Abstract
In the developing brain, the three major cell types, i.e., neurons, astrocytes and oligodendrocytes, are generated from common multipotent neural stem cells (NSCs). In particular, astrocytes eventually occupy a great fraction of the brain and play pivotal roles in the brain development and functions. However, NSCs cannot produce the three major cell types simultaneously from the beginning; e.g., it is known that neurogenesis precedes astrogenesis during brain development. How is this fate switching achieved? Many studies have revealed that extracellular cues and intracellular programs are involved in the transition of NSC fate specification. The former include growth factor- and cytokine-signaling, and the latter involve epigenetic machinery, including DNA methylation, histone modifications, and non-coding RNAs. Accumulating evidence has identified a complex array of epigenetic modifications that control the timing of astrocytic differentiation of NSCs. In this review, we introduce recent progress in identifying the molecular mechanisms of astrogenesis underlying the tight regulation of neuronal-astrocytic fate switching of NSCs.
Keywords: astrogenesis, central nerve system (CNS), epigenetics, neural stem cells (NSCs)
Introduction
Neural stem cells (NSCs) have the ability of self-renewal and of differentiation into neurons and two types of glial cells (astrocytes and oligodendrocytes).1) Glial cells constitute a large part of the cells in the adult human brain.2) Astrocytes are the most abundant cells among the three cell types derived from NSCs2) and reside throughout the entire central nervous system (CNS), playing important roles in brain development and function.3,4) For example, astrocytes provide neurons with physical and metabolic support by being integrated into neural networks, regulating extracellular ion balance and neurotransmission. Moreover, vascular endfeet of astrocytes that surround brain capillaries contribute to the formation of the blood-brain barrier.3,5)
NSCs produce neurons and glial cells in a unique manner. During early gestation, NSCs divide symmetrically to expand their own pool, and then switch to asymmetric divisions to give rise to each cell type: (1) NSCs acquire the potential to differentiate into neurons at mid-gestation, (2) they obtain the gliogenic capacity to generate astrocytes and oligodendrocytes in the late-gestation to perinatal periods.6) Tight regulation of neurogenesis-to-astrogenesis switching is critical for the generation of a balanced number of each neural cell type, and proper neuronal circuit formation. Many studies have shown that extracellular factors such as Notch (for NSC maintenance), Wingless/int (Wnt, for neurons), leukemia inhibitory factor (LIF, for astrocytes), bone morphogenetic protein (BMP, for astrocytes), and sonic hedgehog (Shh, for oligodendrocytes) are required for differentiation of NSCs into each cell type.7–11)
However, the responsiveness of NSCs to extracellular factor is altered as development proceeds. Interestingly, late-gestational (lg) NSCs can differentiate to astrocytes in response to LIF, whereas mid-gestational (mg) NSCs cannot do so even though the LIF-downstream Janus kinase-signal transducer and activator of transcription 3 (JAK-STAT3) signaling cascade is activated in the cells.12) In this regard, we have previously shown that the insensitivity of mgNSCs to LIF stimulation is due to the DNA methylation of the Glial fibrillary acidic protein (Gfap) promoter, including the STAT3 binding site, precluding STAT3 binding to the promoter to induce astrocytic differentiation. These findings demonstrate that the program of neurogenic to astrogenic transition required for appropriate CNS development proceeds in concert with extracellular factors and intracellular mechanisms (epigenetics). As described above, epigenetic machinery, including DNA methylation, histone modifications, and non-coding RNA (ncRNA), contributes to the fate determination of NSCs. In the following sections, we provide up-to-date molecular insights into how both extracellular cues and epigenetic mechanisms regulate astrogenesis of NSCs.
1. Extracellular signals for astrogenesis
In late-gestation, the JAK-STAT3 signaling pathway plays an important role in astrogenesis (Fig. 1). This pathway is activated by members of the interleukin (IL)-6 family cytokines, including LIF, ciliary neurotrophic factor (CNTF), and cardiotrophin-1 (CT-1). These cytokines bind to their cognate receptors and induce either homodimerization of gp130 (common signal transducer) or heterodimerization of gp130 and one of its partners, such as LIFRβ.13) Once they dimerize, receptor-associating JAKs autophosphorylate and become activated. The activated JAKs in turn phosphorylate tyrosine residues on the intracellular domain of these receptors, where STAT3 is recruited and phosphorylated by JAKs. STAT3 activated by the phosphorylation forms homodimers and is translocated into the nucleus to induce the expression of astrocytic genes, such as Gfap, and thereby promotes astrocytic differentiation.14) Mice deficient for the genes in this pathway (Lif, gp130, Lifr, or Stat3) show impairment of astrocytic differentiation of NSCs in vivo, indicating that JAK-STAT3 signaling is indispensable for the production of astrocytes during brain development.12,14–16)
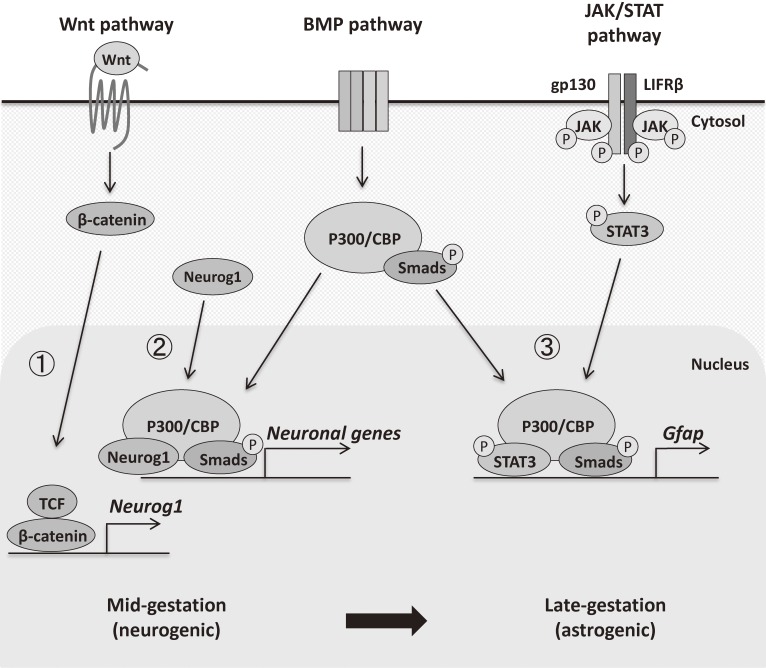
Figure 1.
Schematic illustration of cytokine signaling pathways related to transition from neurogenic to astrogenic competence of NSCs. ① During mid-gestation, by the activation of Wnt/β-catenin signaling, a complex consisting of β-catenin and T-cell factor (TCF) is formed in the nucleus to evoke Neurog1 expression. Then, ② Neurog1 binds to form a complex with P300/CBP, and to upregulate neuronal genes such as NeuroD. During late-gestation, ③ both the JAK-STAT3 pathway and BMP signaling cooperate and synergistically induce the target genes, in which STAT3 and Smads form a complex via P300/CBP. Neurog1 no longer deprives P300/CBP-Smad1 complex of STAT3, due to decreased Neurog1 expression in late-gestation, promoting interaction of STAT3 with P300/CBP.
BMPs, which belong to the transforming growth factor β (TGFβ) superfamily of cytokines, are one type of the well-known extracellular factors related to astrocytic differentiation of NSCs.10) The BMP signal is transduced through heterotetrameric serine/threonine kinase receptors that phosphorylate downstream pathway-restricted transcription factors Smad1, 5 and 8. Activated Smad1/5/8 forms a hetero-oligomer with a common Smad, Smad4, and then this complex translocates into the nucleus to initiate the transcription of its target genes. BMP2 alters the developmental pathway of NSCs from neurogenesis to astrogenesis by inducing negative helix-loop-helix (HLH) factors Id1, Id3, and a homologue of hairy and enhancer of split 5 (Hes5).17) Interestingly, STAT3 and Smad1 form a complex bridged by a transcriptional coactivator, p300 or CREB binding protein (CBP), and synergistically induce astrocytic gene expression.18) During mid-gestation, when mainly neurons are produced from NSCs, Wnt/β-catenin signaling induces Neurogenin1 (Neurog1), which encodes a proneural basic helix-loop-helix (bHLH) transcription factor essential for neuronal differentiation, and Neurog1 deprives p300/CBP-Smad1 complex of STAT3 and thereby upregulates neuronal genes such as NeuroD, leading to the inhibition of astrogenesis (Fig. 1).19) Neurog1 expression is suppressed in late-gestation, and this is considered to be one of the mechanisms responsible for the transition from neurogenic to astrogenic potential of NSCs.20)
In addition, the RAF/mitogen-activated protein kinase (MAPK) or extracellular signal regulated kinase (ERK) kinase (MEK)/ERK pathway, one of the most extensively studied signaling cascades that transduce the effects of extracellular factors into the cells, has also been reported to be involved in astrogenesis of NSCs.21) Both Mek1 and Mek2 deficiency lead to severe impairment of gliogenesis in vivo and to a reduction in JAK-STAT3 signal activation in cultured NSCs through a decrease in the expression of gp130, which contributes to mediating the effects of the entire IL-6 family of cytokines. Moreover, the expression of an E26 transformation-specific (ETS) transcription factor family member, ETS variant 5 (ETV5), has been found to be regulated by MEKs, and overexpression of ETV5 could restore the gliogenic competence of MEK1/2-deficient NSCs. These findings suggest that the RAF/MEK/ERK signaling pathway supports astrogenesis of NSCs via the regulation of gp130 expression levels.
2. DNA methylation
As described above, extracellular cues play an important role in astrogenesis of NSCs during late-gestation. Activation of the JAK-STAT3 pathway in NSCs, however, is observed even during the early- and mid-gestation stages, when NSCs differentiate only into neurons. Nevertheless, treatment with IL-6 family cytokines such as LIF cannot induce astrocytic differentiation of mgNSCs.22) These observations suggest that until late-gestation, NSCs are insensitive to cytokines related to astrogenesis, that is, have not obtained astrogenic potential yet.
The acquisition of this potential by NSCs is attributed to DNA demethylation at astrocyte-specific genes. The promoters of astrocyte-specific genes, including Gfap, S100β, Aqp4, and Clu, are highly methylated before late-gestation, suppressing transcription of these genes.23,24) We have previously shown that Notch ligands such as delta like 1 (Dll1) and jagged1 (Jag1) are expressed in NSC-produced neuroblasts or immature neurons, and they activate Notch signaling in adjacent remaining NSCs.24) When Notch signal is activated, Notch intracellular domain (NICD) is cleaved, translocated into the nucleus, and then forms a complex with recombinant signal sequence-binding protein Jκ (RBP-Jκ) to transcribe Notch-target genes such as nuclear factor I/A (Nfia). Overexpression of NFIA induced precocious GFAP expression in mgNSCs (Fig. 2A–C). We discovered that NFIA induces demethylation of the Gfap promoter, including the STAT3 binding site (Fig. 2D, E). Moreover, we revealed by chromatin immunoprecipitation (ChIP) assays that NFIA induces dissociation of the maintenance DNA methyltransferase 1 (DNMT1) from the Gfap promoter (Fig. 2F), resulting in the demethylation of the STAT3 binding site to activate Gfap expression.24) During DNA replication, DNMT1 reproduces 5-methylcytosine (5mC) on newly synthesized DNA to maintain tissue- or cell-specific methylation patterns, so that dissociation of DNMT1 induces passive demethylation as cells divide. In fact, deletion of DNMT1 accelerates demethylation of the genes encoding the components of the JAK-STAT3 pathway as well as astrocytic genes, leading to precocious astrocytic differentiation in vivo.25) Collectively, our investigations have revealed that Notch signal activation induces Nfia expression, and it thereby leads to the DNA demethylation allowing STAT3 to access these gene promoters, followed by transcriptional activation by the bound STAT3 (Fig. 3).
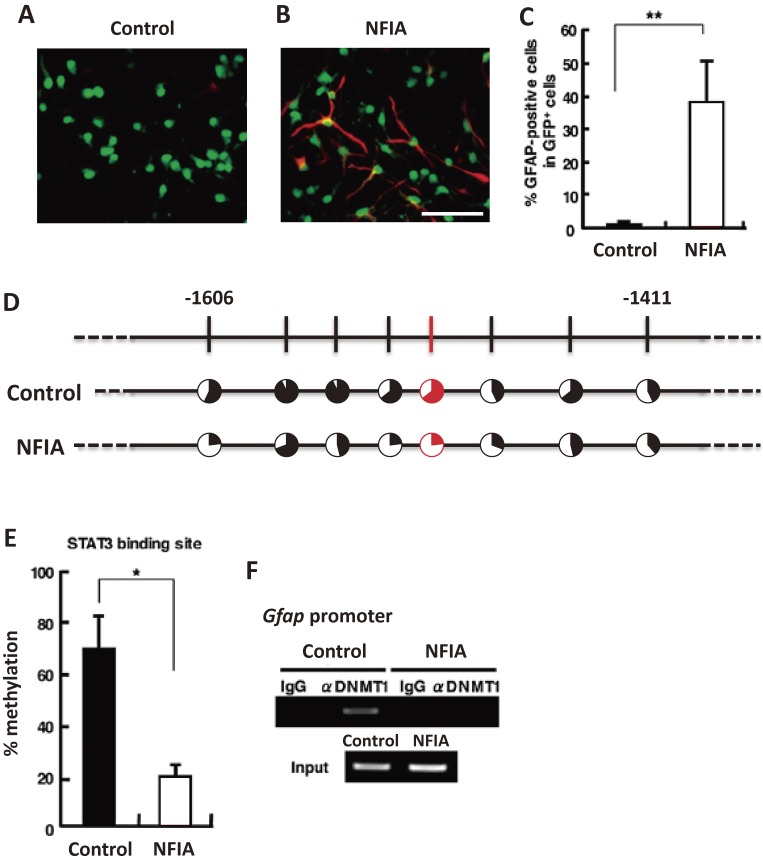
Figure 2.
NFIA potentiates astrocytic differentiation of mgNSCs. A, B. NSCs derived from mouse telencephalons at embryonic day 11.5 (E11.5) were infected with retroviruses engineered to express green fluorescent protein (GFP) alone (A) or GFP together with NFIA (B), cultured for 24 hr in the presence of bFGF, and then stimulated with LIF for a further 3 days to induce astrocytic differentiation. The cells were stained with antibodies against GFP (green) and GFAP (red). Scale bar = 50 µm. C. GFAP-positive astrocytes in GFP control (GFP) and GFP-NFIA-expressing (NFIA) cells were quantified. Data are shown as means ± SD. Statistical significance was examined by Student’s t test (**p < 0.01). D. NSCs derived from E11.5 mouse telencephalons were infected with GFP control (GFP) or GFP-NFIA-expressing (NFIA) retroviruses, and cultured for 4 days with bFGF. After cell sorting based on GFP fluorescence, genomic DNA was extracted, and the methylation status of the Gfap promoter including the STAT3 binding site was examined by bisulfate sequencing. Red indicates STAT3 binding site. Filled portion of circles indicates the percentage of methylation at each CpG site. E. Methylation frequency of the CpG site within the STAT3 binding sequence in the Gfap promoter. Data are shown as means ± SD (n = 3). Statistical significance was examined by Student’s t test (*p < 0.05). F. ChIP assay with a specific antibody for DNMT1 from GFP- and GFP-NFIA-expressing retrovirus-infected NSCs, cultured as in A and B. (Reproduced with modification from Namihira et al., 2009.24))
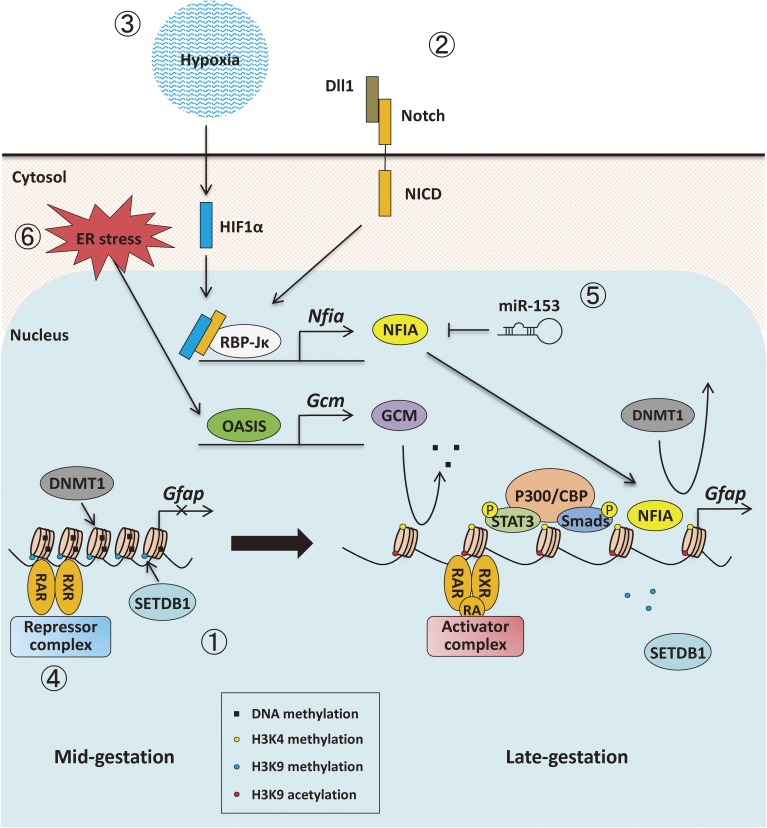
Figure 3.
Schematic illustration of the alterations of epigenetic modifications in NSCs from mid- to late-gestation. ① SETDB1 associates with Gfap promoter, leading to transcriptional repression of the gene mediated by repressive methylation of H3K9. ② The neuronal-committed precursors (neuroblasts) and newly generated immature neurons expressing Notch ligands such as DLL1 activate Notch signaling in neighboring NSCs, producing cleaved Notch (NICD). Then, released NICD binds to RBP-Jκ and activates Nfia expression. NFIA dissociates DNMT1 from the Gfap promoter, resulting in demethylation at the region. ③ In a hypoxic environment, stabilized HIF1α associates with NICD and enhances the transcriptional activity of RBP-Jκ/NICD complex. ④ RAR/RXR forms a complex with transcriptional repressors, leading to formation of a closed chromatin structure. Once RA binds to RAR, the repressor complex is replaced with the activator complex, inducing a relaxed chromatin structure through H3K9ac. ⑤ miR-153 negatively regulates the expression of Nfia. ⑥ The ER stress-transducing protein OASIS induces GCM, which may contribute to active demethylation of the Gfap promoter.
Furthermore, we have demonstrated that hypoxia stabilizes oxygen sensor hypoxia-inducible factor 1α (HIF1α), which associates with NICD to enhance transcriptional activity of RBP-Jκ, enhances Nfia expression, leading to the demethylation of astrocytic genes in mgNSCs (Fig. 3).26) It is known that embryonic tissues, including brain, are in a hypoxic environment, and taken all together these findings indicate that an appropriate oxygen level is crucial for the proper fate-determination timing for astrogenesis through epigenetic alterations.
3. Active DNA demethylation
Until recently, it had been believed that DNA demethylation occurs only in a passive manner through the dissociation of maintenance DNMT1 from methylated regions after cell division associated with DNA replication. However, some reports have suggested recently that active demethylation participates in astrogenesis of NSCs.
The unfolded protein response (UPR), which is induced by unfolded protein accumulation-induced endoplasmic reticulum (ER) stress, has been found to be involved in astrogenesis. The ER stress transducer protein Old Astrocyte Specifically Induced Substance (OASIS), a member of the CREB/ATF family transcription factors, transmits UPR signaling in a cell- or tissue-specific manner.27) In Oasis knockout mice, fewer astrocytes were observed in the cerebral cortex compared with that of wild-type mice, and primary culture of Oasis-deficient NSCs resulted in delayed production of astrocytes with a hypermethylated Gfap promoter.28) The glial cell missing 1 (Gcm1) gene, a mammalian homolog of Drosophila GCM encoding a transcription factor that is required for astrogenesis, is a target of OASIS, and introduction of GCM1 into Oasis-deficient NSCs accelerated the demethylation of the Gfap promoter and rescued the delayed astrogenesis.28) GCM1/2 are also reported to induce demethylation of the Hes5 promoter, including the RBP-Jκ binding site, which is the Notch signal responsive element, and to upregulate the expression of Hes5, resulting in maintenance of the NSC pools.29) Moreover, it has been shown that this demethylation is accomplished without cell division, suggesting that GCM1/2 induce DNA demethylation in an active rather than a passive fashion (Fig. 3).
Recently, Ten-eleven-translocation (TET) family proteins, a family of α-ketoglutarate (α-KG) and Fe (II)-dependent dioxygenases, have been identified as mammalian homologs of the trypanosome proteins J-binding protein (JBP1) and JBP2, and they are able to convert 5mC to 5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC), and 5-carboxylcytosine (5caC) through iterative oxidative reactions.30) 5fC and 5caC are glycosylated by thymine DNA glycosylase (TDG) and 5hmC is converted to 5hmU by activation-induced deaminase (AID), followed by replacement with un-methylated cytosine via the base excision repair (BER) mechanism, in which cell division is not required.31,32)
One report showed that TET proteins are related to neurogenesis.33) In that report, during neurogenesis in the mouse embryonic brain, the 5hmC level increased in gene bodies of neuron-specific genes as differentiation proceeded, and it was inversely correlated with histone H3 lysine 27 (H3K27) methylation levels. Intriguingly, enriched 5hmC was not followed by substantial DNA demethylation, suggesting that 5hmC also served as a stable epigenetic mark. Moreover, knockdown of Enhancer of zeste homolog 2 (Ezh2) an H3K27-specific methyltransferase, disturbed neuronal differentiation of NSCs during development, while such differentiation was promoted by overexpression of TET2 and TET3, raising the possibility that gain of 5hmC and a reduction of H3K27me3 play an important role in brain development.
4. Histone modifications and chromatin accessibility
As described briefly above, histone modifications are involved in the regulation of gene expression via chromatin remodeling, which determines the accessibility of transcription factors to their binding sites. Histones are classified into five types: histone 1 (H1), histone 2A (H2A), histone 2B (H2B), histone 3 (H3), and histone 4 (H4). Chromatin consists of repeated units of nucleosomes, each composed of a histone complex containing two copies each of H2A, H2B, H3, and H4, and 147 bp of DNA, and H1 binds to DNA linking nucleosomes. Histones receive various modifications such as acetylation, methylation, phosphorylation, and ubiquitination.
During mid-gestation, Wnt/β-catenin signaling induces Neurog1 and promotes neuronal differentiation.19) In spite of persistent activation of Wnt/β-catenin signaling, Neurog1 expression is gradually suppressed by late-gestation, when NSCs acquire astrogenic potential. A change in histone modification on the Neurog1 promoter contributes to this different responsiveness. Polycomb repressive complex 2 (PRC2) catalyzes H3K27 methylation since PRC2 includes EZH2, leading to transcriptional repression through the formation of heterochromatin. In fact, the H3K27me3 level in Neurog1 increases with development, indicating that PRC2 indirectly regulates the switch toward astrogenesis.20)
Regarding astrogenesis, when treated with basic fibroblast growth factor (bFGF) and IL-6 family cytokine CNTF, cultured rat lgNSCs produced more astrocytes compared with lgNSCs treated only with CNTF, although bFGF alone cannot induce astrogenesis.34) Treatment with bFGF prior to CNTF strongly enhanced Gfap expression in response to subsequent CNTF treatment, but not vice versa, indicating that bFGF alters the responsiveness of NSCs to CNTF. In fact, that study found that bFGF stimulation increased and decreased H3 lysine 4 (H3K4) methylation (active mark) and H3K9 methylation (suppressive mark), respectively at the Gfap promoter, enabling STAT3 to access this region more easily.
In addition, an H3K9 methyltransferase, SETDB1, that is highly expressed during early gestation and downregulated as development progresses, is important for proper timing of astrogenesis.35) In the Setdb1 mutant mouse brain, neurogenesis was impaired and precocious astrocyte induction was observed. SETDB1 was also found to bind to the Gfap promoter and methylate H3K9. Taken together, these studies suggest that histone modifications are indeed involved in the regulation of Gfap expression (Fig. 3).
Moreover, our studies revealed that retinoic acid (RA) and LIF synergistically activate the promoter of Gfap and facilitate LIF-induced astrogenesis of NSCs.36) RA receptors (RARs), members of the nuclear receptor superfamily, form complexes with retinoid X receptors (RXRs), and RAR/RXR binds to RA response elements (RAREs) in the promoter region of the cognate target genes. In the absence of RA, RAR/RXR recruits transcriptional repressors such as nuclear receptor corepressor (N-CoR) and neuron-restrictive silencer factor/repressor element-1 silencing transcription factor (NRSF/REST) into their complex with histone deacetylases (HDACs), which bring about a closed chromatin structure and suppress the transcription. Conversely, RA-bound RAR/RXR releases HDAC and forms a complex with transcriptional coactivators possessing histone acetyltransferase (HAT) activity (Fig. 3). As mentioned above, once RA-bound RAR/RXR associates with the Gfap promoter (−2.5 kb from the transcription start site), the chromatin structure is relaxed through H3 acetylation (H3ac), promoting STAT3 access to activate Gfap expression in concert with LIF stimulation.
In addition to histone modifications, histone variants and chromatin remodeling complexes also contribute to altering chromatin structure.37,38) We showed that, in spite of the presence of LIF-induced activated STAT3 (LIF is usually supplemented in the embryonic stem cell (ESC) maintenance medium), both wild type ESCs and ESCs deficient for all DNA methyltransferase genes (Dnmt1, Dnmt3a, and Dnmt3b) (Dnmt-TKO ESCs) failed to express Gfap, although the Gfap promoter of Dnmt-TKO ESCs was almost completely demethylated (Fig. 4A–C).39) By micrococcal nuclease (MNase) assays to examine chromatin accessibility,40) we revealed that the region around the STAT3 binding site in the Gfap promoter was digested well in lgNSCs, which are capable of Gfap expression with LIF stimulation, whereas this region in both WT and TKO ESCs cells and in mgNSCs was protected from the digestion (Fig. 4D).39) These findings suggest that chromatin accessibility around the STAT3-binding site in the Gfap promoter is altered during development and regulates the proper astrocyte differentiation potential of NSCs.
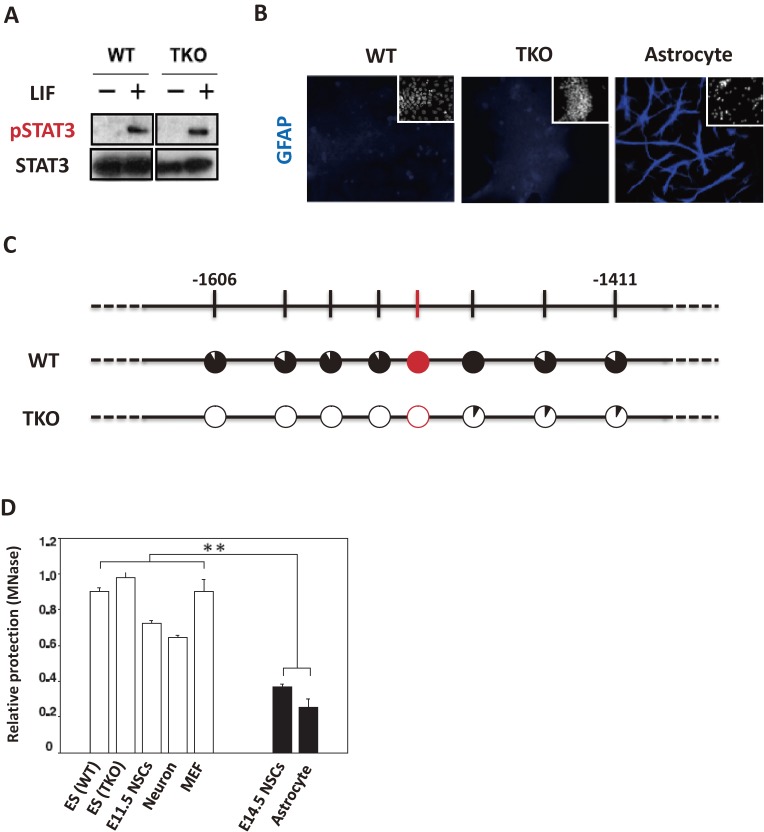
Figure 4.
Chromatin accessibility is important for the GFAP expression. (A) Western blot analysis of tyrosine-phosphorylated STAT3 and total STAT3 in wild-type (WT) and triple knockout (TKO) ESCs. (B) The expression of GFAP in each cell type. WT and TKO ESCs were stained with antibodies against GFAP (blue). E14 NSCs-derived astrocytes were used as a positive control for staining. Nuclei were stained with Hoechst 33258 (white). (C) The DNA methylation status of the Gfap promoter in wild-type (WT) and triple-knockout (TKO) ESCs. Red indicates STAT3 binding site. Filled portion of circles indicates the percentage of methylation at each CpG site. (D) MNase digestion status of Gfap promoter around STAT3 binding site. Quantitative PCR against MNase-digested products from WT ESCs (WT), TKO ESCs (TKO), E11.5 NSCs, E14.5 NSCs, neurons, astrocytes, and mouse embryonic fibroblasts (MEFs) was performed. Amounts of genomic DNA remaining after MNase digestion were determined by qPCR. Each mean value was normalized by that of a linker histone H1foo gene. The H1foo gene is silent in all cells except for oocytes, and the region of its locus used here was completely resistant to MNase digestion in all the tested cell types. Error bars represent standard deviation from the mean of three independent experiments. **P < 0.01, *P < 0.05. (Reproduced with modification from Urayama et al., 201339))
Using a genome-wide enhanced circular chromosomal conformation capture (e4C) technique to investigate inter-chromosomal interactions,41) we have recently demonstrated that several genes expressed during astrocyte differentiation were specifically associated with Gfap, raising the possibility that gene clustering may play some roles in transcriptional regulation during NSCs differentiation.42)
5. ncRNA
As opposed to mRNA, ncRNAs are defined as RNAs that are not translated into proteins. Among many different types of ncRNAs, microRNA (miRNA) and long non-coding RNA (lncRNA) are known to have tissue-specific expression patterns and profound relationships to development and diseases through the regulation of gene expression at both the transcriptional and posttranscriptional levels. Recently, it has been reported that miRNAs are in fact associated with brain development and functions. In the last part of this review, we introduce some examples of miRNAs and a lncRNA related to astrogenesis.
Chicken ovalbumin upstream promoter-transcription factor I and II (Coup-tfI and Coup-tfII), are transiently expressed in the ventricular zone in a relatively early phase of the developing brain, and have been suggested to be involved in the acquisition of gliogenic competence by NSCs. The double knockdown of Coup-tfI/II caused maintenance of DNA hypermethylation at the Gfap promoter in NSCs derived from ESCs and inhibited the initiation of astrogenesis in the developing mouse forebrain.43) Although the detailed mechanism by which Coup-tfI/II regulates NSC differentiation remains elusive, the same research group has recently identified microRNA-17/106 (miR-17/106) as downstream effectors of COUP-TFI/II.44) They have shown that miR-17/106 target the mRNA encoding p38 (also known as MAPK14), which is critical for astrogenesis, suggesting that the miR-17/106-p38 axis plays an important role in the transition of NSCs’ fate from neurogenic to astrogenic.
miR-153 was also found to be crucial for the expression of key regulators of astrocytic differentiation.45) miR-153 targets mRNA of nuclear factor I/A (Nfia) and nuclear factor I/B (Nfib), both of which are essential for initiation of gliogenesis (Fig. 3). In the forebrain, miR-153 expression decreases as the developmental stage progresses from mid- to late-gestation, followed by increased expression of NFIA and NFIB, suggesting that miR-153 is important for the acquisition of gliogenic competence by NSCs.
Moreover, it has been revealed that miR-124, which is highly expressed in the brain, balances the specification of NSCs between neurons and glial cells.46) Several neuron-specific genes are repressed by EZH2, but in mid-gestation, miR-124 decreases the expression level of Ezh2, thus promoting neuronal differentiation of NSCs. Overexpression of Ezh2 mRNA lacking a miR-124 binding site in the 3′-untranslated region (3′-UTR) prevents neuronal differentiation and promotes astrocytic differentiation.
Growing evidence has revealed that enhancer RNA (eRNA), a sort of ncRNAs, is transcribed from enhancers associated with specific histone modifications such as H3K4me147) and H3K27ac,48) and suggested to be involved in the activation of gene (mRNA) expression through enhancing chromatin accessibility.49) For instance, recent report has shown that eRNA-like transcript utNgn1, a lncRNA transcribed from a Neurog1 enhancer element located 5.8–7.0 kb upstream of the Neurog1 transcription start site, positively regulates the transcription of Neurog1.50) In fact, during neuronal differentiation, the expression level of utNgn1 is highly correlated with that of Neurog1, and knockdown of utNgn1 attenuates the transcription of Neurog1. These findings indicate that ncRNAs modify the competence of NSC differentiation in various manners.
Concluding remarks and perspectives
Since the discovery of the multipotency of NSCs,51,52) researchers have been tackling the question of how the differentiation of NSCs is regulated. Owing to extensive investigations, our knowledge about the mechanisms has been rapidly accumulating. Many transcription factors have been identified as key regulators for fate-determination of NSCs. We found that NFIA is a critical molecule conferring astrogenic competence on NSCs during development through the induction of the epigenetic change,24) while a growing body of evidence suggests that the molecular mechanism underlying astrogenesis is regulated by a complex array of extracellular signals and epigenetic mechanisms. Therefore, to verify the mechanisms in detail will require comprehensive analyses using now remarkably advancing technologies such as whole genome bisulfite sequencing (WGBS-seq), RNA-seq, and chromatin immunoprecipitation sequencing (ChIP-seq).
Astrocytes are abundantly present in the brain and are involved in neurological dysfunctions including Alexander disease, neurotrauma, stroke, and Alzheimer’s disease (AD).53) In AD pathology, reactive astrocytes characterized by high GFAP expression and hypertrophied processes, surround amyloid plaques. Although, reactive astrocytes have hitherto been considered to be miscreants since they secrete various cytokines that enhance inflammation and neurodegeneration, recent reports have suggested that reactive astrocytes degrade amyloid plaques through releasing protease and taking up β-amyloid. In addition, deletion of Gfap and Vimentin in mice, both of which are components of cytoplasmic intermediate filaments and are markedly assembled in reactive astrocytes, deprives astrocytes of the ability to develop the hypertrophied processes that interact with amyloid plaques, causing progressive accumulation of amyloid plaques. Thus far, most basic research has addressed NSCs derived from animal models, and few studies have investigated astrogenesis of human NSCs because of the limited access to human cells. However, now we have relatively easy access to induced pluripotent cells (iPSCs) derived from humans. In this context, we have been working on astrocyte differentiation using human iPSC-derived NSCs, and this work has revealed, at least in part, some similarity between human and mouse astrogenesis; for instance, demethylation of the GFAP promoter is critical for hiPSC-derived NSCs to differentiate into astrocytes.54) Further investigations regarding human astrocytes will be required for understanding the pathology and physiology of neurological disorders.
Recently, we and others have reported new techniques that permit us to artificially manipulate the epigenetic modifications in a locus-specific manner.55,56) Based on a modified clustered regularly interspaced short palindromic repeats/CRISPR associated proteins (CRISPR/Cas) system, in which dCas9, a nuclease-deficient form of Cas9 (DNA-binding molecule), is fused with TET1 or DNMT3A and expressed together with guide RNAs, dCas9-TET1 or -DNMT3A achieves demethylation or methylation of targeted promoter regions, respectively. The reports of findings using these techniques show alteration of target gene expressions accompanied by changed chromatin modifications. The combination of our efforts and the advancement of techniques holds promise to pave the way for further understanding of astrocytes’ development and clinical applications.
Acknowledgments
We thank all of the members of the Department of Stem Cell Biology and Medicine, Kyushu University, for valuable comments, and Elizabeth Nakajima for critical reading of this manuscript. This work was supported by grants from the Japan Agency for Medical Research and Development, Core Research for Evolutional Science and Technology (AMED-CREST), JSPS KAKENHI (15K14452) and MEXT KAKENHI (16H06527) to K. N.
Abbreviations
- 3′-UTR
3′-untranslated region
- 5caC
5-carboxylcytosine
- 5fC
5-formylcytosine
- 5hmC
5-hydroxymethylcytosine
- 5mC
5-methylcytosine
- AD
Alzheimer’s disease
- AID
activation-induced deaminase
- BER
base excision repair
- bFGF
basic fibroblast growth factor
- bHLH
basic helix-loop-helix
- BMP
bone morphogenetic protein
- CBP
CREB binding protein
- ChIP-seq
chromatin immunoprecipitation sequencing
- CNS
central nervous system
- CNTF
ciliary neurotrophic factor
- Coup-tf
chicken ovalbumin upstream promoter-transcription factor
- CRISPR/Cas
clustered regularly interspaced short palindromic repeats/CRISPR associated proteins
- CT-1
cardiotrophin-1
- Dll1
delta like 1
- DNMT1
DNA methyltransferase 1
- e4C
enhanced circular chromosomal conformation capture
- eRNA
enhancer RNA
- ER
endoplasmic reticulum
- ERK
extracellular signal regulated kinase
- ESCs
embryonic stem cells
- ETS
E26 transformation-specific
- ETV5
ETS variant 5
- Ezh2
enhancer of zeste homolog 2
- GCM1
glial cell missing 1
- Gfap
glial fibrillary acidic protein
- GFP
green fluorescent protein
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
- Hes5
hairy and enhancer of split 5
- HIF1α
hypoxia activated oxygen sensor hypoxia-inducible factor 1α
- HLH
helix-loop-helix
- IL-6
interleukin-6
- iPSCs
induced pluripotent stem cells
- Jag1
jagged1
- JAK-STAT3
Janus kinase/signal transducer and activator of transcription 3
- JBP
J-binding protein
- lg
late-gestational
- LIF
leukemia inhibitory factor
- lncRNA
long non-coding RNA
- MAPK
mitogen-activated protein kinase
- md
mid-gestational
- MEK
MAPK/ERK kinase
- miRNA
microRNA
- mRNA
messenger RNA
- N-CoR
nuclear receptor corepressor
- ncRNA
non-coding RNA
- Neurog1
neurogenin1
- Nfia
nuclear factor I/A
- Nfib
nuclear factor I/B
- NICD
notch intracellular domain
- NRSF/REST
neuron-restrictive silencer factor/repressor element-1 silencing transcription factor
- NSCs
neural stem cells
- OASIS
old astrocyte specifically induced substance
- PRC2
polycomb repressive complex 2
- RA
retinoic acid
- RAREs
retinoic acid response elements
- RARs
retinoic acid receptors
- RBP-Jκ
recombinant signal sequencing-binding protein Jκ
- RXRs
retinoid X recepters
- Shh
sonic hedgehog
- TCF
T-cell factor
- TDG
thymine DNA glycosylase
- TET
Ten-eleven-translocation
- TGFβ
transforming growth factor β
- UPR
unfolded protein response
- WGBS-seq
whole genome bisulfite sequencing
- Wnt
Wingless/int
- α-KG
α-ketoglutarate
Biographies
Profile
Jun Takouda received Bachelor of Pharmacy degree from Faculty of Pharmaceutical Sciences, Tohoku University in 2016 and is a graduate student in the doctoral course in Department of Stem Cell Biology and Medicine, Graduate School of Medical Sciences, Kyushu University, Japan. His research interest is epigenetic regulation of neural stem cell differentiation.
Sayako Katada, Ph.D. is an Assistant Professor in Department of Stem Cell Biology and Medicine, Graduate School of Medical Sciences, Kyushu University, Japan. She received her Ph.D. in Frontier Science from the Tokyo University in 2006. She did her postdoc at RIKEN BSI (2006–2007) and University of California Irvine (2007–2011) and became an assistant professor at Nara Institute of Science and Technology (NAIST) in 2012 and obtained the present position in 2013.
Kinichi Nakashima, Ph.D. is a Professor in Department of Stem Cell Biology and Medicine, Graduate School of Medical Sciences, Kyushu University, Japan. He received his Ph.D. in Chemistry from the Kyushu University in 1995. He did postdoc at Osaka University and Tokyo Medical and Dental University (1995–1997) and became an assistant professor at Tokyo Medical and Dental University in 1998. He then became an associate professor at Kumamoto University in 2000. He moved to the Salk Institute as a research fellow (2002–2004), and become a professor at Nara Institute of Science and Technology (NAIST) and obtained the present position in 2013. Dr. Nakashima’s research focuses on neural stem cell regulation by epigenetic programs.
References
- 1).Taupin P., Gage F.H. (2002) Adult neurogenesis and neural stem cells of the central nervous system in mammals. J. Neurosci. Res. 69, 745–749. [DOI] [PubMed] [Google Scholar]
- 2).Azevedo F.A., Carvalho L.R., Grinberg L.T., Farfel J.M., Ferretti R.E., Leite R.E., Jacob Filho W., Lent R., Herculano-Houzel S. (2009) Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 513, 532–541. [DOI] [PubMed] [Google Scholar]
- 3).Araque A., Parpura V., Sanzgiri R.P., Haydon P.G. (1999) Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22, 208–215. [DOI] [PubMed] [Google Scholar]
- 4).Molofsky A.V., Krencik R., Ullian E.M., Tsai H.H., Deneen B., Richardson W.D., Barres B.A., Rowitch D.H. (2012) Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 26, 891–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Nedergaard M., Ransom B., Goldman S.A. (2003) New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 26, 523–530. [DOI] [PubMed] [Google Scholar]
- 6).Hirabayashi Y., Gotoh Y. (2005) Stage-dependent fate determination of neural precursor cells in mouse forebrain. Neurosci. Res. 51, 331–336. [DOI] [PubMed] [Google Scholar]
- 7).Kageyama R., Shimojo H., Imayoshi I. (2015) Dynamic expression and roles of Hes factors in neural development. Cell Tissue Res. 359, 125–133. [DOI] [PubMed] [Google Scholar]
- 8).Hirabayashi Y., Itoh Y., Tabata H., Nakajima K., Akiyama T., Masuyama N., Gotoh Y. (2004) The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development 131, 2791–2801. [DOI] [PubMed] [Google Scholar]
- 9).Bonni A., Sun Y., Nadal-Vicens M., Bhatt A., Frank D.A., Rozovsky I., Stahl N., Yancopoulos G.D., Greenberg M.E. (1997) Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science 278, 477–483. [DOI] [PubMed] [Google Scholar]
- 10).Gross R.E., Mehler M.F., Mabie P.C., Zang Z., Santschi L., Kessler J.A. (1996) Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron 17, 595–606. [DOI] [PubMed] [Google Scholar]
- 11).Orentas D.M., Hayes J.E., Dyer K.L., Miller R.H. (1999) Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development 126, 2419–2429. [DOI] [PubMed] [Google Scholar]
- 12).Nakashima K., Wiese S., Yanagisawa M., Arakawa H., Kimura N., Hisatsune T., Yoshida K., Kishimoto T., Sendtner M., Taga T. (1999) Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J. Neurosci. 19, 5429–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Ernst M., Jenkins B.J. (2004) Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet. 20, 23–32. [DOI] [PubMed] [Google Scholar]
- 14).He F., Ge W., Martinowich K., Becker-Catania S., Coskun V., Zhu W., Wu H., Castro D., Guillemot F., Fan G., de Vellis J., Sun Y.E. (2005) A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat. Neurosci. 8, 616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Bugga L., Gadient R.A., Kwan K., Stewart C.L., Patterson P.H. (1998) Analysis of neuronal and glial phenotypes in brains of mice deficient in leukemia inhibitory factor. J. Neurobiol. 36, 509–524. [DOI] [PubMed] [Google Scholar]
- 16).Koblar S.A., Turnley A.M., Classon B.J., Reid K.L., Ware C.B., Cheema S.S., Murphy M., Bartlett P.F. (1998) Neural precursor differentiation into astrocytes requires signaling through the leukemia inhibitory factor receptor. Proc. Natl. Acad. Sci. U.S.A. 95, 3178–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Nakashima K., Takizawa T., Ochiai W., Yanagisawa M., Hisatsune T., Nakafuku M., Miyazono K., Kishimoto T., Kageyama R., Taga T. (2001) BMP2-mediated alteration in the developmental pathway of fetal mouse brain cells from neurogenesis to astrocytogenesis. Proc. Natl. Acad. Sci. U.S.A. 98, 5868–5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Nakashima K., Yanagisawa M., Arakawa H., Kimura N., Hisatsune T., Kawabata M., Miyazono K., Taga T. (1999) Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science 284, 479–482. [DOI] [PubMed] [Google Scholar]
- 19).Sun Y., Nadal-Vicens M., Misono S., Lin M.Z., Zubiaga A., Hua X., Fan G., Greenberg M.E. (2001) Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell 104, 365–376. [DOI] [PubMed] [Google Scholar]
- 20).Hirabayashi Y., Suzki N., Tsuboi M., Endo T.A., Toyoda T., Shinga J., Koseki H., Vidal M., Gotoh Y. (2009) Polycomb limits the neurogenic competence of neural precursor cells to promote astrogenic fate transition. Neuron 63, 600–613. [DOI] [PubMed] [Google Scholar]
- 21).Li X., Newbern J.M., Wu Y., Morgan-Smith M., Zhong J., Charron J., Snider W.D. (2012) MEK is a key regulator of gliogenesis in the developing brain. Neuron 75, 1035–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Takizawa T., Nakashima K., Namihira M., Ochiai W., Uemura A., Yanagisawa M., Fujita N., Nakao M., Taga T. (2001) DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev. Cell 1, 749–758. [DOI] [PubMed] [Google Scholar]
- 23).Namihira M., Nakashima K., Taga T. (2004) Developmental stage dependent regulation of DNA methylation and chromatin modification in a immature astrocyte specific gene promoter. FEBS Lett. 572, 184–188. [DOI] [PubMed] [Google Scholar]
- 24).Namihira M., Kohyama J., Semi K., Sanosaka T., Deneen B., Taga T., Nakashima K. (2009) Committed neuronal precursors confer astrocytic potential on residual neural precursor cells. Dev. Cell 16, 245–255. [DOI] [PubMed] [Google Scholar]
- 25).Fan G., Martinowich K., Chin M.H., He F., Fouse S.D., Hutnick L., Hattori D., Ge W., Shen Y., Wu H., ten Hoeve J., Shuai K., Sun Y.E. (2005) DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development 132, 3345–3356. [DOI] [PubMed] [Google Scholar]
- 26).Mutoh T., Sanosaka T., Ito K., Nakashima K. (2012) Oxygen levels epigenetically regulate fate switching of neural precursor cells via hypoxia-inducible factor 1alpha-notch signal interaction in the developing brain. Stem Cells 30, 561–569. [DOI] [PubMed] [Google Scholar]
- 27).Kondo S., Murakami T., Tatsumi K., Ogata M., Kanemoto S., Otori K., Iseki K., Wanaka A., Imaizumi K. (2005) OASIS, a CREB/ATF-family member, modulates UPR signalling in astrocytes. Nat. Cell Biol. 7, 186–194. [DOI] [PubMed] [Google Scholar]
- 28).Saito A., Kanemoto S., Kawasaki N., Asada R., Iwamoto H., Oki M., Miyagi H., Izumi S., Sanosaka T., Nakashima K., Imaizumi K. (2012) Unfolded protein response, activated by OASIS family transcription factors, promotes astrocyte differentiation. Nat. Commun. 3, 967. [DOI] [PubMed] [Google Scholar]
- 29).Hitoshi S., Ishino Y., Kumar A., Jasmine S., Tanaka K.F., Kondo T., Kato S., Hosoya T., Hotta Y., Ikenaka K. (2011) Mammalian Gcm genes induce Hes5 expression by active DNA demethylation and induce neural stem cells. Nat. Neurosci. 14, 957–964. [DOI] [PubMed] [Google Scholar]
- 30).Cimmino L., Aifantis I. (2016) Alternative roles for oxidized mCs and TETs. Curr. Opin. Genet. Dev. 42, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Nabel C.S., Jia H., Ye Y., Shen L., Goldschmidt H.L., Stivers J.T., Zhang Y., Kohli R.M. (2012) AID/APOBEC deaminases disfavor modified cytosines implicated in DNA demethylation. Nat. Chem. Biol. 8, 751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Guo J.U., Su Y., Zhong C., Ming G.L., Song H. (2011) Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell 145, 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Hahn M.A., Qiu R., Wu X., Li A.X., Zhang H., Wang J., Jui J., Jin S.G., Jiang Y., Pfeifer G.P., Lu Q. (2013) Dynamics of 5-hydroxymethylcytosine and chromatin marks in Mammalian neurogenesis. Cell Rep. 3, 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Song M.R., Ghosh A. (2004) FGF2-induced chromatin remodeling regulates CNTF-mediated gene expression and astrocyte differentiation. Nat. Neurosci. 7, 229–235. [DOI] [PubMed] [Google Scholar]
- 35).Tan S.L., Nishi M., Ohtsuka T., Matsui T., Takemoto K., Kamio-Miura A., Aburatani H., Shinkai Y., Kageyama R. (2012) Essential roles of the histone methyltransferase ESET in the epigenetic control of neural progenitor cells during development. Development 139, 3806–3816. [DOI] [PubMed] [Google Scholar]
- 36).Asano H., Aonuma M., Sanosaka T., Kohyama J., Namihira M., Nakashima K. (2009) Astrocyte differentiation of neural precursor cells is enhanced by retinoic acid through a change in epigenetic modification. Stem Cells 27, 2744–2752. [DOI] [PubMed] [Google Scholar]
- 37).Boyarchuk E., Montes de Oca R., Almouzni G. (2011) Cell cycle dynamics of histone variants at the centromere, a model for chromosomal landmarks. Curr. Opin. Cell Biol. 23, 266–276. [DOI] [PubMed] [Google Scholar]
- 38).Clapier C.R., Cairns B.R. (2009) The biology of chromatin remodeling complexes. Annu. Rev. Biochem. 78, 273–304. [DOI] [PubMed] [Google Scholar]
- 39).Urayama S., Semi K., Sanosaka T., Hori Y., Namihira M., Kohyama J., Takizawa T., Nakashima K. (2013) Chromatin accessibility at a STAT3 target site is altered prior to astrocyte differentiation. Cell Struct. Funct. 38, 55–66. [DOI] [PubMed] [Google Scholar]
- 40).Carey M., Smale S.T. (2007) Micrococcal nuclease-southern blot assay: I. MNase and restriction digestions. CSH Protoc. 2007, pdb prot4890. [DOI] [PubMed] [Google Scholar]
- 41).Schoenfelder S., Sexton T., Chakalova L., Cope N.F., Horton A., Andrews S., Kurukuti S., Mitchell J.A., Umlauf D., Dimitrova D.S., Eskiw C.H., Luo Y., Wei C.L., Ruan Y., Bieker J.J., Fraser P. (2010) Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells. Nat. Genet. 42, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Ito K., Sanosaka T., Igarashi K., Ideta-Otsuka M., Aizawa A., Uosaki Y., Noguchi A., Arakawa H., Nakashima K., Takizawa T. (2016) Identification of genes associated with the astrocyte-specific gene Gfap during astrocyte differentiation. Sci. Rep. 6, 23903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Naka H., Nakamura S., Shimazaki T., Okano H. (2008) Requirement for COUP-TFI and II in the temporal specification of neural stem cells in CNS development. Nat. Neurosci. 11, 1014–1023. [DOI] [PubMed] [Google Scholar]
- 44).Naka-Kaneda H., Nakamura S., Igarashi M., Aoi H., Kanki H., Tsuyama J., Tsutsumi S., Aburatani H., Shimazaki T., Okano H. (2014) The miR-17/106-p38 axis is a key regulator of the neurogenic-to-gliogenic transition in developing neural stem/progenitor cells. Proc. Natl. Acad. Sci. U.S.A. 111, 1604–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Tsuyama J., Bunt J., Richards L.J., Iwanari H., Mochizuki Y., Hamakubo T., Shimazaki T., Okano H. (2015) MicroRNA-153 regulates the acquisition of gliogenic competence by neural stem cells. Stem Cell Rep. 5, 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Neo W.H., Yap K., Lee S.H., Looi L.S., Khandelia P., Neo S.X., Makeyev E.V., Su I.H. (2014) MicroRNA miR-124 controls the choice between neuronal and astrocyte differentiation by fine-tuning Ezh2 expression. J. Biol. Chem. 289, 20788–20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Heintzman N.D., Stuart R.K., Hon G., Fu Y., Ching C.W., Hawkins R.D., Barrera L.O., Van Calcar S., Qu C., Ching K.A., Wang W., Weng Z., Green R.D., Crawford G.E., Ren B. (2007) Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 39, 311–318. [DOI] [PubMed] [Google Scholar]
- 48).Rada-Iglesias A., Bajpai R., Swigut T., Brugmann S.A., Flynn R.A., Wysocka J. (2011) A unique chromatin signature uncovers early developmental enhancers in humans. Nature 470, 279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Kim T.K., Hemberg M., Gray J.M. (2015) Enhancer RNAs: a class of long noncoding RNAs synthesized at enhancers. Cold Spring Harb. Perspect. Biol. 7, a018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Onoguchi M., Hirabayashi Y., Koseki H., Gotoh Y. (2012) A noncoding RNA regulates the neurogenin1 gene locus during mouse neocortical development. Proc. Natl. Acad. Sci. U.S.A. 109, 16939–16944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Temple S. (1989) Division and differentiation of isolated CNS blast cells in microculture. Nature 340, 471–473. [DOI] [PubMed] [Google Scholar]
- 52).Reynolds B.A., Weiss S. (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255, 1707–1710. [DOI] [PubMed] [Google Scholar]
- 53).Pekny M., Pekna M., Messing A., Steinhauser C., Lee J.M., Parpura V., Hol E.M., Sofroniew M.V., Verkhratsky A. (2016) Astrocytes: a central element in neurological diseases. Acta Neuropathol. 131, 323–345. [DOI] [PubMed] [Google Scholar]
- 54).Yasui T., Uezono N., Nakashima H., Noguchi H., Matsuda T., Noda-Andoh T., Okano H., Nakashima K. (2017) Hypoxia epigenetically confers astrocytic differentiation potential on human pluripotent cell-derived neural precursor cells. Stem Cell Rep. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Morita S., Noguchi H., Horii T., Nakabayashi K., Kimura M., Okamura K., Sakai A., Nakashima H., Hata K., Nakashima K., Hatada I. (2016) Targeted DNA demethylation in vivo using dCas9-peptide repeat and scFv-TET1 catalytic domain fusions. Nat. Biotechnol. 34, 1060–1065. [DOI] [PubMed] [Google Scholar]
- 56).Liu X.S., Wu H., Ji X., Stelzer Y., Wu X., Czauderna S., Shu J., Dadon D., Young R.A., Jaenisch R. (2016) Editing DNA methylation in the mammalian genome. Cell 167, 233–247 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]