Abstract
Objective
The purpose of this study was to investigate the prognostic value of lymph node ratio (LNR) in patients with stage III ovarian high-grade serous carcinoma (HGSC).
Methods
A multicenter, retrospective department database review was performed to identify patients with ovarian HGSC at 6 gynecologic oncology centers in Turkey. A total of 229 node-positive women with stage III ovarian HGSC who had undergone maximal or optimal cytoreductive surgery plus systematic lymphadenectomy followed by paclitaxel plus carboplatin combination chemotherapy were included. LNR, defined as the percentage of positive lymph nodes (LNs) to total nodes recovered, was stratified into 3 groups: LNR1 (<10%), LNR2 (10%≤LNR<50%), and LNR3 (≥50%). Kaplan-Meier method was used to generate survival data. Factors predictive of outcome were analyzed using Cox proportional hazards models.
Results
Thirty-one women (13.6%) were classified as stage IIIA1, 15 (6.6%) as stage IIIB, and 183 (79.9%) as stage IIIC. The median age at diagnosis was 56 (range, 18–87), and the median duration of follow-up was 36 months (range, 1–120 months). For the entire cohort, the 5-year overall survival (OS) was 52.8%. An increased LNR was associated with a decrease in 5-year OS from 65.1% for LNR1, 42.5% for LNR2, and 25.6% for LNR3, respectively (p<0.001). In multivariate analysis, women with LNR≥0.50 were 2.7 times more likely to die of their tumors (hazard ratio [HR]=2.7; 95% confidence interval [CI]=1.42–5.18; p<0.001).
Conclusion
LNR seems to be an independent prognostic factor for decreased OS in stage III ovarian HGSC patients.
Keywords: Analysis, Survival; Epithelial Ovarian Cancer; Lymph Node; Serous Cystadenecarcinom
INTRODUCTION
Lymph node ratio (LNR) is defined as the number of metastatic lymph nodes (LNs) divided by the total number of LNs removed. LNR has been shown to be useful in identifying prognostic subgroups within colorectal [1], gastric [2], breast [3], esophageal [4] cancer patients. Several recent studies have focused on the clinical relevance of LNR in assessing prognosis of gynecologic malignancies but the information of its prognostic significance is scant [5,6,7,8,9].
Today, it is well-known that epithelial ovarian cancer (EOC) is not a single disease but rather a group of diseases — each with different morphology and biologic behavior [10]. At least 5 main types are currently distinguished: high-grade serous carcinoma (HGSC; 70%), endometrioid carcinoma (10%), clear-cell carcinoma (10%), mucinous carcinoma (3%), and low-grade serous carcinoma (LGSC; <5%) [11]. Patients with EOC of different histological types and grades have different probabilities of LN metastasis [12,13,14,15]. Especially patients with ovarian HGSC have an increased risk for LN metastasis [12,13,14,15]. Thus, distinct histopathological features might characterize tumor cells metastasizing through the lymphatic route.
However, previous studies which have investigated the prognostic impact of LNR in EOC analyzed all histologic types and grades simultaneously [7,8,9]. The number of prior studies investigating the prognostic significance of LNR in EOC seems to be limited [7,8,9], and the prognostic impact of LNR in ovarian HGSC has not been clearly delineated. Under the current perspective that the different histological subtypes in ovarian cancer probably represent different disease entities [16], we conducted this multicenter retrospective analysis aiming at a better understanding of the prognostic impact of LNR in ovarian HGSC.
The purpose of this study was to investigate the prognostic value of LNR in patients with stage III ovarian HGSC who have undergone systematic LN dissection during primary upfront surgery resulting in maximal or optimal cytoreduction.
MATERIALS AND METHODS
1. Study design and eligibility
After Institutional Review Board approval of Zekai Tahir Burak Women's Health Training and Research Hospital, Faculty of Medicine, University of Health Sciences, Ankara, Turkey (IRB approval number: 15, date: May 29, 2017), patients with EOC who had been treated with upfront surgery between January 2007 and December 2016 at 6 gynecologic oncology centers from Turkey were retrospectively reviewed. All patients gave informed consent for the surgical procedure and research use of their medical information at admission.
The study population included women who had high-grade serous EOC with histopathologically proven International Federation of Gynecology and Obstetrics (FIGO) stage III [17] disease. Women were included if they had undergone primary surgical treatment including total hysterectomy plus bilateral salpingo-oopherectomy, with bilateral pelvic and para-aortic lymphadenectomy and other surgical procedures resulting in maximal or optimal debulking. Since this study focused only on women having high-grade serous EOC; women with LGSC, those with non-serous EOC (i.e., endometrioid, clear-cell, mucinous, and mixed subtypes) were excluded. Exclusion criteria also included absence of LN dissection, absence of metastatic LNs, patients with suboptimal cytoreduction, and women receiving non-systematic lymphadenectomy. We also excluded patients who received neoadjuvant chemotherapy, women with synchronous malignancies, women with stage IV disease, and those with incomplete medical records.
2. Clinical information
Patient data were extracted from 6 institutions with maintained EOC databases. With the eligible cases, the following information was abstracted from medical records: demographic characteristics, date and type of surgical procedure, presence or absence of ascites, the status of peritoneal cytology examination (negative or positive), size of the primary tumor, size of residual tumor after surgery, stage of disease, the date of diagnosis, length of follow-up, and survival. Tumor characteristics were abstracted from original pathology reports. Data were collected from centers with an online standardized form.
Data on the extent of surgery included number of total LNs removed, number of pelvic LNs removed, and number of para-aortic LNs removed and number of metastatic LNs. All operations were performed by gynecologic oncologists with intent to achieve optimal cytoreduction. Lymphadenectomy was performed after completion of other cytoreductive procedures.
All surgical specimens were examined and interpreted by gynecologic pathologists. Serous EOC was diagnosed based on the World Health Organization (WHO) classification [11] after examination of permanent sections. Architectural grading was defined by standard FIGO criteria. All tumors were staged according to the 2014 FIGO staging system [17]. In patients treated before 2014, stage was determined retrospectively on the basis of surgical and pathologic assessment.
The treatment policies were decided by the attending physician or by the multidisciplinary tumor board at each participating institution. Adjuvant chemotherapy was administered to all patients. The standard primary chemotherapy regimen included paclitaxel (Taxol®; Bristol-Myers Squibb Company, Princeton, NJ, USA) 175 mg/m2 plus carboplatin (Paraplatin®; Bristol-Myers Squibb Company) dosed at an area under curve of 5 or 6 every 21 days for 6 cycles.
Patients returned for follow-up evaluation every 3 months for the first 2 years, every 6 months for the next 3 years, and annually thereafter. Computed tomography or magnetic resonance imaging was performed annually. Survival data were last calculated on 31 December 2016. The survival status of the patients was determined as alive or dead at the time of the last follow-up. For all study subjects with a recorded death, this was confirmed by performing a social security death index search.
3. Definitions
LNR, defined as the percentage of positive LNs to total nodes recovered, was stratified into 3 groups: LNR1 (<10%), LNR2 (10%≤LNR<50%), and LNR3 (≥50%) as described by Mahdi et al. [7].
Maximal debulking was defined as no gross residual disease after primary cytoreductive surgery. Optimal cytoreduction was defined as less than or equal to 1 cm maximal diameter of the largest residual tumor nodule at the completion of the primary operation whereas suboptimal debulking was defined as >1 cm of residual disease after primary cytoreductive surgery. Systematic lymphadenectomy was defined as the removal of at least 20 pelvic and 10 para-aortic LNs [18].
Overall survival (OS) was calculated as the time period between primary cytoreductive surgery to the date of death or the last follow-up. Surviving patients were censored at their last known follow-up.
4. Statistical analysis
Statistical analyses were performed using the statistical software package SPSS version 23.0 (SPSS Inc., Chicago, IL, USA). The data were expressed as median and range for continuous variables. The continuous variables such as age and tumor size have been divided into categories according to the median values. Binary variables were reported as counts and percentages.
Survival curves were generated using the Kaplan-Meier method, and the differences between survival curves were calculated using the log-rank test. In order to evaluate the prognostic factors for OS, a univariate Cox-regression model was used. Any p-value of less than 0.05 in the univariate analysis was subjected to multivariate analysis. A p-value <0.05 was considered to indicate statistical significance.
RESULTS
A total of 229 women met the inclusion criteria; 31 (13.6%) were classified as stage IIIA1, 15 (6.6%) as stage IIIB, and 183 (79.9%) as stage IIIC. The median age at diagnosis was 56 (range, 18–87) and the median duration of follow-up was 36 months (range, 1–120 months). Table 1 demonstrates the clinical and pathological characteristics of node-positive women with stage III ovarian HGSC.
Table 1. Baseline characteristics of the patients.
| Characteristic | Values | |
|---|---|---|
| Age (yr) | 56 (18–87) | |
| ≤56 | 119 (52) | |
| >56 | 10 (48) | |
| Menopausal status | ||
| Premenopausal | 66 (28.8) | |
| Postmenopausal | 163 (71.2) | |
| Grade | ||
| 2 | 51 (22.3) | |
| 3 | 178 (77.7) | |
| Tumor size (cm) | 12 (4–40) | |
| <12 | 133 (58.1) | |
| ≥12 | 96 (41.9) | |
| Ascites | ||
| Present | 171 (74.7) | |
| Absent | 58 (25.3) | |
| Peritoneal cytology | ||
| Positive | 186 (81.2) | |
| Negative | 43 (18.8) | |
| LNs removed | 63 (32–132) | |
| Pelvic LNs | 36 (20–79) | |
| Para-aortic LNs | 23 (10–85) | |
| Positive LNs | 5 (1–89) | |
| Pelvic LNs | 2 (0–50) | |
| Para-aortic LNs | 3 (0–46) | |
| LNR (%) | 8.4 (0.9–90.4) | |
| <10 | 120 (52.4) | |
| 10–49 | 81 (35.4) | |
| ≥50 | 28 (12.5) | |
| FIGO stage | ||
| IIIA1 | 31 (13.6) | |
| IIIB | 15 (6.6) | |
| IIIC | 183 (79.9) | |
| Cytoreduction | ||
| Maximal | 144 (62.9) | |
| Optimal | 85 (37.1) | |
| Status | ||
| Alive | 143 (62.4) | |
| Dead | 86 (37.6) | |
Values are presented as number (median) or number (%).
FIGO, International Federation of Gynecology and Obstetrics; LN, lymph node; LNR, lymph node ratio.
The median number of total LNs harvested was 63 (range, 32–132). The median number of pelvic and para-aortic LNs removed was 36 (range, 20–79), and 23 (range, 10–85), respectively. There were 187 patients with pelvic LN involvement (81.6%), and 194 patients with para-aortic LN involvement (84.7%). Isolated pelvic LN metastasis was detected in 35 women (15.2%). There were 42 patients with isolated para-aortic LN metastasis (18.3%). One hundred fifty-two patients (66.3%) had pelvic and para-aortic LN metastases at the same time. The median number of metastatic pelvic and para-aortic LNs was 2 (range, 0–50) and 3 (range, 0–46), respectively. Finally, the median number of total metastatic LNs was 5 (range, 1–89).
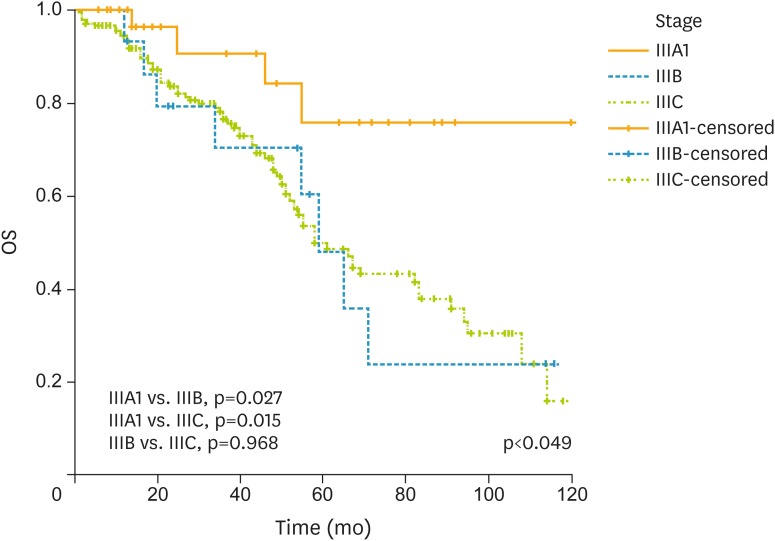
For the entire cohort, the median LNR was 8.4% (range, 0.9%–90.4%). There were 120 (52.4%) women with LNR1, 81(35.4%) women with LNR2, and 28 (12.2%) with LNR3. The median LNR was 6.9% (range, 1.3%–7.6%) for stage IIIA1, 5.7% (range, 1.7%–5.8%) for stage IIIB, and 9.7% (range, 0.9%–90.4%) for stage IIIC. The 5-year OS was 52.8% for the entire cohort. This figure was found to be 75.5% for stage IIIA1 disease, 48.1% for stage IIIB, and 49.9% for stage IIIC (p<0.049) (Fig. 1).
Fig. 1.
OS analysis of patients with regard to stage of disease. Number of patients was 31 as stage IIIA1, 15 as stage IIIB, and 183 as stage IIIC.
OS, overall survival.
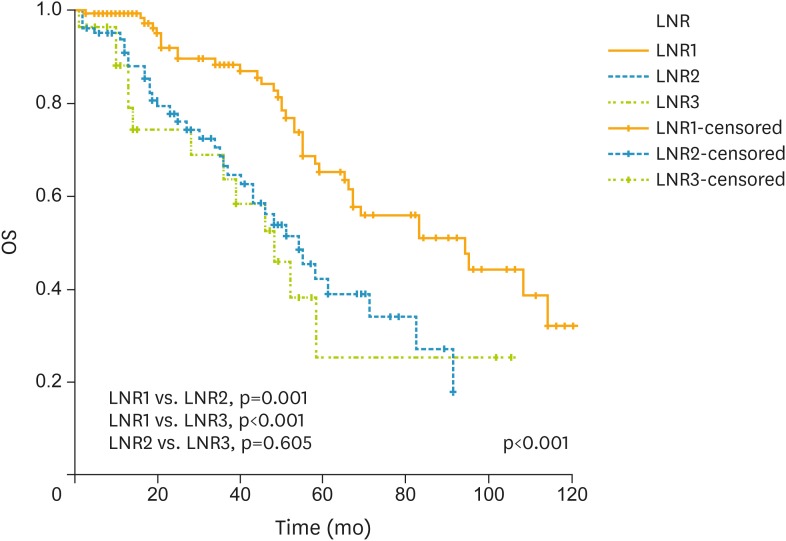
An increased LNR was associated with a decrease in 5-year OS from 65.1% for LNR1, 42.5% for LNR2, and 25.6% for LNR3, respectively (p<0.001; Fig. 2). Further prognostic factors in univariate analysis were stage, and omental involvement (Table 2). In multivariate Cox regression analysis; LNR was an independent prognostic factor for OS after adjusting for other clinically relevant factors in univariate analysis (Table 2). Women with LNR2 were 2.3 times more likely to die of their tumors (hazard ratio [HR]=2.3; 95% confidence interval [CI]=1.46–3.8; p=0.003) whereas patients with LNR3 had a worse prognosis (HR=2.7; 95% CI=1.42–5.18; p<0.001). At the time of reporting, of 229 node-positive women with ovarian HGSC, 143 (62.4%) were alive and 86 (37.6%) were dead.
Fig. 2.
OS analyses with regard to LNR. Number of patients were 120, 81, and 28 for LNR1 (<10%), LNR2 (10%≤LNR<50%), and LNR3 (≥50%).
LNR, lymph node ratio; OS, overall survival.
Table 2. Univariate and multivariate analyses of all patients for OS.
| Characteristic | OS* | Univariate analyses | Multivariate analyses | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | CI 95% | p | HR | CI 95% | p | ||||
| Age (yr) | 1.12 | 0.734–1.719 | 0.590 | ||||||
| ≤56 | 49.3 | ||||||||
| >56 | 56.2 | ||||||||
| Menopausal status | 1.04 | 0.654–1.664 | 0.850 | ||||||
| Premenopausal | 46.3 | ||||||||
| Postmenopausal | 55.4 | ||||||||
| Grade | 1.40 | 0.870–2.280 | 0.160 | ||||||
| Grade 2 | 58.5 | ||||||||
| Grade 3 | 50.3 | ||||||||
| Tumor size (cm) | 1.37 | 0.885–2.132 | 0.150 | ||||||
| ≤12 | 50.2 | ||||||||
| >12 | 56.3 | ||||||||
| Ascites | 1.40 | 0.822–2.439 | 0.200 | ||||||
| Yes | 49.8 | ||||||||
| No | 62.9 | ||||||||
| Malign cytology | 1.15 | 0.649–2.052 | 0.600 | ||||||
| Yes | 51.4 | ||||||||
| No | 58.9 | ||||||||
| Cytoreduction | 1.27 | 0.828–1.957 | 0.260 | ||||||
| Maximal | 54.7 | ||||||||
| Optimal | 51.0 | ||||||||
| Omental involvement | 2.5 | 1.210–5.189 | 0.010 | 1.9 | 0.700–5.150 | 0.200 | |||
| Yes | 48.8 | ||||||||
| No | 71.4 | ||||||||
| FIGO stage | |||||||||
| IIIA1 | 75.5 | ||||||||
| IIIB | 48.1 | ||||||||
| IIIC | 49.9 | ||||||||
| IIIA1 vs. IIIB | 3.2 | 1.121–10.750 | 0.027 | 1.7 | 0.380–8.280 | 0.46 | |||
| IIIA1 vs. IIIC | 3.2 | 1.194–8.944 | 0.015 | 1.7 | 0.450–7.020 | 0.40 | |||
| LNR | |||||||||
| LNR1 (<10%) | 65.1 | ||||||||
| LNR2 (10%≤LNR<50%) | 42.5 | ||||||||
| LNR3 (≥50%) | 25.6 | ||||||||
| LNR1 vs. LNR2 | 2.2 | 1.423–3.673 | 0.001 | 2.3 | 1.460–3.803 | 0.003 | |||
| LNR1 vs. LNR3 | 2.7 | 1.425–5.140 | <0.001 | 2.7 | 1.420–5.188 | <0.001 | |||
CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; LNR, lymph node ratio; OS, overall survival.
*Five-year OS (%).
DISCUSSION
The key findings of the current study indicate that LNR seems to be an independent prognostic factor for OS in stage III ovarian HGSC. Women with LNR2 were 2.3 times more likely to die of their tumors (HR=2.3; 95% CI=1.46–3.8; p=0.003) whereas patients with LNR3 had a worse prognosis (HR=2.7; 95% CI=1.42–5.18; p<0.001).
However, we should underline some limitations of the current study. First, our study is retrospective in nature and thus confounding factors might have been missed. Second, though the differences in survival among groups were statistically significant, causation cannot be proven based on our data. Third, our study was also restricted by a lack of central pathology review. Despite above limitations, our findings provide additional information to the limited body of knowledge on this topic.
There is no numerical significance of the harvested LNs and the positive LNs in the FIGO staging system. The number of harvested LNs is dependent on the surgeon, the pathologist, and the patient (i.e., age, and performance status) [19,20]. Zhou et al. [9] suggested that LNR may reduce the potential bias due to variations of surgeons and pathologists which may affect accurate assessment of LN status. However, Ataseven et al. [8] reported that the potential value of LNR may be based on its composition of both tumor biology and extent of surgical effort and the authors emphasized that the latter may be seen as one of the theoretical weaknesses of this parameter.
Three retrospective studies have reported that high LNR is independently associated with decreased OS in patients with advanced EOC [7,8,9]. Mahdi et al. [7] used the Surveillance, Epidemiology, and End Results (SEER) database and analyzed the prognostic significance of LNR for 6,310 patients with FIGO stages III and IV EOC who underwent surgery between 1988 and 2006. Patients who had positive LNs were divided into 3 groups according to LNR; LNR1 (<10%), LNR2 (10%–50%), and LNR3 (>50%). They found out that LNR was an independent prognostic factor for survival, especially in patients with no macroscopic peritoneal disease [7]. Based on the percentage of metastatic LNs, an increased ratio of metastatic LNs was reported to be associated with a decrease in 5-year survival from 51.5% for LNR1, 38.1% for LNR2, and 27.0% for LNR3, respectively (p<0.001) [7]. These figures were found to be 65.1%, 42.5%, and 25.6%, respectively (p<0.001) in our study; comparable to those of Mahdi et al. [7].
Although Mahdi et al. [7] reported that the prognostic significance of LNR persisted irrespective of the extent of LN dissection; the extent of lymphadenectomy seems to be the major limitation of their study. In their cohort, 58.0% and 21.1% of patients had ≤10 and 11–20 LNs removed, respectively. It should be emphasized that just a small amount of patients (20.8%) had comprehensive lymphadenectomy with >20 LNs removed in their study [7]. Additionally, approximately 35.2% of their cohort exhibited non-serous histology [7].
Zhou et al. [9] also used the SEER database and examined 5,926 patients with stage IIIC EOC. The authors reported LNR to be a good prognostic marker for stage IIIC EOC patients after stratification by the number of resected LNs, tumor histology and tumor grade. They used ROC analysis and determined 0.42 as the optimal cut-off point for LNR [9]. Zhou et al. [9] emphasized that LNR had a more significant impact on survival in EOC than the number of removed LNs and the number of positive LNs. However, SEER database lacks several important variables such as extent and outcome of initial surgery including the size of residual disease, type of adjuvant chemotherapy, and recurrence. Additionally, 18.2% of their cohort exhibited non-serous histology [7].
Ataseven et al. [8] reported on 809 patients with FIGO stage I–IV EOC of whom 398 had node-positive disease. Their results indicated that LNR provided additional information compared to LN status alone. The authors emphasized that patients with LN metastasis presented with a median OS of 44 months. Patients with a LNR≤0.25 presented with a median OS of 52 months compared to a median OS of 30 months for patients having a LNR>0.25 [8]. Ataseven et al. [8] concluded that LNR could be an easily assessable parameter in operated EOC, which allows a favorable and further prognostic discrimination of node-positive disease. It should be emphasized that 27.2% of their study population exhibited non-serous histology.
Compared to previous studies [7,8,9], our cohort seems to be more homogenous with all patients having high-grade serous histotype, and all patients having undergone maximal or optimal cytoreduction as well as systematic LN dissection. Additionally, all patients were treated with the standard paclitaxel/carboplatin regimen postoperatively. These factors seem to reduce the possibility of confounding, and enhance the reliability of the prognostic effects those have been estimated.
Few previous studies have investigated the role of LNR in EOC, so there is no standard LNR cut-off point for comparisons of groups with lower and higher LNRs [9]. Instead of reporting a new cut-off value for LNR, we preferred to stratify our cohort by the LNR cut-offs described by Mahdi et al. [7] in order to make a comparison. In addition to this, we defined systematic lymphadenectomy as the removal of at least 20 pelvic and 10 para-aortic LNs as described in the lymphadenectomy in ovarian neoplasms (LION) study [18]. Although Harter et al. [18] have recently reported that systematic pelvic and para-aortic lymphadenectomy in patients with advanced ovarian cancer with both intra-abdominal complete resection and clinically negative LNs improve neither overall nor progression-free survival despite detecting sub-clinical retroperitoneal LN metastases in 56% of their patients, our data indicated that LNR seems to be an independent prognostic factor for decreased OS in node-positive stage III ovarian HGSC.
To the best of our knowledge, this is the first study that investigated the prognostic impact of LNR in stage III high-grade serous EOC. The strengths of the current study lie in its multicenter nature with a large number of node-positive patients with stage III ovarian HGSC. Our study has the advantage that its time period encompasses the last 10 years, during which all included patients were treated with standard paclitaxel/carboplatin regimen. Additionally, all of the patients in the current study underwent systematic LN dissection.
We conclude that LNR seems to be an independent prognostic factor for decreased OS in stage III ovarian HGSC patients. However, our findings may be confirmed or denied if the prognostic impact of LNR is investigated in a subgroup analysis of node-positive stage III ovarian HGSC patients included in the LION study [18].
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: A.A., M.M.M.
- Data curation: O.N.T., S.M.E., Ş.H., M.M.M.
- Formal analysis: S.M.E, M.M.M.
- Investigation: A.A., O.N.T., S.M.E., C.H., D.M., A.Ö., G.K., Ş.H., H.A., G.T., A.M., M.M.M.
- Methodology: A.A., O.N.T., S.M.E., C.H., D.M., A.Ö., G.K., Ş.H., H.A., G.T., A.M., M.M.M.
- Project administration: M.M.M.
- Resources: A.A., O.N.T., S.M.E., Ş.H., M.M.M.
- Software: S.M.E., M.M.M.
- Supervision: A.A., M.M.M.
- Validation: S.M.E., M.M.M.
- Visualization: O.N.T., S.M.E., M.M.M.
- Writing - original draft: A.A., O.N.T., S.M.E., Ş.H., M.M.M.
- Writing - review & editing: A.A., O.N.T., S.M.E., C.H., D.M., A.Ö., .G.K., Ş.H., H.A., G.T., A.M., M.M.M.
References
- 1.Qiu HB, Zhang LY, Li YF, Zhou ZW, Keshari RP, Xu RH. Ratio of metastatic to resected lymph nodes enhances to predict survival in patients with stage III colorectal cancer. Ann Surg Oncol. 2011;18:1568–1574. doi: 10.1245/s10434-010-1528-8. [DOI] [PubMed] [Google Scholar]
- 2.Deng J, Sun D, Pan Y, Zhang L, Zhang R, Wang D, et al. Ratio between negative and positive lymph nodes is suitable for evaluation the prognosis of gastric cancer patients with positive node metastasis. PLoS One. 2012;7:e43925. doi: 10.1371/journal.pone.0043925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen LJ, Chung KP, Chang YJ, Chang YJ. Ratio and log odds of positive lymph nodes in breast cancer patients with mastectomy. Surg Oncol. 2015;24:239–247. doi: 10.1016/j.suronc.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Hou X, Wei JC, Xu Y, Luo RZ, Fu JH, Zhang LJ, et al. The positive lymph node ratio predicts long-term survival in patients with operable thoracic esophageal squamous cell carcinoma in China. Ann Surg Oncol. 2013;20:1653–1659. doi: 10.1245/s10434-012-2794-4. [DOI] [PubMed] [Google Scholar]
- 5.Polterauer S, Khalil S, Zivanovic O, Abu-Rustum NR, Hofstetter G, Concin N, et al. Prognostic value of lymph node ratio and clinicopathologic parameters in patients diagnosed with stage IIIC endometrial cancer. Obstet Gynecol. 2012;119:1210–1218. doi: 10.1097/AOG.0b013e318255060c. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Zhang L, Tian J, Fu X, Ren X, Hao Q. Significance of the absolute number and ratio of metastatic lymph nodes in predicting postoperative survival for the International Federation of Gynecology and Obstetrics stage IA2 to IIA cervical cancer. Int J Gynecol Cancer. 2013;23:157–163. doi: 10.1097/IGC.0b013e3182778bcf. [DOI] [PubMed] [Google Scholar]
- 7.Mahdi H, Thrall M, Kumar S, Hanna R, Seward S, Lockhart D, et al. The prognostic impact of the ratio of positive lymph nodes on survival of epithelial ovarian cancer patients. J Surg Oncol. 2011;103:724–729. doi: 10.1002/jso.21869. [DOI] [PubMed] [Google Scholar]
- 8.Ataseven B, Grimm C, Harter P, Prader S, Traut A, Heitz F, et al. Prognostic value of lymph node ratio in patients with advanced epithelial ovarian cancer. Gynecol Oncol. 2014;135:435–440. doi: 10.1016/j.ygyno.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J, He ZY, Li FY, Sun JY, Lin HX, Wu SG, et al. Prognostic value of lymph node ratio in stage IIIC epithelial ovarian cancer with node-positive in a SEER population-based study. Oncotarget. 2016;7:7952–7959. doi: 10.18632/oncotarget.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012;460:237–249. doi: 10.1007/s00428-012-1203-5. [DOI] [PubMed] [Google Scholar]
- 11.Kurman RJ, Carcangiu ML, Herrington CS, Young RH, editors. WHO classification of tumours of female reproductive organs. 4th ed. Lyon: International Agency for Research on Cancer; 2014. Tumors of the ovary; pp. 11–86. [Google Scholar]
- 12.Powless CA, Aletti GD, Bakkum-Gamez JN, Cliby WA. Risk factors for lymph node metastasis in apparent early-stage epithelial ovarian cancer: implications for surgical staging. Gynecol Oncol. 2011;122:536–540. doi: 10.1016/j.ygyno.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Takeshima N, Hirai Y, Umayahara K, Fujiwara K, Takizawa K, Hasumi K. Lymph node metastasis in ovarian cancer: difference between serous and non-serous primary tumors. Gynecol Oncol. 2005;99:427–431. doi: 10.1016/j.ygyno.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 14.Kleppe M, Wang T, Van Gorp T, Slangen BF, Kruse AJ, Kruitwagen RF. Lymph node metastasis in stages I and II ovarian cancer: a review. Gynecol Oncol. 2011;123:610–614. doi: 10.1016/j.ygyno.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Kim HS, Park NH, Chung HH, Kim JW, Song YS, Kang SB. Significance of preoperative serum CA-125 levels in the prediction of lymph node metastasis in epithelial ovarian cancer. Acta Obstet Gynecol Scand. 2008;87:1136–1142. doi: 10.1080/00016340802478158. [DOI] [PubMed] [Google Scholar]
- 16.Vaughan S, Coward JI, Bast RC, Jr, Berchuck A, Berek JS, Brenton JD, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11:719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prat J, FIGO Committee on Gynecologic Oncology Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124:1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Harter P, Sehouli J, Lorusso D, Reuss A, Vergote I, Marth C, et al. LION: lymphadenectomy in ovarian neoplasms—a prospective randomized AGO study group led gynecologic cancer intergroup trial. J Clin Oncol. 2017;35 suppl:abstr 5500. [Google Scholar]
- 19.Carnino F, Fuda G, Ciccone G, Iskra L, Guercio E, Dadone D, et al. Significance of lymph node sampling in epithelial carcinoma of the ovary. Gynecol Oncol. 1997;65:467–472. doi: 10.1006/gyno.1997.4633. [DOI] [PubMed] [Google Scholar]
- 20.Panici PB, Maggioni A, Hacker N, Landoni F, Ackermann S, Campagnutta E, et al. Systematic aortic and pelvic lymphadenectomy versus resection of bulky nodes only in optimally debulked advanced ovarian cancer: a randomized clinical trial. J Natl Cancer Inst. 2005;97:560–566. doi: 10.1093/jnci/dji102. [DOI] [PubMed] [Google Scholar]