Abstract
The concept of neurodegenerative diseases and the therapeutics targeting these intractable diseases are changing rapidly. Protein aggregation as the top of pathological cascade is now challenged, and many alternative ideas are proposed. Early molecular pathologies before microscopic detection of diseases protein aggregates, which I propose to call “Ultra-Early Phase pathologies or phase 0 pathologies”, are the focus of research that might explain the failures of clinical trials with anti-Aβ antibodies against Alzheimer’s disease. In this review article, I summarize the critical issues that should be successfully and consistently answered by a new concept of neurodegeneration. For reevaluating old concepts and reconstructing a new concept of neurodegeneration that will replace the old ones, non-biased comprehensive approaches including proteome combined with systems biology analyses will be a powerful tool. I introduce our recent efforts in this orientation that have reached to the stage of non-clinical proof of concept applicable to clinical trials.
Keywords: Ultra-Early Phase pathology, amyloid hypothesis, intracellular amyloid, Alzheimer’s disease, neurodegeneration, Huntington’s disease
Neurodegeneration or neurodegenerative diseases are still confusing terms and used in various ways by different ideas. However, the core concept and diagnosis criteria in neuropathology are that they are the pathological processes associated with aggregation of abnormal proteins inside or outside of cells. Historically, the concept for pathological diagnosis was directly translated to a religious concept of the molecular pathomechanism that protein aggregation is the essential and indispensable event to trigger neurodegeneration at the top of the pathological cascade, and many researches and even human clinical trials have been conducted based on the assumption. However, it is yet unclear whether the protein aggregation is the cause or the result of neurodegeneration. Actually, a number of recent results from clinical trials to remove amyloid aggregates in Alzheimer’s disease turned out to be almost non-effective on the patient symptoms and recent findings by new techniques such as comprehensive proteome analysis revealed molecular changes before protein aggregation in the brain. The aims of this review are to summarize the accumulating evidences against the previous concept, to revise the working hypothesis, and to prospect future approaches to the therapeutics against neurodegenerative diseases.
1. The criteria for pathological diagnosis of neurodegenerative diseases
In 1901, Professor Alois Alzheimer described clinical symptoms of the first case of Alzheimer’s disease, a 51 years old woman Auguste Deter. She died in 1905 and the brain autopsy findings were reported in 1906. At that time there was no technique of immunohistochemistry, and no knowledge exists about amyloid peptides. The pure morphological analysis mainly by silver stain method revealed neurofibrillary changes and amyloid plaques. In the case of plaques, concentrating degenerative neurofibrils to the core substance were stained by such sliver stains. A recent comparison of various silver stain methods and amyloid immunohistochemistry1) indicated that smaller plaques are difficult to detect with such silver stain methods, thus intracellular amyloid accumulation and the following small plaques might have been overlooked, which will be discussed later in this review. After the long history, the insoluble substance in the Alzheimer’s disease brain was purified, the amino acid sequence was determined by protein sequencing and named as as amyloid beta (Aβ). Subsequently, cDNA cloning was performed and amyloid precursor protein (APP) was discovered in 1986–1987.2–5) The antibody against Aβ clearly stains the plaques,6) and Alzheimer’s disease was finally defined as the disease possessing extracellular amyloid deposition in the cerebrum. Based on these historical backgrounds, neuropathological diagnosis of Alzheimer’s disease is now made by existence of extracellular amyloid aggregation in the brain.
Similarly neuropathological diagnoses of the other neurodegenerative diseases including frontotemporal lobar degeneration (FTLD), Parkinson’s disease, Huntington’s disease, spinocerebellar ataxias (SCAs), and amyotrophic lateral sclerosis (ALS) are all defined with intracellular aggregates of tau/TDP43/FUS, alpha-synuclein, huntingtin, polyglutamine proteins and TDP43, respectively.
2. Confusing relationship between pathomechanism hypothesis and diagnosis criteria
The abovementioned diagnostic criteria of neurodegenerative diseases have been directly used as a research hypothesis of Alzheimer’s disease and other neurodegenerative diseases. The typical case is “amyloid hypothesis”. In this famous hypothesis, “amyloid aggregation” is located at the top of pathological cascade. Nothing will happen in the absence of Aβ plaques in the brain, if we faithfully believe this hypothesis. Similarly, the initial hypothesis in polyglutamine diseases was “aggregation hypothesis”. This direct translation from diagnosis criteria to research hypothesis might be successful, while it is also possible that the neglect of other possible hypothesis by this direct translation might lead to final failure in understanding and therapeutics development of human neurodegeneration.
Results from genetics of familial Alzheimer’s disease seem supporting the “aggregation hypothesis”, since the major causative genes of Alzheimer’s disease are amyloid precursor protein (APP) and enzymes cleaving APP to produce Aβ. A proportion among several species of Aβ seem changed, and aggregation-prone species Aβ42/43 are predominantly (though not exclusively and the extents were variable) accumulated in comparison to Aβ40 in human AD brains7) and in mouse models simulating APP or PS1/2 mutations.8)
However, we should remember that a large part of Alzheimer’s disease is non-familial, such patients do not carry APP/PS1/PS2 mutations, and contributing/modifier genes for them are largely unknown. Therefore, in most of Alzheimer’s disease patients, we have no direct proof that molecular events are similar to familial AD and it is possible that molecular events distant from or even unrelated to Aβ cleavage trigger the independent pathological cascades that finally reach or affect Aβ aggregation.
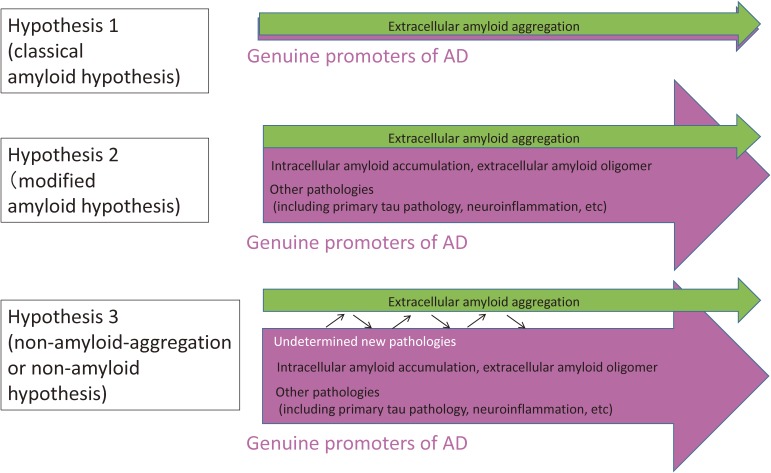
In Fig. 1, I summarize the hypothetical concepts between diagnostic criteria of AD (extracellular Aβ aggregation) and pathomechanism(s) that actually promote AD (Fig. 1). Hypothesis 1 is “classical” amyloid hypothesis, and hypothesis 2 is “modified” amyloid hypothesis in which more researchers are getting to believe. Hypothesis 3 could be the most advanced or aggressive hypothesis partially based on the results from human clinical trials described in the following chapter.
Figure 1.
Prototypes of hypotheses for Alzheimer’s disease (AD) molecular pathology. Hypothesis 1 assumes that extracellular amyloid aggregation is the only one pathological process essential and sufficient for AD. Progression can be explained by extracellular amyloid aggregation and the other pathologies are not necessary for the progression of AD. Hypothesis 2 assumes that several other pathologies play essential and critical roles in the AD progression. The relationships of pathological domains to extracelluar amyloid aggregation are variable. Intracellular amyloid accumulation and amyloid oligomer are relatively close to extracellular amyloid aggregation. Meanwhile the relationship of tau or inflammation to amyloid aggregation is more indirect, and the exact mechanisms connecting them to amyloid aggregation are not understood completely. Hypothesis 3 is more aggressive. In this case, although amyloid aggregation occurs in the group of various Alzheimer’s diseases as diagnostic criteria, many and even undetermined pathologies exist other than extracellular amyloid aggregation, and their contributions to the AD progression are larger than extracellular amyloid aggregation or might be even the main or sufficient promoter of AD.
3. Contradiction of the aggregation-based hypothesis in clinical trials
Based on “classical amyloid hypothesis”, therapeutics such as γ-secretase inhibitors, BACE inhibitors, anti-amyloid antibody therapy, have been developed and applied to clinical trials (Table 1). Most of the candidate drugs are currently facing difficulties. First, clinical trials of γ-secretase inhibitors have failed. The representative of this category, Semagacestat, showed no clinical improvement of cognitive functions in Phase III clinical trial, in addition it induced decline of the symptoms when used at a high dose.9) Other trials such as Flurizan could not show improvement.10) BACE1 inhibitors (AZD3292, E2609), whose suppression induces neurite growth inhibition in experiments, is ongoing in Phase III clinical trial so far without inducing large side effects.
Table 1.
Clinical trials for Alzheimer's disease
| Investigational Products | Mechanism of action | Clinical trials | Results | Remarks |
|---|---|---|---|---|
| AN-1792 (Elan, Wyeth) | Active immunotherapy | Phase I/II France: 97 patients U.S.A.: 298 patients | Withdrawal | Adverse event: Encephalitis (6–7%) Fatalities: France 4 patients U.S.A. 18 patients (Nat Med, 2003) |
| Bapineuzumab (Pfizer, J&J) | Passive Immunotherapy | Phase III North America/Europe Mild-moderate AD (2,452 patients) | Futility Stop (ADAS-Dog, ADA) | ARIA-E Aβ-PET: cleared cerebral Aβ by week 78 4 hundred million dollar loss |
| Solanezumab LY2062430 (Eli Lilly) | Passive Immunotherapy | Phase III Mild-moderate AD (2,052 patients) Expedition-1, -2, -3 | Futility Stop Failure in Exp-1, Exp-2, and Exp3 | Reduction of Aβ in Aβ-PET |
| Aducanumab (Biogen) | Passive Immunotherapy | Phase I/II 26 patients/group | Slightly improved in Phase I/II (CDR-SB, MMSE) | Phase III trial |
| Flurizan (Myriad Genetics & Laboratories) | γ-secretase inhibitor | Phase III | Futility Stop | Green et al., 200910) |
| Semagacestat (Eli Lilly) | γ-secretase inhibitor | Phase III | Futility Stop | Doody et al., 20139) |
| LY2886721 (Eli Lilly) | β-secretase inhibitor | Phase II | Withdrawal | Adverse event: Hepatopathy |
| Verubecestat (Merck) | β-secretase inhibitor | Phase III | EPOCH (mild-moderate case): Futility Stop APECS (prodromal AD): Phase III | |
| AZD3293 (AstraZeneca) | β-secretase inhibitor | Phase III | On going | |
| E2609 (Eisai & Biogen) | β-secretase inhibitor | Phase III | On going |
ARIA-E: Amyloid-related imaging abnormalities-vasogenic edema.
Second, removal of Aβ aggregates with anti-amyloid antibody has not been successful. Solanezumab is the representative in this category, which underwent three times of Phase III clinical trial and failed. The most recent trial (Expedition 3) was press-released in November 2016. Important observation from this drug is that it was successful to reduce Aβ aggregates in the brain judging from the results of amyloid PET.11) This was also the case with bapineuzumab that had been failed to slow down the cognitive decline. More importantly, the similar scheme that decrease of Aβ aggregates did not correspond to improvement of cognitive decline had been reported in the active immunization with Aβ, whose clinical trial was stopped at Phase II due to the severe side effect encephalitis.12) All these data in human trials indicated the discrepancy between the amount of Aβ aggregates and cognitive symptoms.
One possible explanation is that in such clinical trials anti-amyloid antibodies were injected to objects after the stage when Aβ aggregates had induced irreversible changes in the brain. If this is the case, injection of anti-amyloid antibody should be started in preclinical stages. Following this idea, clinical trials with A4, A3, DIAN and so on have been started (A4,13) A3,14) DIAN15)). Especially DIAN is performed with mutation carriers before the onset of familial Alzheimer’s disease, thus outcome is expected.
4. Changing concepts of the amyloid roles in the AD pathology
The other explanations for the failure in clinical trials with ant-Aβ antibodies are also possible. There might be some other molecule that changes the pathology in parallel with Aβ collaboratively or independently. In this line of idea, tau would be the first candidate. According to amyloid hypothesis, tau is located in the downstream of Aβ aggregation. However, it is becoming recognized that there is a group of human dementia patients possessing Primary Age-Related Tauopathy (PART) whose clinical symptoms are sometimes difficult to distinguish from Alzheimer’s disease.16) In this PART, a portion of patients in whom Aβ is aggregated in the brain later are diagnosed as Alzheimer’s disease in autopsy because of the presence of senile plaques in addition to tau accumulation. In this case, tau accumulates in the brain before Aβ, and they continue to accumulate in parallel while they could influence each other.
In addition to the clinical scheme of PART, results from basic experiments have changed the view of amyloid hypothesis. The group of Professor Selkoe proposed the toxicity of Aβ oligomers on synapse.17,18) In this case, amyloid oligomer binds to some membrane receptors and triggers signaling pathway that leads to tau phosphorylation. Possible membrane receptors for Aβ oligomers could be EphB2, PirB, PrPc and so on, but others are also possible.
5. Chronological relationship between cell death and Aβ aggregation
Another issue is the chronological relationship between cell death and Aβ aggregation. According to “amyloid hypothesis”, cell death is induced by extracellular Aβ aggregation. However, a number of results regarding intracellular Aβ accumulation19–23) provide a different viewpoint. Immunohistochemistry with anti-Aβ antibody stains a part of neurons in the brain of PS1-linked and non-familial Alzheimer’s disease patients Alzheimer’s disease.19) It was reported subsequently that a clinically AD patient in a Japanese family with APP(E693Δ) mutation did not have extracellular Aβ aggregation in PET.24) As aggregation-resistant and degradation-resistant characteristics of the deduced mutant Aβ were suggested,24) it is possible that the patients possessed intracellular Aβ accumulation. Unfortunately, autopsy case has not been reported in this family. Intracellular Aβ accumulation is also implicated as a seed of extracellular Aβ aggregation21) and actually mouse models of Alzheimer’s disease (5xFAD mice) show extracellular Aβ aggregation overlapping DAPI stains after necrotic cell death.25) The chronological relationship between cell death and Aβ aggregation is obviously reversed in this situation.
6. New approaches to “Ultra-Early Phase” pathology
The concept of “preclinical stage” of Alzheimer’s disease is the basis for performing clinical trials such as A4, A3 and DIAN in which anti-amyloid antibodies will be used for objects before their onset. But the concept of preclinical stage is currently defined as the duration between the extracellular Aβ aggregation detected by amyloid PET and the onset of clinical symptoms.26,27) In other words, the concept is still dependent on amyloid hypothesis and carries the limitation of amyloid hypothesis that extracellular Aβ aggregation is the top of the cascade. Though the possibility that some other molecular events may precede extracellular Aβ aggregation is not excluded in the scientific sense, this kind of upstream pathologies will not be examined if the objects of A4, A3 and DIAN only include people with Aβ aggregation in the brain diagnosed by PET.
With these backgrounds we intended to perform comprehensive analysis of Alzheimer’s disease pathology including the stages before onset and before extracellular Aβ aggregation. We employed comprehensive phosphoproteome analysis using the high-end mass spectrometer that could identify 60,000–100,000 peptides and 1,000–1,500 proteins at fidelity more than 95% from brain tissues, and tried to identify the earliest molecular signature in the brains of four types of Alzheimer’s disease model mice by putting the data on the protein-protein interaction (PPI) databases.28) In parallel we performed the same analyses with autopsy brains of human Alzheimer’s disease patients to examine whether such early changes continue to the terminal stage in human.28)
First, the changed phosphoproteins were mapped on the protein-protein interaction (PPI) databases to draw the signaling network that could occur in each model mouse at each time point. Then, identified changes of phosphoproteins in the four types of Alzheimer’s disease model mice were further subjected to systems biology analyses to extract common changes among the different mouse models. Consequently, we found 17 proteins that were changed more than two model mice and/or changed at the timing around initiation of extracellular Aβ aggregation. Unexpectedly, most of the 17 proteins were directly connected in PPI database.
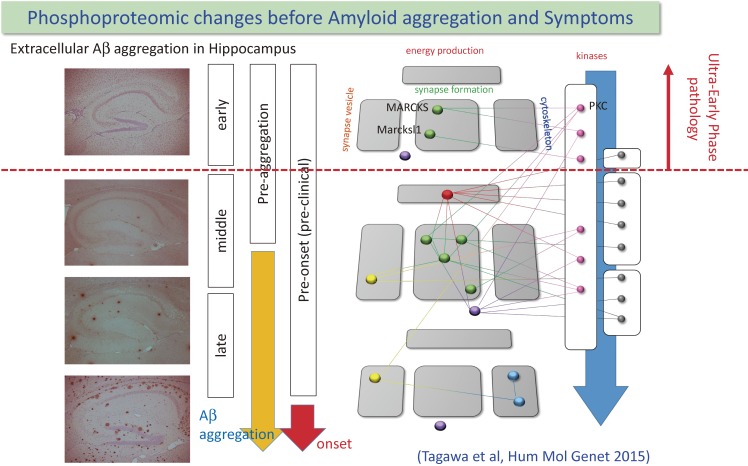
The most important finding was that 3 of 17 proteins were changed at the stage when extracellular Aβ aggregation was not detected (Fig. 2).28) This is the direct evidence for that certain molecular events are initiated before extracellular Aβ aggregation and that extracellular Aβ aggregation is not the top of the cascade of Alzheimer’s disease pathology.
Figure 2.
Chronological relationship among extracellular amyloid aggregation, cognitive/memory symptom and protein phosphorylation changes that were identified from comprehensive phosphoproteome analysis is shown. Surprisingly, Phosphorylation of two synapse-related phosphoproteins and one splicing related protein were changed before initiation of Amyloid-beta aggregation. Protein-protein interactions among identified proteins or between kinases and substrates are indicated with lines. Reproduced from Tagawa et al., Hum Mol Genet 2015 with modifications.
7. Molecular events and synaptic changes at Ultra-Early Phase
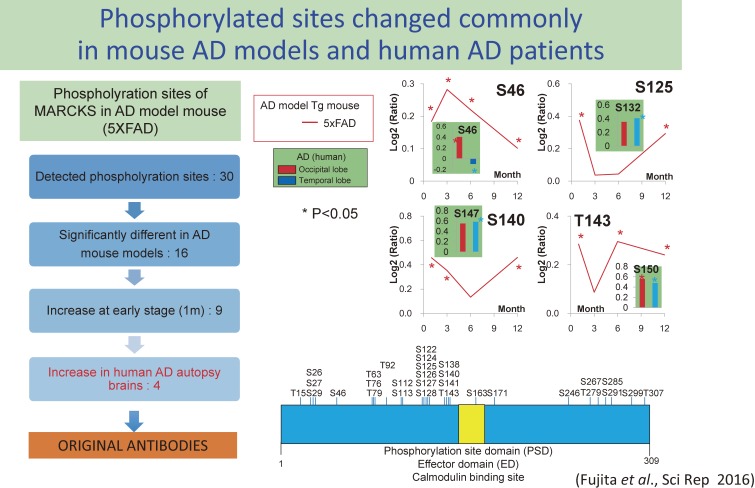
Among the three proteins, we selected MARCKS (Myristoylated Alanine Rich C-Kinase/protein kinase-c Substrate) as the first target and conducted further investigation. It is because another protein among the three proteins was Marcksl1 (MARCKS-like protein 1) partially homologous to MARCKS and two of three could be related to a similar functional group. Therefore, we went back to phosphorylation sites detected by mass spectrometry and checked whether each site was change at the pathological stage before extracellular Aβ aggregation and whether phosphorylation at each site is similarly changed in human AD autopsy brains. From these analyses, four phosphorylation sites were selected (Fig. 3) for which we made original antibodies.29)
Figure 3.
Strategies to narrow down the important phosphorylation sites of MARCKS in the AD pathology.29) We detected 30 phospho-sites in MARCKS by our mass spectroscopy analysis (right lower scheme). 16 phospho-sites were significantly changed in AD model mice, and 9 phospho-sites among them were changed at 1 month of age in 5xFAD mice. 4 in 9 phspho-sites were commonly changed in human postmortem brains (left scheme). Chronological changes of phosphorylation at the 4 phospho-sites are shown (right upper scheme).
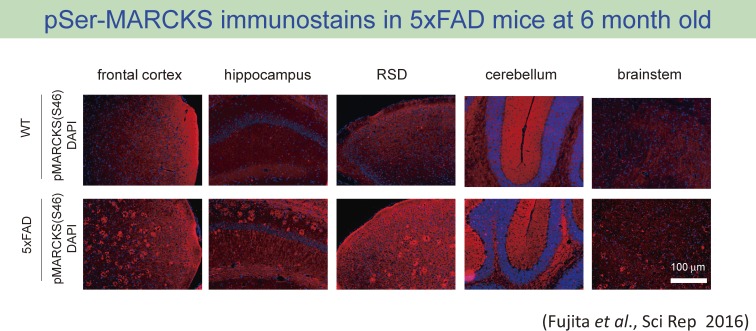
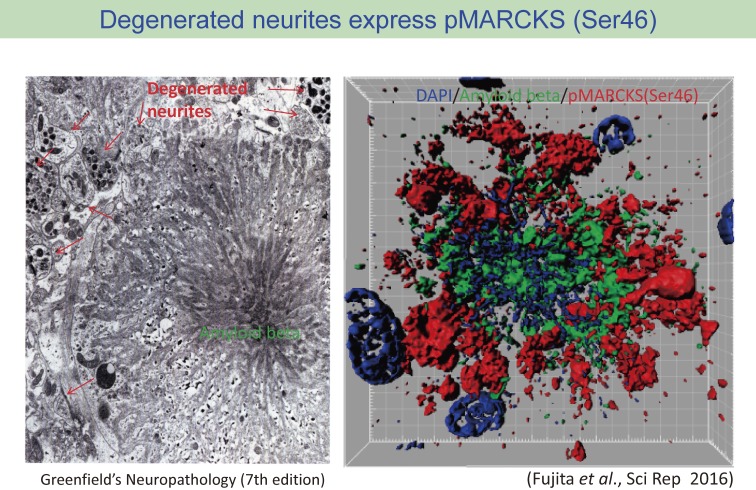
One of these antibodies (anti-pSer46 antibody) revealed an interesting pattern of stains in immunohistochemistry that seemed amyloid plaques.29) However, the detailed analysis with co-staining with anti-Aβ antibody indicated that the immunostain pattern was not due to the cross-reaction to Aβ cbut the anti-pSer46 antibody stained the structure surrounding extracellular Aβ aggregation (Fig. 4). The most plausible structure reactive to the anti-pSer46 antibody was degenerative neurite surrounding Aβ aggregates, which are a historically famous component of amyloid plaque but little is known about the significance and roles in the AD pathology (Fig. 5). To support this idea, we also detected some neurites (axons and dendrites) distant from amyloid plaques are reciprocally but continuously stained with their markers like tau and MAP2.29)
Figure 4.
Immunohistochemistry of 5xFAD mice with the antibody against phospho-Ser46-MARCKS showed amyloid plaque-like staining pattern.29) The pattern was not detected in age-matched background mice.
Figure 5.
Comparison between electron microscopy image of amyloid plaque and immunohistochemistry image from co-staining of amyloid-beta and pSer46-MARCKS. pSr46-MARCKS stains correspond to the localization of degenerative neurites surrounding amyloid aggregates. The image are reproduced from Greenfield’s Neuropathology Seventh Edition (Volume II, page 210)66) and Fujita et al., Scientific Reports 2016.29)
MARCKS is a submembrane protein attaching to PIP2 and connecting the actin network to the plasma membrane of cells to keep and change the shape of membrane. The function is also used for formation of dendritic spine (post-synapse structure in excitatory synapses) and phosphorylation at the binding domain of MARCKS is known to dissociate the interaction between actin network and PIP2.30)
Ser46 is located to the N-terminal region in the upstream of the PIP2 binding domain, which is distinct from the previously reported site.30) Therefore, we investigated the effect of phosphorylation at Ser46 on actin-MARCKS interaction and found it reduces the interaction.29) However, questions remain such as the details of structure changes of MARCKS by phosphorylation at Ser46 or the mechanisms how the phosphorylation reduces the interaction with actin. These issues should be investigated in the future.
Another interesting result was that anti-Aβ antibody and anti-pSer46 antibody stained different but neighboring neurons.29) Before the stage of extracellular Aβ aggregation, it is suggested that some neurons are positively stained with anti-Aβ antibody. Such neurons with intracellular Aβ accumulation are considered vulnerable, undergoing necrotic cell death, and whose intracellular Aβ accumulation could be seeds of extracellular Aβ aggregation after the cell death.29) Based on the consideration, we speculated that such neurons with intracellular Aβ accumulation could release certain substances that trigger phosphorylation of MARCKS at Ser46.
One of the candidates of such triggering substances would be a molecular group called as DAMPs (damage associated molecular patterns) including Aβ and HMGB1. We tested whether Aβ and HMGB1 could trigger phosphorylation of MARCKS at Ser46 in primary neurons by adding them to the culture medium. HMGB1 rather than Aβ at a lower concentration activated MAPKs in the downstream of TLR4 and phosphorylated MARCKS at Ser46. The in vitro data might suggest that neurons damaged by intracellular Aβ accumulation expand damaging effect to neighboring cells and accelerate the AD pathology in vivo at the stage even before extracellular Aβ aggregation.
8. Differential roles of HMGB1 inside and outside of neurons
Interestingly, we also reached to HMGB1 by another comprehensive proteome analysis to identify target molecules in two types of polyglutamine diseases, Huntington’s disease (HD) and Spinocerebellar ataxia type 1 (SCA1). We established a primary neuron-based system expressing huntingtin (Htt) and ataxin-1 (Atxn1) after infection of adenovirus vectors that mimics cellular pathologies of HD and SCA1.31,32) With soluble nuclear proteins prepared from such neuron sets expressing normal/mutant Htt or normal/mutant Atxn1, we performed comprehensive proteome analysis.33) Consequently we identified HMGB1 and HMGB2 as commonly decreased nuclear proteins across HD and SCA1 pathologies.33) They are degraded upon interaction with mutant Htt or Atxn1 or sequestered into inclusion bodies of mutant Htt or Atxn1.33) HMGB1 and HMGB2 are highly homologous DNA architectural proteins that relax double-strand DNA from histone complexes and essential for various nuclear functions such as transcription, DNA damage repair and DNA duplication.34) HMGB1 is also implicated in autophagy35) and mitochondrial DNA quality control.36) Therefore, the reduction was expected to lead to decreased cell viability possibly through DNA damage accumulation and/on abnormal transcription and so on in non-dividing neurons. Actually we observed that DNA damage was increased both in mouse and Drosophila models mimicking HD and SCA1 pathologies.33,36) Important thing is again that HMGB1 was decreased and DNA damage was increased before polyQ protein aggregation was detected microscopically.33,36)
We extended the finding to therapeutics development. AAV vector for expression of HMGB1 was generated and injected to the CSF space at cerebellar surface of mutant Atxn1-knockin mice 1 week after the onset of symptoms.36) As expected, the increased DNA damage and abnormal transcription were partially rescued.36) Most surprising thing was that their lifespans were extended to 170% only by nearly 2 folds increase of HMGB1.36)
Together with the results from AD and polyQ disease models, the shift of HMGB1 from inside to outside of cells is critically linked to neurodegeneration (Fig. 6). Interestingly, we also observed the intracellular HMGB1 was reduced in neurons during ageing.37) Though we have not confirmed the increase of extracellular HMGB1 in the brain, if it is the case, we might be able to speculate that the accelerated shift in neurodegenerative diseases enhances ageing process based on the balance between intracellular and extracellular HMGB1.
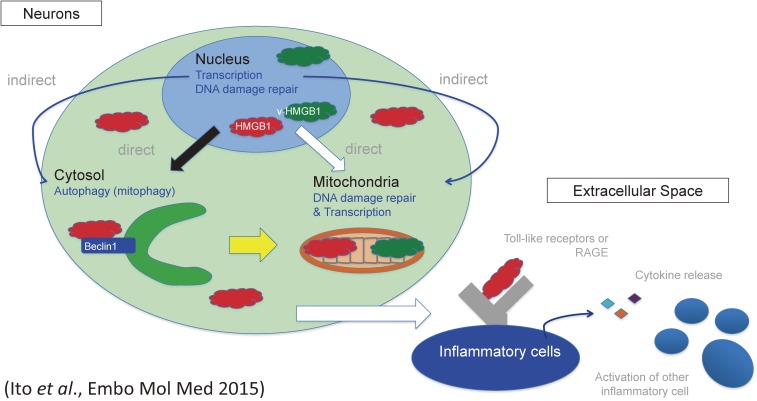
Figure 6.
Multiple functions of HMGB1 inside and outside of cells. HMGB1 accelerates autophagy and contributes to nuclear transcription and nuclear/mitochondrial DNA damage repair. Meanwhile HMGB1 interacts with membrane receptors of inflammatory cells like TLRs and RAGE and triggers inflammation. Thus HMGB1 protects cells from inside but damages cells from outside. The scheme was reproduced from Ito et al., EMBO Mol Med 2015.36)
9. Paradigm shift of disease protein aggregation
In the previous section, I referred our recent original data and discussed about the Ultra-Early Phase AD pathology before extracellular Aβ aggregation. Likewise, “amyloid hypothesis” has been challenged from other aspects. An important question is whether disease protein aggregates are really insoluble or not? This is different from the working hypothesis of anti-Aβ antibody therapy in which they expect phagocytosis mediated by Fc region of the antibody. Independently from such phagocytosis, there has been an idea that disease proteins are not irreversibly insoluble in aggregates. There exist results indicating that so-called aggregates are still reversible and soluble.
For instance, in the field of polyglutamine diseases, the Paulson group revealed that proteins in aggregates are shuttling inside and outside of aggregates by using fluorescence recovery after photobleaching (FRAP) and fluorescence loss in photobleaching (FLIP) on aggregates formed by green fluorescent protein-tagged polyglutamine disease proteins.38) Fluorescent fusion proteins of Ataxin-3 (disease protein of spinocerebellar ataxia type-3) and huntingtin (disease protein of Huntington’s disease) recover after photobleaching was very slow, while FLIP did not show protein release from aggregates. In the case of Ataxin-1 (disease protein of spinocerebellar ataxia type-1), however, showed both rapid recovery and rapid release from aggregates. Their findings indicated difference among so-called aggregates of different polyglutamine disease proteins. The similar experiments by the Mancini group reconfirmed the data of the Paulson group. In addition, they revealed that larger inclusions of mutant ataxin-1 showed slower exchange of the disease proteins.39) Using the similar approaches, the Morimoto group observed how different protein components exchange at the same aggregate and concluded that elongated polyglutamine proteins are tightly associated with the inclusion body structure, while other proteins in inclusion dynamically shuttle from the same inclusion body.
Another important results came from transgenic mouse models using Tet-regulated system. Yamamoto and colleagues revealed that addition of Dox to the Tet-off transgenic mice induced disappearance of intracellular aggregates of mutant Htt and ameliorated the symptoms.40) The Orr group performed similar experiments with Ataxin-1 transgenic mice, and reported the recovery of neuronal phenotypes.41) These results indicated that continuous expression of disease proteins is essential for existence of aggregates in the brain and that equivalent levels of aggregation and dissociation of disease proteins occur continuously in the brain. It means that so-called aggregates are soluble in vivo.
10. Intrinsically disordered proteins/high complexity proteins
Another paradigm shift of so-called aggregates also undergoes in the field of front-temporal lobar degeneration (FTLD). FTLDs are the group of neurodegenerative diseases that predominantly affect frontal and/or temporal lobes of cerebral cortex. As mentioned in a previous chapter, FTLDs possess intracellular aggregates whose major component is tau, TDP43, FUS or other unknown protein. These FTLD disease proteins possess Q/N-rich sequence that enhances self-aggregation,42) and the region is recently called prion-domain.
Basically such domain is very flexible and does not form fixed tertiary structures, but the flexible or high complexity domain is used for self-interaction or interaction with other proteins. The Q/N-rich prion domain is utilized for self-assembly under physiological state of normal form of these disease proteins. The typical example is Atxin-1 and/or PQBP1 nuclear speckle.43) The assembly are called nuclear speckles or nuclear bodies and function as the subcellular domain of proteins necessary for their synergic actions in transcription, RNA-splicing and maybe for other nuclear functions. This is also the case with PQBP1 (Fig. 7). The details of possible roles of such nuclear domains and neurodegeneration were reviewed and discussed previously.44) In brief, the morphological features and functional features of nuclear speckles and those of disease proteins such as Ataxin-1 are highly homologous.44)
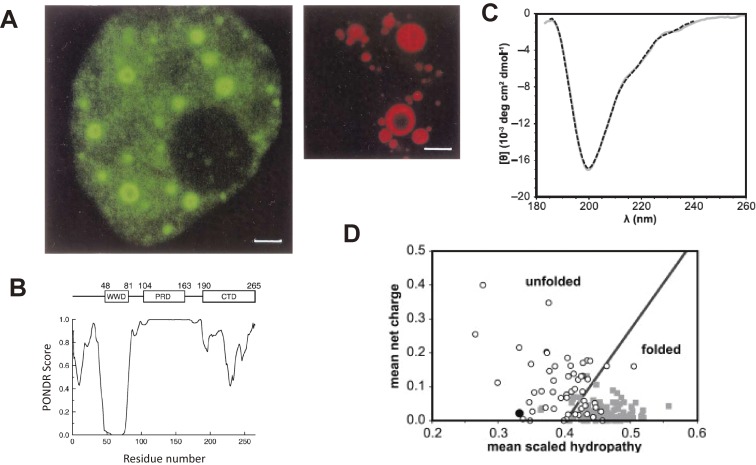
Figure 7.
(A) Nuclear bodies of PQBP1. PQBP1-EGFP (left) and PQBP1-RFP (right) are expressed in cell lines. Reproduced from Okazawa et al., Brain Res Bullet 2001.45) (B) PONDR analysis of the PQBP-1 that predicts C-terminal domain is disordered. Reproduced from Takahashi et al., Biochem Biophys Acta 2009.67) (C) The result from CD analysis of PQBP1 is typical for intrinsically disordered protein. Reproduced from Rees et al., Biophys J 2012.68) (D) Net charge versus hydropathy analysis predict PQBP1 is disordered. Plots are generated from database of folded (gray squares) and unfolded (open circles) proteins. PQBP-1 indicated with a black circle. Reproduced from Rees et al., Biophys J 2012.68)
Another typical example came from our research in polyglutamine diseases.45) We screened binding proteins to the polyglutamine tract amino acid sequence by yeast two hybrid method.46) Consequently, we identified three new proteins named polyglutamine binding proteins (PQBP1, PQBP3, PQBP5) and TERA/p97/VCP.46) Among them, we have continued researches of PQBP1 from functional, structural and pathological aspects. In brief, PQBP is a multi-functional protein; it regulates transcription and RNA splicing through interaction with RNA polymerase II and splicing factor U5-15kD,43,47,48) and the target of transcription and RNA splicing are involved in neural stem cell proliferation,49) neuronal dendrite extension,50) and neuronal cilia.51) Moreover, PQBP1 is recently implicated to function in immune dendritic cell as an intracellular HIV receptor52) and to a major risk factor of coronary diseases.53) Mutations of PQBP1 leads to mental retardation/intellectual disability syndromes called Renpenning syndrome, Golabi-Ito-Hall syndrome, Southerland-Haan syndrome, Hamel syndrome and so on that accompany microcephaly at 80–90% frequency and other minor anomalies at a low frequency.54–57) We have developed Nestin-Cre-mediated conditional knockout mice that show microcephaly and recovered by re-expression of PQBP149) and proved that loss of function of PQBP1 mostly explain the phenotype of these X-linked intellectual disability (XLID) with PQBP1 mutations.
Structural analyses of PQBP1 revealed that PQBP1 has two domains, one is WW domain and another is C-terminal domain that turned out to be an intrinsically denatured sequence later.45) The structural analyses of two research groups confirmed that the C-terminal domain is essential for interaction with the splicing factor U5-15KD,58) and human mutations of Renpenning syndrome is reported in the motif sequence critical for interaction with U5-15KD.58) PQBP1 also forms nuclear speckle based on self-assembly that overlaps with mutant Ataxin-1, the disease protein of spinocerebellar ataxia type-1.43)
The assembly of disease proteins such as TDP43 and FUS based on intrinsically denatured protein domains are recently reported to become solid and difficult to dissociate under repeated changes of temperature.59,60) The similar scheme will be applicable to other intrinsically disordered proteins like PQBP1. The concept of intrinsically disordered disease proteins will fundamentally change the landscape of protein aggregation in neurodegeneration. The structure seemed to be functional but possibly become unusable under some conditions.
11. Intracellular amyloid
Going back to Alzheimer’s disease, which is believed to be a disease of extracellular protein by many researchers, we need to cast light again on intracellular amyloid. This was probably firstly reported by the Yankner group, and they described the intracellular accumulation in neurons of transgenic mice expressing Swedish mutant APP.61) Our group firstly reported that cortical neurons of human non-familial AD as well as PS1-linked familial AD patients also show intracellular Aβ accumulation.19) In addition, we revealed JNK activation in such neurons possessing intracellular Aβ accumulation.19)
Such intracellular Aβ accumulation has been repeatedly reported and now become the generally accepted pathological findings.21) Intracellular expression of Aβ was reconfirmed to be toxic for neurons,20) and suggested to be linked to synaptic dysfunction.22,23) Moreover, intracellular amyloid accumulation in culture system induces cell death and the accumulated amyloid was released extracellularly.62) This finding was also confirmed in transgenic mice expressing mutant APP and PS1 in our study, and the accumulated inside of cells becomes the seed for plaque formation.25)
Presenilins are localized to endosome systems. Strictly, PS1 is localized to recycling endosome system, while PS2 is involved in endosome-lysosome pathway.63,64) The mutation of PS1/2 may lead to the increased activity,65) suggesting that the enhanced activity of PS1/2 under mutation leads to intracellular accumulation. The complementary relationship between PS1 and PS2 might affect such function of PS2 in the case of PS1 mutation.
These findings of intracellular Aβ accumulation might unite the AD pathology, which has been considered rather unique from the aspect of extracellular aggregation, to other neurodegenerative diseases with intracellular accumulation of disease proteins, leading to a general concept in neurodegeneration.
12. Towards therapies targeting on Ultra-Early Phase pathology of neurodegenerative diseases
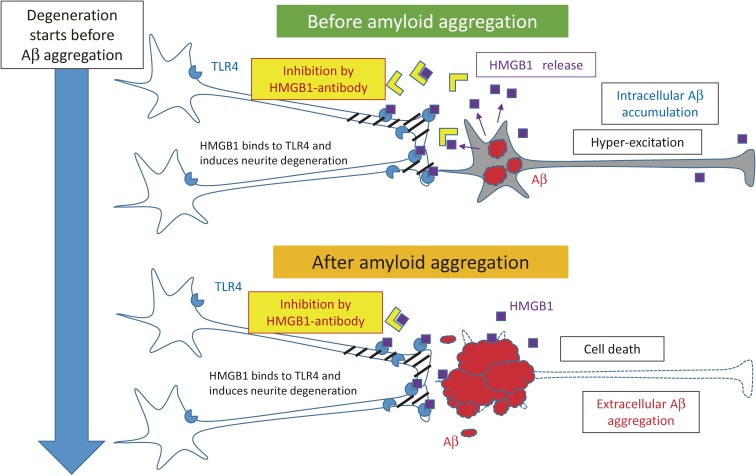
Based on our hypothesis about Ultra-Early Phase pathology that HMGB1 induces neurite degeneration via TLR4-pSer46MARCKS pathway, we developed antibody therapy against HMGB1 to inhibit the damage propagation from neuron to neuron in AD.29) We injected the monoclonal anti-HMGB1-antibody to AD model mice (5xFAD mice) from 1 month when extracellular Aβ aggregation was not detected by immunohistochemistry in 5xFAD mice, and successfully delayed the onset of cognitive symptom in the model mice.29) The result in turn supported and strengthened our hypothesis of Ultra-Early Phase pathology mediated by HMGB1-TLR4-Ser46pMARCKS (Fig. 8).
Figure 8.
Concept of the therapy with anti-HMGB1 antibody against AD.29) Before formation of amyloid aggregates in the brain, HMGB1 is released from neurons accumulating intracellular amyloid or from hyper-excitatory neurons. The released HMGB1 trigger MARCKS phosphorylation at Ser46 through TLR4 and induces the synapse and neurite instability that leads to cognitive impairment. Anti-HMGB1 antibody blocks the action of HMGB1 and inhibits the progressive neurite degeneration. The similar pathological process based on the HMGB1-TLR4-MARCKS axis continues aftre cell death and extracellular amyloid aggregation.
To ensure the Ultra-Early Phase pathology hypothesis, it would be necessary to address several issues. The first issue is the whole chronological scheme of molecular events that precede HMGB1 release to extracellular space. Some trigger molecules should have damaged neurons already and we need to know whether the trigger is intracellular amyloid or other molecules. Related to this question, we have to understand the structural state of intracellular Aβ below or above the detection level of immunohistochemistry. Aβ should be changed structurally in the cell even it is not detected by immunohistochemistry. We also need to know how such changes of intracellular Aβ below the detection level of immunohistochemistry affect cellular functions.
Another point is preceding molecular events other than intracellular Aβ. As I have proposed in this review, Aβ accumulation even inside of cells may not be the cause but the result of AD. To find out such molecular events, analyses of iPS cells or of other models that bridge the chronology and species gaps between iPS cells and animal models (mouse models) are essential. Discovery of such molecular triggers related and non-related to intracellular Aβ would solve the initial AD pathology and will provide us the definite solution of this major intractable neurodegenerative disease.
The second issue is to elucidate the down stream signaling that forces cells to release HMGB1 or other toxic molecules to extracellular space and to clarify the down stream cellular dysfunctions of neurons and/or glia. The first and second issues collectively demand a big science to be solved. Beyond currently prevailing sciences, we need the next generation sciences including comprehensive analysis, multiple new models, new genome techniques and computer sciences for solution of neurodegenerative diseases such as AD, one of the most critical problems for human being.
13. Conclusion
Targeting on the Ultra-Early Phase pathology is getting more and more important across many neurodegenerative diseases. Unsuccessful results in clinical trials and accumulating data on the molecular events before protein aggregation changed our focus to the earlier time point during the progression of neurodegenerative diseases. Obviously intervention after the onset is insufficient. The changing concept from protein aggregation to protein assembly, the complex intracellular signals before disease protein aggregation, the released substances from damaged cells with disease protein accumulation like HMGBs or alarmins, intercellular communication including inflammatory responses are highly important. For development of therapies targeting on the Ultra-Early Phase pathology, we need to focus on such new frontiers of neurodegenerative disease researches to which sufficient efforts have not been concentrated so far.
In this review, I did not mention some critical issues in the research field such as the prion hypothesis, which assumes cell-to-cell transmission of disease proteins. However, the up-take of disease proteins from extracellular space into neurons is directly linked to intracellular accumulation of disease proteins, and the downstream pathways after transmission would be the same as described in this review.
Acknowledgement
This work was supported by the Strategic Research Program for Brain Sciences (SRPBS), Brain Mapping by Integrated Neurotechnologies for Disease Studies from Japan agency for Medical research and Development (Brain/MINDS) from Japan agency for Medical research and Development (AMED), Grant-in-Aid for Scientific Research on Innovative Areas (Foundation of Synapse and Neurocircuit Pathology, 22110001/22110002) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), Grant-in-Aid for Scientific Research A, B, and C (07558233, 10670574, 12670596, 14370213, 16650076, 16390249, 17025017, 18650097, 18023014, 18390254, 20058010, 20023011, 21390265, 26640037, 16H02655) from Japan Society for the Promotion of Science (JSPS), and CREST/PRESTO from Japan Science and Technology Agency (JST). I deeply thank many excellent collaborators, especially Dr. Masaaki Waragai, Dr. Kazuhiko Tagawa, and Professor Ichiro Kanazawa.
Abbreviations
- PQBP1
polyglutamine binding protein1
- Aβ
amyloid beta
- FTLD
frontotemporal lobar degeneration
- ALS
amyotrophic lateral sclerosis
- AD
Alzheimer’s diseases
- PART
primary age-related tauopathy
Profile
Hitoshi Okazawa was born in 1959. He graduated from The University of Tokyo School of Medicine in 1984. After completing his medical intern training, he joined Department of Neurology in The University of Tokyo Hospital in 1986. He received a Ph.D. in 1991 for his work on discovery, characterization, and gene regulation of Oct-3/Oct-4 (Cell 1990; EMBO J 1991, 1993), a switching molecule of stem cell differentiation and the most important factor for generation of iPS cells, from The University of Tokyo Graduate School of Medicine in 1991. He was appointed a staff scientist of Max-Planck Institute for Psychiatry at Munich in Germany, and discovered neurotrophin receptors with Professor Yves-Alain Barde. On returning to Japan, as an assistant professor at The University of Tokyo Hospital, then as a department head at Tokyo Metropolitan Institute for Neuroscience, he continued his research on Alzheimer’s disease, Huntington’s disease and polyglutamine diseases. Notable research contributions include the discovery of polyglutamine binding protein 1 (PQBP1) that is a major causative gene for human intellectual disabilities and a pathological mediator of neurodegeneration, intracellular accumulation of amyloid beta in Alzheimer’s disease, and impairment of DNA damage repair as a common pathology across neurodegenerative diseases. These results were obtained from his pioneering works in disease neuroscience that have combined comprehensive analyses and systems biology. Some of his discoveries are reaching to clinical trials of human patients with supports from AMED, MEXT and so on.
He was appointed as Professor and Chair of Neuropathology at Tokyo Medical and Dental University in 2003 and also serves as Director of Center for Brain Integration Research TMDU since 2012. He was awarded the Narabayashi Prize (2011; Japanese Society of Neurology) and is a director of Japanese Neuroscience Society and a trustee of Japanese Society of Neurology, Japan Society of Neuropathology and many others.
References
- 1).Halliday G., Flowers D., Baum L. (1994) Analysis of staining methods for different cortical plaques in Alzheimer’s disease. Acta Neuropathol. 87, 174–186. [DOI] [PubMed] [Google Scholar]
- 2).Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. (1988) A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature 331, 525–527. [DOI] [PubMed] [Google Scholar]
- 3).Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., Ito H. (1988) Novel precursor of Alzheimer’s disease amyloid protein shows protease inhibitory activity. Nature 331, 530–532. [DOI] [PubMed] [Google Scholar]
- 4).Salbaum J.M., Weidemann A., Lemaire H.G., Masters C.L., Beyreuther K. (1988) The promoter of Alzheimer’s disease amyloid A4 precursor gene. EMBO J. 7, 2807–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Manning R.W., Reid C.M., Lampe R.A., Davis L.G. (1988) Identification in rodents and other species of an mRNA homologous to the human beta-amyloid precursor. Brain Res. 427, 293–297. [DOI] [PubMed] [Google Scholar]
- 6).Yamaguchi H., Hirai S., Morimatsu M., Shoji M., Ihara Y. (1988) A variety of cerebral amyloid deposits in the brains of the Alzheimer-type dementia demonstrated by beta protein immunostaining. Acta Neuropathol. 76, 541–549. [DOI] [PubMed] [Google Scholar]
- 7).Iwatsubo T., Odaka A., Suzuki N., Mizusawa H., Nukina N., Ihara Y. (1994) Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43). Neuron 13, 45–53. [DOI] [PubMed] [Google Scholar]
- 8).Kokjohn T.A., Roher A.E. (2009) Amyloid precursor protein transgenic mouse models and Alzheimer’s disease: understanding the paradigms, limitations, and contributions. Alzheimers Dement. 5, 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Doody R.S., Raman R., Farlow M., Iwatsubo T., Vellas B., Joffe S., Kieburtz K., He F., Sun X., Thomas R.G., Aisen P.S., Alzheimer’s Disease Cooperative Study Steering, C. Siemers E., Sethuraman G., Mohs R., Semagacestat Study, G. (2013) A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N. Engl. J. Med. 369, 341–350. [DOI] [PubMed] [Google Scholar]
- 10).Green R.C., Schneider L.S., Amato D.A., Beelen A.P., Wilcock G., Swabb E.A., Zavitz K.H., Tarenflurbil Phase 3 Study, G. (2009) Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA 302, 2557–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). http://www.alzforum.org/news/research-news/pib-pet-biomarker-study-confirms-bapineuzumab-lowers-amyloid
- 12).Nicoll J.A., Wilkinson D., Holmes C., Steart P., Markham H., Weller R.O. (2003) Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat. Med. 9, 448–452. [DOI] [PubMed] [Google Scholar]
- 13). http://a4study.org/
- 14). http://grantome.com/grant/NIH/R01-AG054029-01
- 15). http://www.dian-info.org/
- 16).Crary J.F., Trojanowski J.Q., Schneider J.A., Abisambra J.F., Abner E.L., Alafuzoff I., Arnold S.E., Attems J., Beach T.G., Bigio E.H., Cairns N.J., Dickson D.W., Gearing M., Grinberg L.T., Hof P.R., Hyman B.T., Jellinger K., Jicha G.A., Kovacs G.G., Knopman D.S., Kofler J., Kukull W.A., Mackenzie I.R., Masliah E., McKee A., Montine T.J., Murray M.E., Neltner J.H., Santa-Maria I., Seeley W.W., Serrano-Pozo A., Shelanski M.L., Stein T., Takao M., Thal D.R., Toledo J.B., Troncoso J.C., Vonsattel J.P., White C.L., 3rd, Wisniewski T., Woltjer R.L., Yamada M., Nelson P.T. (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 128, 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Walsh D.M., Klyubin I., Fadeeva J.V., Cullen W.K., Anwyl R., Wolfe M.S., Rowan M.J., Selkoe D.J. (2002) Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539. [DOI] [PubMed] [Google Scholar]
- 18).Walsh D.M., Selkoe D.J. (2004) Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron 44, 181–193. [DOI] [PubMed] [Google Scholar]
- 19).Shoji M., Iwakami N., Takeuchi S., Waragai M., Suzuki M., Kanazawa I., Lippa C.F., Ono S., Okazawa H. (2000) JNK activation is associated with intracellular beta-amyloid accumulation. Brain Res. Mol. Brain Res. 85, 221–233. [DOI] [PubMed] [Google Scholar]
- 20).Kienlen-Campard P., Miolet S., Tasiaux B., Octave J.N. (2002) Intracellular amyloid-beta 1–42, but not extracellular soluble amyloid-beta peptides, induces neuronal apoptosis. J. Biol. Chem. 277, 15666–15670. [DOI] [PubMed] [Google Scholar]
- 21).LaFerla F.M., Green K.N., Oddo S. (2007) Intracellular amyloid-beta in Alzheimer’s disease. Nat. Rev. Neurosci. 8, 499–509. [DOI] [PubMed] [Google Scholar]
- 22).Bayer T.A., Wirths O. (2010) Intracellular accumulation of amyloid-Beta — a predictor for synaptic dysfunction and neuron loss in Alzheimer’s disease. Front. Aging Neurosci. 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Ripoli C., Cocco S., Li Puma D.D., Piacentini R., Mastrodonato A., Scala F., Puzzo D., D’Ascenzo M., Grassi C. (2014) Intracellular accumulation of amyloid-β (Aβ) protein plays a major role in Abeta-induced alterations of glutamatergic synaptic transmission and plasticity. J. Neurosci. 34, 12893–12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Tomiyama T., Nagata T., Shimada H., Teraoka R., Fukushima A., Kanemitsu H., Takuma H., Kuwano R., Imagawa M., Ataka S., Wada Y., Yoshioka E., Nishizaki T., Watanabe Y., Mori H. (2008) A new amyloid beta variant favoring oligomerization in Alzheimer’s-type dementia. Ann. Neurol. 63, 377–387. [DOI] [PubMed] [Google Scholar]
- 25).Chen X., Kondo K., Motoki K., Homma H., Okazawa H. (2015) Fasting activates macroautophagy in neurons of Alzheimer’s disease mouse model but is insufficient to degrade amyloid-beta. Sci. Rep. 5, 12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Vos S.J., Xiong C., Visser P.J., Jasielec M.S., Hassenstab J., Grant E.A., Cairns N.J., Morris J.C., Holtzman D.M., Fagan A.M. (2013) Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 12, 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Fagan A.M., Vos S.J. (2013) Preclinical Alzheimer’s disease criteria. Lancet Neurol. 12, 1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Tagawa K., Homma H., Saito A., Fujita K., Chen X., Imoto S., Oka T., Ito H., Motoki K., Yoshida C., Hatsuta H., Murayama S., Iwatsubo T., Miyano S., Okazawa H. (2015) Comprehensive phosphoproteome analysis unravels the core signaling network that initiates the earliest synapse pathology in preclinical Alzheimer’s disease brain. Hum. Mol. Genet. 24, 540–558. [DOI] [PubMed] [Google Scholar]
- 29).Fujita K., Motoki K., Tagawa K., Chen X., Hama H., Nakajima K., Homma H., Tamura T., Watanabe H., Katsuno M., Matsumi C., Kajikawa M., Saito T., Saido T., Sobue G., Miyawaki A., Okazawa H. (2016) HMGB1, a pathogenic molecule that induces neurite degeneration via TLR4-MARCKS, is a potential therapeutic target for Alzheimer’s disease. Sci. Rep. 6, 31895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Calabrese B., Halpain S. (2005) Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron 48, 77–90. [DOI] [PubMed] [Google Scholar]
- 31).Tagawa K., Hoshino M., Okuda T., Ueda H., Hayashi H., Engemann S., Okado H., Ichikawa M., Wanker E.E., Okazawa H. (2004) Distinct aggregation and cell death patterns among different types of primary neurons induced by mutant huntingtin protein. J. Neurochem. 89, 974–987. [DOI] [PubMed] [Google Scholar]
- 32).Tagawa K., Marubuchi S., Qi M.L., Enokido Y., Tamura T., Inagaki R., Murata M., Kanazawa I., Wanker E.E., Okazawa H. (2007) The induction levels of heat shock protein 70 differentiate the vulnerabilities to mutant huntingtin among neuronal subtypes. J. Neurosci. 27, 868–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Qi M.L., Tagawa K., Enokido Y., Yoshimura N., Wada Y., Watase K., Ishiura S., Kanazawa I., Botas J., Saitoe M., Wanker E.E., Okazawa H. (2007) Proteome analysis of soluble nuclear proteins reveals that HMGB1/2 suppress genotoxic stress in polyglutamine diseases. Nat. Cell Biol. 9, 402–414. [DOI] [PubMed] [Google Scholar]
- 34).Bianchi M.E., Agresti A. (2005) HMG proteins: dynamic players in gene regulation and differentiation. Curr. Opin. Genet. Dev. 15, 496–506. [DOI] [PubMed] [Google Scholar]
- 35).Tang D., Kang R., Livesey K.M., Cheh C.W., Farkas A., Loughran P., Hoppe G., Bianchi M.E., Tracey K.J., Zeh H.J., 3rd, Lotze M.T. (2010) Endogenous HMGB1 regulates autophagy. J. Cell Biol. 190, 881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Ito H., Fujita K., Tagawa K., Chen X., Homma H., Sasabe T., Shimizu J., Shimizu S., Tamura T., Muramatsu S., Okazawa H. (2015) HMGB1 facilitates repair of mitochondrial DNA damage and extends the lifespan of mutant ataxin-1 knock-in mice. EMBO Mol. Med. 7, 78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Enokido Y., Yoshitake A., Ito H., Okazawa H. (2008) Age-dependent change of HMGB1 and DNA double-strand break accumulation in mouse brain. Biochem. Biophys. Res. Commun. 376, 128–133. [DOI] [PubMed] [Google Scholar]
- 38).Chai Y., Shao J., Miller V.M., Williams A., Paulson H.L. (2002) Live-cell imaging reveals divergent intracellular dynamics of polyglutamine disease proteins and supports a sequestration model of pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 99, 9310–9315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Stenoien D.L., Mielke M., Mancini M.A. (2002) Intranuclear ataxin1 inclusions contain both fast- and slow-exchanging components. Nat. Cell Biol. 4, 806–810. [DOI] [PubMed] [Google Scholar]
- 40).Yamamoto A., Lucas J.J., Hen R. (2000) Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell 101, 57–66. [DOI] [PubMed] [Google Scholar]
- 41).Zu T., Duvick L.A., Kaytor M.D., Berlinger M.S., Zoghbi H.Y., Clark H.B., Orr H.T. (2004) Recovery from polyglutamine-induced neurodegeneration in conditional SCA1 transgenic mice. J. Neurosci. 24, 8853–8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Okazawa, H. (2007) Protein Misfolding, Aggregation, and Conformational Diseases. In Protein Reviews (eds. Uversky, V.N. and Fink, A.L.). Springer, New York, pp. 451–463. [Google Scholar]
- 43).Okazawa H., Rich T., Chang A., Lin X., Waragai M., Kajikawa M., Enokido Y., Komuro A., Kato S., Shibata M., Hatanaka H., Mouradian M.M., Sudol M., Kanazawa I. (2002) Interaction between mutant ataxin-1 and PQBP-1 affects transcription and cell death. Neuron 34, 701–713. [DOI] [PubMed] [Google Scholar]
- 44).Okazawa H. (2003) Polyglutamine diseases: a transcription disorder? Cell. Mol. Life Sci. 60, 1427–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Okazawa H., Sudol M., Rich T. (2001) PQBP-1 (Np/PQ): a polyglutamine tract-binding and nuclear inclusion-forming protein. Brain Res. Bull. 56, 273–280. [DOI] [PubMed] [Google Scholar]
- 46).Imafuku I., Waragai M., Takeuchi S., Kanazawa I., Kawabata M., Mouradian M.M., Okazawa H. (1998) Polar amino acid-rich sequences bind to polyglutamine tracts. Biochem. Biophys. Res. Commun. 253, 16–20. [DOI] [PubMed] [Google Scholar]
- 47).Waragai M., Junn E., Kajikawa M., Takeuchi S., Kanazawa I., Shibata M., Mouradian M.M., Okazawa H. (2000) PQBP-1/Npw38, a nuclear protein binding to the polyglutamine tract, interacts with U5-15kD/dim1p via the carboxyl-terminal domain. Biochem. Biophys. Res. Commun. 273, 592–595. [DOI] [PubMed] [Google Scholar]
- 48).Zhang Y., Lindblom T., Chang A., Sudol M., Sluder A.E., Golemis E.A. (2000) Evidence that dim1 associates with proteins involved in pre-mRNA splicing, and delineation of residues essential for dim1 interactions with hnRNP F and Npw38/PQBP-1. Gene 257, 33–43. [DOI] [PubMed] [Google Scholar]
- 49).Ito H., Shiwaku H., Yoshida C., Homma H., Luo H., Chen X., Fujita K., Musante L., Fischer U., Frints S.G., Romano C., Ikeuchi Y., Shimamura T., Imoto S., Miyano S., Muramatsu S.I., Kawauchi T., Hoshino M., Sudol M., Arumughan A., Wanker E.E., Rich T., Schwartz C., Matsuzaki F., Bonni A., Kalscheuer V.M., Okazawa H. (2015) In utero gene therapy rescues microcephaly caused by Pqbp1-hypofunction in neural stem progenitor cells. Mol. Psychiatry 20, 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Wang Q., Moore M.J., Adelmant G., Marto J.A., Silver P.A. (2013) PQBP1, a factor linked to intellectual disability, affects alternative splicing associated with neurite outgrowth. Genes Dev. 27, 615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Ikeuchi Y., de la Torre-Ubieta L., Matsuda T., Steen H., Okazawa H., Bonni A. (2013) The XLID protein PQBP1 and the GTPase Dynamin 2 define a signaling link that orchestrates ciliary morphogenesis in postmitotic neurons. Cell Reports 4, 879–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Yoh S.M., Schneider M., Seifried J., Soonthornvacharin S., Akleh R.E., Olivieri K.C., De Jesus P.D., Ruan C., de Castro E., Ruiz P.A., Germanaud D., des Portes V., Garcia-Sastre A., Konig R., Chanda S.K. (2015) PQBP1 is a proximal sensor of the cGAS-dependent innate response to HIV-1. Cell 161, 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Talukdar H.A., Foroughi Asl H., Jain R.K., Ermel R., Ruusalepp A., Franzen O., Kidd B.A., Readhead B., Giannarelli C., Kovacic J.C., Ivert T., Dudley J.T., Civelek M., Lusis A.J., Schadt E.E., Skogsberg J., Michoel T., Bjorkegren J.L. (2016) Cross-tissue regulatory gene networks in coronary artery disease. Cell Syst. 2, 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Kalscheuer V.M., Freude K., Musante L., Jensen L.R., Yntema H.G., Gecz J., Sefiani A., Hoffmann K., Moser B., Haas S., Gurok U., Haesler S., Aranda B., Nshedjan A., Tzschach A., Hartmann N., Roloff T.C., Shoichet S., Hagens O., Tao J., Van Bokhoven H., Turner G., Chelly J., Moraine C., Fryns J.P., Nuber U., Hoeltzenbein M., Scharff C., Scherthan H., Lenzner S., Hamel B.C., Schweiger S., Ropers H.H. (2003) Mutations in the polyglutamine binding protein 1 gene cause X-linked mental retardation. Nat. Genet. 35, 313–315. [DOI] [PubMed] [Google Scholar]
- 55).Lenski C., Abidi F., Meindl A., Gibson A., Platzer M., Frank Kooy R., Lubs H.A., Stevenson R.E., Ramser J., Schwartz C.E. (2004) Novel truncating mutations in the polyglutamine tract binding protein 1 gene (PQBP1) cause Renpenning syndrome and X-linked mental retardation in another family with microcephaly. Am. J. Hum. Genet. 74, 777–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Cossee M., Demeer B., Blanchet P., Echenne B., Singh D., Hagens O., Antin M., Finck S., Vallee L., Dollfus H., Hegde S., Springell K., Thelma B.K., Woods G., Kalscheuer V., Mandel J.L. (2006) Exonic microdeletions in the X-linked PQBP1 gene in mentally retarded patients: a pathogenic mutation and in-frame deletions of uncertain effect. Eur. J. Hum. Genet. 14, 418–425. [DOI] [PubMed] [Google Scholar]
- 57).Lubs H., Abidi F.E., Echeverri R., Holloway L., Meindl A., Stevenson R.E., Schwartz C.E. (2006) Golabi-Ito-Hall syndrome results from a missense mutation in the WW domain of the PQBP1 gene. J. Med. Genet. 43, e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Mizuguchi M., Obita T., Serita T., Kojima R., Nabeshima Y., Okazawa H. (2014) Mutations in the PQBP1 gene prevent its interaction with the spliceosomal protein U5-15kD. Nat. Commun. 5, 3822. [DOI] [PubMed] [Google Scholar]
- 59).Murakami T., Qamar S., Lin J.Q., Schierle G.S., Rees E., Miyashita A., Costa A.R., Dodd R.B., Chan F.T., Michel C.H., Kronenberg-Versteeg D., Li Y., Yang S.P., Wakutani Y., Meadows W., Ferry R.R., Dong L., Tartaglia G.G., Favrin G., Lin W.L., Dickson D.W., Zhen M., Ron D., Schmitt-Ulms G., Fraser P.E., Shneider N.A., Holt C., Vendruscolo M., Kaminski C.F., St George-Hyslop P. (2015) ALS/FTD mutation-induced phase transition of FUS liquid droplets and reversible hydrogels into irreversible hydrogels impairs RNP granule function. Neuron 88, 678–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Molliex A., Temirov J., Lee J., Coughlin M., Kanagaraj A.P., Kim H.J., Mittag T., Taylor J.P. (2015) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Martin B.L., Schrader-Fischer G., Busciglio J., Duke M., Paganetti P., Yankner B.A. (1995) Intracellular accumulation of beta-amyloid in cells expressing the Swedish mutant amyloid precursor protein. J. Biol. Chem. 270, 26727–26730. [DOI] [PubMed] [Google Scholar]
- 62).Friedrich R.P., Tepper K., Ronicke R., Soom M., Westermann M., Reymann K., Kaether C., Fandrich M. (2010) Mechanism of amyloid plaque formation suggests an intracellular basis of Abeta pathogenicity. Proc. Natl. Acad. Sci. U.S.A. 107, 1942–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Sannerud R., Esselens C., Ejsmont P., Mattera R., Rochin L., Tharkeshwar A.K., De Baets G., De Wever V., Habets R., Baert V., Vermeire W., Michiels C., Groot A.J., Wouters R., Dillen K., Vints K., Baatsen P., Munck S., Derua R., Waelkens E., Basi G.S., Mercken M., Vooijs M., Bollen M., Schymkowitz J., Rousseau F., Bonifacino J.S., Van Niel G., De Strooper B., Annaert W. (2016) Restricted location of PSEN2/gamma-secretase determines substrate specificity and generates an intracellular Aβ pool. Cell 166, 193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Wolfe M.S., Yankner B.A. (2016) Sorting out presenilins in Alzheimer’s disease. Cell 166, 13–15. [DOI] [PubMed] [Google Scholar]
- 65).De Strooper B. (2007) Loss-of-function presenilin mutations in Alzheimer disease. Talking point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 8, 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66).Mirra, S.S. and Hyman, B.T. (2002) Aging and dementia. In Greenfield’s Neuropathology Seventh Edition, Volume II (eds. Graham, D.I. and Lantos, P.L.). Arnold, London, pp. 210. [Google Scholar]
- 67).Takahashi M., Mizuguchi M., Shinoda H., Aizawa T., Demura M., Okazawa H., Kawano K. (2009) Polyglutamine tract binding protein-1 is an intrinsically unstructured protein. Biochim. Biophys. Acta 1794, 936–943. [DOI] [PubMed] [Google Scholar]
- 68).Rees M., Gorba C., de Chiara C., Bui T.T., Garcia-Maya M., Drake A.F., Okazawa H., Pastore A., Svergun D., Chen Y.W. (2012) Solution model of the intrinsically disordered polyglutamine tract-binding protein-1. Biophys. J. 102, 1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]