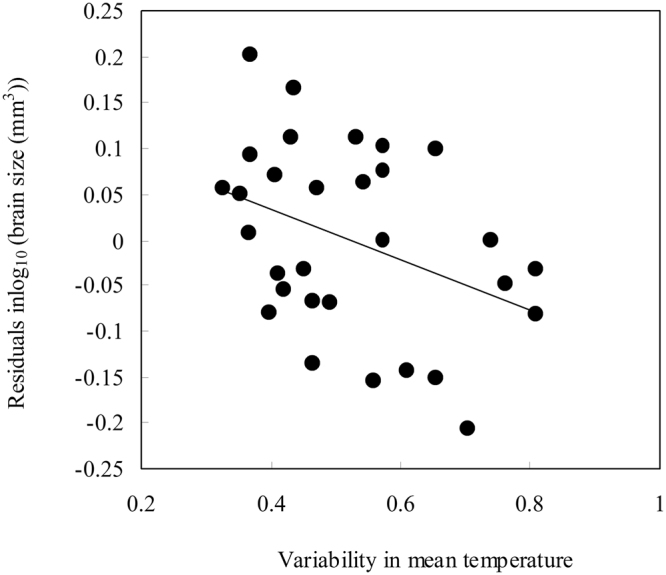
Relationship between relative brain size and variability in mean temperature across 30 anurans species.
The multiple linear regressions showed a qualitatively and quantitatively very similar result, as relative brain size was also negatively associated with variability in monthly temperature, but not CV in precipitation and P2T (Table 1). We further found no effects of the CV on temperature among years on relative brain size (λ = 0.2320.189, <0.0001; β = −0.255, t = −0.662, P = 0.634).
Table 1.
Regression models of brain size and size of brain regions in relation to various predictor variables for males across 30 species anurans when controlling for phylogeny.
| Source | λ | Predictor | β | t | P |
|---|---|---|---|---|---|
| Brain | 0.3200.266,0.023 | CV in temperature | −0.098 | −3.123 | 0.022 |
| CV in precipitation | −0.154 | −1.292 | 0.209 | ||
| P2T | 0.075 | 0.473 | 0.640 | ||
| Body size | 2.134 | 3.781 | <0.001 | ||
| Body mass | −0.177 | −1.006 | 0.324 | ||
| Olfactory nerves | 0.7300.051,<0.001 | CV in temperature | −0.106 | −1.156 | 0.260 |
| CV in precipitation | −0.028 | −0.434 | 0.668 | ||
| P2T | −0.003 | 0.657 | 0.518 | ||
| Rest of brain | 0.278 | 5.838 | <0.001 | ||
| Olfactory bulbs | 0.7130.001,0.077 | CV in temperature | −0.080 | −1.621 | 0.118 |
| CV in precipitation | −0.002 | −0.063 | 0.950 | ||
| P2T | −0.001 | −0.052 | 0.959 | ||
| Rest of brain | 0.138 | 5.411 | <0.001 | ||
| Telencephalon | 0.4790.015,<0.001 | CV in temperature | −0.007 | −0.389 | 0.700 |
| CV in precipitation | 0.011 | 0.813 | 0.424 | ||
| P2T | 0.001 | 0.683 | 0.501 | ||
| Rest of brain | 0.078 | 8.20 | <0.001 | ||
| Optic tecta | 0.8700.149,<0001 | CV in temperature | −0.103 | −3.653 | 0.001 |
| CV in precipitation | −0.017 | −1.486 | 0.150 | ||
| P2T | 0.019 | 1.107 | 0.278 | ||
| Rest of brain | 0.094 | 10.360 | <0.001 | ||
| Cerebellum | <0.0011.0,<0.001 | CV in temperature | −0.021 | −0.562 | 0.579 |
| CV in precipitation | −0.028 | −0.986 | 0.337 | ||
| P2T | <0.001 | 0.023 | 0.982 | ||
| Rest of brain | 0.132 | 7.058 | <0.001 |
Rest of brain was added as a covariate and was significantly positively related to the size of different brain regions in all models. The partial regression slopes (β) for the predictor variable; Phylogenetic signal (λ), t- and P-values are presented for each model.
For brain regions, the PGLS controlling for body size revealed that variation in relative size of optic tectum was negatively correlated with variability in monthly temperature (Fig. 2). The fact that we controlled for the effect of decreasing brain size with seasonality (by using brain size as covariate) indicates that frog brains, in addition to getting smaller in overall size, get disproportionally less composed of optic tectum with increasing seasonality. The sizes of all other brain regions (olfactory nerves, olfactory bulbs, telencephalon and cerebellum) were not affected by variability in mean temperature (Table 1). Brain regions sizes were further unrelated to CV in mean precipitation and P2T (Table 1; Appendix file: Figure S2). PGLS revealed that none of the tested brain regions were significantly associated with CV of temperature among years (λ ≤ 0.5220.001,<0.001; β ≤ 0.224, t ≤ 0.546, P ≥ 0.342).
Figure 2.
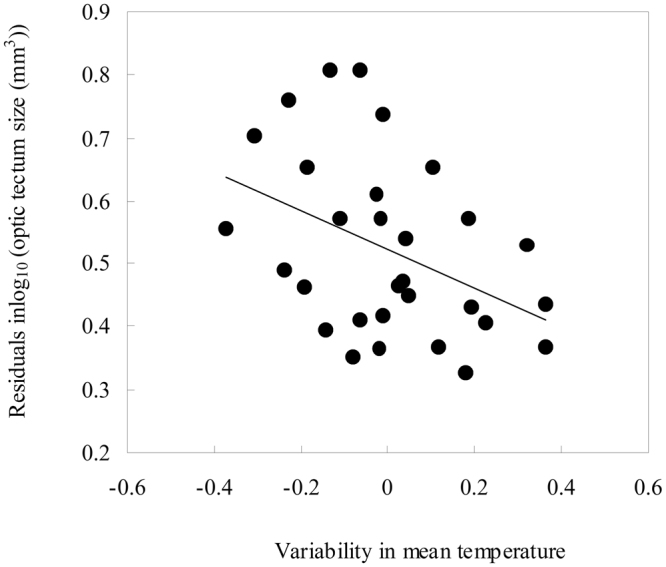
Relationship between relative size of optic tectum and variability in mean temperature across 30 anurans species.
Discussion
Here, we investigated the patterns of brain evolution in the context of seasonality for the first time comparatively in poikilothermic vertebrates. We found a negative association between relative brain size and variability in mean temperature while controlling for possibly confounding variables such as body size and phylogenetic relatedness. Our results are hence consistent with the Expensive Brain Framework. Anurans may tackle recurring food shortages by decreasing brain size in an effort to preserve energy. For brain regions we found only an effect in optic tectum size, which even after the effect of overall brain size was controlled for, was negatively correlated with the variability in mean temperature.
Cognitive buffering of seasonality enables large-brained animals to live in habitats with scare food as exploiting novel and/or changing food sources likely requires behavioral flexibility7,23. Seasonality may select for a relatively larger brain, which may be a special case of the CBH21,58. In the 30 species of frogs we sampled from areas with considerable variation in seasonality, relative brain size was not associated with variability in mean precipitation and the length of the dry season, indicating that those aspects of seasonality do not impact brain size evolution in anurans. However, we did find a decrease in relative brain size with increasing variability in environmental temperature, which is opposite to what would be expected in a CBH context and instead supports the EBF. This is the first comparative support for the EBF in a seasonality context in non-homoeothermic vertebrates. Currently there is a lack of data showing a clear association between brain size and cognitive abilities in anurans, which is why the CBH should be addressed with caution in anurans (see below). Especially so because Amiel et al. (2011) found in amphibians and squamates that successful invaders have relatively larger brains than unsuccessful invaders59. Hence, it is too early to conclusively refute the CBH for anurans in a seasonality context.
It is feasible that recurring periods of food scarcity constrain the evolution of brain size because net energy availability is reduced during these times. Anurans exposed to high variability in environmental temperature adapt to a shorter period of climate favorable for foraging, growth and reproduction by increasing the length of hibernation60. This may help explain our results, as smaller brains should consume less energy and so allow for longer hibernation periods. However, additional studies are needed to clarify whether hibernation is linked to brain evolution. Importantly our findings corroborate circumstantial evidence from a single species study in Bufo andrewsi; these toads occur along a seasonal gradient and specimens collected at different sites revealed a negative relationship between relative brain size and the degree of environmental seasonality28. The effect of seasonality on anuran brain evolution is hence evident in both micro-evolutionary process and macro-evolutionary patterns.
The evolutionary response of the brain to the challenges of seasonality seems qualitatively different between poikilothermic and homeothermic animals; birds and mammals seem to largely show a positive association between brain size and seasonality16,18,20,35, while the brains of anurans seem negatively associated with seasonality28. In any case the benefits of saving energy must outweigh the potential cognitive costs of a decreased brain size, which offers two conceivable explanations to the potential discrepancy between homeothermic and ectothermic species. First, brain size and behavioral flexibility may not be linked in anurans. However, behavioral flexibility and brain size correlates positively in birds and mammals18–20,35, and such a relationship may also be inferred in amphibians and squamates59. Also the fact that brain size is positively associated with several aspects of cognition in fishes14,61–64 and that poison frogs show advanced forms of behavioral flexibility65 renders such a scenario unlikely. Second, the brains of ectothermic animals are relatively more expensive than those of homeothermic animals: per unit of mass, ectotherms’ brain tissues consume as much energy as that of homeotherms25, but ectotherms have a more than 10-fold lower whole-body metabolic rate66. Also, their brain metabolism is less responsive to ambient temperature than that of their bodies67. Decreasing investment into brain mass should hence save relatively more energy in ectotherms than homeotherms. We speculate that this relative difference in costliness of brain tissue underlies the opposing evolutionary response of brain size to seasonality in ectotherms versus homeotherms.
We found that the size of most brain regions seemed unaffected by seasonality with exception of the optic lobes, which showed a similar relationship to the variability of mean temperature as overall brain size; the lobes were larger in frogs from stable environments. As selection on whole brain size usually leads to a concerted change in all brain regions68, this suggests that a specific selection pressure for visual abilities is in place as it is likely that decreasing optic tectum size concurs with reduced visual abilities1. It has been suggested that in anurans a larger optic tectum should allow for better detection of potential predators, which might be an adaptation to high predation risk8,28. If seasonality and predator pressure are linked, i.e. there are less predators in habitats with high variability in temperature, it may be possible to decrease optic tectum size while keeping predation risk constant. It is well established that general biodiversity increases with decreasing seasonality69, whether this translates into decreased predation pressure with increasing seasonality in anurans remains to be tested. Moreover, the role of the optic tectum in prey localization and capture is a classic topic of neuroethology8,70–72. While those studies focus on prey capture it is evident that the optic tectum is responsible for detecting moving objects in the visual field72, which may be extrapolated to predator detection Additionally, based on brain morphological comparisons, Taylor et al. (1995) suggested a potential trade-off between olfaction and vision across anurans, which may indicate that habitat preference could play a role in anuran sensory system evolution72. For instance, if more seasonal habitats would boast a greater number of fossorial (burying) species and a fossorial lifestyle impacts brain anatomy this may impact our results. However, we did not find any indication of uneven distribution of habitat preferences across our samples as fossorial species seemed evenly distributed over the climatic range.
In anuran brain evolution additional aspects may play a role in a seasonality context. Some behavioral features may render anurans more susceptible to the constraints of temperature variability than other vertebrates such as birds, fish or lizards. For example frog movements, including dispersal distances and home range sizes are generally smaller than in other vertebrates73–75, frogs may therefore have a decreased ability of leaving unfavorable habitats. In line their habitat preferences are usually highly specific75,76, which may imply that the likelihood of the CBH affecting their brain evolution should be low.
Why was relative brain size negatively associated with variability in temperature but not with precipitation or length of the dry season? We may speculate that this relationship is be mediated by insect abundance as the frogs in our sample predominantly feed on insects and other invertebrates76 and it is commonly observed that insect biomass depends more strongly on temperature characteristics than on aspects of moisture43,77,78. Among other reasons this is thought to be because insects can behaviorally buffer the effects of varying moisture/precipitation by moving towards more favorable microclimates (e.g. by moving deeper into the leaf litter79), while temperature effects may be harder to buffer. Hence that temperature, but not moisture aspects were related to brain anatomy is in line with the hypothesis that energetic limitations (in our case low availability of insect prey) underlie the negative association between seasonality and relative brain size. What may speak against this argument is the fact that variability in environmental precipitation has previously been shown to affect abundance of food resource in anurans42,43. At this stage more detailed analyses of anuran diet and it’s relationship with seasonality parameters are needed to elucidate the role of food abundance in anuran brain evolution. Especially because previous studies in anurans have shown that environmental variables, such as temperature can be associated with dietary preference80,81, because relative brain size and gut size are positively associated31, and because gut size sometimes covaries with temperature variability in some populations80,82. Integrated studies are therefore needed to fully understand the interplay between diet, seasonality, and gut size in the evolution of anuran brains.
We conclude that our findings are consistent with the EBF and suggest that for anurans variation in temperature can be an energetic challenge that likely constrains brain evolution.
Materials and Methods
Ethical approval
All experiments protocols were approved by the Animal Ethics Committee at China West Normal University. All experimental methods were carried out in accordance with the current laws of China concerning animal experimentation, and permission to collect amphibians was received from the ethical committee for animal experiments in China Council on Animal Care (CCAC) guidelines.
Field sampling
Most frog species exhibit heavily male-biased sex ratios and females are often difficult to obtain8, we therefore collected only male individuals. A total of 171 adult male individuals from 30 species of anurans were collected during the breeding seasons 2007–2014 at the Hengduan Mountains in China (Appendix file: Table S1). Each species was sampled in a single site and we recorded the location’s altitude, longitude and latitude as characteristic for each species. All individuals were confirmed to be adult males on the basis of examination of secondary sexual characters83, and were transferred to the laboratory and kept in individual rectangular tanks (0.5 m × 0.4 m × 0.4 m) with food for one week10. We anesthetized and euthanized them using benzocaine and double-pithing84,85. Finally, we preserved all specimens in 4% phosphate buffered formalin for tissue fixation. After two to eight weeks of preservation, we measured the body size (snout-vent length: SVL) to the nearest 0.01 mm with calipers, and body mass to the nearest 0.1 mg with an electronic balance.
Brains were dissected out and weighed to the nearest 0.1 mg using an electronic balance. The number of days samples spent in the buffered formalin prior to dissection does not influence brain mass31. We measured total brain volume and the volumes of five major brain regions (olfactory nerves, olfactory bulbs, telencephalon, optic tectum and cerebellum; Table S1). Medulla volume was not determined because pithing damaged the structural integrity of the brain stem. Whole brain mass is not affected by this method28. Brain anatomy within a single species can show signs of local adaptation86–88. Indeed, when estimating the climatic variation experienced by our frog species across the entire range we found that each species experiences variations in the parameters of seasonality we used (see below for parameter descriptions; all P < 0.0001). As each species was collected in a single locality (see below), the potential subtle effects of microevolution and the likely more pronounced cross-species differences are additive in our data set. The strength of this single-location approach lies within the high precision with which climatic variation can be determined for each cohort of animals.
Brain measurements
All dissections, digital imaging and measurements were performed by two persons (Lou SL & Liao WB) and all measurements were taken blind to the species identity because specimens were coded by uninformative ID-number. We took digital images of the dorsal, ventral, left and right sides of the brain and brain regions using a Motic Images 3.1 digital camera mounted on a Moticam 2006 light microscope at a 400 × magnification (Figure S3 in Appendix file). For dorsal and ventral views, we ensured that the view of the photographed brain was horizontal. We also confirmed that the brain was symmetrically positioned such that one hemisphere did not appear larger than the other. For paired regions, we only measured the width of the right hemisphere and doubled the volume estimate. We measured the length (L), width (W) and height (H) of the brain and the five brain regions from the digital photographs using tpsDig 1.37 software. We defined brain and brain regions as the greatest distance enclosed by the given region and exhibited the used landmarks28. Finally, the volumetric estimates of different brains were obtained using an ellipsoid model: volume = (L*W*H) π / (6*1.43)8. For all species, there are very high both intra- and inter-measurer repeatabilities of the inter-measurer repeatability for all brain traits8. Average size of brain and average size of brain regions for given species were used in all analyses. All variables were log10-transformed to meet distributional assumptions before all analyses. To avoid negative values after log transformation, all data were multiplied by 1000 prior to log transformation, as some measurements were smaller than one89. The heterogeneity in variability across the five brain regions would not bias the results8.
Phylogeny reconstruction
To reconstruct the phylogeny, we obtained the sequences of six nuclear, three mitochondrial and three mitochondrial ribosome genes from GenBank (for GenBank accession numbers and sequence coverage see Table S2 in Appendix file). The nuclear sequences comprised the recombination-activating gene 1 (RAG1), rhodopsin (RHOD) and tyrosinase (TYR), the mitochondrial genes included cytochrome b (CYTB) and the large and small subunits of the mitochondrial ribosome genes (12 S/16 S). For each locus, we aligned the sequences of all species using multiple sequence alignment (MUSCLE) as implemented in MEGA v.7.090 and determined its best nucleotide substitution model using jModelTest v.2.1.791,92. The best substitution models were GTR + G for 16 S and TYR, HKY + G for RAG1 and RHOD and HKY + G + I for 12 S and CYTB, respectively.
Subsequently, we reconstructed the phylogeny (Appendix file: Figure S4) based on Bayesian inference using Mrbayes V3.2.693. Due to a lack of fossil dates in our sample of species and because the absolute timing of speciation events was deemed less important for our analyses than the relative branch lengths, we omitted the time calibration points. The Markov Chain Monte Carlo (MCMC) simulation was allowed to run for 20 million generations and we sampled a tree every 2000th generation. We used Tracer v.1.6.094 to examine the convergence of the Bayesian chain and the stationary states of all parameters, considering effective sample sizes (ESSs) greater than 200 to be adequate. Finally, we generated a maximum clade credibility tree with mean node heights and a 10% burn-in using Tree Annotator v.1.8.395.
Data analyses
According to the location of each collected species, we retrieved data on average monthly temperature and precipitation from https://www.meteoblue.com (Table S1)96. In this study, we used two standard measures of precipitation variation: i) the coefficient of variation (CV = SD/mean); ii) P2T as a measure of the length of the dry season, a dry month is defined when its total precipitation is less than two times the mean temperature97. We also used the coefficient of variation in monthly mean temperature. We also collected everyday average temperature at each location and calculated CV of temperature among years from Chinese Meteorological Stations (http://www.lishi.tianqi.com) between 2011 and 2015. A greater CV of temperature among years may affected brain size, as it might expose animals to higher cognitive demands if they have to behaviorally respond to unexpected changes. Physiologically, anurans can respond to seasonality by increasing the length of hibernation60. All collected species hibernate and hibernation length may impact brain evolution. However, it was beyond the scope of the current project to collect data on hibernation length for all species.
The relationships between brain size, size of five brain regions, and the degree of environmental seasonality (i.e. precipitation variation: CV in monthly precipitation and P2T; temperature variation: CV in monthly temperature) were assessed in a series of phylogenetically controlled linear models. To account for the evolutionary relationships among species, we used log10-transformed data in the APE-package98 in R software package (V.2.13.1)99 to perform phylogenetically controlled generalized least-squared (PGLS) regression analyses100. According to maximum-likelihood method, the PGLS regression estimates a phylogenetic scaling parameter λ. The parameter λ estimates the effect of phylogenetic signal on the relationships between brain size and environmental factors analyzed (λ = 0 indicating no phylogenetic signal, and λ = 1 indicating strong phylogenetic signal).First we analyzed the relationship between SVL (log-transformed) and seasonal variation in temperature to test whether seasonality is associated with smaller brains or larger bodies. Since brains are subject to a wide range of selective pressures that act simultaneously, we used multiple regressions of phylogenetically controlled linear models treating brain volume as response variable, and environmental seasonality as independent variables, and SVL as covariate to test for relationships between brain volume and the degree of environmental seasonality. To test the relationships between brain regions and degree of environmental seasonality, we used PGLS treating brain regions as response variable (s), environmental seasonality as independent variables, and rest of brain (brain minus respective regions) as covariate. Controlling for potential collinearity between CV in monthly temperature and CV of temperature among years, we used a separate model treating brain regions as response variable, CV of temperature among years as independent variables, and rest of brain as covariate to investigate the effect CV of temperature among years on brain regions.
Electronic supplementary material
Acknowledgements
We thank Shang Ling Lou, Ao Jiang, Long Jin and Cheng Chen help with the fieldwork. The study was supported by the National Natural Sciences Foundation of China (31471996; 31772451) and Sichuan Province Department of Education Innovation Team Project (15TD0019).
Author Contributions
W.B.L. conceived and designed the experiments; M.J.Z. analyzed the data; Y.L. collected data; W.B.L. wrote the manuscript; A.K. advised W.B.L. and revised and reviewed the manuscript. All authors discussed the results and commented on the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-16921-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Striedter, G. F. Principles of brain evolution. (Sinauer Associates Inc, 2005).
- 2.Harvey PH, Clutton-Brock TH, Mace GM. Brain size and ecology in small mammals and primates. Proc. Natl. Acad. Sci. USA. 1980;77:4387–4389. doi: 10.1073/pnas.77.7.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotrschal K, van Staaden MJ, Huber R. Fish brains: evolution and environmental relationships. Rev. Fish Biol. Fisher. 1998;8:373–408. doi: 10.1023/A:1008839605380. [DOI] [Google Scholar]
- 4.Marino L. A comparison of encephalization between Odontocete Cetaceans and anthropoid primates. Brain Behav. Evol. 1998;51:230–238. doi: 10.1159/000006540. [DOI] [PubMed] [Google Scholar]
- 5.Day LB, Westcott DA, Olster DH. Evolution of bower complexity and cerebellum size in bowerbirds. Brain Behav. Evol. 2005;66:62–72. doi: 10.1159/000085048. [DOI] [PubMed] [Google Scholar]
- 6.Aviles JM, Garamszegi LZ. Egg rejection and brain size among potential hosts of the common cuckoo. Ethology. 2007;113:562–572. doi: 10.1111/j.1439-0310.2007.01359.x. [DOI] [Google Scholar]
- 7.Van Woerden JT, Willems EP, van Schaik CP, Isler K. Large brains buffer energetic effects of seasonal habitats in catarrhine primates. Evolution. 2012;66:191–199. doi: 10.1111/j.1558-5646.2011.01434.x. [DOI] [PubMed] [Google Scholar]
- 8.Liao WB, Lou SL, Zeng Y, Merilä J. Evolution of anuran brains: disentangling ecological and phylogenetic sources of variation. J. Evol. Biol. 2015;28:1986–1996. doi: 10.1111/jeb.12714. [DOI] [PubMed] [Google Scholar]
- 9.Wu QG, Lou SL, Zeng Y, Liao WB. Spawning location promotes evolution of bulbus olfactorius size in anurans. Herpetol. J. 2016;26:247–250. [Google Scholar]
- 10.Zeng Y, Lou SL, Liao WB, Jehle R, Kotrschal A. Sexual selection impacts brain anatomy in frogs and toads. Ecol. Evol. 2016;6:7070–7079. doi: 10.1002/ece3.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomasello, M. The cultural origins of human cognition. (Harvard University Press, 1999).
- 12.Deaner RO, Isler K, Burkart J, van Schaik CP. Overall brain size and not encephalization quotient, best predicts cognitive ability across non-human primates. Brain Behav. Evol. 2007;70:115–124. doi: 10.1159/000102973. [DOI] [PubMed] [Google Scholar]
- 13.Reader SM, Hager Y, Laland KN. The evolution of primate general and cultural intelligence. Phil. Trans. R. Soc. B. 2011;366:1017–1027. doi: 10.1098/rstb.2010.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotrschal A, et al. Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Curr. Biol. 2013;23:168–171. doi: 10.1016/j.cub.2012.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allmann J, McLaughlin T, Hakeem A. Brain-weight and life-span in primate species. Proc. Natl. Acad. Sci. USA. 1993;90:118–122. doi: 10.1073/pnas.90.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sol D. Revisiting the cognitive buffer hypothesis for the evolution of large brains. Biol. Lett. 2009;5:130–133. doi: 10.1098/rsbl.2008.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefebvre L, Whittle P, Lascaris E, Finkelstein A. Feeding innovations and forebrain size in birds. Anim. Behav. 1997;53:549–560. doi: 10.1006/anbe.1996.0330. [DOI] [Google Scholar]
- 18.Sol D, Duncan RP, Blackburn TM, Cassey P, Lefebvre L. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl. Acad. Sci. USA. 2005;102:5460–5465. doi: 10.1073/pnas.0408145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sol D, Bacher S, Reader SM, Lefebvre L. Brain size predicts the success of mammal species introduced into novel environments. Am. Nat. 2008;172:S63–S71. doi: 10.1086/588304. [DOI] [PubMed] [Google Scholar]
- 20.Sayol F, et al. Environmental variation and the evolution of large brains in birds. Nature Commucat. 2016;7:13971. doi: 10.1038/ncomms13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuck-Paim C, Alonso WJ, Ottoni EB. Cognition in an ever-changing world: climatic variability is associated with brain size in neotropical parrots. Brain Behav. Evol. 2008;71:200–215. doi: 10.1159/000119710. [DOI] [PubMed] [Google Scholar]
- 22.Winkler H, Leisler B, Bernroider G. Ecological constraints on the evolution of avian brains. J. Ornithol. 2004;145:238–244. doi: 10.1007/s10336-004-0040-y. [DOI] [Google Scholar]
- 23.Sol D, Lefebvre L, Rodriguez-Teijeiro JD. Brain size, innovative propensity and migratory behaviour in temperate Palaearctic birds. Proc. R. Soc. B. 2005;272:1433–1441. doi: 10.1098/rspb.2005.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sol D, et al. Evolutionary divergence in brain size between migratory and resident birds. PLoS One. 2010;5:e9617. doi: 10.1371/journal.pone.0009617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mink JW, Blumenschine RJ, Adams DB. Ratio of central nervous-system to body metabolism in vertebrates - its constancy and functional basis. Am. J. Physiol. 1981;241:R203–R212. doi: 10.1152/ajpregu.1981.241.3.R203. [DOI] [PubMed] [Google Scholar]
- 26.Raichle ME, Gusnard DA. Appraising the brain’s energy budget. Proc. Natl. Acad. Sci. USA. 2002;99:10237–10239. doi: 10.1073/pnas.172399499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isler K, van Schaik CP. The expensive brain: A framework for explaining evolutionary changes in brain size. J. Hum. Evol. 2009;57:392–400. doi: 10.1016/j.jhevol.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Jiang A, Zhong MJ, Yang RL, Liao WB, Jehle R. Seasonality and age is positively related to brain size in Andrew’s toad (Bufo andrewsi) Evol. Biol. 2015;42:339–348. doi: 10.1007/s11692-015-9329-4. [DOI] [Google Scholar]
- 29.Lukas WD, Campbell BC. Evolutionary and ecological aspects of early brain malnutrition in humans. Hum. Nat. 2000;11:1–26. doi: 10.1007/s12110-000-1000-8. [DOI] [PubMed] [Google Scholar]
- 30.Tsuboi M, et al. Comparative support for the expensive tissue hypothesis: Big brains are correlated with smaller gut and greater parental investment in Lake Tanganyika cichlids. Evolution. 2015;69:190–200. doi: 10.1111/evo.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao WB, Lou SL, Zeng Y, Kotrschal A. Large brains, small guts: The expensive tissue hypothesis supported in anurans. Am. Nat. 2016;188:693–700. doi: 10.1086/688894. [DOI] [PubMed] [Google Scholar]
- 32.Kotrschal A, Kolm N, Penn DJ. Selection for brain size impairs innate, but not adaptive immune responses. Proc. R. Soc. B. 2016;283(20152857):20152857. doi: 10.1098/rspb.2015.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozlovsky DY, Brown SL, Branch CL, Roth I, Pravosudov VV. Chickadees with bigger brains have smaller digestive tracts: A multipopulation comparison. Brain Behav. Evol. 2014;84:172–180. doi: 10.1159/000363686. [DOI] [PubMed] [Google Scholar]
- 34.Aiello LC, Wheeler P. The expensive-tissue hypothesis - The brain and the digestive system in human and primate evolution. Curr. Anthropol. 1995;36:199–221. doi: 10.1086/204350. [DOI] [Google Scholar]
- 35.Van Woerden JT, van Schaik CP, Isle K. Effects of seasonality on brain size evolution: evidence from strepsirrhine primates. Am. Nat. 2010;176:758–767. doi: 10.1086/657045. [DOI] [PubMed] [Google Scholar]
- 36.Filin I, Ziv Y. New theory of insular evolution: unifying the loss of dispersability and body-mass change. Evol. Ecol. Res. 2004;6:115–124. [Google Scholar]
- 37.Köhler M, Moyà-Solà S. Reduction of brain and sense organs in the fossil insular bovid Myotragus. Brain Behav. Evol. 2004;63:125–140. doi: 10.1159/000076239. [DOI] [PubMed] [Google Scholar]
- 38.Lomolino MV. Body size evolution in insular vertebrates: generality of the island rule. J. Biogeogr. 2005;32:1683–1699. doi: 10.1111/j.1365-2699.2005.01314.x. [DOI] [Google Scholar]
- 39.Weston EM, Lister AM. Insular dwarfism in hippos and a model for brain size reduction in Homo floresiensis. Nature. 2009;459:85–88. doi: 10.1038/nature07922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bunge J, Fitzpatrick M. Estimating the number of species: a review. J. Am. Statist. Assoc. 1993;88:364–373. [Google Scholar]
- 41.Sol D, Sayol F, Ducatez S, Lefebvre L. The life-history basis of behavioural innovations. Phil. Trans. R. Soc. B. 2016;371:20150187. doi: 10.1098/rstb.2015.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen WZ, Zhang YJ. Modelling of the relationship between the frequency of large-scale outbreak of the beet armyworm, Spodopter aexigua (Lepidoptera: Noctuidae) and the wide-area temperature and rainfall trends in China. Acta Entomol. Sin. 2010;53:1367–1381. [Google Scholar]
- 43.Shi PJ, Ikemoto T, Ge F. Development and application of models for describing the effects of temperature on insects’ growth and development. Chin. J. Appl. Entomol. 2011;48:1149–1160. [Google Scholar]
- 44.Savage VM, Gillooly JF, Brown JH, West GB, Charnov EL. Effects of body size and temperature on population growth. Am. Nat. 2004;163:429–441. doi: 10.1086/381872. [DOI] [PubMed] [Google Scholar]
- 45.Fitt GP. The ecology of Heliothis species in relation to agroecosystems. Annu. Rev. Entomol. 1989;l34:17–153. doi: 10.1146/annurev.en.34.010189.000313. [DOI] [Google Scholar]
- 46.Morecroft MD, Bealey CE, Howells E, Rennie S, Woiwod IP. Effects of drought on contrasting insect and plant species in the UK in the mid—1990s. Global Ecol. Biol. 2002;11:7–22. doi: 10.1046/j.1466-822X.2002.00174.x. [DOI] [Google Scholar]
- 47.Joannat S, Simon RM, Michael DM, Valerie KB, Gregory JM. Summer drought alters plant-mediated competition between foliar-and root-feeding insects. Global Change Biol. 2007;13:866–877. [Google Scholar]
- 48.Dang ZH, Chen FJ. Responses of insects to rainfall and drought. Chin. J. App. Entomol. 2011;8:1161–1169. [Google Scholar]
- 49.Clutton-Brock TH, Harvey PH. Primates, brains and ecology. J. Zool. 1980;190:309–323. doi: 10.1111/j.1469-7998.1980.tb01430.x. [DOI] [Google Scholar]
- 50.Huber R, Van Staaden MJ, Kaufman LS, Liem KF. Microhabitat use, trophic patterns, and the evolution of brain structure in African cichlids. Brain Behav. Evol. 1997;50:167–182. doi: 10.1159/000113330. [DOI] [PubMed] [Google Scholar]
- 51.Pollen AA, et al. Environmental complexity and social organization sculpt the brain in lake Tanganyikan cichlid fish. Brain Behav. Evol. 2007;70:21–39. doi: 10.1159/000101067. [DOI] [PubMed] [Google Scholar]
- 52.Safi K, Dechmann D. Adaptations of brain regions to habitat complexity: a comparative analysis in bats (Chiroptera) Proc. Roy. Soc. B. 2005;272:179–186. doi: 10.1098/rspb.2004.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunbar RI, Shultz S. Evolution in the social brain. Science. 2007;317:1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- 54.Yopak EK, et al. A conserved pattern of brain scaling from sharks to primates. Proc. Natl. Acad. Sci. USA. 2010;107:12946–12951. doi: 10.1073/pnas.1002195107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West RJD. The evolution of large brain size in birds is related to social, not genetic, monogamy. Biol. J. Linn. Soc. 2014;111:668–678. doi: 10.1111/bij.12193. [DOI] [Google Scholar]
- 56.Kotrschal A, Deacon AE, Magurran EA, Kolm N. Predation pressure shapes brain anatomy in the wild. Evol. Ecol. 2017;31:619–633. doi: 10.1007/s10682-017-9901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Portavella M, Vargas J, Torres B, Salas C. The effects of telencephalicpallial lesions on spatial, temporal, and emotional learning in goldfish. Brain Res. Bull. 2002;57:397–399. doi: 10.1016/S0361-9230(01)00699-2. [DOI] [PubMed] [Google Scholar]
- 58.Sol D, Székely T, Liker A, Lefebvre L. Big-brained birds survive better in nature. Proc. Roy. Soc. B. 2007;274:763–769. doi: 10.1098/rspb.2006.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amiel JJ, Tingley R, Shine R. Smart moves: Effects of relative brain size on establishment success of invasive amphibians and reptiles. PLoS ONE. 2011;6(4):e18277. doi: 10.1371/journal.pone.0018277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wells, K. D. The ecology and behavior of amphibians. (University of Chicago Press, 2007).
- 61.Kotrschal A, et al. The benefit of evolving a larger brain: big-brained guppies perform better in a cognitive task. Anim. Behav. 2013;86:e4–e6. doi: 10.1016/j.anbehav.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kotrschal A, Corral-Lopez A, Amcoff M, Kolm N. A larger brain confers a benefit in a spatial mate search learning task in male guppies. Behav. Ecol. 2014;26:527–532. doi: 10.1093/beheco/aru227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kotrschal A, et al. Brain size affects female but not male survival under predation threat. Ecol. Lett. 2015;18:646–652. doi: 10.1111/ele.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Bijl W, Thyselius M, Kotrschal A, Kolm N. Brain size affects the behavioral response to predators in female guppies (Poecilia reticulata) Proc. Roy. Soc. B. 2015;282:20151132. doi: 10.1098/rspb.2015.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu YX, Day LB, Summers K, Burmeister SS. Learning tolearn: advanced behavioural flexibility in a poison frog. Anim. Behav. 2016;111:167–172. doi: 10.1016/j.anbehav.2015.10.018. [DOI] [Google Scholar]
- 66.White CR, Blackburn TM, Martin GR, Butler PJ. Basal metabolic rate of birds is associated with habitat temperature and precipitation, not primary productivity. Proc. R. Soc. B. 2007;274:287–293. doi: 10.1098/rspb.2006.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heath AG. Anaerobic and aerobic energy metabolism in brain and liver tissue from rainbow trout (Salmo gairdneri) and bullhead catfish (Ictalurus nebulosus) J. Exp. Zool. Part A. 1988;248:140–146. doi: 10.1002/jez.1402480203. [DOI] [Google Scholar]
- 68.Kotrschal, A. et al. Evolution of brain region volumes during artificial selection for relative brain size. Evolution, 10.1111/evo.13373 (2017b). [DOI] [PubMed]
- 69.Rohde K. Latitudinal gradients in species diversity: the search for the primary source. Oikos. 1992;65:514–527. doi: 10.2307/3545569. [DOI] [Google Scholar]
- 70.Garamszegi, L. Z., Møller, A. P. & Erritzøe, J. Coevolving avian eye size and brain size in relation to prey capture and nocturnality. Proc. R. Soc. B, 269, 358 961–967 (2002). [DOI] [PMC free article] [PubMed]
- 71.Cronin, T. W., Johnsen, S., Marshall, N. J. & Warrant, E. J. Visual ecology. Princeton Univ Press, Princeton (2014).
- 72.Taylor GM, Nol E, Boire D. Brain regions and encephalization in anurans: adaptation or stability? Brain Behav. Evol. 1995;45:96–109. doi: 10.1159/000113543. [DOI] [PubMed] [Google Scholar]
- 73.Duellman, W. E. & Trueb, D. L. Biology of amphibians. (McGraw-Hill Inc, 1986).
- 74.Roff, D. A. The evolution of life histories. (Chapman & Hall, 1992).
- 75.Liao WB. Site fidelity in the Sichuan Torrent Frog (Amolops mantzorum) in a montane region in western China. Acta Herpetol. 2011;6:131–136. [Google Scholar]
- 76.Fei, L. et al. Fauna Sinica. Amphibia, Vol. 2, Anura. (Science Press, 2009).
- 77.Frith CB, Frith DW. Seasonality of insect abundance in an Australian upland tropical rainforest. Austr. J. Ecol. 1985;10:237–248. doi: 10.1111/j.1442-9993.1985.tb00886.x. [DOI] [Google Scholar]
- 78.Guo K, Hao SG, Sun JXO, Kang L. Differential responses to warming and increased precipitation among three contrasting grasshopper species. Global Change Biol. 2009;15:2539–2548. doi: 10.1111/j.1365-2486.2009.01861.x. [DOI] [Google Scholar]
- 79.Ferro DN, Chapman RB, Penman DR. Observations on insect microclimate and insect pest management. Envir. Entomol. 1979;8:1000–1003. doi: 10.1093/ee/8.6.1000. [DOI] [Google Scholar]
- 80.Naya DE, Veloso C, Bozinovic F. Gut size variation among Bufo spinulosus populations along an altitudinal (and dietary) gradient. Ann. Zool. Fenn. 2009;46:16–20. doi: 10.5735/086.046.0102. [DOI] [Google Scholar]
- 81.Lou SL, et al. Altitudinal variation in digestive tract length in Yunnan Pond Frog (Pelophylax pleuraden) Asian Herpetol. Res. 2013;4:263–267. [Google Scholar]
- 82.Roff, D. A. Life-history evolution. (Sinauer Associates, 2002).
- 83.Liao WB, Liu WC, Merilä J. Andrew meets Rensch: Sexual size dimorphism and the inverse of Rensch’s rule in Andrew’s toad (Bufo andrewsi) Oecologia. 2015;177:389–399. doi: 10.1007/s00442-014-3147-8. [DOI] [PubMed] [Google Scholar]
- 84.Jin L, Liu WC, Li YH, Zeng Y, Liao WB. Evidence for the expensive-tissue hypothesis in the Omei Wood Frog (Rana omeimontis) Herpetol. J. 2015;25:127–130. [Google Scholar]
- 85.Mai CL, Liao J, Zhao L, Liu SM, Liao WB. Brain size evolution in the frog Fejervarya limnocharis does neither support the cognitive buffer nor the expensive brain framework hypothesis. J. Zool. 2017;302:63–72. doi: 10.1111/jzo.12432. [DOI] [Google Scholar]
- 86.Pravosudov VV, Clayton NS. A test of the adaptive specialization hypothesis: population differences in caching, memory, and the hippocampus in black-capped chickadees (Poecile atricapilla) Behav. Neurosci. 2002;116:515–522. doi: 10.1037/0735-7044.116.4.515. [DOI] [PubMed] [Google Scholar]
- 87.Gonda A, Herczeg G, Merilä J. Population variation in brain size of nine-spined sticklebacks (Pungitius pungitius)–local adaptation or environmentally induced variation? BMC Evol. Biol. 2011;11:75. doi: 10.1186/1471-2148-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kotrschal A, Rogell B, Maklakov AA, Kolm N. Sex-specific plasticity in brain morphology depends on social environment of the guppy. Poecilia reticulata. Behav. Ecol. Sociobiol. 2012;66:1485–1492. doi: 10.1007/s00265-012-1403-7. [DOI] [Google Scholar]
- 89.Sokal, R. R. & Rohlf, F. J. Biometry. (Freeman and Company, 1995).
- 90.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lüpold S, Jin L, Liao WB. Population density and structure drive differential investment in pre- and postmating sexual traits in frogs. Evolution. 2017;71:1686–1699. doi: 10.1111/evo.13246. [DOI] [PubMed] [Google Scholar]
- 93.Ronquist F, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rambaut, A. & Drummond, A. Tracer v1.6 http://tree.bio.ed.ac.uk/software/tracer/ (2014).
- 95.Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jin L, Yang SN, Liao WB, Lüpold S. Altitude underlies variation in the mating system, somatic condition and investment in reproductive traits in male Asian grass frogs (Fejervarya limnocharis) Behav. Ecol. Sociobiol. 2016;70:1197–1208. doi: 10.1007/s00265-016-2128-9. [DOI] [Google Scholar]
- 97.Walter, H. Ecology of tropical and subtropical vegetation. (Oliver and Boyd, 1971).
- 98.Paradis E, Claude J, Strimmer K. Bioinformatics. 2004. APE: Analyses of phylogenetics and evolution in R language; pp. 289–290. [DOI] [PubMed] [Google Scholar]
- 99.R Development Core Team 2015. R: A Language and Environment for Statistical Computing. Computing. 2015;1:12–21. [Google Scholar]
- 100.Freckleton RP. On the misuse of residuals in ecology: regression of residuals vs. multiple regression. J. Anim. Ecol. 2002;71:542–545. doi: 10.1046/j.1365-2656.2002.00618.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


