Abstract
The safety and biological effects of a long-term dose of D-allulose were evaluated in healthy dogs. For 12 weeks, the dogs were administered D-allulose (0.2 g/kg) or placebo daily. Plasma total cholesterol concentrations in the D-allulose group were significantly lower than those in the control group at and after week 2 (P<0.05). D-Allulose administration did not cause clinical signs or changes in hematological and biochemical levels, except for lipids. D-Allulose administration also did not influence body weight. Plasma glucose and insulin concentrations in the glucose tolerance test, performed one day after the termination of D-allulose administration, were not different between groups, suggesting no cumulative effects of D-allulose on glucose metabolism in healthy dogs. In conclusion, long-term administration of D-allulose caused no harmful effects in dogs.
Keywords: D-allulose, dog, safety
D-allulose, also called as D-psicose, is a rare sugar, which is a monosaccharide rarely exist in nature [7]. Recently, D-allulose has been suggested to exert antiobesity and antihyperglycemic effects [7]. D-allulose has been shown to decrease blood glucose concentrations in healthy rats [13], genetically obese rats [8, 10], humans ingesting maltodextrin [11], and humans with borderline diabetes [5]. The anti-hyperglycemic effects of D-allulose may be caused by the inhibition of digestive enzymes such as α-glucosidase [7], the inhibition of glucose absorption in the intestine [6], and the induction of hepatic glucokinase, increasing glucose utilization towards glycogen synthesis [10]. In addition to antihyperglycemic effects, D-allulose has been shown to exert antiobesity effects in rodents with diet-induced obesity [2, 9, 17, 20]. The antiobesity effects of D-allulose have been reported to be caused by increased energy expenditure [9], suppressed dietary intake [20], and increased fatty acid oxidation [17]. Furthermore, some reports have shown that D-allulose administration ameliorates dyslipidemia in rats [1, 7], although the mechanism underlying the lipid-lowering effect remains unexplained [7].
In recent years, obesity, hyperlipidemia, and diabetes have become significant clinical problems in companion dogs [3, 16, 22]. The reported effects of D-allulose in rodents and humans may help the management of these pathologic conditions in dogs. In fact, D-allulose has been shown to suppress the rise in blood glucose following oral or intravenous glucose administration in dogs [19].
D-allulose has been classified as an ordinary substance, with an oral LD50 value of 16 g/kg in rats [15], and its long-term safety has already been confirmed in humans [5] and rats [14, 23]. In dogs, oral administration of a high dose of D-allulose (1 and 4 g/kg) has been shown to cause only self-limiting gastrointestinal symptoms and a transient rise in plasma alkaline phosphatase activity [18]. These results suggested that a single-dose administration of D-allulose does not cause acute toxicity in dogs. However, repeated, long-term safety or biological effects of D-allulose are currently unknown for this species. In the present study, the safety and biological effects of a 12-week administration of D-allulose were evaluated in healthy dogs.
This study was approved by the institutional Animal Experiment Committee of Gifu University. Ten healthy beagle dogs were randomly divided into 2 groups of 5 dogs each, the D-allulose and control groups. The D-allulose and control groups both consist of 1 castrated male and 4 spayed female, and age, body weight, and body condition score (9-point scale) were 5.2 ± 2.2 and 6.0 ± 2.2 years, 14.2 ± 2.5 and 13.7 ± 1.6 kg, and 5.4 ± 0.9 and 5.2 ± 0.4, respectively. There were no differences in age, body weight, and body condition score between groups (Welch’s t-test). All the dogs were confirmed to be healthy by a veterinary clinician (NN) based on a physical examination.
The experiment lasted 12 weeks, during which the dogs were fed commercially available dry food (Royal Canin Medium Adult, Royal Canin Japon, Tokyo, Japan) once daily. The feeding amount was calculated by the equation for maintenance of adult dogs: 1.2 × 95 × body weight (kg)0.75 kcal. Dogs were allowed free access to water during the experiment. The D-allulose group received a D-allulose solution (0.2 g/kg), and the control group received equivalent amounts of water with food once daily. Because D-allulose is a zero-calorie sweetener, there was no difference of calorie intake between the two groups [7]. D-allulose was provided by the Kagawa University Rare Sugar Research Center, Japan. The dose rate of D-allulose was determined according to a previous study [19]. Food consumption, feces characteristics, activity, and clinical signs, if any, were recorded daily. Body weight was measured at 0, 2, 4, 8 and 12 weeks. EDTA blood samples for complete blood count were collected at the 0 and 12 week. Biochemical analyses (plasma alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, total bilirubin, urea nitrogen, creatinine, total protein, albumin, total cholesterol, triglyceride, total calcium, inorganic phosphorus, sodium, potassium, and chlorine concentrations) were conducted using heparinized plasma at 0, 2, 4 and 12 weeks. Before the experiment and one day after the termination of D-allulose administration or placebo, an intravenous glucose tolerance test was performed. A 50% glucose solution (0.5 g/kg) (Otsuka Pharmaceutical, Tokyo, Japan) was intravenously injected, and heparinized plasma samples were collected at 0, 5, 10, 15, 30 and 60 min after the glucose injection for the measurement of glucose and insulin concentrations.
Complete blood count was obtained by an automated analyzer (Celltac α, Nihon Kohden, Tokyo, Japan). The biochemical parameters were measured by an automated biochemical analyzer (Labospect 003; Hitachi High-Technologies, Tokyo, Japan). Plasma insulin concentration was assayed using the LBIS dog insulin enzyme-linked immunosorbent assay kit (Shibayagi, Gunma, Japan) [19].
The area under the curves (AUC) for plasma glucose and insulin concentrations was calculated by the trapezoid method. Differences between the groups were analyzed using Welch’s t-test. Differences from values at week 0 were analyzed using a repeated measures one-way ANOVA, with Bonferroni correction. Values of P<0.05 were considered significant. Statistical analyses were conducted using EZR (Saitama Medical Center, Jichi Medical University) [12], which is a graphical user interface for R (The R Foundation for Statistical Computing, version 3.0.2).
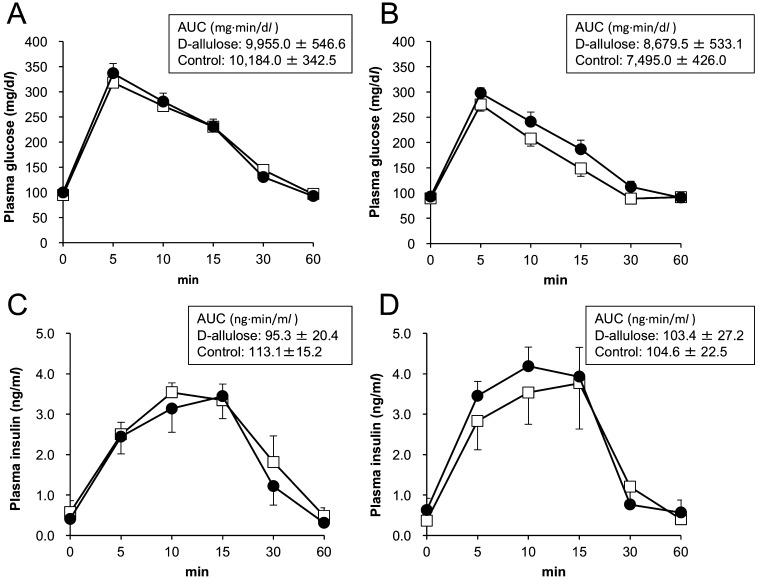
During the experimental period, the dogs consumed all the food provided, evacuated normal feces, and remained active with no clinical signs. Body weight was stable in both the groups, with no difference between the groups (body weight at week 12, 14.5 ± 1.2 and 13.6 ± 0.8 kg for the D-allulose and control groups, respectively). Hematological parameters did not change during the study period in both groups (Supplementary data). Platelet count levels in the D-allulose group were lower than those in the control group at both week 0 and 12 (P<0.05). At and after week 2, plasma total cholesterol concentrations become lower in the D-allulose group than in the control group (P<0.05) (Table 1). During the course of the experimental period, plasma triglyceride concentrations increased in the control group, whereas they remained low in the D-allulose group (Table 1). A significant difference in plasma triglyceride concentrations between groups was observed at and after week 2 (P<0.05). There were no significant differences between groups in other biochemical parameters. There were no differences in plasma glucose and insulin concentrations in the intravenous glucose tolerance test, either before or after the experimental period (Fig. 1). AUC for plasma glucose and insulin concentrations did not significantly differ between groups either before or after the experimental period (Fig. 1).
Table 1. Changes in blood chemistry parameters in dogs during the 12-week administration of D-allulose.
| Parameters | Groups | Week 0 | Week 2 | Week 4 | Week 12 | Reference range | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alanine aminotransferase (U/l) | D-allulose | 42.4 | ± | 3.6 | 30.2 | ± | 9.0 | 25.4 | ± | 4.4 b) | 32.8 | ± | 18.6 | 20–99 |
| Control | 47.8 | ± | 27.9 | 31.2 | ± | 21.1 | 27.8 | ± | 19.1 | 42.8 | ± | 32.2 | ||
| Aspartate aminotransferase (U/l) | D-allulose | 33.6 | ± | 6.7 | 28.4 | ± | 5.0 | 24.2 | ± | 1.8 | 29.2 | ± | 1.8 | |
| Control | 26.4 | ± | 5.9 | 24.4 | ± | 2.6 | 22.6 | ± | 3.2 | 27.4 | ± | 5.9 | ||
| Alkaline phosphatase (U/l) | D-allulose | 127.6 | ± | 29.9 | 150.8 | ± | 30.3 | 181.6 | ± | 29.9 | 289.8 | ± | 55.0 b) | 49–298 |
| Control | 163.2 | ± | 60.8 | 231.2 | ± | 88.7 | 289.8 | ± | 55.0 | 442.8 | ± | 197.8 | ||
| Total bilirubin (mg/dl) | D-allulose | 0.3 | ± | 0.0 | 0.1 | ± | 0.0 b) | 0.1 | ± | 0.0 b) | 0.1 | ± | 0.0 b) | |
| Control | 0.2 | ± | 0.0 | 0.1 | ± | 0.0 b) | 0.1 | ± | 0.0 b) | 0.1 | ± | 0.0 b) | ||
| Urea nitrogen (mg/dl) | D-allulose | 14.6 | ± | 3.0 | 10.0 | ± | 3.4 | 9.0 | ± | 2.0 | 10.6 | ± | 2.1 | 6–31 |
| Control | 13.0 | ± | 2.9 | 8.6 | ± | 1.1 | 9.0 | ± | 1.9 | 9.6 | ± | 1.9 | ||
| Creatinine (mg/dl) | D-allulose | 0.6 | ± | 0.1 | 0.9 | ± | 0.1 b) | 0.9 | ± | 0.1 b) | 0.9 | ± | 0.1 b) | 0.4–1.6 |
| Control | 0.5 | ± | 0.1 | 0.8 | ± | 0.1 b) | 0.8 | ± | 0.1 b) | 0.8 | ± | 0.1 b) | ||
| Total protein (g/dl) | D-allulose | 6.7 | ± | 0.3 | 6.2 | ± | 0.4 | 6.3 | ± | 0.2 | 6.2 | ± | 0.2 | 5.5–7.7 |
| Control | 6.8 | ± | 0.7 | 6.8 | ± | 0.6 | 6.7 | ± | 0.5 | 6.7 | ± | 0.5 | ||
| Albumin (g/dl) | D-allulose | 3.3 | ± | 0.1 | 3.3 | ± | 0.2 | 3.3 | ± | 0.1 | 3.1 | ± | 0.2 | 2.5–3.8 |
| Control | 3.3 | ± | 0.3 | 3.3 | ± | 0.4 | 3.3 | ± | 0.3 | 3.2 | ± | 0.3 | ||
| Total cholesterol (mg/dl) | D-allulose | 157.4 | ± | 36.6 | 121.4 | ± | 29.9 a,b) | 125.2 | ± | 22.2 a,b) | 119.0 | ± | 19.5 a,b) | 85–337 |
| Control | 163.6 | ± | 21.0 | 156.6 | ± | 19.2 | 159.4 | ± | 30.1 | 171.6 | ± | 29.6 | ||
| Triglyceride (mg/dl) | D-allulose | 27.4 | ± | 11.0 | 26.0 | ± | 13.4 a) | 23.0 | ± | 11.7 a) | 40.0 | ± | 11.6 a) | 26–137 |
| Control | 48.2 | ± | 17.6 | 84.2 | ± | 32.6 | 65.6 | ± | 16.1 | 116.4 | ± | 24.4 b) | ||
| Calcium (mg/dl) | D-allulose | 10.3 | ± | 0.4 | 9.8 | ± | 0.5 | 10.0 | ± | 0.4 | 10.1 | ± | 0.3 | 8.9–11.4 |
| Control | 10.5 | ± | 0.5 | 10.4 | ± | 0.4 | 10.3 | ± | 0.4 | 10.5 | ± | 0.5 | ||
| Inorganic phosphate (mg/dl) | D-allulose | 3.0 | ± | 0.7 | 3.9 | ± | 1.0 | 4.0 | ± | 0.4 | 4.9 | ± | 0.9 | 2.0–5.3 |
| Control | 3.2 | ± | 1.0 | 4.1 | ± | 0.3 | 4.0 | ± | 1.1 | 5.0 | ± | 0.3 | ||
| Sodium (mEq/l) | D-allulose | 149.0 | ± | 1.2 | 147.2 | ± | 2.9 | 149.0 | ± | 2.1 | 147.6 | ± | 0.5 | 141–151 |
| Control | 149.6 | ± | 1.3 | 148.6 | ± | 1.8 | 148.0 | ± | 2.0 | 146.6 | ± | 1.3 | ||
| Potassium (mEq/l) | D-allulose | 3.9 | ± | 0.4 | 4.3 | ± | 0.2 | 4.4 | ± | 0.1 | 4.4 | ± | 0.2 | 3.5–5.4 |
| Control | 4.3 | ± | 0.2 | 4.5 | ± | 0.2 | 4.6 | ± | 0.2 | 4.5 | ± | 0.1 | ||
| Chlorine (mEq/l) | D-allulose | 111.8 | ± | 1.1 | 115.4 | ± | 1.8 | 115.8 | ± | 2.2 | 116.6 | ± | 1.1 | 107–121 |
| Control | 112.2 | ± | 1.3 | 113.0 | ± | 3.3 | 112.6 | ± | 1.3 | 115.0 | ± | 2.4 | ||
Data were presented as the mean ± SD of 5 dogs. a) P<0.05 vs. the value in the control group. b) P<0.05 vs. the value at the week 0.
Fig. 1.
Plasma glucose and insulin concentrations before (A and C) and one day after termination (B and D) of D-allulose and placebo administrations. Closed circles and open squares represent data from dogs in the D-allulose and control groups, respectively. Data are shown as the mean ± SEM (n=5). There was no significant difference between groups. AUC: area under the curve.
A 12-week administration of D-allulose (0.2 g/kg once daily) in healthy dogs did not cause clinical signs. In addition, no significant differences were found in liver enzymes, renal function markers, and electrolytes between the D-allulose and control groups. Plasma alkaline phosphatase level in the D-allulose group increased within 12 weeks. Because the change in the plasma alkaline phosphatase level in the D-allulose group was within the reference range, similar increase was also observed in the control group, and plasma alanine aminotransferase and aspartate aminotransferase levels did not increase. Therefore, we concluded that a mild increase in the plasma alkaline phosphatase level does not suggest D-allulose toxicity. In rats, an 18-month administration of 1.28 g/kg D-allulose has been known to show no adverse effects [23]. The authors have previously reported that a single high dose (up to 4 g/kg) of D-allulose is well tolerated in healthy dogs [18]; however, long-term safety remains unknown. The present results suggest that long-term administration of D-allulose at the dose rate of 0.2 g/kg/day does not cause harmful effects in dogs.
In the present study, plasma total cholesterol and triglyceride concentrations were lower in the D-allulose group than in the control group. In rodents, D-allulose has been considered to exert antihyperlipidemic effects; however, it has been inconsistently reported [7], with some studies that report lower blood triglyceride levels [1, 7], whereas others that report unchanged [2] or even higher triglyceride levels [17, 20] after D-allulose administration. Furthermore, blood cholesterol has been reported to decrease [17, 20], remain unchanged [1] or increase [2] after administration of D-allulose. Baek et al. [1] have reported unchanged total cholesterol levels, with a lower low-density lipoprotein cholesterol/high-density lipoprotein cholesterol ratio. The mechanisms underlying the change in triglyceride or cholesterol levels by D-allulose remain unknown; it has been hypothesized that it may be mediated by effects on lipogenic and lipolytic enzymes [7]. The present study is the first to demonstrate the lipid-lowering effect of D-allulose in dogs, although the mechanisms underlying the effect are not yet well understood even in dogs. However, plasma triglyceride concentration in the D-allulose group was lower than that in the control group because of the elevation of plasma triglyceride concentration in the control group. Therefore, it might be inappropriate to conclude that D-allulose suppressed plasma triglyceride concentration.
The body weight of the dogs was not affected by the administration of D-allulose during the experimental period. This is consistent with the results of a previous clinical study performed with nonobese humans, which showed that a 12-week oral administration of D-allulose (5 g thrice a day, approximately 0.25 g/kg/day) did not alter body weight [5]. In contrast, D-allulose administration in rodents has resulted in significant weight loss [2, 4, 8,9,10, 17, 20, 23]. The reason for the discrepancy among species remains unclear. One possibility is that the discrepancy is caused by differences in the diet or in the adipose tissue of animals. In rodent studies [2, 4, 8,9,10, 17, 20, 23], a high-fat or high-carbohydrate diet was fed or obese animals were used. In addition, the rodents received 5% D-allulose in food (w/w), which is a higher dose than that used in the present study (approximately 1.3% of food, w/w). These differences might account for the discrepancies among studies.
In the present study, D-allulose administration did not affect the results of the glucose tolerance test. In contrast, a previous study has reported that a single administration of D-allulose (0.2 g/kg), 60 min before the intravenous glucose injection significantly lowers plasma glucose and insulin concentrations in dogs [19]. In the present study, the postadministration experiment was conducted one day after termination of D-allulose administration to evaluate the long-term cumulative effect of D-allulose on glucose metabolism. Ingested D-allulose has been shown to be well absorbed and rapidly excreted in urine, with a halflife of 57 min [21]. The liver has been found to be the only organ that accumulates D-allulose [21]. The rapid elimination of D-allulose may contribute to the absence of effects on glucose metabolism in the present study. We conclude that D-allulose has no cumulative effects on glucose metabolism in dogs.
One limitation of this study was the fluctuation of parameters in the control group. Although the control group received equivalent amounts of water with food, some biochemical parameters, such as total bilirubin, creatinine, and triglyceride, fluctuated during the 12 weeks. The reason for this fluctuation was unclear, and nutritional or environmental changes might be implicated. Despite this limitation, a parallel comparison between groups enabled the evaluation of the effects of D-allulose administration.
In conclusion, long-term administration of D-allulose at the dose rate of 0.2 g/kg/day is well tolerated in dogs. In addition, long-term administration of D-allulose showed antihyperlipidemic effects in dogs. The mechanisms underlying these antihyperlipidemic effects of D-allulose remain to be elucidated. Based on the results of the present study, clinical investigation of D-allulose administration in dogs with hyperlipidemia, diabetes mellitus, and obesity may be warranted. These further studies can demonstrate the clinical efficacy of D-allulose administration in dogs.
Supplementary
REFERENCES
- 1.Baek S. H., Park S. J., Lee H. G.2010. D-psicose, a sweet monosaccharide, ameliorate hyperglycemia, and dyslipidemia in C57BL/6J db/db mice. J. Food Sci. 75: H49–H53. doi: 10.1111/j.1750-3841.2009.01434.x [DOI] [PubMed] [Google Scholar]
- 2.Chung Y. M., Hyun Lee J., Youl Kim D., Hwang S. H., Hong Y. H., Kim S. B., Jin Lee S., Hye Park C.2012. Dietary D-psicose reduced visceral fat mass in high-fat diet-induced obese rats. J. Food Sci. 77: H53–H58. doi: 10.1111/j.1750-3841.2011.02571.x [DOI] [PubMed] [Google Scholar]
- 3.Guptill L., Glickman L., Glickman N.2003. Time trends and risk factors for diabetes mellitus in dogs: analysis of veterinary medical data base records (1970−1999). Vet. J. 165: 240–247. doi: 10.1016/S1090-0233(02)00242-3 [DOI] [PubMed] [Google Scholar]
- 4.Han Y., Han H. J., Kim A. H., Choi J. Y., Cho S. J., Park Y. B., Jung U. J., Choi M. S.2016. d-Allulose supplementation normalized the body weight and fat-pad mass in diet-induced obese mice via the regulation of lipid metabolism under isocaloric fed condition. Mol. Nutr. Food Res. 60: 1695–1706. doi: 10.1002/mnfr.201500771 [DOI] [PubMed] [Google Scholar]
- 5.Hayashi N., Iida T., Yamada T., Okuma K., Takehara I., Yamamoto T., Yamada K., Tokuda M.2010. Study on the postprandial blood glucose suppression effect of D-psicose in borderline diabetes and the safety of long-term ingestion by normal human subjects. Biosci. Biotechnol. Biochem. 74: 510–519. doi: 10.1271/bbb.90707 [DOI] [PubMed] [Google Scholar]
- 6.Hishiike T., Ogawa M., Hayakawa S., Nakajima D., O’Charoen S., Ooshima H., Sun Y.2013. Transepithelial transports of rare sugar D-psicose in human intestine. J. Agric. Food Chem. 61: 7381–7386. doi: 10.1021/jf401449m [DOI] [PubMed] [Google Scholar]
- 7.Hossain A., Yamaguchi F., Matsuo T., Tsukamoto I., Toyoda Y., Ogawa M., Nagata Y., Tokuda M.2015. Rare sugar D-allulose: Potential role and therapeutic monitoring in maintaining obesity and type 2 diabetes mellitus. Pharmacol. Ther. 155: 49–59. doi: 10.1016/j.pharmthera.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 8.Hossain A., Yamaguchi F., Matsunaga T., Hirata Y., Kamitori K., Dong Y., Sui L., Tsukamoto I., Ueno M., Tokuda M.2012. Rare sugar D-psicose protects pancreas β-islets and thus improves insulin resistance in OLETF rats. Biochem. Biophys. Res. Commun. 425: 717–723. doi: 10.1016/j.bbrc.2012.07.135 [DOI] [PubMed] [Google Scholar]
- 9.Hossain A., Yamaguchi F., Hirose K., Matsunaga T., Sui L., Hirata Y., Noguchi C., Katagi A., Kamitori K., Dong Y., Tsukamoto I., Tokuda M.2015. Rare sugar D-psicose prevents progression and development of diabetes in T2DM model Otsuka Long-Evans Tokushima Fatty rats. Drug Des. Devel. Ther. 9: 525–535. doi: 10.2147/DDDT.S71289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hossain M. A., Kitagaki S., Nakano D., Nishiyama A., Funamoto Y., Matsunaga T., Tsukamoto I., Yamaguchi F., Kamitori K., Dong Y., Hirata Y., Murao K., Toyoda Y., Tokuda M.2011. Rare sugar D-psicose improves insulin sensitivity and glucose tolerance in type 2 diabetes Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biochem. Biophys. Res. Commun. 405: 7–12. doi: 10.1016/j.bbrc.2010.12.091 [DOI] [PubMed] [Google Scholar]
- 11.Iida T., Kishimoto Y., Yoshikawa Y., Hayashi N., Okuma K., Tohi M., Yagi K., Matsuo T., Izumori K.2008. Acute D-psicose administration decreases the glycemic responses to an oral maltodextrin tolerance test in normal adults. J. Nutr. Sci. Vitaminol. (Tokyo) 54: 511–514. doi: 10.3177/jnsv.54.511 [DOI] [PubMed] [Google Scholar]
- 12.Kanda Y.2013. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48: 452–458. doi: 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuo T., Izumori K.2006. Effects of dietary D-psicose on diurnal variation in plasma glucose and insulin concentrations of rats. Biosci. Biotechnol. Biochem. 70: 2081–2085. doi: 10.1271/bbb.60036 [DOI] [PubMed] [Google Scholar]
- 14.Matsuo T., Ishii R., Shirai Y.2012. The 90-day oral toxicity of d-psicose in male Wistar rats. J. Clin. Biochem. Nutr. 50: 158–161. doi: 10.3164/jcbn.11-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuo T., Tanaka T., Hashiguchi M., Izumori K., Suzuki H.2002. Effects of oral acute administration and subchronic feeding of several levels of D-psicose in rats. J. Nutr. Sci. Vitaminol. (Tokyo) 48: 512–516. doi: 10.3177/jnsv.48.512 [DOI] [PubMed] [Google Scholar]
- 16.McGreevy P. D., Thomson P. C., Pride C., Fawcett A., Grassi T., Jones B.2005. Prevalence of obesity in dogs examined by Australian veterinary practices and the risk factors involved. Vet. Rec. 156: 695–702. doi: 10.1136/vr.156.22.695 [DOI] [PubMed] [Google Scholar]
- 17.Nagata Y., Kanasaki A., Tamaru S., Tanaka K.2015. D-psicose, an epimer of D-fructose, favorably alters lipid metabolism in Sprague-Dawley rats. J. Agric. Food Chem. 63: 3168–3176. doi: 10.1021/jf502535p [DOI] [PubMed] [Google Scholar]
- 18.Nishii N., Nomizo T., Takashima S., Matsubara T., Tokuda M., Kitagawa H.2016. Single oral dose safety of D-allulose in dogs. J. Vet. Med. Sci. 78: 1079–1083. doi: 10.1292/jvms.15-0676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishii N., Nomizo T., Takashima S., Matsubara T., Tokuda M., Kitagawa H.2016. Effects of D-allulose on glucose metabolism after the administration of sugar or food in healthy dogs. J. Vet. Med. Sci. 78: 1657–1662. doi: 10.1292/jvms.16-0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochiai M., Nakanishi Y., Yamada T., Iida T., Matsuo T.2013. Inhibition by dietary D-psicose of body fat accumulation in adult rats fed a high-sucrose diet. Biosci. Biotechnol. Biochem. 77: 1123–1126. doi: 10.1271/bbb.130019 [DOI] [PubMed] [Google Scholar]
- 21.Tsukamoto I., Hossain A., Yamaguchi F., Hirata Y., Dong Y., Kamitori K., Sui L., Nonaka M., Ueno M., Nishimoto K., Suda H., Morimoto K., Shimonishi T., Saito M., Song T., Konishi R., Tokuda M.2014. Intestinal absorption, organ distribution, and urinary excretion of the rare sugar D-psicose. Drug Des. Devel. Ther. 8: 1955–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xenoulis P. G., Steiner J. M.2015. Canine hyperlipidaemia. J. Small Anim. Pract. 56: 595–605. doi: 10.1111/jsap.12396 [DOI] [PubMed] [Google Scholar]
- 23.Yagi K., Matsuo T.2009. The study on long-term toxicity of d-psicose in rats. J. Clin. Biochem. Nutr. 45: 271–277. doi: 10.3164/jcbn.08-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.